Antioxidant and Antibacterial Activities of a Purified Polysaccharide Extracted from Ceratonia siliqua L. and Its Involvement in the Enhancement Performance of Whipped Cream
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Carob Kibble Water-Soluble Polysaccharide (KWSP)
2.3. Physico-Chemical Analysis of KWSP
2.4. Water and Oil Holding Capacities of KWSP
2.5. Emulsifying Properties of KWSP
2.6. Spectroscopic Analysis of KWSP
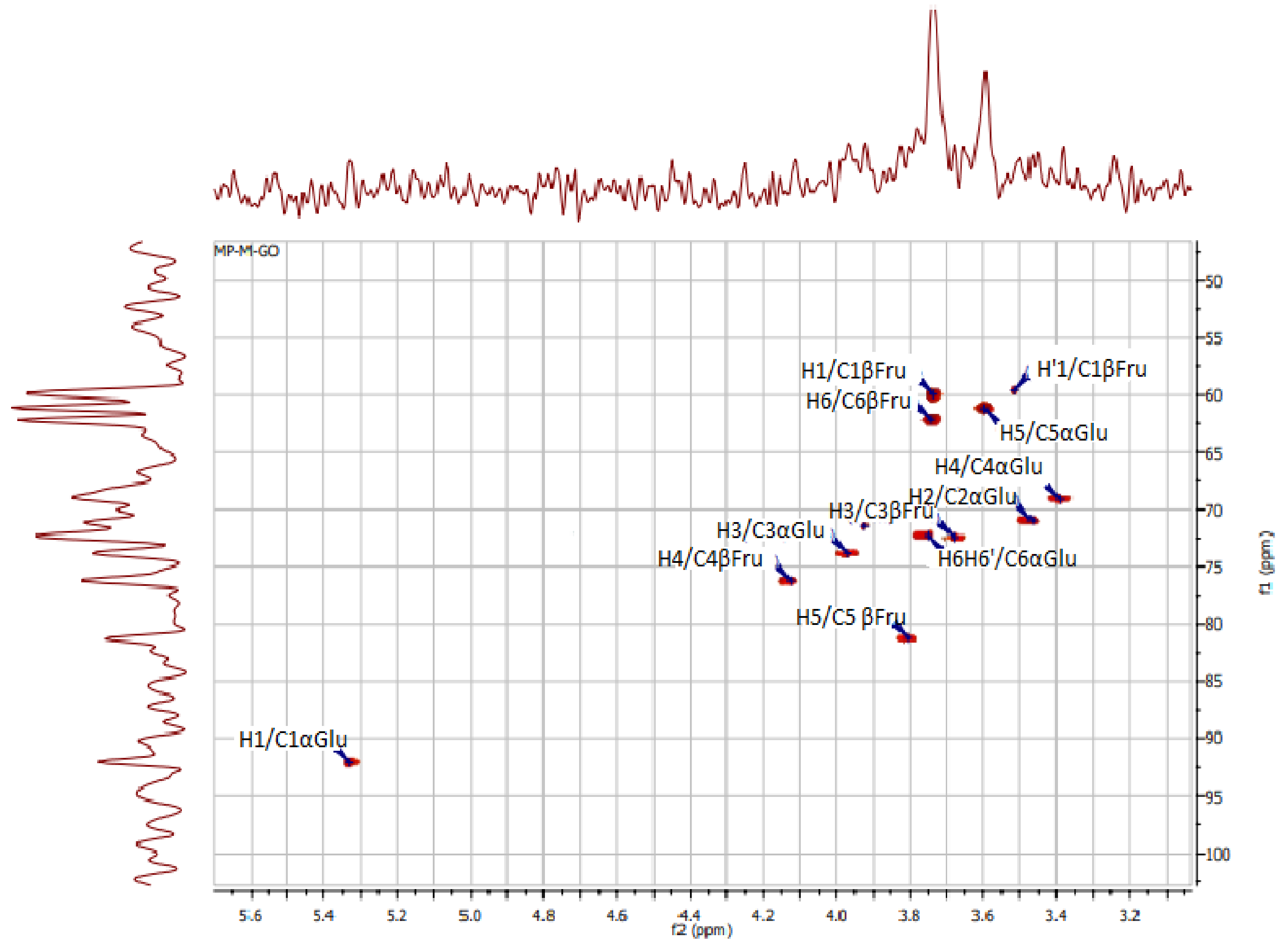
2.6.1. Nuclear Magnetic Resonance (NMR) Analysis
2.6.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis of KWSP
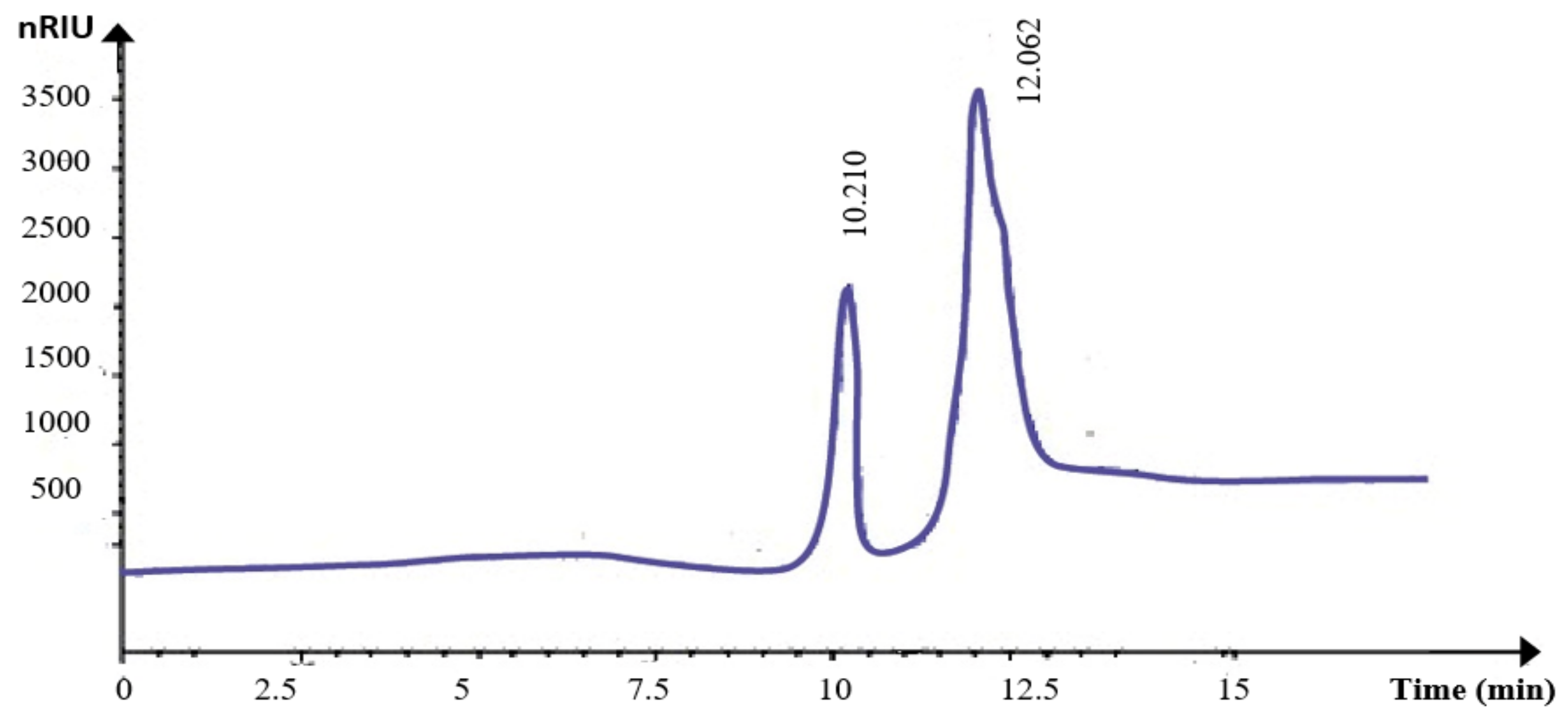
2.6.3. HPLC and TLC Analysis of KWSP
2.6.4. Average Molecular Weight (Mw) of KWSP
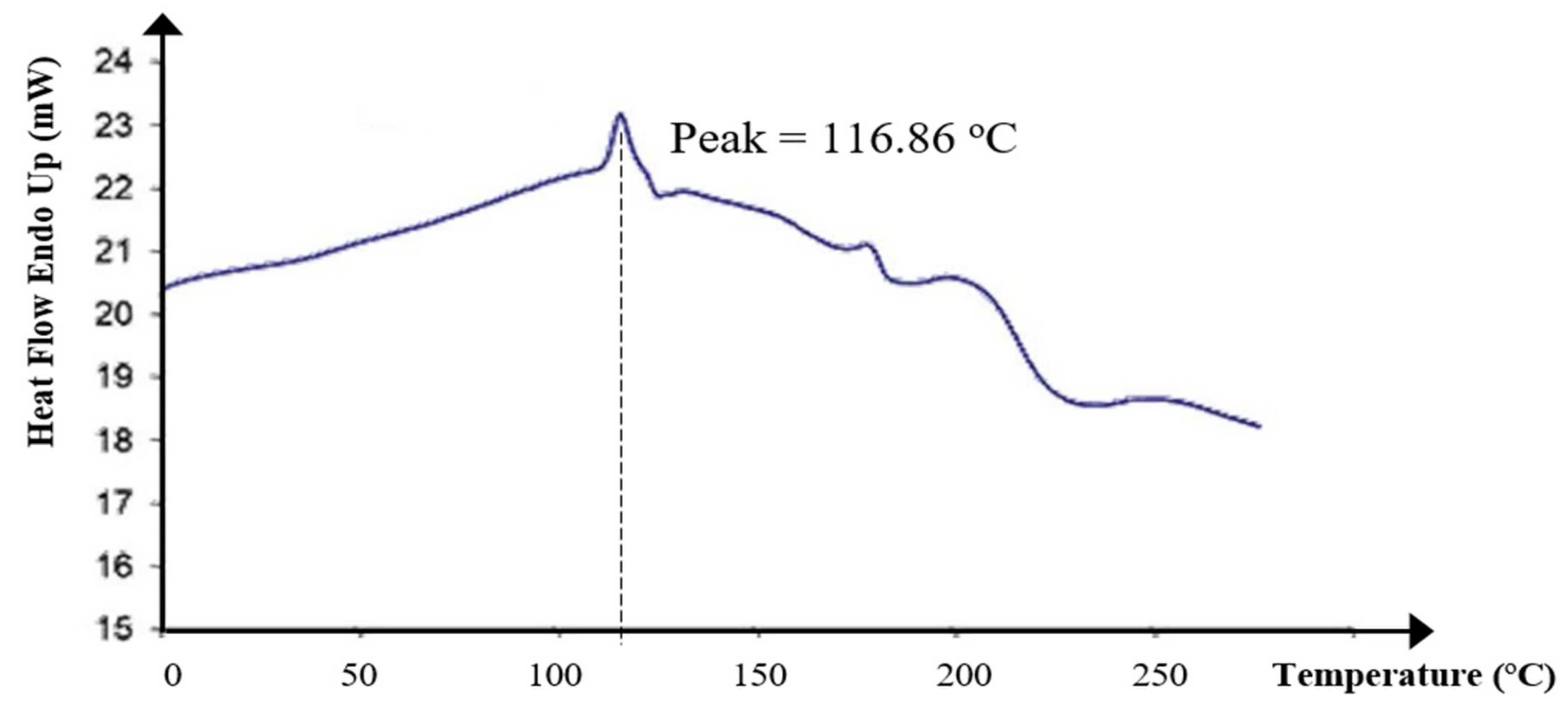
2.6.5. Differential Scanning Calorimetry (DSC) Measurement
2.7. Antioxidant Activities
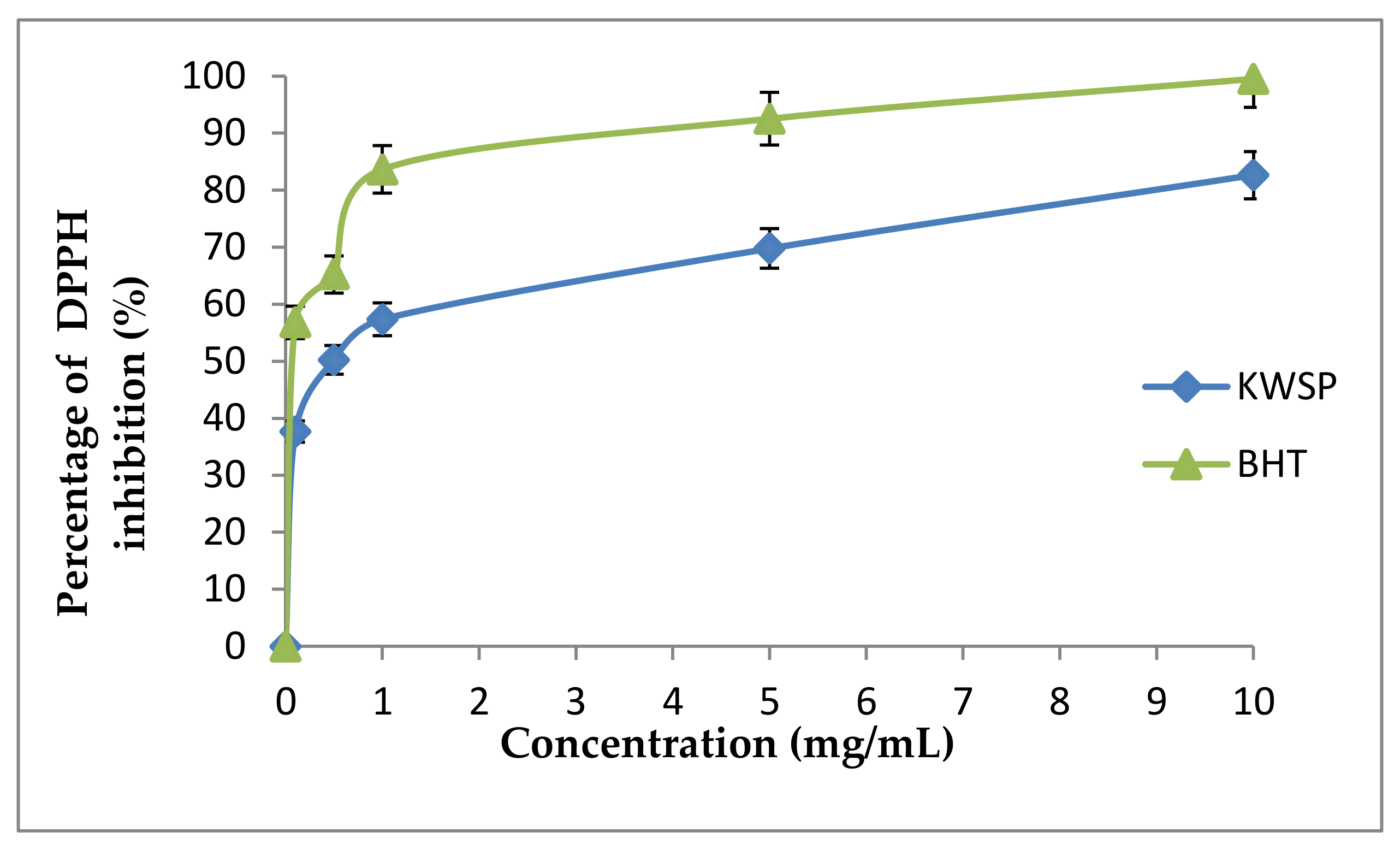
2.7.1. DPPH Radical-Scavenging Assay
2.7.2. ABTS⋅+ Radical Scavenging Activity
2.7.3. Total Antioxidant Activity
2.8. Antibacterial Activity of KWSP
2.9. Incorporation of KWSP in Whipped Cream
2.9.1. Antioxidant Activities of Whipped Cream
- Reducing power assay: The potential of peptidic fractions of ZBPH-Z to reduce iron (III) was defined, as clarified by the method of Yildirim et al. [9]. An amount of 1 mL aliquot of peptidic fractions at concentrations from 1 to 5 mg/mL was added to 2.5 mL 0.2 M Na phosphate buffer (Sigma Chemical) (pH 6.6) and 2.5 mL of 1% (w/v) potassium ferricyanide solution (Sigma Chemical). These mixtures were incubated for 30 min at 50 °C. Subsequently, they were mixed with 2.5 mL 10% (w/v) of trichloro acetic acid (TCA), and the reaction mixture was then centrifuged for 10 min at 6000× g. Finally, 2.5 mL of the supernatant solution from each sample mixture was added to 2.5 mL distilled water and 0.5 mL 0.1% (w/v) ferric chloride. After 10 min, the absorbance was determined at 700 nm. The higher absorbance of the reaction mixture suggested a higher reducing power. Control was carried out following the same method, but distilled water was used instead of the sample. The butylated hydroxyanisole (BHA) was used as a positive control;
- The ferrous chelating activity of KWSP: 15 mg/mL was determined following the method of Dinis et al. [9]. An amount of 0.5 mL of sample and 1.6 mL of distilled water were added to 0.05 mL of FeCl2 (2 mM), following the addition of 0.1 mL of ferrozine (5 mM) after15 min. After 10 min, the Fe2+-ferrozine complex absorbance was defined at 562 nm. The Ethylenediaminetetraacetic acid (EDTA) was used as a reference. The chelating antioxidant activity for Fe2+ was measured in terms of Equation (5):
2.9.2. Effect of KWSP on K232 and K270 Values
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of KWSP
3.2. Functional Properties of KWSP
3.2.1. Water-Holding and Oil-Binding Capacities of KWSP
3.2.2. Emulsification Capacity of KWSP
3.3. Spectroscopic Analysis Results of KWSP
