Comparative Study of the Selective Sorption of Organic Dyes on Inorganic Materials—A Cost-Effective Method for Waste Treatment in Educational and Small Research Laboratories
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Stock Solutions
2.3. Preparation of Activated Carbon from Coffee Grounds (ACCG)
2.4. Measurement and Characterization of the Adsorbent Materials
2.5. Adsorption Experiments
2.6. Selective Adsorption of Dyes from Mixed-Dye Solutions
2.7. Adsorption Kinetics Studies
2.7.1. Pseudo-First-Order Kinetic Model
2.7.2. Pseudo-Second-Order Kinetic Model
2.7.3. Intra-Particle Diffusion Model
2.8. Thermodynamic Studies
2.9. Desorption and Regeneration of Sorbent Materials
3. Results
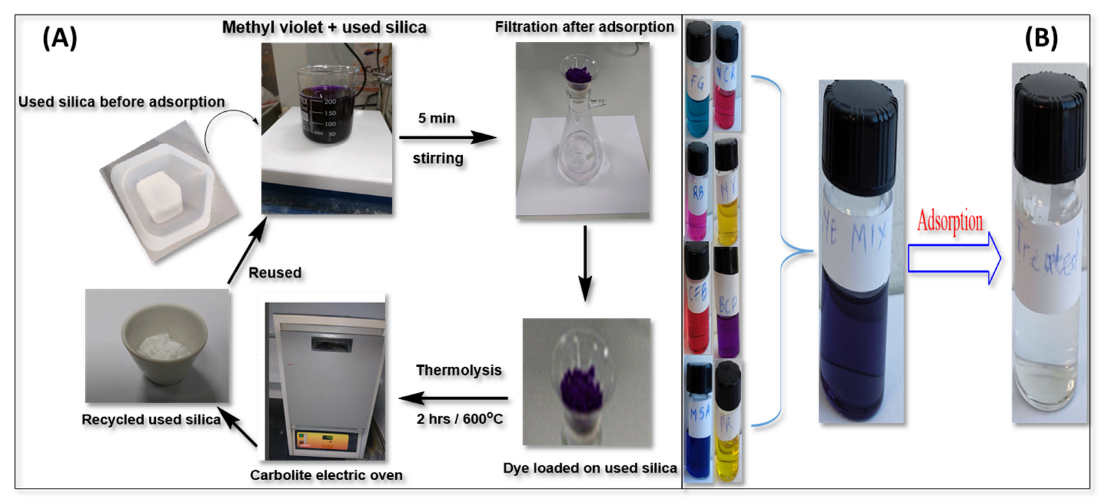
3.1. General Experimental Overview
3.2. Characterization of the Adsorbent Materials
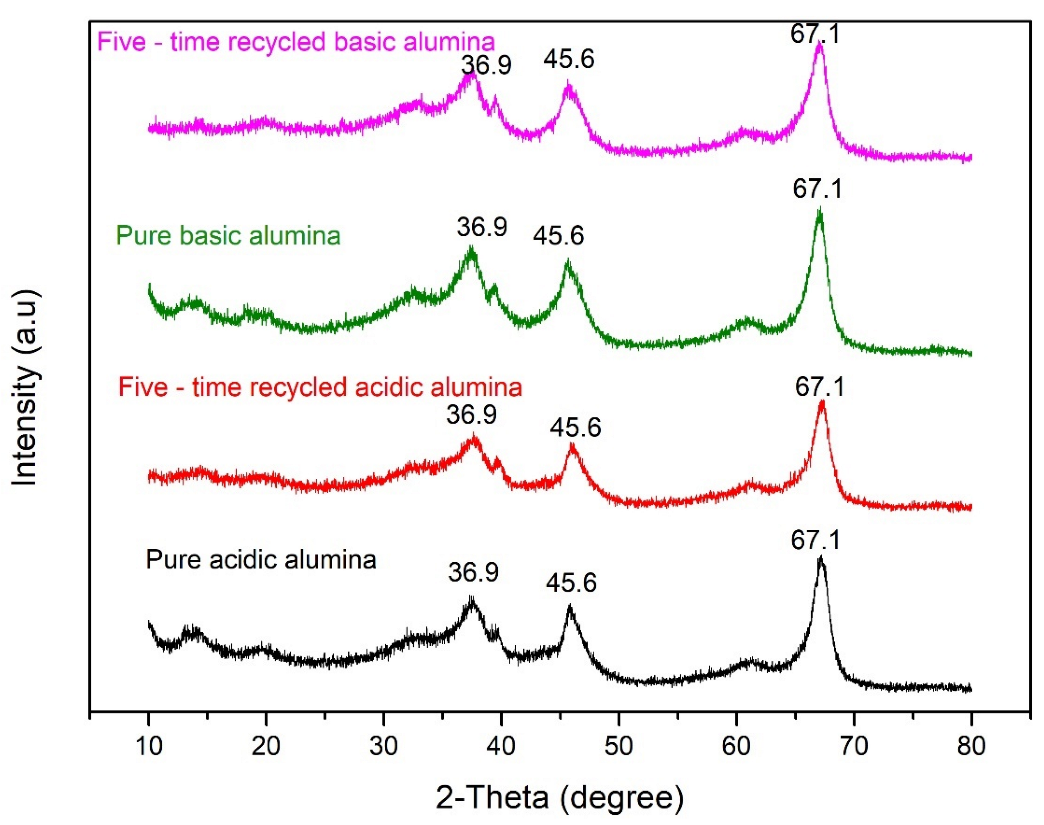
3.2.1. X-ray Diffraction
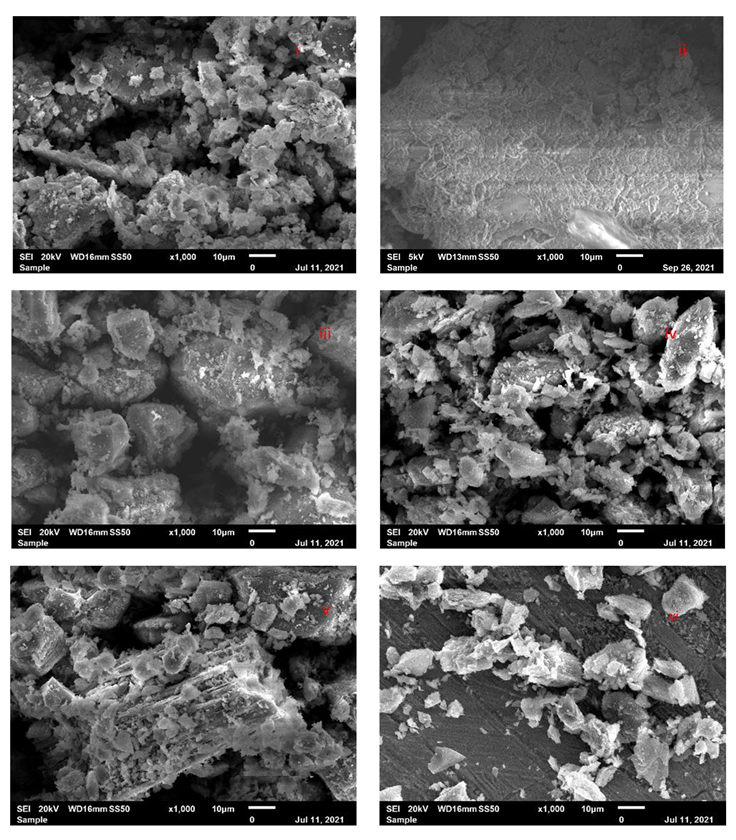
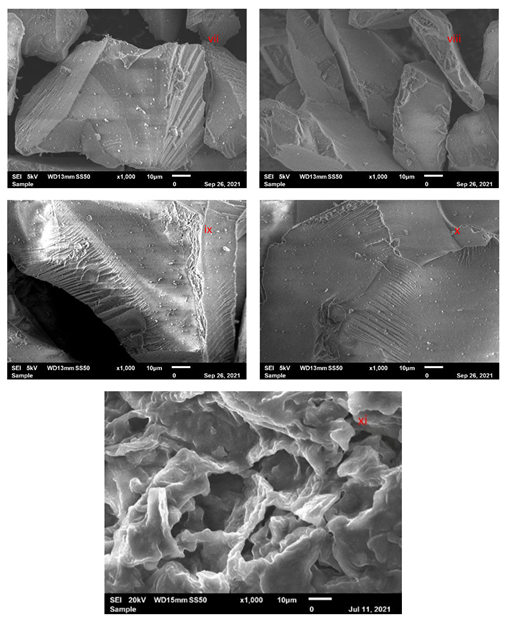
3.2.2. SEM Characterization of Adsorbent Material
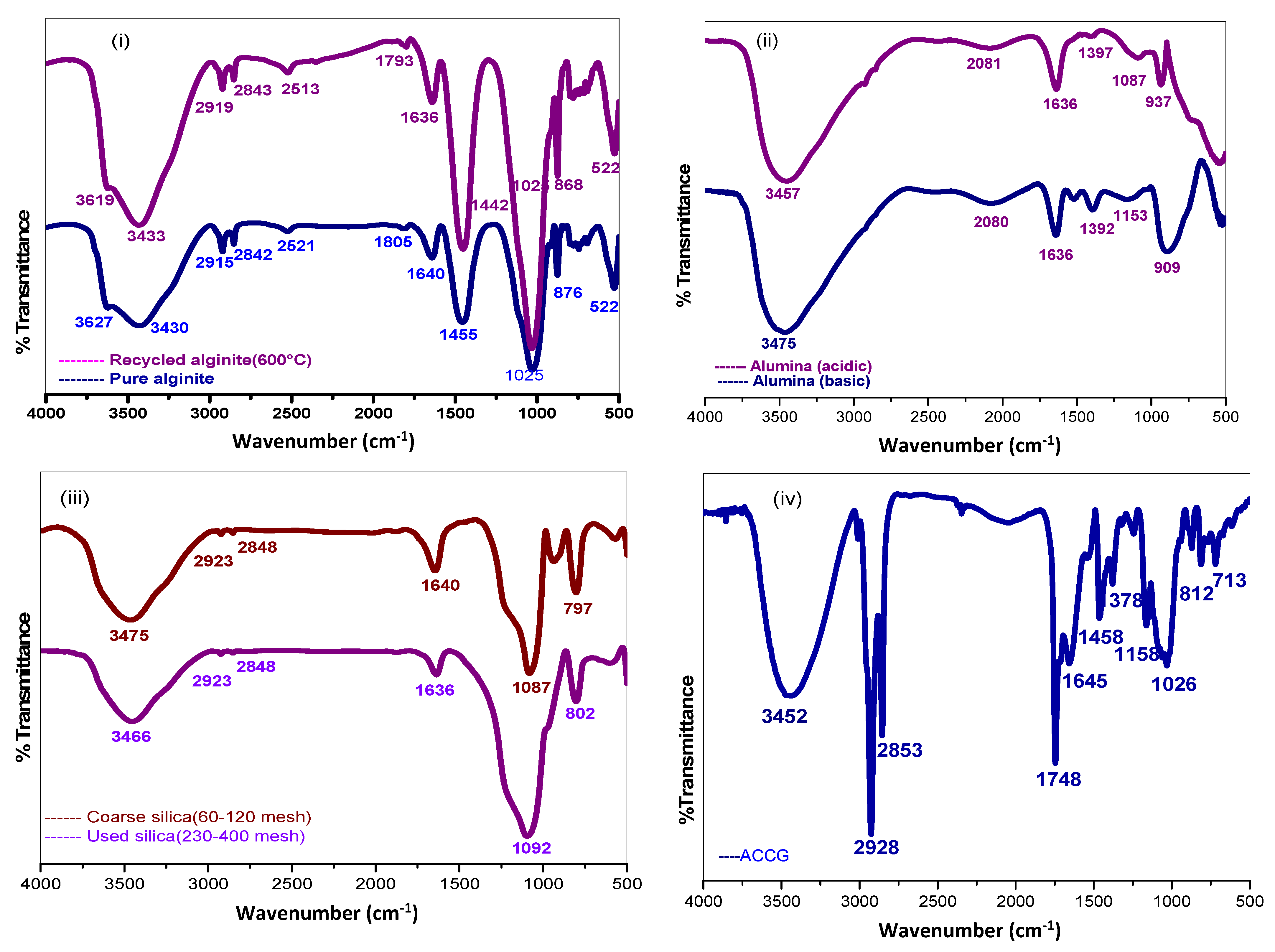
3.2.3. FT-IR Characterization of the Adsorbent Materials
3.3. Adsorption Studies
3.4. Optimization Studies of Organic Pollutant Removal Using Adsorbent Material
3.4.1. Effect of Contact Time and Initial Concentration
3.4.2. Effect of pH on the Adsorption of Dyes
3.4.3. Effect of Sorbent Dosage on the Adsorption of Dyes
3.4.4. Effect of Temperature on the Adsorption of Dyes
3.5. Thermodynamic Analysis
3.6. Activation Energy Studies
3.7. Adsorption Kinetics
3.8. Intra-Particle Diffusion Model
3.9. Selective Adsorption of Dye Mixture
3.10. Regeneration of the Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goh, H.Y.; Wong, W.W.C.; Ong, Y.Y. A Study To Reduce Chemical Waste Generated in Chemistry Teaching Laboratories. J. Chem. Educ. 2020, 97, 87–96. [Google Scholar] [CrossRef]
- de Souza Nascimento, E.; Tenuta Filho, A. Chemical waste risk reduction and environmental impact generated by laboratory activities in research and teaching institutions. Braz. J. Pharm. Sci. 2010, 46, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Wahshi, F.S.; Alqahtani, M.D.; Abdulla, M.; Al-Hemyari, A.; Bufaroosha, M.; Ramachandran, T.; Hamed, F.; Thiemann, T. Our