Abstract
The extractive distillation of a methanol and dimethyl carbonate (DMC) azeotrope system was taken as an example, and the simulation and optimization of the conventional extractive process (CEP) and extractive dividing wall column (EDWC) were carried out by Aspen Plus software. In order to meet the requirements of separation, lower energy consumption and investment cost were obtained by using a univariate analysis of the optimal operating parameters of the EDWC. The coupling mechanism of the EDWC was described. The results showed that the number of theoretical plates of EDWC was 36, which was lower than the sum of theoretical plates in the two columns of CEP. At the same time, compared with the CEP, the energy consumption of the EDWC could save up to 16.09% and 11.85%, respectively.
1. Introduction
A large number of chemical raw materials for chemical production are toxic, which will do great harm to human beings and the environment. For the sustainable development of the human living environment, at the end of the 1960s, human beings began to pay attention to environmental problems. Various countries have also promulgated environmental protection laws and regulations and formulated emission standards for various pollutants. However, this terminal control of pollution can only play a role in controlling pollution for a certain range of time. The fundamental solution to pollution control is to use “green technology” chemical production, as far as possible to choose non-toxic or low toxicity chemical raw materials and as far as possible to use non-pollutant chemical processes [1]. Therefore, it is urgent to find non-toxic and harmless basic chemical raw materials. Dimethyl carbonate (DMC), as a non-toxic and non-corrosive liquid, is one of the green chemical products with development prospects in recent years. It can be used as an important component of polycarbonate and other organic syntheses, methylating agent, solvent and fuel additive for lithium-ion batteries, etc. In the synthesis process of DMC, the most promising synthesis route is to use vinyl carbonate as the intermediate and then vinyl carbonate and methanol to further synthesize DMC and glycerol. The efficient separation of methanol and DMC azeotrope system is involved in the synthesis process [2,3]. In industry, special distillation methods such as extractive distillation [2,4,5], azeotropic distillation [6], membrane separation [7,8] and variable pressure distillation [9,10] are often used to separate methanol and DMC. The selection of extractant is the key to extractive distillation separation. Extractants such as vinyl carbonate, aniline and ethylene glycol have been reported [3,11], among which aniline as an extraction agent can save 25.46% of equipment investment and 42.30% of the total cost [11]. Therefore, aniline was selected as the extraction agent despite its toxicity. However, rectification column energy consumption is huge, thermodynamic efficiency is low, so more and more experts are committed to the development of high energy efficiency of the process, in order to reduce energy consumption and CO2 emissions, to help achieve the “dual carbon” goal.
A dividing wall column (DWC) is a special structure of thermocouple distillation [12,13,14]. Partitions are installed in the column to prevent the back-mixing effect and greatly reduce energy consumption [14]. In recent years, researchers have focused on the application of dividing wall column technology in special distillation systems, such as reactive distillation [15], extractive distillation [16], azeotropic distillation [17] and other new fields, in order to minimize energy consumption. In this paper, the methanol and DMC azeotrope system will be separated by the organic fusion of an extractive distillation column and the introduction technology of the DWC, namely, the extractive dividing wall column (EDWC) [18,19,20,21,22,23]. Peng et al. [20] studied the separation of pentane mixture by EDWC, the results showed that the back-mixing could be avoided effectively and the distillation efficiency could be improved by EDWC. Compared with the conventional process, EDWC could save energy by 18.1% and reduce the investment in related column equipment. Shen [22] found that the separation of vinyl acetate–methanol by EDWC could save total energy consumption by 46.8% and total cost by RMB 11.797 million per year. In this paper, the simulation and optimization of the conventional extractive process (CEP) and extractive dividing wall column (EDWC) were carried out by Aspen Plus software. Simultaneously, in order to meet the requirements of separation, lower energy consumption and investment cost were obtained by using a univariate analysis of the optimal operating parameters of the EDWC.