3.3.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.3.2. FT-IR Spectrometric Analysis of KWSP
3.3.3. HPLC Analysis and TLC Analysis of KWSP
3.3.4. Average Molecular Weight (Mw) of KWSP
3.3.5. DSC Measurement
3.4. Antioxidant Activities Results
3.4.1. DPPH Radical-Scavenging Experiment
3.4.2. ABTS⋅+ Radical Scavenging Activity
3.4.3. Total Antioxidant Activity
3.5. Antibacterial Activity Results
3.6. Incorporation of KWSP in Whipped Cream Results
3.6.1. Antioxidant Properties of KWSP in Whipped Cream
3.6.2. Amelioration of K232 and K270
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Bourhia, M.; Bouothmany, K.; Bakrim, H.; Hadrach, S.; Salamatullah, A.M.; Alzahrani, A.; Khalil Alyahya, H.; Albadr, N.A.; Gmouh, S.; Laglaoui, A.; et al. Chemical Profiling, Antioxidant, Antiproliferative, and Antibacterial Potentials of Chemically Characterized Extract of Citrullus colocynthis L. Seeds. Separations 2021, 8, 114. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Gramia, D.; Amri, M.; Etod, B.; El-benna, J.; Sebai, H.; Marzouki, L. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract. J. Biomed. Pharmacother. 2017, 93, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Batlle, I.; Tous, J. Carob Tree. Ceratonia siliqua L.; Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetic Resources Institute: Rome, Italy, 1997; Available online: https://www.bioversityinternational.org/fileadmin/_migrated/uploads/tx_news/Carob_tree_Ceratonia_siliqua_L._347.pdf (accessed on 16 April 2022).