Experience of Using Thermally Recycled Silica Gel in a Teaching and Small Research Laboratory Setting. Proceedings 2018, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Sales, M.G.F.; Delerue-Matos, C.; Martins, I.B.; Serra, I.; Silva, M.R.; Morais, S. A waste management school approach towards sustainability. Resour. Conserv. Recycl. 2006, 48, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; Bachiri, A.E.; Harfi, A.E. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Paul, B.; Xu, W.; Yuan, Y.; Liu, E.; Ke, X.; Wellard, R.M.; Guo, C.; Xu, Y.; Sun, Y.; et al. Alumina nanofibers grafted with functional groups: A new design in efficient sorbents for removal of toxic contaminants from water. Water Res. 2010, 44, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, M.; Zablouk, M.; Abid-Alameer, A. Experimental Study of Dye Removal from Industrial Wastewater by Membrane Technologies of Reverse Osmosis and Nanofiltration. Iran. J. Environ. Health Sci. Eng. 2012, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Malakootian, M.; Shahesmaeili, A.; Faraji, M.; Amiri, H.; Silva Martinez, S. Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process Saf. Environ. Prot. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Al-Abri, M.; Al-Ghafri, B.; Bora, T.; Dobretsov, S.; Dutta, J.; Castelletto, S.; Rosa, L.; Boretti, A. Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. NPJ Clean Water 2019, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Derouich, G.; Younssi, S.A.; Bennazha, J.; Achiou, B.; Ouammou, M.; El Hassani IE, E.A.; Albizane, A. Adsorption study of cationic and anionic dyes onto Moroccan natural pozzolan. Application for removal of textile dyes from aqueous solutions. Desalination Water Treat. 2019, 145, 348–360. [Google Scholar] [CrossRef]
- Banerjee, S.; Dubey, S.; Gautam, R.K.; Chattopadhyaya, M.C.; Sharma, Y.C. Adsorption characteristics of alumina nanoparticles for the removal of hazardous dye, Orange G from aqueous solutions. Arab. J. Chem. 2019, 12, 5339–5354. [Google Scholar] [CrossRef]