2. Method
2.1. Design Method
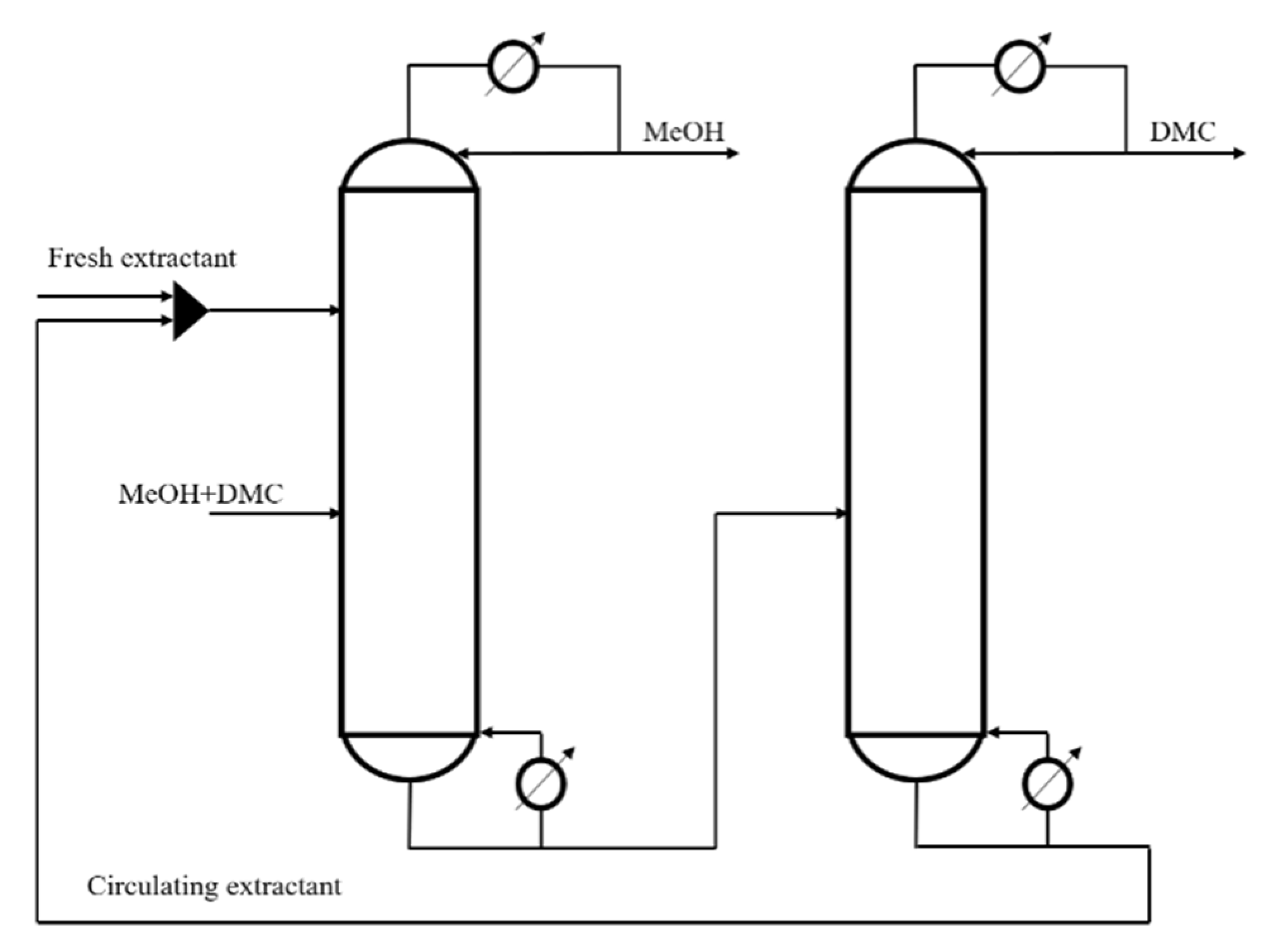
The conventional extractive distillation process (CEP) generally adopts double column operation, as shown in Figure 1, including extractive distillation column (EDC) and extraction agent recovery column (ERC). The extractant is fed from the upper EDC, the methanol and DMC liquid are fed from the lower part of the EDC. Here, high purity methanol is obtained at top of the EDC, the bottom of the EDC feeds the extractant and DMC mixture into the ERC, then high purity DMC is obtained at the top of the EDC and the bottom receives high purity extractant, which is fed into the EDC for recycling.
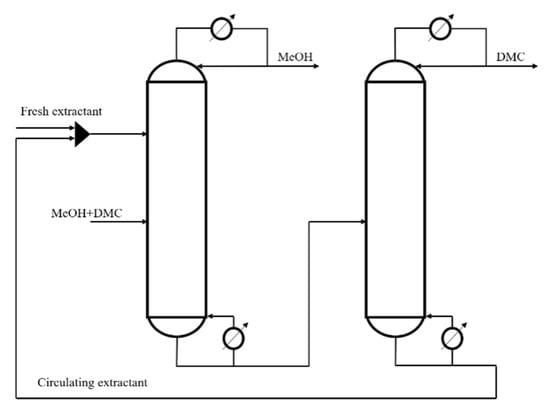
Figure 1.
