Isotopic Characterization of Italian Industrial Hemp (Cannabis sativa L.) Intended for Food Use: A First Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Cultivation Sites
2.2. Stable Isotope Ratio Analysis
2.3. Statistical Analysis
3. Results and Discussion
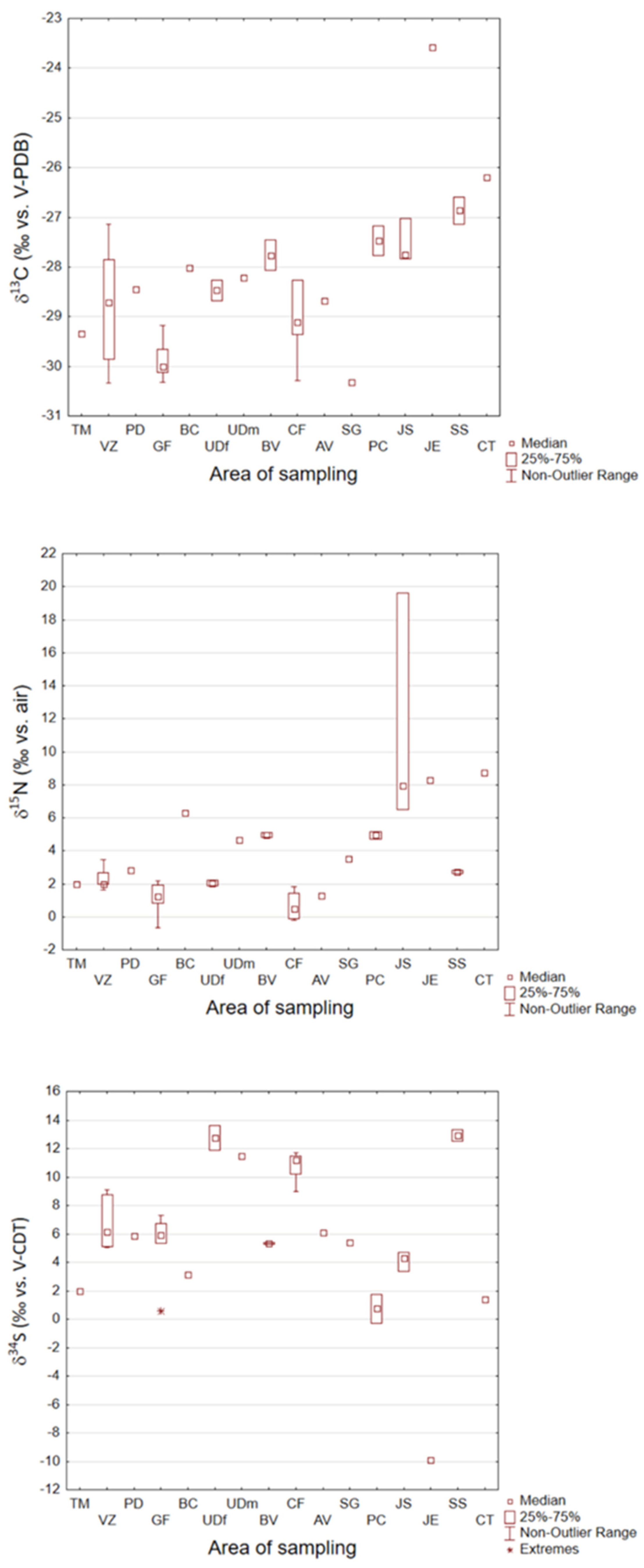