- Alpat, S.K.; Özbayrak, Ö.; Alpat, Ş.; Akçay, H. The adsorption kinetics and removal of cationic dye, Toluidine Blue O, from aqueous solution with Turkish zeolite. J. Hazard. Mater. 2008, 151, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wahshi, F.S.; Alqahtani, M.D.; Abdulla, M.; Ramachandran, T.; Hamed, F.; Thiemann, T. Adsorption of Model Dyes on Recycled Silica Gel. Proceedings 2019, 48, 10. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Zhao, Y.; Zhang, B.; Liu, J.; Yang, Y.; Liu, J. Study on the adsorption of Neutral Red from aqueous solution onto halloysite nanotubes. Water Res. 2010, 44, 1489–1497. [Google Scholar] [CrossRef]
- Ali, F.F. Adsorption of Toluidine blue dye from industrial waste water on the remnants of tea leaf. Int. J. ChemTech Res. 2017, 10, 497–506. [Google Scholar]
- Kurniawan, T.A.; Lo, W.H. Removal of refractory compounds from stabilized landfill leachate using an integrated H2O2 oxidation and granular activated carbon (GAC) adsorption treatment. Water Res. 2009, 43, 4079–4091. [Google Scholar] [CrossRef]
- Titchou, F.E.; Akbour, R.A.; Assabbane, A.; Hamdani, M. Removal of cationic dye from aqueous solution using Moroccan pozzolana as adsorbent: Isotherms, kinetic studies, and application on real textile wastewater treatment. Groundw. Sustain. Dev. 2020, 11, 100405. [Google Scholar] [CrossRef]
- Benafqir, M.; Hsini, A.; Laabd, M.; Laktif, T.; Ait Addi, A.; Albourine, A.; El Alem, N. Application of Density Functional Theory computation (DFT) and Process Capability Study for performance evaluation of Orthophosphate removal process using Polyaniline@Hematite-titaniferous sand composite (PANI@HTS) as a substrate. Sep. Purif. Technol. 2020, 236, 116286. [Google Scholar] [CrossRef]
- Abdulrazak, S.; Hussaini, K.; Sani, H.M. Evaluation of removal efficiency of heavy metals by low-cost activated carbon prepared from African palm fruit. Appl. Water Sci. 2017, 7, 3151–3155. [Google Scholar] [CrossRef] [Green Version]
- Bushra, R.; Mohamad, S.; Alias, Y.; Jin, Y.; Ahmad, M. Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review. Microporous Mesoporous Mater. 2021, 319, 111040. [Google Scholar] [CrossRef]