- Roseiro, L.B.; Duarte, L.C.; Oliveira, D.L.; Roque, R.; Bernardo-Gil, M.G.; Martins, A.I.; Sepúlveda, C.; Almeida, J.; Meireles, M.; Gírio, F.M.A. Pilar Rauter, Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. J. Ind. Crops Prod. 2013, 47, 132–138. [Google Scholar] [CrossRef]
- De la Fuente-Fernández, M.; González-Hedström, D.; Amor, S.; Tejera-Muñoz, A.; Fernández, N.; Monge, L.; Almodóvar, P.; Andrés-Delgado, L.; Santamaría, L.; Prodanov, M.; et al. Supplementation with a Carob (Ceratonia siliqua L.) Fruit Extract Attenuates the Cardiometabolic Alterations Associated with Metabolic Syndrome in Mice. Antioxidants 2020, 9, 339. [Google Scholar]
- Emilia Brassesco, M.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob bean (Ceratonia siliqua L.): A new perspective for functional food. J. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices, Antioxidant activity and health effects. J. Funct. Foods 2015, 78, 820–897. [Google Scholar] [CrossRef]
- Abidar, S.; Stefan Boiangiu, R.; Stefan Boiangiu, G.; Todirascu-Ciornea, E.; Amakran, A.; Cioanca, O.; Cioanca, L.; Nhiri, M. The Aqueous Extract from Ceratonia siliqua Leaves Protects against 6-Hydroxydopamine in Zebrafish: Understanding the Underlying Mechanism. Antioxidants 2020, 9, 304. [Google Scholar] [CrossRef] [Green Version]
- Hamzaoui, A.; Ghariani, M.; Sellem, I.; Hamdi, M.; Feki, A.; Jaballi, I.; Nasri, M.; Ben Amara, I. Extraction, characterization and biological properties of polysaccharide derived from green seaweed “Chaetomorpha linum” and its potential application in Tunisian beef sausages. Int. J. Biol. Macromol. 2020, 148, 1156–1168. [Google Scholar] [CrossRef]
- Sila, A.; Bayar, N.; Ghazala, I.; Bougatef, A.; Ellouze-Ghorbel, R.; Ellouze-Chaabouni, S. Water-soluble polysaccharides from agro-industrial by-products: Functional and biological Properties. Int. J. Biol. Macromol. 2014, 69, 236–243. [Google Scholar] [CrossRef]
- Lakkab, I.; El Hajaji, H.; Lachkar, N.; Lefter, R.; Ciobica, A.; El Balia, B.; Lachkar, M. Ceratonia siliqua L. seed peels: Phytochemical profile, antioxidant activity, and effect on mood disorders. J. Funct. Foods 2019, 54, 457–465. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Carbas, B.; Salinas, M.V.; Serrano, C.; Passarinho, J.A.; Puppo, M.C.; Ricardo, C.P.; Brites, C. Chemical composition and antioxidant activity of commercial flours from Ceratonia siliqua and Prosopis spp. J. Food Meas. Charact. 2019, 13, 305–311. [Google Scholar] [CrossRef]
- Benković, M.; Belščak-Cvitanović, A.; Bauman, I.; Komes, D.; Srečec, S. Flow properties and chemical composition of carob (Ceratonia siliqua L.) flours as related to particle size and seed presence. Food Res. Int. 2017, 100, 211–218. [Google Scholar] [CrossRef]