3.1. Carbon, Nitrogen, and Sulfur Stable Isotope Ratios in Hemp
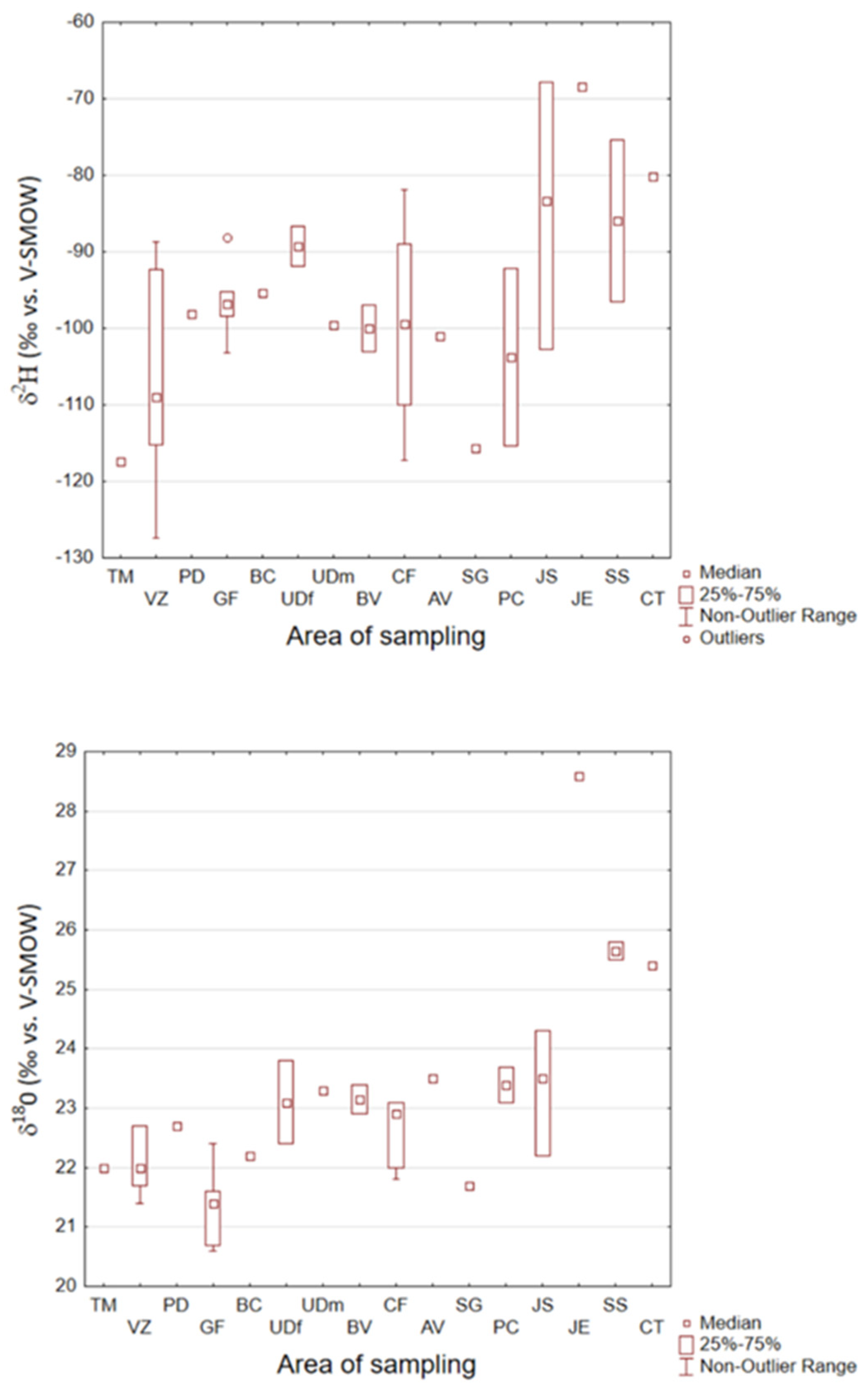
3.2. Hydrogen and Oxygen Stable Isotope Ratios in Hemp
3.3. Hydrogen and Oxygen Stable Isotope Ratios in Hemp Seed Oil
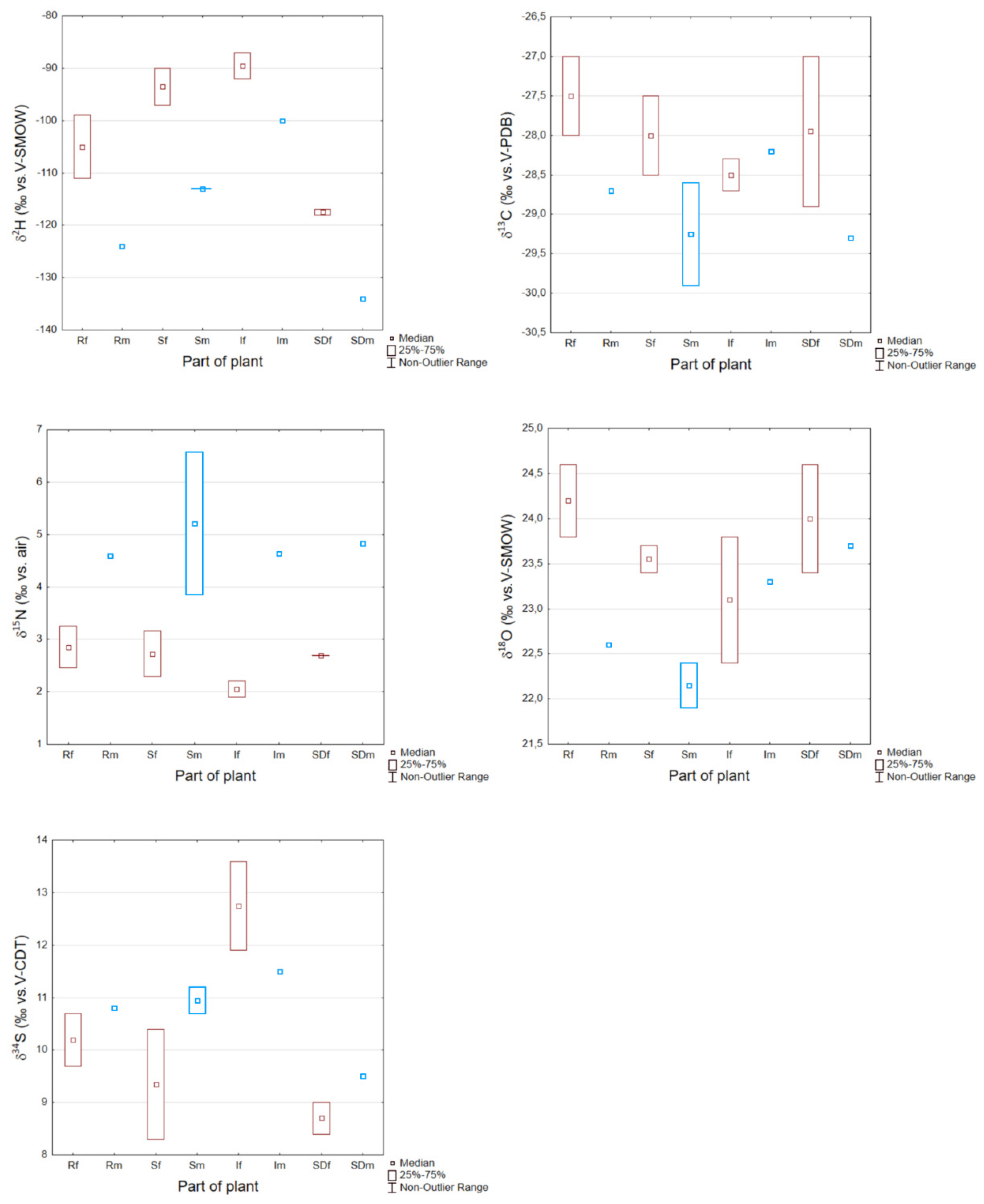
3.4. Insight on Stable Isotope Ratios in Hemp Collected at Udine Sampling Site
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, K. Agronomy and Photosynthesis Physiology of Hemp (Cannabis sativa L.). Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 2018. Available online: https://research.wur.nl/en/publications/agronomy-and-photosynthesis-physiology-of-hemp-cannabis-sativa-l (accessed on 18 October 2021).