- Hu, Q.; Bin, L.; Li, P.; Fu, F.; Guan, G.; Hao, X.; Tang, B. Highly efficient removal of dyes from wastewater over a wide range of pH value by a self-adaption adsorbent. J. Mol. Liq. 2021, 331, 115719. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Hameed, B.H.; Ok, Y.S.; Omirou, M. A review on waste-derived adsorbents from sugar industry for pollutant removal in water and wastewater. J. Mol. Liq. 2017, 240, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Goswami, A.; Purkait, M.K. The defluoridation of water by acidic alumina. Chem. Eng. Res. Des. 2012, 90, 2316–2324. [Google Scholar] [CrossRef]
- Revathi, M.; Kavitha, B.; Vasudevan, T. Removal of nickel ions from industrial plating effluents using activated alumina as adsorbent. J. Environ. Sci. Eng. 2005, 47, 1–6. [Google Scholar]
- Frišták, V.; Pipíška, M.; Lesný, J. Cd2+ Ions Removal from Aqueous Solutions Using Alginite. Int. J. Chem. Biomol. Metall. Mater. Sci. Eng. 2014, 8, 237. [Google Scholar]
- Hippmann, S.; Ahmed, S.S.; Fröhlich, P.; Bertau, M. Demulsification of water/crude oil emulsion using natural rock Alginite. Colloids Surf. Physicochem. Eng. Asp. 2018, 553, 71–79. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Zur Theorie der sogenannten Adsorption gelőster Stoffe. Sven. Vetenskapsakad. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Schieber, J.; Mastalerz, M. Petrographic and micro-FTIR study of organic matter in the Upper Devonian New Albany shale during thermal maturation: Implications for kerogen transformation. In Mudstone Diagenesis: Research Perspectives for Shale Hydrocarbon Reservoirs, Seals, and Source Rocks, AAPG Memoir; The American Association of Petroleum Geologists: Tulsa, OK, USA, 2020; Chapter 10; Volume 120. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Xu, Y.; Han, B.; Wang, Y.; Liu, X.; Yan, Z. Synthesis and characterization of mesoporous Si-modified alumina with high thermal stability. Microporous Mesoporous Mater. 2017, 238, 84–89. [Google Scholar] [CrossRef]
- Toledo, R.R.; Santoyo, V.R.; Sánchez, D.M.; Rosales, M.M. Effect of aluminum precursor on physicochemical properties of Al2O3 by hydrolysis/precipitation method. Nova Sci. 2018, 10, 83–99. [Google Scholar] [CrossRef]
- An, D.; Wang, Z.; Zhao, X.; Liu, Y.; Guo, Y.; Ren, S. A new route to synthesis of surface hydrophobic silica with long-chain alcohols in water phase. Colloids Surf. Physicochem. Eng. Asp. 2010, 1–3, 218–222. [Google Scholar] [CrossRef]