Conventional extractive process.
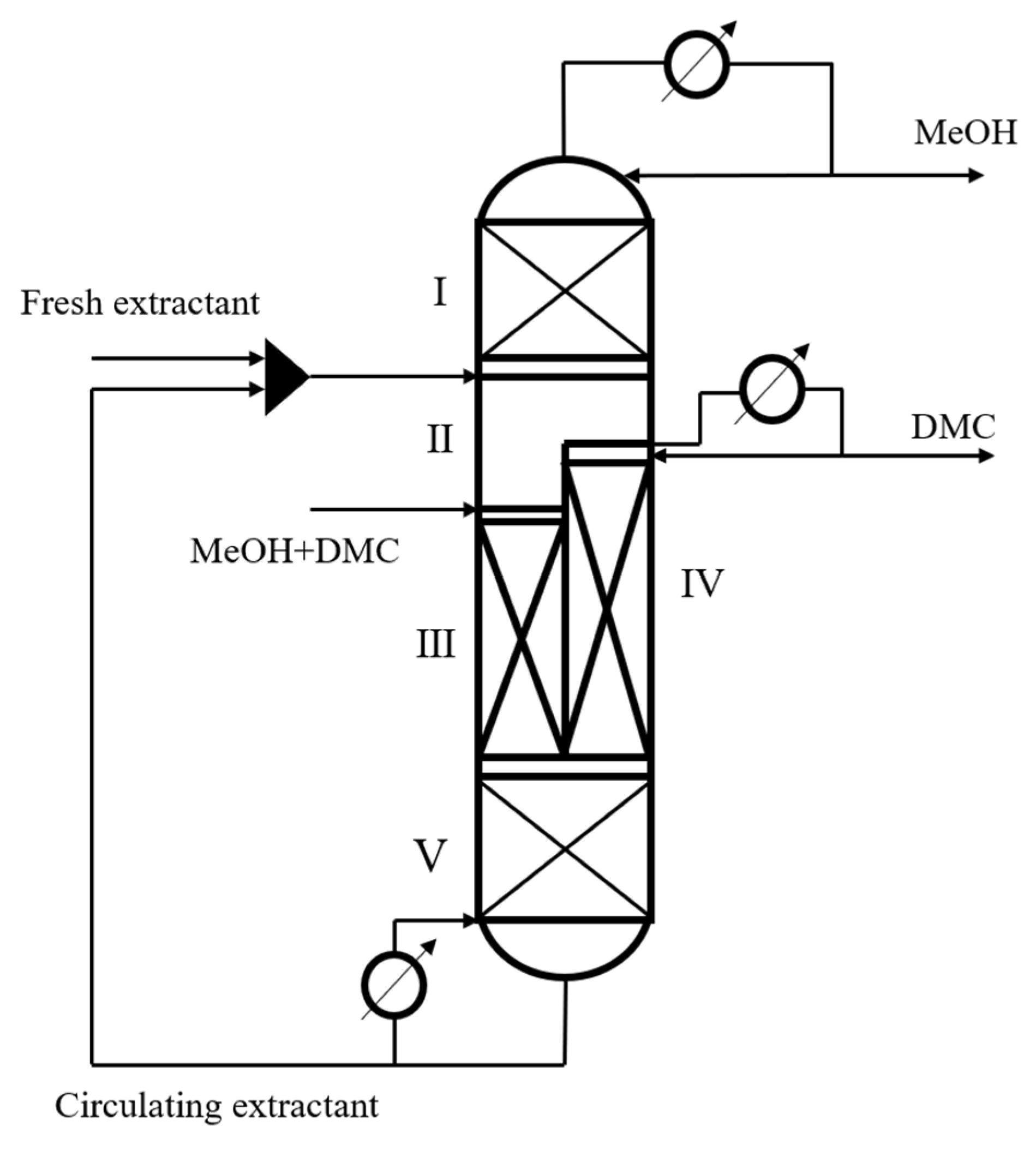
On the basis of CEP, the performance of EDWC was investigated, whose structure [12] is shown in Figure 2. A partition is set in the middle of the column, and the whole column is divided into 5 different zones according to the feeding position of extractant and raw material. Zone (I) is the extraction agent recovery section, which mainly realizes the removal of the extraction agent aniline and obtains high purity methanol products at the top of the column, which is equivalent to the distillation section above the feeding position of the extraction agent of the EDC in the CEP. Zone (II) is the distillation section, which mainly realizes the extraction of DMC from the raw material liquid by the extraction agent aniline, which is equivalent to the distillation section of the EDC in the CEP between the extraction agent feeding position and the raw material liquid feeding position. Zone (III) is the extractive distillation section, which mainly removes a small amount of methanol and is equivalent to the extractive distillation section of the EDC in the CEP. Zone (IV) is the side-line distillation section, which mainly achieves the separation of DMC and the extraction agent aniline, and obtains DMC products with a mass fraction higher than 99.8% at the top, which is equivalent to the distillation section of the ERC in the CEP. Zone (V) is the extraction and distillation section of the extractant, which mainly realizes the concentration of the extraction agent aniline. This zone is equivalent to the extraction and distillation section of the ERC in the CEP. Zones (I), (II), (III) and (V) are called the main column, and zone (IV) is the attached column.