- Landi, S. Mineral nutrition of Cannabis sativa L. J. Plant Nutr. 1997, 20, 311–326. [Google Scholar] [CrossRef]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis Roots: A Traditional Therapy with Future Potential for Treating Inflammation and Pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.-Y.; Li, S.-H.; Ma, W.; Wu, D.-T.; Li, H.-B.; Xiao, A.-P.; Liu, L.-L.; Zhu, F.; Gan, R.-Y. Cannabis sativa bioactive compounds and their extraction, separation, purification, and identification technologies: An updated review. TrAC Trends Anal. Chem. 2022, 149, 116554. [Google Scholar] [CrossRef]
- Moreno, T.; Montanes, F.; Tallon, S.J.; Fenton, T.; King, J.W. Extraction of cannabinoids from hemp (Cannabis sativa L.) using high pressure solvents: An overview of different processing options. J. Supercrit. Fluids 2020, 161, 104850. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Falconer, J.R. Extraction of medicinal cannabinoids through supercritical carbon dioxide technologies: A review. J. Chromatogr. B 2021, 1167, 122581. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V.; Carrer, M.; Ceciliani, G.; Sala, S.; Panseri, S.; Pavlovic, R.; Giorgi, A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020, 15, 194–205. [Google Scholar] [CrossRef]
- Baldini, M.; Zuliani, F.; Barbiani, G.; Cattivello, C. Coltivazione di varietà di canapa industriale nel medio Friuli: Nuove opportunità per una “vecchia” coltura. Not. ERSA Reg. Agency Rural. Dev. 2016, 3, 41–49. Available online: http://www.ersa.fvg.it/ex-port/sites/ersa/aziende/in-formazione/notiziario/allegati/2016/3/COLTIVAZIONE-CANAPA.pdf (accessed on 18 October 2021). (In Italian).
- Cacchioni, D. Hemp and Industry in Italy: Between Pasts and Present. AGER J. Depopulation Rural. Dev. Stud. 2021, 32, 93–115. [Google Scholar] [CrossRef]
- Law 242/2016. Disposizioni per la Promozione della Coltivazione e della Filiera Agroindustriale della Canapa. Law 2nd December 2016, n. 242; 2016. Available online: https://www.gazzettaufficiale.it/eli/id/2016/12/30/16G00258/sg (accessed on 13 December 2021). (In Italian).
- Coldiretti—Italian National Confederation of Direct Farmers. Via Libera alla Cannabis a Tavola, Campi Decuplicati. Available online: https://www.coldiretti.it/economia/via-libera-alla-cannabis-tavola-campi-decuplicati (accessed on 18 October 2021). (In Italian).
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Federcanapa—Italian Hemp Federation. Linee Guida per la Coltivazione Della Canapa da Estrazione. Available online: https://www.federcanapa.it/coltivazione-canapa-industriale-estrazione (accessed on 18 October 2021). (In Italian).
- Federcanapa—Italian Hemp Federation. Linee Guida per il Seme di Canapa ad Uso Alimentare. Available online: https://www.federcanapa.it/linee-guida-canapa-alimentare (accessed on 18 October 2021). (In Italian).
- Altieri, S.; Saiano, K.; Biondi, M.; Ricci, P.; Lubritto, C. Traceability of ‘Mozzarella di Bufala Campana’ production chain by means of carbon, nitrogen and oxygen stable isotope ratios. J. Sci. Food Agric. 2020, 100, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, L.; Camin, F.; Perini, M.; Ziller, L.; Larcher, R. Isotopic and elemental characterisation of Italian white truffle: A first exploratory study. Food Chem. Toxicol. 2020, 145, 111627. [Google Scholar] [CrossRef] [PubMed]