- Darmakkolla, S.R.; Tran, H.; Gupta, A.; Rananavare, S.B. A method to derivatize surface silanol groups to Si-alkyl groups in carbon-doped silicon oxides. RSC Adv. 2016, 6, 93219–93230. [Google Scholar] [CrossRef]
- Ashu-Arrah, B.A.; Glennon, J.D.; Albert, K. Preparation and characterization of bonded silica hydride intermediate from triethoxysilane and dimethylmethoxysilane using supercritical carbon dioxide and dioxane as reaction medium. J. Chromatogr. A 2012, 1236, 42–50. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [Green Version]
- Block, I.; Günter, C.; Duarte Rodrigues, A.; Paasch, S.; Hesemann, P.; Taubert, A. Carbon Adsorbents from Spent Coffee for Removal of Methylene Blue and Methyl Orange from Water. Materials 2021, 14, 3996. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, P.; Valiyaveettil, S. Successive extraction of As(V), Cu(II) and P(V) ions from water using spent coffee powder as renewable bioadsorbents. Sci. Rep. 2017, 7, 42881. [Google Scholar] [CrossRef] [Green Version]
- Chaouki, Z.; Hadri, M.; Nawdali, M.; Benzina, M.; Zaitan, H. Treatment of a landfill leachate from Casablanca city by a coagulation-flocculation and adsorption process using a palm bark powder (PBP). Sci. Afr. 2021, 12, e00721. [Google Scholar] [CrossRef]
- Deniz, F.; Karaman, S. Removal of Basic Red 46 dye from aqueous solution by pine tree leaves. Chem. Eng. J. 2011, 170, 67–74. [Google Scholar] [CrossRef]
- Li, T.-T.; Liu, Y.; Peng, Q.; Hu, X.; Liao, T.; Wang, H.; Lu, M. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Chem. Eng. J. 2013, 214, 189–197. [Google Scholar] [CrossRef]
- Nanthamathee, C.; Dechatiwongse, P. Kinetic and thermodynamic studies of neutral dye removal from water using zirconium metal-organic framework analogues. Mater. Chem. Phys. 2021, 258, 123924. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Fahanwi, A.N.; Erdem, A.; Yildiz, U. Synergistic removal of Cu(II) and nitrazine yellow dye using an eco-friendly chitosan-montmorillonite hydrogel: Optimization by response surface methodology. J. Appl. Polym. Sci. 2016, 133, 43664. [Google Scholar] [CrossRef]