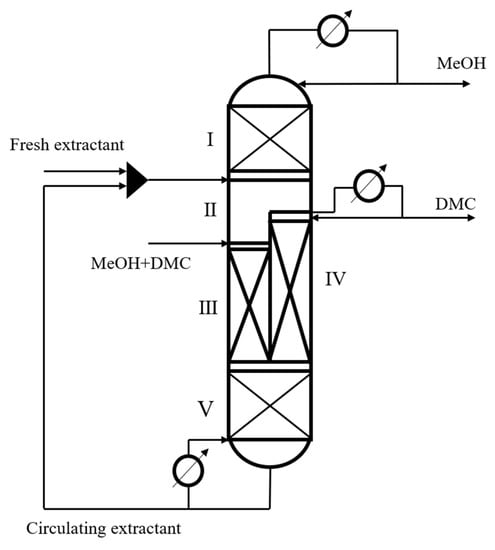
Figure 2.
Extractive dividing wall column structure, (I) the extraction agent recovery section; (II) the distillation section; (III) the extractive distillation section; (IV) the side-line distillation section; (V) the extraction and distillation section.
2.2. Simulation Conditions
The UNIQUAC thermodynamic model is used in this textual method. The corresponding binary parameters were according to reference [5] and related physical parameters are shown in Table 1. Process conditions: operating pressure was normal pressure, DMC molar fraction was 0.154, methanol molar fraction was 0.844, other component is 0.002, which were from reference [5]; Separation requirements: the quality purity of DMC is not less than 0.998. The optimal operation parameters of CEP are not described in detail in this paper, which