- Wadood, S.A.; Boli, G.; Yimin, W. Geographical traceability of wheat and its products using multielement light stable isotopes coupled with chemometrics. J. Mass Spectrom. 2019, 54, 178–188. [Google Scholar] [CrossRef]
- Portarena, S.; Gavrichkova, O.; Lauteri, M.; Brugnoli, E. Authentication and traceability of Italian extra-virgin olive oils by means of stable isotopes techniques. Food Chem. 2014, 164, 12–16. [Google Scholar] [CrossRef]
- Booth, A.L.; Wooller, M.J.; Howe, T.; Haubenstock, N. Tracing geographic and temporal trafficking patterns for marijuana in Alaska using stable isotopes (C, N, O and H). Forensic Sci. Int. 2010, 202, 45–53. [Google Scholar] [CrossRef]
- Hurley, J.M.; West, J.B.; Ehleringer, J.R. Tracing retail cannabis in the United States: Geographic origin and cultivation patterns. Int. J. Drug Policy 2010, 21, 222–228. [Google Scholar] [CrossRef]
- Shibuya, E.K.; Sarkis, J.E.S.; Negrini-Neto, O.; Martinelli, L.A. Carbon and nitrogen stable isotopes as indicative of geographical origin of marijuana samples seized in the city of São Paulo (Brazil). Forensic Sci. Int. 2007, 167, 8–15. [Google Scholar] [CrossRef]
- West, J.B.; Hurley, J.M.; Ehleringer, J.R. Stable Isotope Ratios of Marijuana. I. Carbon and Nitrogen Stable Isotopes Describe Growth Conditions. J. Forensic Sci. 2009, 54, 84–89. [Google Scholar] [CrossRef]
- Brand, W.A.; Coplen, T.B. Stable isotope deltas: Tiny, yet robust signatures in nature. Isot. Environ. Health Stud. 2012, 48, 393–409. [Google Scholar] [CrossRef]
- Bontempo, L.; van Leeuwen, K.A.; Paolini, M.; Laursen, K.H.; Micheloni, C.; Prenzler, P.D.; Ryan, D.; Camin, F. Bulk and compound-specific stable isotope ratio analysis for authenticity testing of organically grown tomatoes. Food Chem. 2020, 318, 126426. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.F.; Chesson, L.A. Food Forensics. Stable Isotopes as a Guide to Authenticity and Origin; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9780367782085. [Google Scholar]
- Bateman, A.S.; Kelly, S.D. Fertilizer nitrogen isotope signature. Isot. Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Inácio, C.T.; Chalk, P.M.; Magalhães, A.M.T. Principles and Limitations of Stable Isotopes in Differentiating Organic and Conventional Foodstuffs: 1. Plant Products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Paolini, M. Development and Implementation of Stable Isotope Ratio Analysis in Bulk Products and Sub-Components to Ensure Food Traceability. Ph.D. Thesis, University of Udine, Udine, Italy, 2017. Available online: https://hdl.handle.net/10449/37884 (accessed on 18 October 2021).
- Zazzo, A.; Monahan, F.J.; Moloney, A.P.; Green, S.; Schmidt, O. Sulphur isotopes in animal hair track distance to sea. Rapid Commun. Mass Spectrom. 2011, 25, 2371–2378. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, H.; Pan, J.; Chen, H.; Jin, B.; Yan, Z.; Xie, L.; Rogers, K.M. Geographical traceability of south-east Asian durian: A chemometric study using stable isotopes and elemental compositions. J. Food Compos. Anal. 2021, 101, 103940. [Google Scholar] [CrossRef]
- Costantini, E.A.C.; L’Abate, G.; Barbetti, R.; Fantappié, M.; Lorenzetti, R.; Magini, S. Soil Map of Italy; National Research Council and Minister of Agriculture Food and Forestry: Rome, Italy, 2012. Available online: https://esdac.jrc.ec.europa.eu/content/carta-dei-suoli-ditalia-soil-map-italy (accessed on 18 October 2021).