- Lafi, R.; Rezma, S.; Hafiane, A. Removal of toluidine blue from aqueous solution using orange peel waste (OPW). Desalin. Water Treatm. 2015, 56, 2754–2765. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Comparisons of porous and adsorption properties of carbons activated by steam and KOH. J. Colloid Interface Sci. 2005, 283, 49–56. [Google Scholar] [CrossRef] [PubMed]


| Organic Compound | λmax (nm) | Type |
|---|---|---|
| Nitrazine yellow | 460 | Azo dye |
| Malachite green oxalate | 620 | Cationic triphenylmethane dye |
| Fast green | 625 | Cationic dye |
| Methylene blue | 665 | Cationic dye |
| Novacron® yellow | 415 | Reactive textile dye |
| Novacron® cherry red | 530 | Reactive textile dye |
| Phenol red | 430 | Anionic dye |
| Neutral red | 520 | Cationic dye |
| Rose bengal | 550 | Anionic dye |
| Crystal violet | 590 | Cationic dye |
| Methyl green | 635 | Cationic triphenylmethane dye |
| Alizarin red | 510 | Anionic dye |
| Toluidine blue O | 635 | Basic cationic dye |
| Chromotrope FB | 510 | Azo dye |
| Bromophenol blue | 590 | Triphenylmethane dye |
| Methylsulphonazo iii | 580 | Azo dye |
| Methyl violet | 585 | Triphenylmethane dye |
| Adsorbent | Before Adsorption | After Five-Time Regeneration | ||||
|---|---|---|---|---|---|---|
| Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) | |
| Acidic alumina | 109.97 | 0.23 | 84.63 | 105.84 | 0.24 | 90.03 |
| Basic alumina | 109.73 | 0.22 | 80.40 | 104.33 | 0.22 | 85.79 |
| Used silica gel | 286.28 | 0.61 | 85.40 | 302.01 | 0.65 | 85.62 |
| Dyes Removed | Basic Alumina | Acidic Alumina | Used Silica Gel 230–400 | Silica Gel 60–120 | Alginite | Activated Coffee |
|---|---|---|---|---|---|---|
| Alizarine red S | 81.7% | 99.9% | (-) | (-) | 55.5% | 94.9% |
| Bromocresol purple | (-) | 100% | (-) | (-) | (-) | (-) |
| Bromophenol blue | (-) | 97.1% | (-) | (-) | (-) | (-) |
| Chromotrope TB | (-) | 99.9% | (-) | (-) | (-) | |
| Crystal violet | (-) | (-) | 99.9% | (--+) | 99.9% | 90.1% |
| Fast green | (-) | 99.2% | 91.2% | (-) | (-) | (-) |
| Malachite green oxalate | (-) | (-) | 98.9% | 88.4% | 98.8% | 66.9% |
| Methylene blue | (-) | (-) | 99.8% | 99.0% | 99.8% | 92.1% |
| Methyl green | (-) | (-) | 98.9% | 95.8% | 98.7% | 73.3% |