were obtained by univariate analysis optimization with the objective function of similar separation requirements of EDWC. When one of the variables is analyzed, the others are all optimal.

Table 1.
Physical parameters.
2.3. Process Modeling
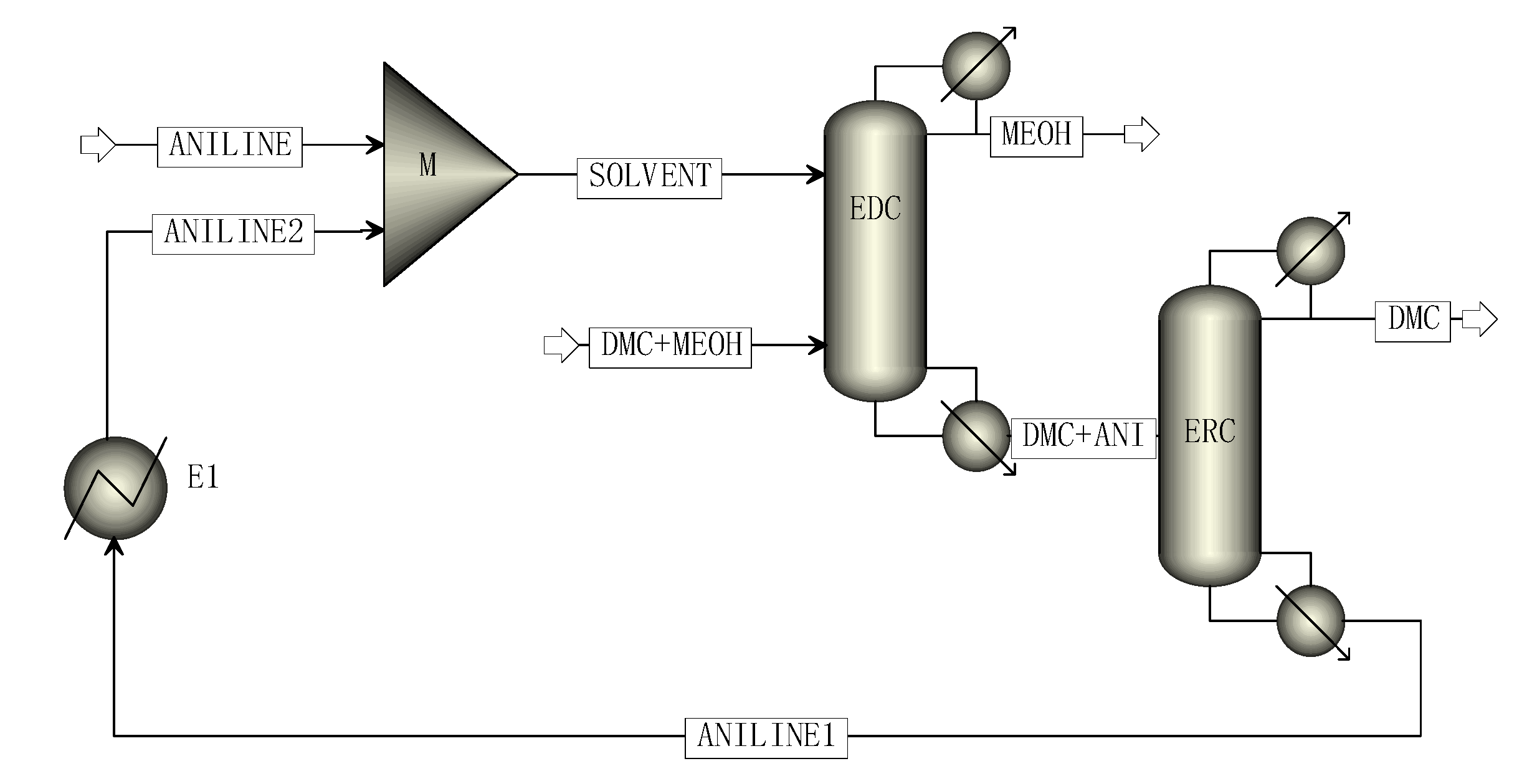
The two-column simulation process of CEP is shown in Figure 3, including the extractive distillation column (EDC) and extractant recovery column (ERC). Finally, high purity methanol products were obtained at the top of the EDC column and high purity DMC products were obtained at the top of the ERC column. At the bottom of the ERC column was the extraction agent aniline, which was cooled by a heat exchanger and mixed with fresh extraction agent to enter the EDC for recycling.
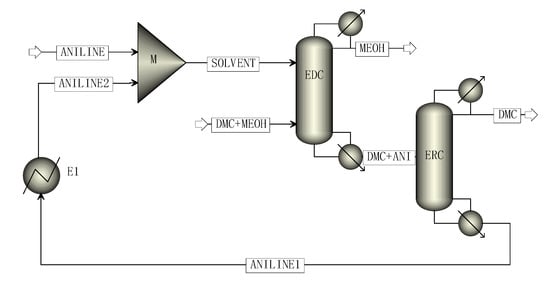
Figure 3.
The simulation process of CEP.