- Liu, G.; Xu, S.; Chen, L. Chemical composition and bioactivities of a water-soluble polysaccharide from the endodermis of shaddock. Int. J. Biol. Macromol. 2012, 51, 763–766. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Method of Analysis Gaithersburg; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef]
- Slima, S.B.; Ktari, N.; Trabelsi, I.; Moussa, H.; Makni, I.; Salah, R.B. Purification, characterization and antioxidant properties of a novel polysaccharide extracted from Sorghum bicolor (L.) seeds in sausage. Int. J. Biol. Macromol. 2018, 106, 168–178. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Torres, C.A.; Cruz, M.; Sousa, I.; Melo, M.J.; Reis, M.A. Fucose-containing exopolysaccharide produced by the newly isolated Enterobacter strain A47 DSM 23139. Carbohydr. Polym. 2011, 83, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Ktari, N.; Bkhairia, I.; Nasri, M.; Salah, R.B. Structure and biological activities of polysaccharide purified from Senegrain seed. Int. J. Biol. Macromol. 2020, 144, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ezernieks, V.; Rochfort, S.; Cocks, B. Comparison of methylation methods for fatty acid analysis of milk fat. Food Chem. 2018, 261, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, I.B.; Márquez-Ruiz, G.; Holgado, F.; Sonda, A.; Skalicka-Wozniak, K.; Bouaziz, M. RP-UHPLC–DAD-QTOF-MS as a Powerful Tool of Oleuropein and Ligstroside Characterization in Olive-Leaf Extract and Their Contribution to the Improved Performance of Refined Olive-Pomace Oil during Heating. J. Agric. Food Chem. 2020, 68, 12039–12047. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef]
- El Gerssifi, M. Les défauts des produits de pâtisserie et biscuiterie au cours du stockage: La prévention par la formulation. Ind. Aliment. Agric. 1998, 115, 82–88. [Google Scholar]
- Petkova, N.; Petrova, I.; Ivanov, I.; Mihov, R.; Hadjikinova, R.; Ognyanov, M.; Nikolova, V. Nutritional and antioxidant potential of carob (Ceratonia siliqua) flour and evaluation of functional properties of its polysaccharide fraction. J. Pharm. Sci. Res. 2017, 9, 2189–2195. [Google Scholar]
- Sciarini, L.S.; Maldonado, F.; Ribotta, P.D.; Pérez, G.T.; León, A.E. Chemical composition and functional properties of Gleditsia triacanthos gum. Food Hydrocoll. 2009, 23, 306–313. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Barber, X.; Pérez-Álvarez, J.A.; Fernández-López, J. Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Ind. Crops Prod. 2015, 69, 472–479. [Google Scholar] [CrossRef]
- Ktari, N.; Feki, A.; Trabelsi, I.; Triki, M.; Maalej, H.; Slima, S.B.; Salah, R.B. Structure, functional and antioxidant properties in Tunisian beef sausage of a novel polysaccharide from Trigonella foenum-graecum seeds. Int. J. Biol. Macromol. 2017, 98, 169–181. [Google Scholar] [CrossRef]