| Methyl violet | (+--) | (-) | 100% | 96.9% | 100% | 99.1% |
| Methylsulphonazo III | (-) | 99.8% | (-) | (-) | (-) | (-) |
| Neutral red | (-) | (-) | 96.2% | 86% | 94.6% | 88.6% |
| Nitrazene yellow | (-) | 98.1% | (-) | (-) | (-) | |
| Novacron® cherry red | (-) | 99.7% | (-) | (-) | (-) | (-) |
| Novacron® yellow | (-) | 97.5% | (-) | (-) | (-) | (-) |
| Phenol red | (-) | 94% | 36.2% | (-) | (-) | (-) |
| Rose bengal | (-) | 97.9% | (-) | (-) | (-) | (-) |
| Toluidine blue | (-) | (-) | 100% | 100% | 100% | 99.6% |
| T (K) | KL | ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (J/mol·K) | R (Square) |
|---|---|---|---|---|---|
| 283 | 0.534 | −13.039 | 14.428 | 46.127 | 0.9651 |
| 288 | 0.659 | −13.270 | |||
| 301 | 0.794 | −13.869 | |||
| 308 | 0.914 | −14.192 |
| Dyes | Types of Sorbent | Pseudo-First-Order Parameters | Pseudo-Second-Order Parameters | Intra-Particle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe exp (mg/g) | Qe cal (mg/g) | K1 (min1) | R2 | Qe exp (mg/g) | Qe cal (mg/g) | K1 (min1) | R2 | Kp (mg/gmin1/2) | R2 | ||
| Malachite green oxalate | Used silica | 4.949 | 0.060 | 0.119 | 0.676 | 4.949 | 4.960 | 3.175 | 1 | 0.004 | 0.516 |
| Alginte | 4.940 | 0.246 | 0.139 | 0.975 | 4.940 | 4.965 | 1.200 | 1 | 0.089 | 0.922 | |
| ACCG | 3.440 | 3.415 | 0.092 | 0.996 | 3.344 | 5.068 | 0.012 | 0.992 | 0.162 | 0.937 | |
| Coarse silica | 4.418 | 5.103 | 0.206 | 0.883 | 4.418 | 4.842 | 0.073 | 0.995 | 0.008 | 0.926 | |
| Nitrazine yellow | Acidic Alumina | 4.904 | 0.128 | 0.082 | 0.980 | 4.904 | 4.923 | 0.705 | 1 | 0.007 | 0.997 |
| Novacron Cherry red | Acidic Alumina | 4.486 | 0.095 | 0.128 | 0.970 | 4.486 | 4.498 | 2.823 | 1 | 0.000 | 0.946 |
| Novacron yellow | Acidic Alumina | 4.874 | 0.496 | 0.198 | 0.895 | 4.874 | 4.930 | 0.688 | 0.999 | 0.019 | 0.519 |
| Methysulphon azo iii | Acidic Alumina | 4.990 | 0.034 | 0.125 | 0.996 | 4.990 | 4.997 | 7.024 | 1 | 0.002 | 0.988 |
| Bromophenol blue | Acidic Alumina | 4.990 | 0.005 | 0.081 | 1 | 4.990 | 4.856 | 28.263 | 1 | 0.000 | 0.964 |
| Fast green | Acidic Alumina | 4.959 | 0.004 | 0.138 | 1 | 4.959 | 4.960 | 67.737 | 1 | 0.000 | 0.890 |