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef] [Green Version]
- Giustini, F.; Brilli, M.; Patera, A. Mapping oxygen stable isotopes of precipitation in Italy. J. Hydrol. Reg. Stud. 2016, 8, 162–181. [Google Scholar] [CrossRef] [Green Version]
- Silveira Lobo Sternberg, L. Oxygen and Hydrogen Isotope Ratios in Plant Cellulose: Mechanisms and Applications. In Stable Isotopes in Ecological Research; Rundel, P.W., Ehleringer, J.R., Nagy, K.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 124–141. [Google Scholar] [CrossRef]
- Yakir, D.; DeNiro, M.J. Oxygen and Hydrogen Isotope Fractionation during Cellulose Metabolism in Lemna gibba L. Plant Physiol. 1990, 93, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Tarapoulouzi, M.; Skiada, V.; Agriopoulou, S.; Psomiadis, D.; Rébufa, C.; Roussos, S.; Theocharis, C.R.; Katsaris, P.; Varzakas, T. Chemometric Discrimination of the Geographical Origin of Three Greek Cultivars of Olive Oils by Stable Isotope Ratio Analysis. Foods 2021, 10, 336. [Google Scholar] [CrossRef]
- Schmidt, H.-L.; Werner, R.A.; Eisenreich, W. Systematics of 2H patterns in natural compounds and its importance for the elucidation of biosynthetic pathways. Phytochem. Rev. 2003, 2, 61–85. [Google Scholar] [CrossRef]
- Cucinotta, L.; De Grazia, G.; Micalizzi, G.; Bontempo, L.; Camin, F.; Mondello, L.; Sciarrone, D. Simultaneous evaluation of the enantiomeric and carbon isotopic ratios of Cannabis sativa L. essential oils by multidimensional gas chromatography. Anal. Bioanal. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, L.; Camin, F.; Paolini, M.; Micheloni, C.; Laursen, K.H. Multi-isotopic signatures of organic and conventional Italian pasta along the production chain. J. Mass Spectrom. 2016, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Georgi, M.; Voerkelius, S.; Rossmann, A.; Graßmann, J.; Schnitzler, W.H. Multielement Isotope Ratios of Vegetables from Integrated and Organic Production. Plant Soil 2005, 275, 93–100. [Google Scholar] [CrossRef]
- Laursen, K.H.; Mihailova, A.; Kelly, S.D.; Epov, V.N.; Bérail, S.; Schjoerring, J.K.; Donard, O.F.X.; Larsen, E.H.; Pedentchouk, N.; Marca-Bell, A.D.; et al. Is it really organic?—Multi-isotopic analysis as a tool to discriminate between organic and conventional plants. Food Chem. 2013, 141, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable Isotopes in Plant Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]

| Samples | δ2H | δ13C | δ15N | δ18O | δ34S | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | Type * | n | Mean | S.Dev | Mean | S.Dev | Mean | S.Dev | Mean | S.Dev | Mean | S.Dev |
| Tolmezzo (UD) | I | 1 | −117 | −29.3 | 1.9 | 22.0 | 2.0 | |||||
| Verzegnis (UD) | I | 7 | −107 | 13 | −28.8 | 1.1 | 2.4 | 0.9 | 22.1 | 0.5 | 6.6 | 1.7 |
| S | 7 | −85 | 5 | −28.4 | 0.8 | 0.8 | 0.9 | 23.9 | 0.7 | 3.9 | 1.7 | |
| R | 1 | −94 | −28.5 | 0.9 | 24.1 | 4.7 | ||||||
| Predaia (TN) | O | 1 | −197 | 17.9 | ||||||||
| SD | 1 | −149 | −30.4 | 5.1 | 20.9 | 5.5 | ||||||
| I | 1 | −98 | −28.4 | 2.8 | 22.7 | 5.9 | ||||||
| Gemona del Friuli (UD) | I | 6 | −96 | 5 | −29.9 | 0.4 | 1.1 | 1.0 | 21.4 | 0.7 | 5.3 | 2.4 |
| S | 6 | −103 | 10 | −29.5 | 0.2 | 1.5 | 0.5 | 22.9 | 1.1 | 4.3 | 2.6 | |
| R | 1 | −110 | −29.1 | 1.7 | 23.4 | 4.1 | ||||||
| Baceno (VB) | I | 1 | −95 | −28.0 | 6.3 | 22.2 | 3.1 | |||||