- Sun, M.; Sun, Y.; Li, Y.; Liu, Y.; Liang, J.; Zhang, Z. Physical properties and antidiabetic potential of a novel galactomannan from seeds of Gleditsia japonica var delavayi. J. Funct. Foods 2018, 46, 546–555. [Google Scholar] [CrossRef]
- De Andrade Vieira, É.; Alcântara, M.A.; Dos Santos, N.A.; Gondim, A.D.; Iacomini, M.; Mellinger, C.; de Magalhães Cordeiro, A.M.T. Mucilages of cacti from Brazilian biodiversity: Extraction, physicochemical and technological properties. Food Chem. 2021, 346, 128892. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, X.; Gu, X.; Gong, S.; Wu, J.; Wang, Z.; Wang, S. Emulsifying properties of pectic polysaccharides obtained by sequential extraction from black tomato pomace. Food Hydrocoll. 2020, 100, 105454. [Google Scholar] [CrossRef]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts Magn. Reson. Part A Educ. J. 2003, 19, 1–19. [Google Scholar] [CrossRef]
- Medlej, M.K.; Cherri, B.; Nasser, G.; Zaviska, F.; Hijazi, A.; Li, S.; Pochat-Bohatier, C. Optimization of polysaccharides extraction from a wild species of Ornithogalum combining ultrasound and maceration and their anti-oxidant properties. Int. J. Biol. Macromol. 2020, 161, 958–968. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wu, F.J.; Du, L.; Li, G.Y.; Takahashi, K.; Xue, Y.; Xue, C.H. Effects of polysaccharides from abalone (Haliotis discus hannai Ino) on HepG2 cell proliferation. Int. J. Biol. Macromol. 2014, 66, 354–361. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W.; Yu, J.; Zou, S.; Wang, J.; Yao, W.; Gao, X. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr. Polym. 2013, 98, 8–16. [Google Scholar] [CrossRef]
- Park, F.S. Application of IR Spectroscopy in Biochemistry: Biology and Medicine; Plenum Press: New York, NY, USA, 1971; pp. 100–140. [Google Scholar]
- Krichen, F.; Karoud, W.; Sila, A.; Abdelmalek, B.E.; Ghorbel, R.; Ellouz-Chaabouni, S.; Bougatef, A. Extraction, characterization and antimicrobial activity of sulfated polysaccharides from fish skins. Int. J. Biol. Macromol. 2015, 75, 283–289. [Google Scholar] [CrossRef]
- Ahmad, M.M. Characterization and antioxidant activities of polysaccharides extracted from flageolet bean pods waste. Curr. Res. Green Sustain. Chem. 2021, 4, 100154. [Google Scholar] [CrossRef]
- Boual, Z.; Pierre, G.; Delattre, C.; Benaoun, F.; Petit, E.; Gardarin, C.; El Hadj, M.D.O. Mediterranean semi-arid plant Astragalus armatus as a source of bioactive galactomannan. Bioact. Carbohydr. Diet. Fibre 2015, 5, 10–18. [Google Scholar] [CrossRef]