| Used silica | 4.560 | 4.273 | 0.113 | 0.923 | 4.560 | 5.552 | 0.025 | 0.980 | 0.158 | 0.976 | |
| Methyl green | Used silica | 4.949 | 0.186 | 0.157 | 0.603 | 4.949 | 4.960 | 2.002 | 1 | 0.003 | 0.891 |
| Alginite | 4.936 | 0.096 | 0.084 | 0.933 | 4.936 | 4.948 | 1.963 | 1 | 0.004 | 0.926 | |
| ACCG | 3.667 | 1.392 | 0.014 | 0.720 | 3.667 | 2.770 | 0.320 | 0.993 | 0.109 | 0.664 | |
| Coarse silica | 4.791 | 4.587 | 0.108 | 0.881 | 4.791 | 6.293 | 0.016 | 0.980 | 0.197 | 0.905 | |
| Neutral red | Used silica | 4.810 | 1.319 | 0.189 | 0.936 | 4.81 | 4.930 | 0.285 | 0.999 | 0.033 | 0.796 |
| Coarse silica | 4.300 | 3.819 | 0.096 | 0.954 | 4.30 | 4.332 | 0.782 | 0.999 | 0.011 | 0.956 | |
| Alginite | 4.730 | 0.118 | 0.051 | 0.75 | 4.73 | 4.759 | 1.137 | 1 | 0.008 | 0.856 | |
| ACCG | 4.43 | 2.328 | 0.068 | 0.885 | 4.43 | 4.482 | 0.3659 | 0.999 | 0.023 | 0.874 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bankole, A.A.; Poulose, V.; Ramachandran, T.; Hamed, F.; Thiemann, T. Comparative Study of the Selective Sorption of Organic Dyes on Inorganic Materials—A Cost-Effective Method for Waste Treatment in Educational and Small Research Laboratories. Separations 2022, 9, 144. https://doi.org/10.3390/separations9060144
Bankole AA, Poulose V, Ramachandran T, Hamed F, Thiemann T. Comparative Study of the Selective Sorption of Organic Dyes on Inorganic Materials—A Cost-Effective Method for Waste Treatment in Educational and Small Research Laboratories. Separations. 2022; 9(6):144. https://doi.org/10.3390/separations9060144
Chicago/Turabian StyleBankole, Anifat Adenike, Vijo Poulose, Tholkappiyan Ramachandran, Fathalla Hamed, and Thies Thiemann. 2022. "Comparative Study of the Selective Sorption of Organic Dyes on Inorganic Materials—A Cost-Effective Method for Waste Treatment in Educational and Small Research Laboratories" Separations 9, no. 6: 144. https://doi.org/10.3390/separations9060144
APA StyleBankole, A. A., Poulose, V., Ramachandran, T., Hamed, F., & Thiemann, T. (2022). Comparative Study of the Selective Sorption of Organic Dyes on Inorganic Materials—A Cost-Effective Method for Waste Treatment in Educational and Small Research Laboratories. Separations, 9(6), 144. https://doi.org/10.3390/separations9060144