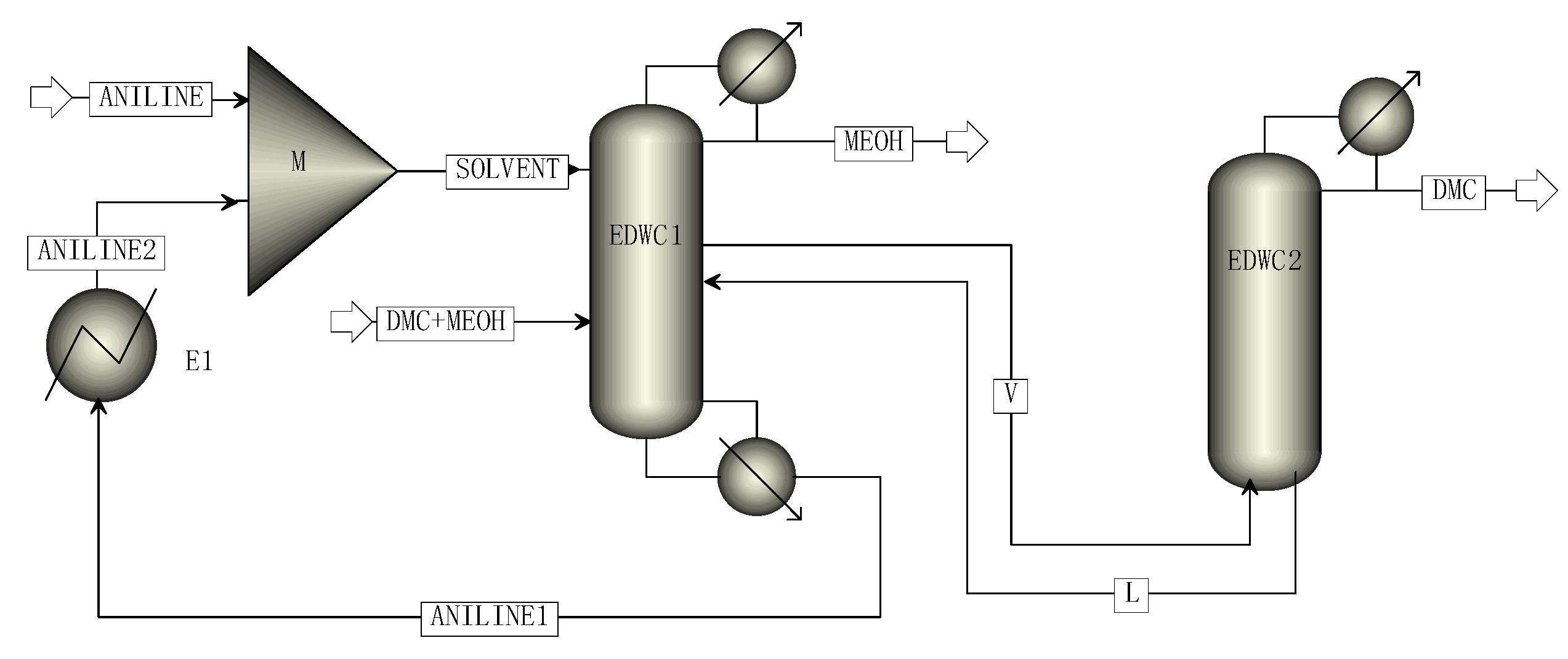
The simulation process of methanol and DMC by EDWC is shown in Figure 4, including the main column (EDWC1) and the attached column (EDWC2). Finally, high purity methanol products were obtained at the top of the main column EDWC1, and high purity DMC products were obtained at the top of the attached column EDWC2. At the bottom of the attached column, the mixture of aniline and DMC was returned to the main column (EDWC1) for further separation.
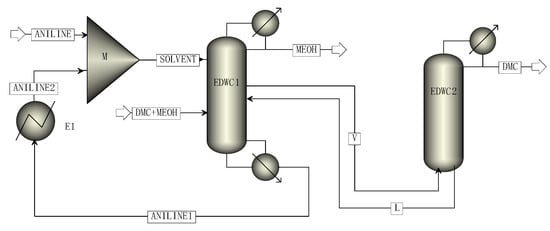
Figure 4.
The simulation process of EDWC.
3. Results and Discussion
3.1. Univariate Analysis
The operation parameters of the column were related to operating cost and equipment cost. In this section, several important parameters affecting the operation performance of the EDWC were analyzed, especially the effects on product purity and energy consumption.
