Evaluation of Greenness of LC-MS Chromatographic Methods for Simultaneous Analysis of Mixtures of Serotonin, Dopamine, Acetylcholine, GABA and Glutamate: AGREE Tool Application
Abstract
:1. Introduction
2. Methods
2.1. Greenness Assessment Methods
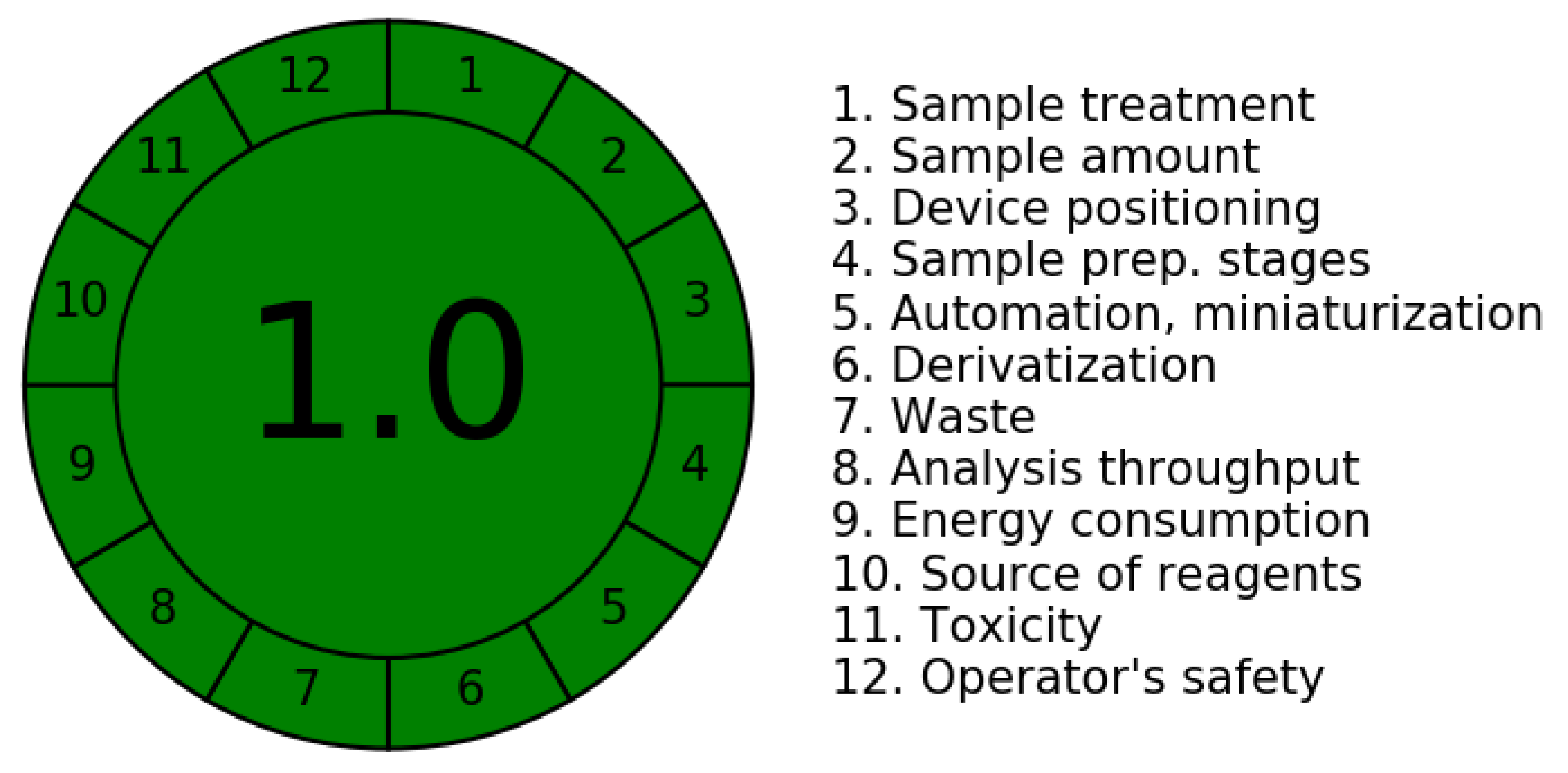
2.1.1. The Analytical GREEnness Metric Tool (AGREE)
- Pretreatment/extraction process or reagents added to the mobile phase to improve chromatographic resolution [24]. Elimination of pretreatment of samples significantly contributes to the greenness by reducing hazard to the environment and operators. The AGREE tool takes into consideration many factors like online, offline, at line, invasiveness and remote sensing in estimating the greenness.
- Minimum quantity and number of samples.
- Location of the device must be as close as possible to the analysis site, which reduces time of analysis.
- Number of steps in the analysis.
- Use of automated and miniaturized devices.
- Circumventing derivatization.
- Volume of waste produced.
- Number of analytes estimated at a time.
- Energy consumption.
- Source of reagents used, as renewable sources are safer.
- Toxicity of the reagents.
- Operator’s safety.