- Mutaillifu, P.; Bobakulov, K.; Abuduwaili, A.; Huojiaaihemaiti, H.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020, 144, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Mzoughi, Z.; Majdoub, H. Pectic polysaccharides from edible halophytes: Insight on extraction processes, structural characterizations and immunomodulatory potentials. Int. J. Biol. Macromol. 2021, 173, 554–579. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, W.; Wen, P.; Shen, M.; Li, H.; Ren, Y.; Xie, J. Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food Hydrocoll. 2020, 100, 105412. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.T.; Zheng, W.; Han, X.X.; Jiang, Y.H.; Hu, P.L.; Shi, L.E. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J. Funct. Foods 2017, 38, 273–279. [Google Scholar] [CrossRef]
- Walayat, N.; Liu, J.; Nawaz, A.; Aadil, R.M.; López-Pedrouso, M.; Lorenzo, J.M. Role of Food Hydrocolloids as Antioxidants along with Modern Processing Techniques on the Surimi Protein Gel Textural Properties, Developments, Limitation and Future Perspectives. Antioxidants 2022, 11, 486. [Google Scholar] [CrossRef]
- Puangmanee, S.; Hayakawa, S.; Sun, Y.; Ogawa, M. Application of whey protein isolate glycated with rare sugars to ice cream. Food Sci. Technol. Res. 2008, 14, 457. [Google Scholar] [CrossRef] [Green Version]
- Bali, O.; Ammar, I.; Ennouri, M.; Attia, H. Physicochemical characteristics and storage stability of clarified butter fat «smen» produced from pasteurized and non-pasteurized milk. J. Pharm. Health Sci. 2017, 5, 195–205. [Google Scholar]







| Parameters | Values |
|---|---|
| Physico-Chemical Properties | |
| Yield (%) | 8.30 ± 0.86 1 |
| pH | 6.59 ± 0.1 1 |
| Moisture (%) | 12.00 ± 0.13 1 |
| Dry matter (%) | 88.00 ± 0.13 1 |
| Ash (%) | 3.66 ± 0.8 1 |
| Proteins (%) | 9.13 ± 0.06 1 |
| Fat (%) | 0.00 |
| Polysaccharides | 86.68 ± 0.02 1 |
| Average molecular weight (kDa) | 65.00 |
| Wa | 0.43 |
| Color | |
| a* | 7.32 |
| b* | 11.71 |
| L | 45.40 |
| FunctionalProperties | |
| WHC | 3.14 ± 0.50 1 |
| OHC | 0.87 ± 0.02 1 |
| Hours | Emulsion Properties 1 |
|---|---|
| 1 | 94.73 ± 0.06 |
| 24 | 72.00 ± 0.08 |
| 168 | 71.00 ± 0.01 |
| KWSP | OP%US Medlej et al. [36] | ||
|---|---|---|---|
| C13 (ppm) | 1H (ppm) | C13 (ppm) | 1H (ppm) |
| C1βFru: 60.05 | H1βFru: H1 3.82/H1′ 3.88 | C1βFru: [59.00 61.48] | H1βFru: [3.7 3.8] |
| C2βFru: 103.63 | - | C2βFru: 103.11 | - |
| C3βFru: 72.50 | H3βFru: 4.22 | C3βFru: [79.05 82.50] | H3βFru: 4.27 |
| C4βFru: 76.24 | H4βFru: 4.13 | C4βFru: [57.3 77.77] | H4βFru: 4.13 |
| C5βFru: 81.19 | H5βFru: 4.08 | C5βFru: 81.20 | H5βFru: 3.89 |
| C6βFru: 62.17 | H6βFru: 3.68 | C6βFru: 73.30–75.15 | H6βFru: [3.68 3.89] |
| C1αGlu: 92.13 | H1αGlu: 5.42 | C1αGlu: 92.15 | H1αGlu: 5.45 |
| C2αGlu: 71.02 | H2αGlu: 3.47 | C2αGlu: 71.18 | H2αGlu: 3.60 |
| C3αGlu: 73.93 | H3αGlu: 4.00 | C3αGlu: 72.52 | H3αGlu: 3.80 |
| C4αGlu: 69.16 | H4αGlu: 3.45 | C4αGlu: 69.32 | H4αGlu: 3.50 |
| C5αGlu: 61.29 | H5αGlu: 3.60 | C5αGlu: 71.67 | H5αGlu: 4.00 |