| Udine | SDf | 2 | −117 | 1 | −27.9 | 1.4 | 2.7 | 0.01 | 24.0 | 0.8 | 8.7 | 0.5 |
| SDm | 1 | −134 | −29.3 | 4.8 | 23.7 | 9.5 | ||||||
| If | 2 | −89 | 4 | −28.5 | 0.3 | 2.0 | 0.2 | 23.1 | 1.0 | 12.7 | 1.2 | |
| Im | 1 | −100 | −28.2 | 4.6 | 23.3 | 11.5 | ||||||
| Sf | 2 | −94 | 5 | −28.0 | 0.7 | 2.7 | 0.6 | 23.6 | 0.2 | 9.3 | 1.5 | |
| Sm | 2 | −113 | 0.1 | −29.2 | 0.9 | 5.2 | 1.9 | 22.2 | 0.4 | 10.9 | 0.4 | |
| Rf | 2 | −105 | 9 | −27.5 | 0.7 | 2.9 | 0.6 | 24.2 | 0.6 | 10.2 | 0.7 | |
| Rm | 2 | −125 | 0.1 | −28.9 | 0.3 | 4.9 | 0.5 | 22.8 | 0.2 | 11.1 | 0.4 | |
| Borgo Valbelluna (BL) | I | 2 | −100 | 4 | −27.8 | 0.4 | 4.9 | 0.2 | 23.2 | 0.4 | 5.3 | 0.1 |
| Campoformido (UD) | I | 6 | −103 | 10 | −29.1 | 0.8 | 0.6 | 0.8 | 22.6 | 0.6 | 10.8 | 1.0 |
| S | 6 | −103 | 8 | −28.7 | 0.5 | 1.1 | 0.5 | 23.2 | 0.3 | 8.6 | 1.6 | |
| R | 1 | −117 | −28.6 | 1.9 | 23.9 | 8.7 | ||||||
| Altopiano della Vigolana (TN) | SD | 1 | −141 | −29.4 | 2.6 | 22.3 | 6.3 | |||||
| I | 1 | −101 | −28.7 | 1.3 | 23.5 | 6.1 | ||||||
| Seren del Grappa (BL) | SD | 1 | −138 | −30.9 | 3.6 | 21.5 | 4.5 | |||||
| I | 1 | −116 | −30.3 | 3.5 | 21.7 | 5.4 | ||||||
| Piacenza | SD + I | 2 | −104 | 16 | −27.5 | 0.4 | 5.0 | 0.3 | 23.4 | 0.4 | 0.7 | 1.4 |
| S | 2 | −84 | 2 | −26.7 | 0.1 | 4.7 | 0.8 | 24.5 | 0.4 | −1.2 | 0.8 | |
| Jolanda di Savoia (FE) | O | 1 | −200 | 20.9 | ||||||||
| I | 3 | −85 | 18 | −27.5 | 0.4 | 11.3 | 7.2 | 23.3 | 1.1 | 4.1 | 0.7 | |
| Jesi (AN) | O | 1 | −181 | 22.4 | ||||||||
| SD | 1 | −118 | −28.5 | 10.7 | 23.7 | 2.8 | ||||||
| I | 1 | −68 | −23.6 | 8.3 | 28.6 | −9.9 | ||||||
| Sassari | O | 1 | −179 | 24.5 | ||||||||
| SD | 2 | −99 | 7 | −27.8 | 0.4 | 3.1 | 0.7 | 27.3 | 0.2 | 10.5 | 0.2 | |
| I | 2 | −86 | 15 | −26.9 | 0.4 | 2.7 | 0.1 | 25.7 | 0.2 | 12.9 | 0.6 | |
| Caltagirone (CT) | O | 1 | −173 | 25.6 | ||||||||
| SD | 1 | −104 | −25.8 | 7.3 | 28.7 | 1.4 | ||||||
| I | 1 | −80 | −26.2 | 8.7 | 25.4 | 1.4 | ||||||
| S | 1 | −88 | −27.6 | 7.9 | 26.5 | 0.5 | ||||||
| R | 1 | −93 | −27.3 | 7.1 | 27.8 | 2.6 | ||||||
| Minimum value | −200 | −30.9 | 0.6 | 17.9 | −9.9 | |||||||
| Maximum value | −68 | −23.6 | 11.3 | 28.7 | 12.9 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvi, M.; Bontempo, L.; Pizzini, S.; Cucinotta, L.; Camin, F.; Stenni, B. Isotopic Characterization of Italian Industrial Hemp (Cannabis sativa L.) Intended for Food Use: A First Exploratory Study. Separations 2022, 9, 136. https://doi.org/10.3390/separations9060136
Calvi M, Bontempo L, Pizzini S, Cucinotta L, Camin F, Stenni B. Isotopic Characterization of Italian Industrial Hemp (Cannabis sativa L.) Intended for Food Use: A First Exploratory Study. Separations. 2022; 9(6):136. https://doi.org/10.3390/separations9060136
Chicago/Turabian StyleCalvi, Marco, Luana Bontempo, Sarah Pizzini, Lorenzo Cucinotta, Federica Camin, and Barbara Stenni. 2022. "Isotopic Characterization of Italian Industrial Hemp (Cannabis sativa L.) Intended for Food Use: A First Exploratory Study" Separations 9, no. 6: 136. https://doi.org/10.3390/separations9060136
APA StyleCalvi, M., Bontempo, L., Pizzini, S., Cucinotta, L., Camin, F., & Stenni, B. (2022). Isotopic Characterization of Italian Industrial Hemp (Cannabis sativa L.) Intended for Food Use: A First Exploratory Study. Separations, 9(6), 136. https://doi.org/10.3390/separations9060136