2.1.2. National Environmental Method Index (NEMI)
2.2. Chromatographic Methods under Investigation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuevas, J. Neurotransmitters and Their Life Cycle. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–7. [Google Scholar]
- Knapp, R.; Rubenzik, M.; Malatynska, E.; Varga, E.; Roeske, W.R.; Yamamura, H.I. Neurotransmitter Receptors. In Encyclopedia of the Neurological Sciences; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: New York, NY, USA, 2003; pp. 602–614. [Google Scholar]
- Stevens, L.; Rodin, I. Introduction to drug treatments. In Psychiatry, 2nd ed.; Stevens, L., Rodin, I., Eds.; Churchill Livingstone: London, UK, 2011; pp. 20–21. [Google Scholar]
- Analytical Abstracts. Available online: http://pubs.rsc.org/lus/analytical-abstracts (accessed on 11 December 2020).
- Tang, Y.B.; Sun, F.; Teng, L.; Li, W.B.; An, S.M.; Zhang, C.; Yang, X.J.; Lv, H.Y.; Ding, X.P.; Zhu, L.; et al. Simultaneous determination of the repertoire of classical neurotransmitters released from embryonal carcinoma stem cells using online microdialysis coupled with hydrophilic interaction chromatography-tandem mass spectrometry. Anal. Chim. Acta 2014, 849, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J.; Pi, Z.; Zheng, Z.; Xing, J.; Song, F.; Shu, L.; Liu, Z. Application of online microdialysis coupled with liquid chromatography-tandem mass spectrometry method in assessing neuroprotective effect of Rhizoma coptidis on diabetic rats. Anal. Methods 2015, 7, 45–52. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Hou, C.; Sun, X.; Wang, Z.; Li, S.; Lv, M.; Chen, X. Neuroprotective effect of total glycosides from paeonies against neurotoxicity induced by strychnos alkaloids related to recovering the levels of neurotransmitters and neuroendocrine hormones in rat serum and brain. RSC Adv. 2018, 8, 29210–29219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.L.; Zhu, R.H.; Li, H.D. Determination of dansylated monoamine and amino acid neurotransmitters and their metabolites in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. Anal. Biochem. 2010, 396, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gamal, M.; Naguib, I.A.; Panda, D.S.; Abdallah, F.F. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N-butyl bromide. Anal. Methods 2021, 13, 369–380. [Google Scholar] [CrossRef] [PubMed]
- León, N.; Miralles, P.; Yusà, V.; Coscollà, C. A green analytical method for the simultaneous determination of 30 tropane and pyrrolizidine alkaloids and their N-oxides in teas and herbs for infusions by LC-Q-Orbitrap HRMS. J. Chromatogr. A 2022, 1666, 462835. [Google Scholar] [CrossRef]
- Park, S.-H.; Yang, C.; Ayaril, N.; Szekely, G. Solvent-Resistant Thin-Film Composite Membranes from Biomass-Derived Building Blocks: Chitosan and 2,5-Furandicarboxaldehyde. ACS Sustain. Chem. Eng. 2022, 10, 998–1007. [Google Scholar] [CrossRef]
- Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Green methodology based on active air sampling followed by solid phase microextraction and gas chromatography-tandem mass spectrometry analysis to determine hazardous substances in different environments related to tire rubber. J. Chromatogr. A 2022, 1668, 462911. [Google Scholar] [CrossRef]
- Hu, J.; Hardian, R.; Gede, M.; Holtzl, T.; Szekely, G. Reversible crosslinking of polybenzimidazole-based organic solvent nanofiltration membranes using difunctional organic acids: Toward sustainable crosslinking approaches. J. Membr. Sci. 2022, 648, 120383. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Cann, M.C.; Dickneider, T.A. Infusing the Chemistry Curriculum with Green Chemistry Using Real-World Examples, Web Modules, and Atom Economy in Organic Chemistry Courses. J. Chem. Educ. 2004, 81, 977. [Google Scholar] [CrossRef]
- Hjeresen, D.L.; Boese, J.M.; Schutt, D.L. Green Chemistry and Education. J. Chem. Educ. 2000, 77, 1543. [Google Scholar] [CrossRef]
- Gionfriddo, E. Green analytical solutions for sample preparation: Solid phase microextraction and related techniques. Phys. Sci. Rev. 2020, 5, 6. [Google Scholar] [CrossRef]
- Ibáñez, E.; Cifuentes, A. Moving forward to greener extraction techniques. TrAC Trends Anal. Chem. 2020, 122, 115698. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green Analytical Chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Sergio, A. Green extraction techniques in green analytical chemistry. Trends Anal. Chem. 2019, 116, 248–253. [Google Scholar]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Zampieri, D.; Gennaro, M.C. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3929–3937. [Google Scholar] [CrossRef]
- Kufahl, P.R.; Watterson, L.R.; Nemirovsky, N.E.; Hood, L.E.; Villa, A.; Halstengard, C.; Zautra, N.; Olive, M.F. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology 2013, 66, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Environmental Methods Index. Available online: www.nemi.gov (accessed on 20 January 2021).