| C6αGlu: 72.34 | H6αGlu: H6 3.74/H6′ 3.76 | C6αGlu: 61.9 | H6αGlu: H6 3.70/H6′ 3.80 |
| Bacterial Strains | Inhibition Zone (mm) 1 | |
|---|---|---|
| KWSP | Gentamicin (Antibiotic) | |
| S. aureus (ATCC 6538) | 8.20 ± 0.25 | 17.00 ± 1.00 |
| L. monocytogenes (ATCCC 19117) | 8.90 ± 0.63 | 15.00 ± 0.30 |
| S. enterica (ATCC 14028) | 10.00 ± 0.46 | 21.00 ± 0.80 |
| P. aeruginosa (ATCC 4912) | no inhibition | 20.00 ± 0.20 |
| Antioxidant Properties | Sample Results of Control | Sample Results with KWSP | Sample Mean Control | Sample Mean KWSP | Sample SD Control | Sample SD KWSP | T-Value | p-Value 1 |
|---|---|---|---|---|---|---|---|---|
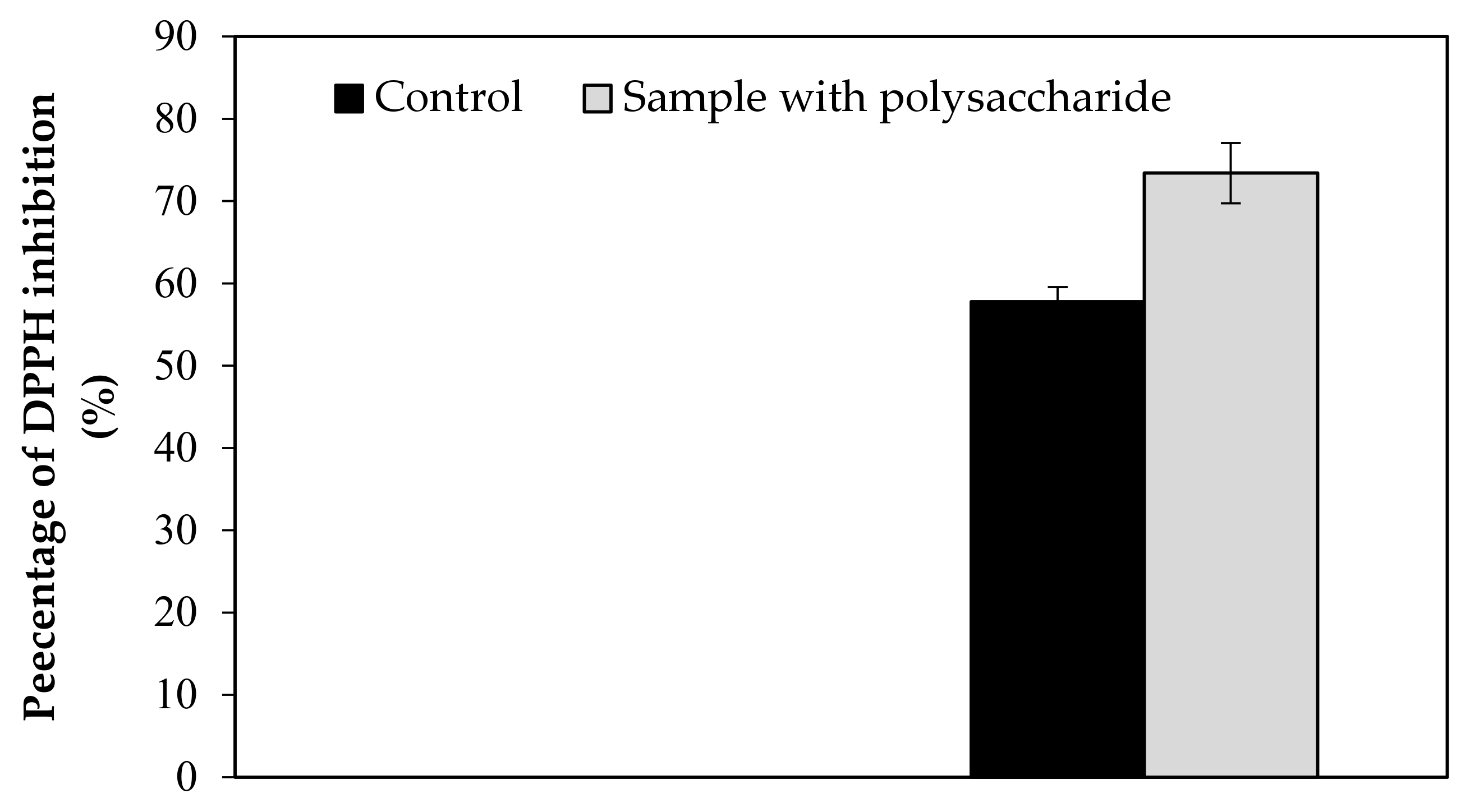
| DPPH | 57.79 | 73.37 | 57.94 | 73.39 | 4.98 | 6.62 | 3.23 | 0.016 |
| 63.00 | 66.78 | |||||||
| 53.04 | 80.02 | |||||||
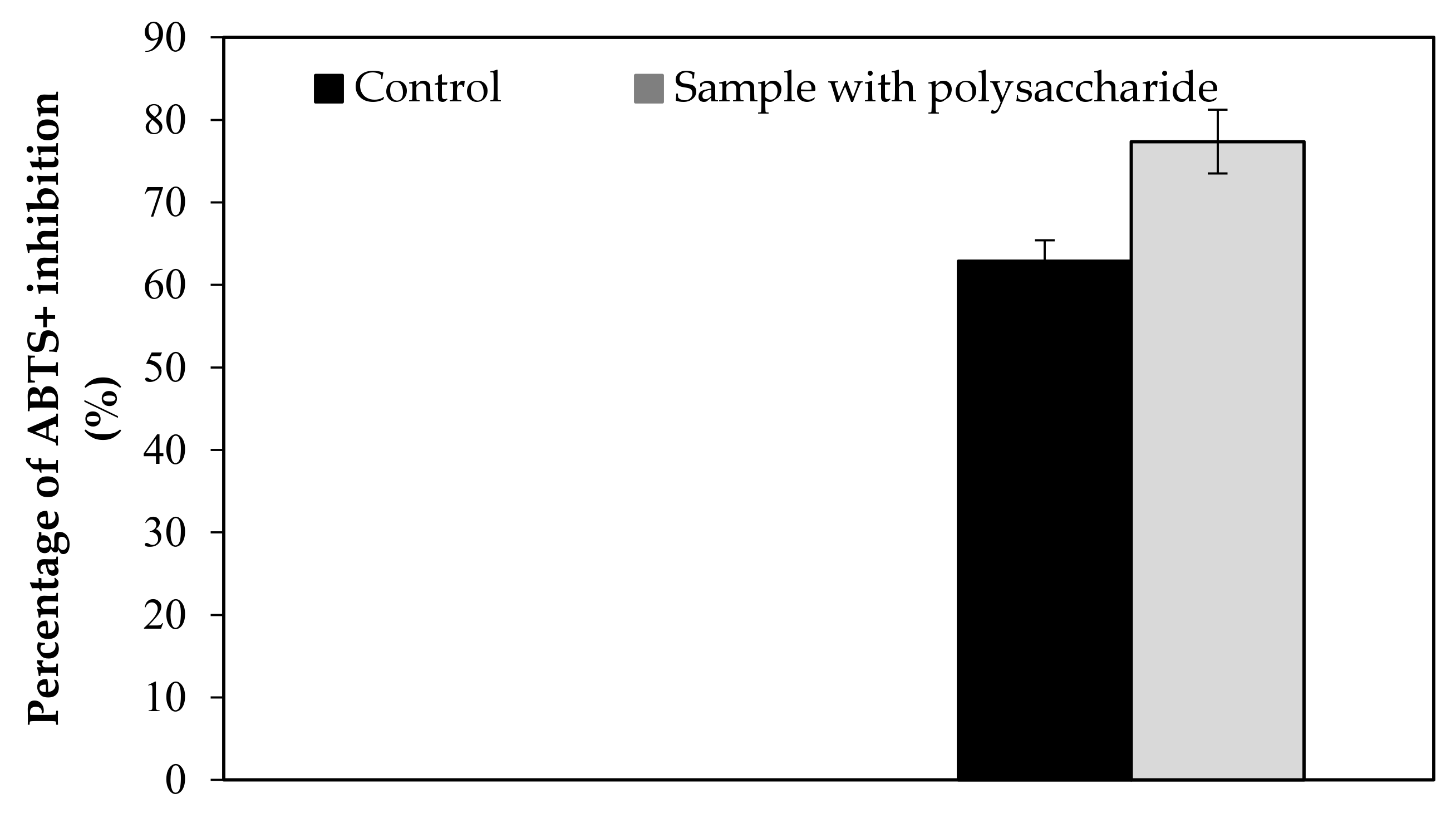
| ABTS+ | 62.89 | 77.37 | 62.90 | 77.39 | 6.30 | 7.70 | 2.52 | 0.033 |
| 69.20 | 85.10 | |||||||
| 56.60 | 69.70 | |||||||
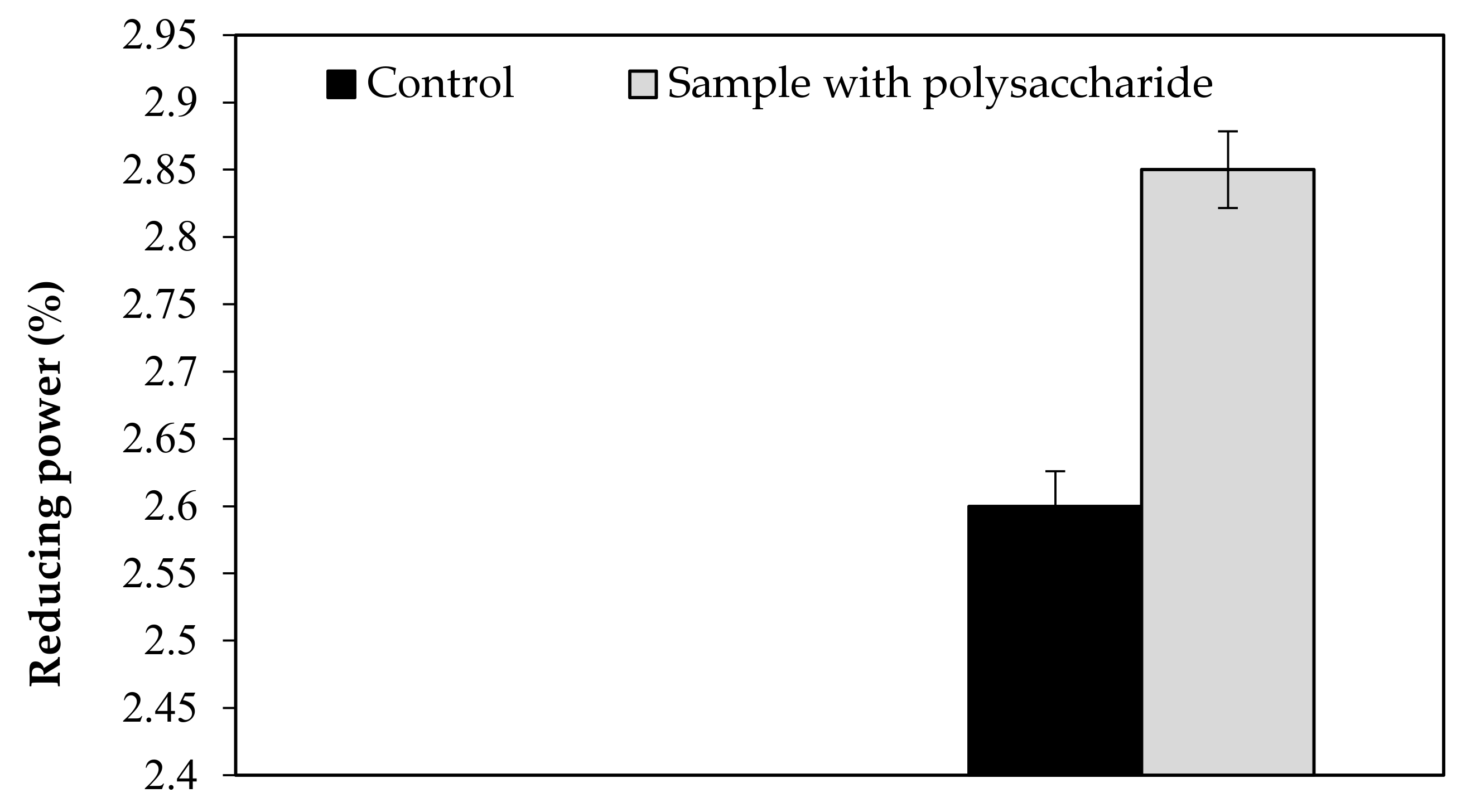
| Reducing power | 2.60 | 2.85 | 2.597 | 2.843 | 0.105 | 0.120 | 2.78 | 0.025 |
| 2.70 | 2.73 | |||||||
| 2.49 | 2.96 | |||||||
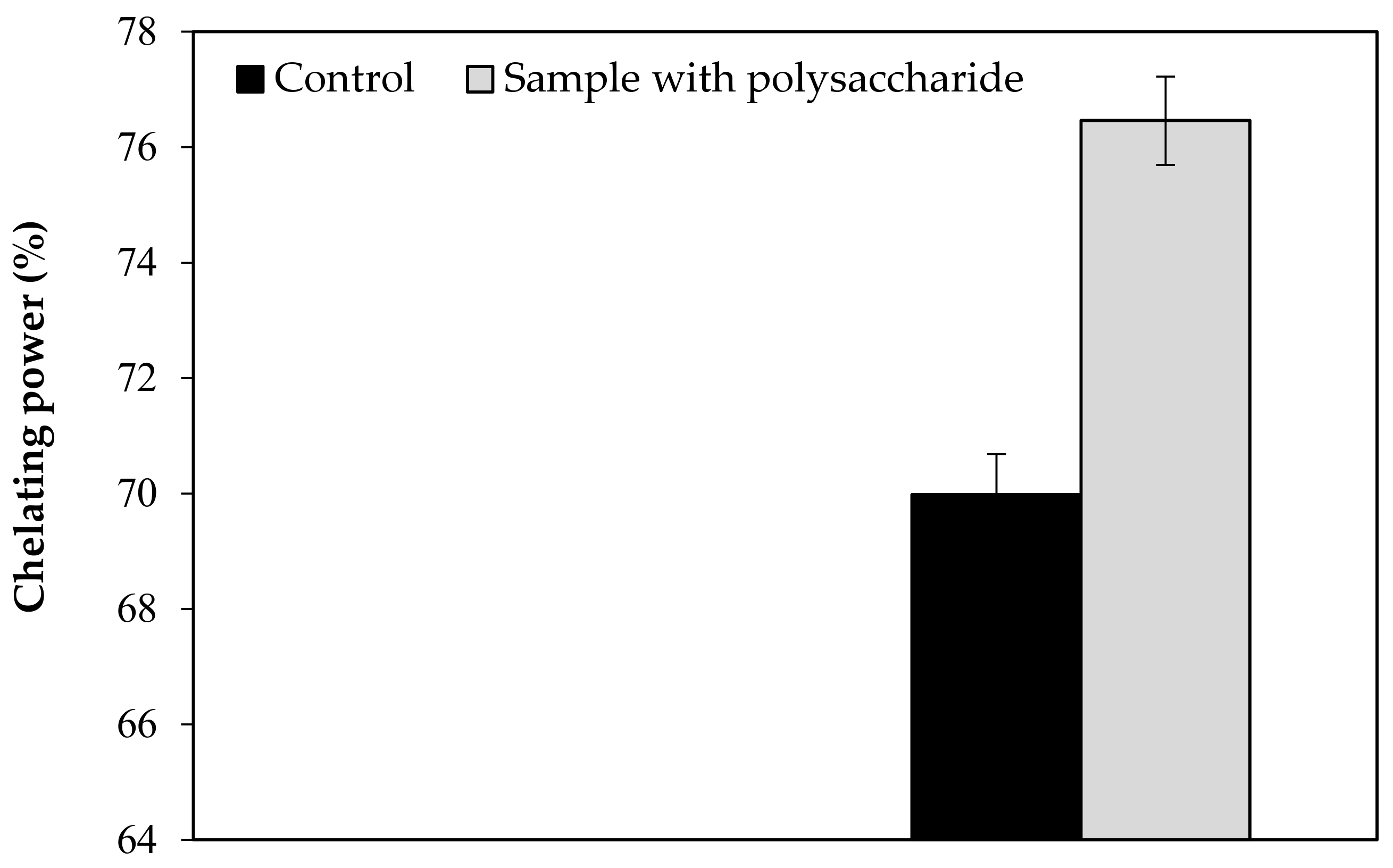
| Chelating power | 69.98 | 76.46 | 69.980 | 76.460 | 2.800 | 3.060 | 2.71 | 0.027 |
| 72.77 | 73.40 | |||||||
| 67.18 | 79.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dammak, A.; Ben Slima, S.; Gomes da Silva, M.D.R.; Ben Salah, R.; Aljuaid, A.M.; Hachicha, W.; Bouaziz, M. Antioxidant and Antibacterial Activities of a Purified Polysaccharide Extracted from Ceratonia siliqua L. and Its Involvement in the Enhancement Performance of Whipped Cream. Separations 2022, 9, 117. https://doi.org/10.3390/separations9050117
Dammak A, Ben Slima S, Gomes da Silva MDR, Ben Salah R, Aljuaid AM, Hachicha W, Bouaziz M. Antioxidant and Antibacterial Activities of a Purified Polysaccharide Extracted from Ceratonia siliqua L. and Its Involvement in the Enhancement Performance of Whipped Cream. Separations. 2022; 9(5):117. https://doi.org/10.3390/separations9050117
Chicago/Turabian StyleDammak, Ameni, Sirine Ben Slima, Marco D. R. Gomes da Silva, Riadh Ben Salah, Awad M. Aljuaid, Wafik Hachicha, and Mohamed Bouaziz. 2022. "Antioxidant and Antibacterial Activities of a Purified Polysaccharide Extracted from Ceratonia siliqua L. and Its Involvement in the Enhancement Performance of Whipped Cream" Separations 9, no. 5: 117. https://doi.org/10.3390/separations9050117
APA StyleDammak, A., Ben Slima, S., Gomes da Silva, M. D. R., Ben Salah, R., Aljuaid, A. M., Hachicha, W., & Bouaziz, M. (2022). Antioxidant and Antibacterial Activities of a Purified Polysaccharide Extracted from Ceratonia siliqua L. and Its Involvement in the Enhancement Performance of Whipped Cream. Separations, 9(5), 117. https://doi.org/10.3390/separations9050117