- Tobiszewski, M.; Mechlińska, A.; Namieśnik, J. Green analytical chemistry—Theory and practice. Chem. Soc. Rev. 2010, 39, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rauch, A.; Lee, H.; Xiao, H.; Rainer, G.; Logothetis, N.K. Capillary hydrophilic interaction chromatography/mass spectrometry for simultaneous determination of multiple neurotransmitters in primate cerebral cortex. Rapid Commun. Mass Spectrom. 2007, 21, 3621–3628. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Canela, C.; Tornero-Cañadas, D.; Prats, E.; Piña, B.; Tauler, R.; Raldúa, D. Comprehensive characterization of neurochemicals in three zebrafish chemical models of human acute organophosphorus poisoning using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 1735–1748. [Google Scholar] [CrossRef]
- Bergh, M.S.-S.; Bogen, I.L.; Lundanes, E.; Øiestad, Å.M.L. Validated methods for determination of neurotransmitters and metabolites in rodent brain tissue and extracellular fluid by reversed phase UHPLC–MS/MS. J. Chromatogr. B 2016, 1028, 120–129. [Google Scholar] [CrossRef]
- Pérez, R.L.; Escandar, G.M. Experimental and chemometric strategies for the development of Green Analytical Chemistry (GAC) spectroscopic methods for the determination of organic pollutants in natural waters. Sustain. Chem. Pharm. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Eldin, A.B.; Shalaby, A.; Abdallah, M.S.; Shaldam, M.A.; Abdallah, M.A. Applying green analytical chemistry (GAC) for development of stability indicating HPLC method for determining clonazepam and its related substances in pharmaceutical formulations and calculating uncertainty. Arab. J. Chem. 2019, 12, 1212–1218. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.B.; Gamal, M.; Naguib, I.A.; Ali, H.M.; Abdallah, F.F. Environmental impact of the reported chromatographic methods for the determination of the first FDA-Approved therapy for COVID-19 Patients, Remdesivir: A comparative study. Microchem. J. 2022, 176, 107242. [Google Scholar] [CrossRef]
- Almalki, A.H.; Naguib, I.A. Application of Three Ecological Assessment Tools in Examining Chromatographic Methods for the Green Analysis of a Mixture of Dopamine, Serotonin, Glutamate and GABA: A Comparative Study. Molecules 2021, 26, 5436. [Google Scholar] [CrossRef]
- Alexovič, M.; Andruch, V.; Balogh, I.S.; Šandrejová, J. A single-valve sequential injection manifold (SV-SIA) for automation of air-assisted liquid-phase microextraction: Stopped flow spectrophotometric determination of chromium(vi). Anal. Methods 2013, 5, 2497–2502. [Google Scholar] [CrossRef]

| Method | Instrument and Chromatographic Settings | AGREE Assessment Method Pictogram Plot | NEMI Assessment Method Pictogram Plot |
|---|---|---|---|
| 1 |
|  |  |
| 2 |
|  |  |
| 3 |
|  |  |
| 4 |
|  |  |
| 5 |
|  |  |
| 6 |
|  |  |
| Evaluation Criteria | Method * | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| Overall Greenness score out of 1 | 0.66 | 0.56 | 0.43 | 0.39 | 0.6 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almalki, A.H.; Alsalahat, I.; Alharthi, M.A.; Panda, D.S.; Almahri, A.; Naguib, I.A. Evaluation of Greenness of LC-MS Chromatographic Methods for Simultaneous Analysis of Mixtures of Serotonin, Dopamine, Acetylcholine, GABA and Glutamate: AGREE Tool Application. Separations 2022, 9, 147. https://doi.org/10.3390/separations9060147
Almalki AH, Alsalahat I, Alharthi MA, Panda DS, Almahri A, Naguib IA. Evaluation of Greenness of LC-MS Chromatographic Methods for Simultaneous Analysis of Mixtures of Serotonin, Dopamine, Acetylcholine, GABA and Glutamate: AGREE Tool Application. Separations. 2022; 9(6):147. https://doi.org/10.3390/separations9060147
Chicago/Turabian StyleAlmalki, Atiah H., Izzeddin Alsalahat, Muath A. Alharthi, Dibya Sundar Panda, Albandary Almahri, and Ibrahim A. Naguib. 2022. "Evaluation of Greenness of LC-MS Chromatographic Methods for Simultaneous Analysis of Mixtures of Serotonin, Dopamine, Acetylcholine, GABA and Glutamate: AGREE Tool Application" Separations 9, no. 6: 147. https://doi.org/10.3390/separations9060147
APA StyleAlmalki, A. H., Alsalahat, I., Alharthi, M. A., Panda, D. S., Almahri, A., & Naguib, I. A. (2022). Evaluation of Greenness of LC-MS Chromatographic Methods for Simultaneous Analysis of Mixtures of Serotonin, Dopamine, Acetylcholine, GABA and Glutamate: AGREE Tool Application. Separations, 9(6), 147. https://doi.org/10.3390/separations9060147