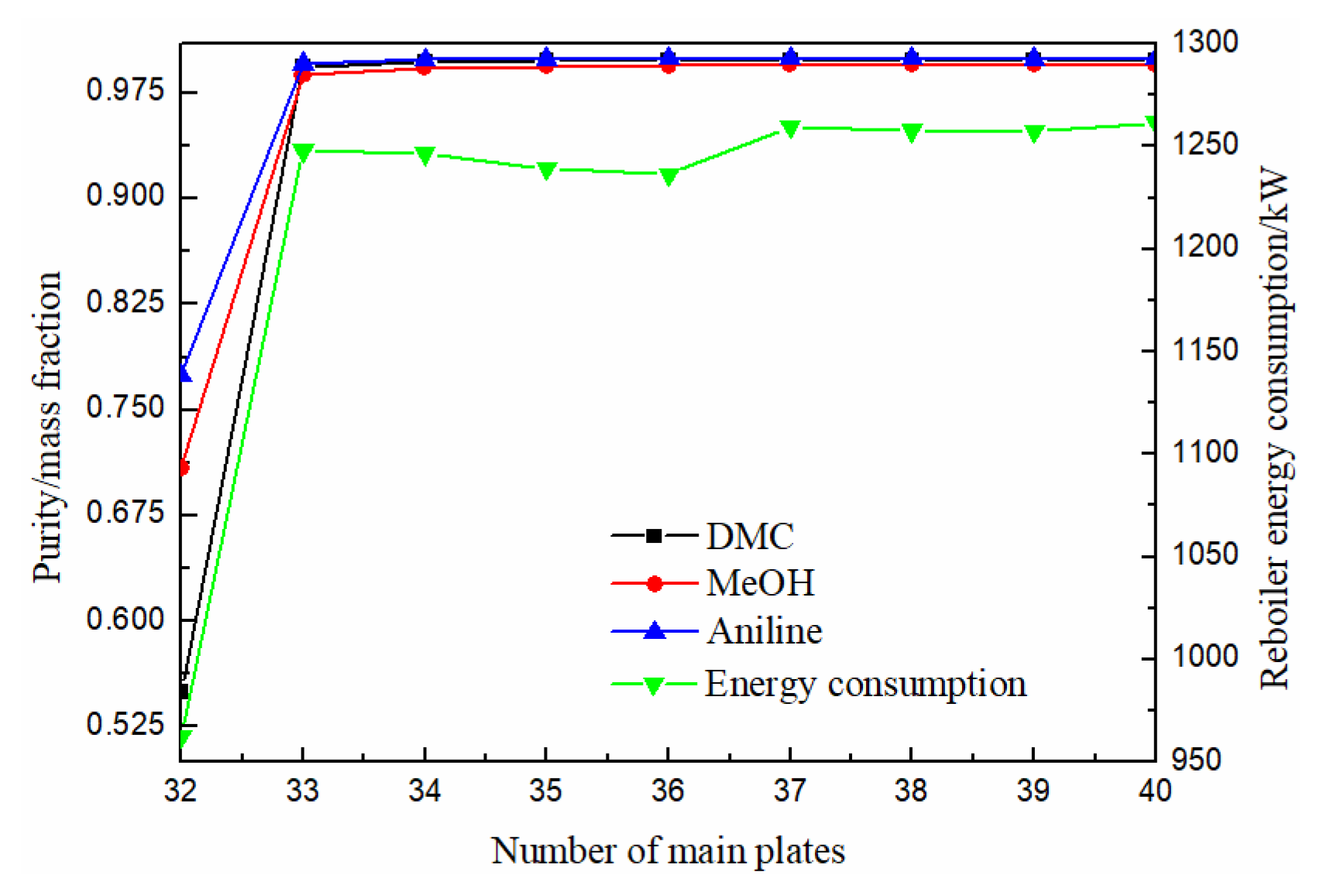
3.1.1. Effect of Number of Main Plates
Relative to the CEP, the structure of EDWC is relatively complex with difficult column internal parts processing and higher installation cost. Therefore, under the premise of separation conditions and low energy consumption, the number of main plates should be as few as possible to reduce the equipment investment cost of the EDWC.
As can be seen from Figure 5, the purity of methanol, DMC and solvent aniline all increased first and then slowly increased with the increase of the number of the main column, and finally tended to be stable. When the theoretical plate number was 36, the purity of DMC reached above 0.998, and the energy consumption column was relatively low. Therefore, the optimal theoretical plate number for the main column was determined to be 36.
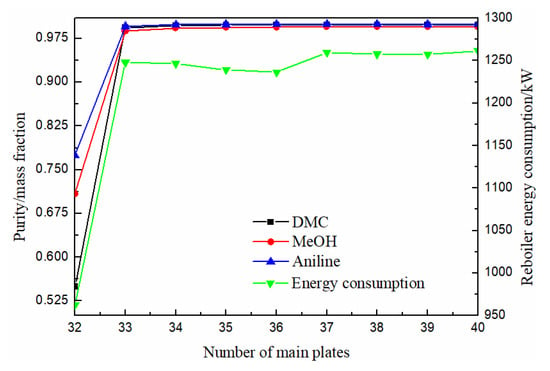
Figure 5.
Effect of number of main plates on product purity and heat duty of reboiler.
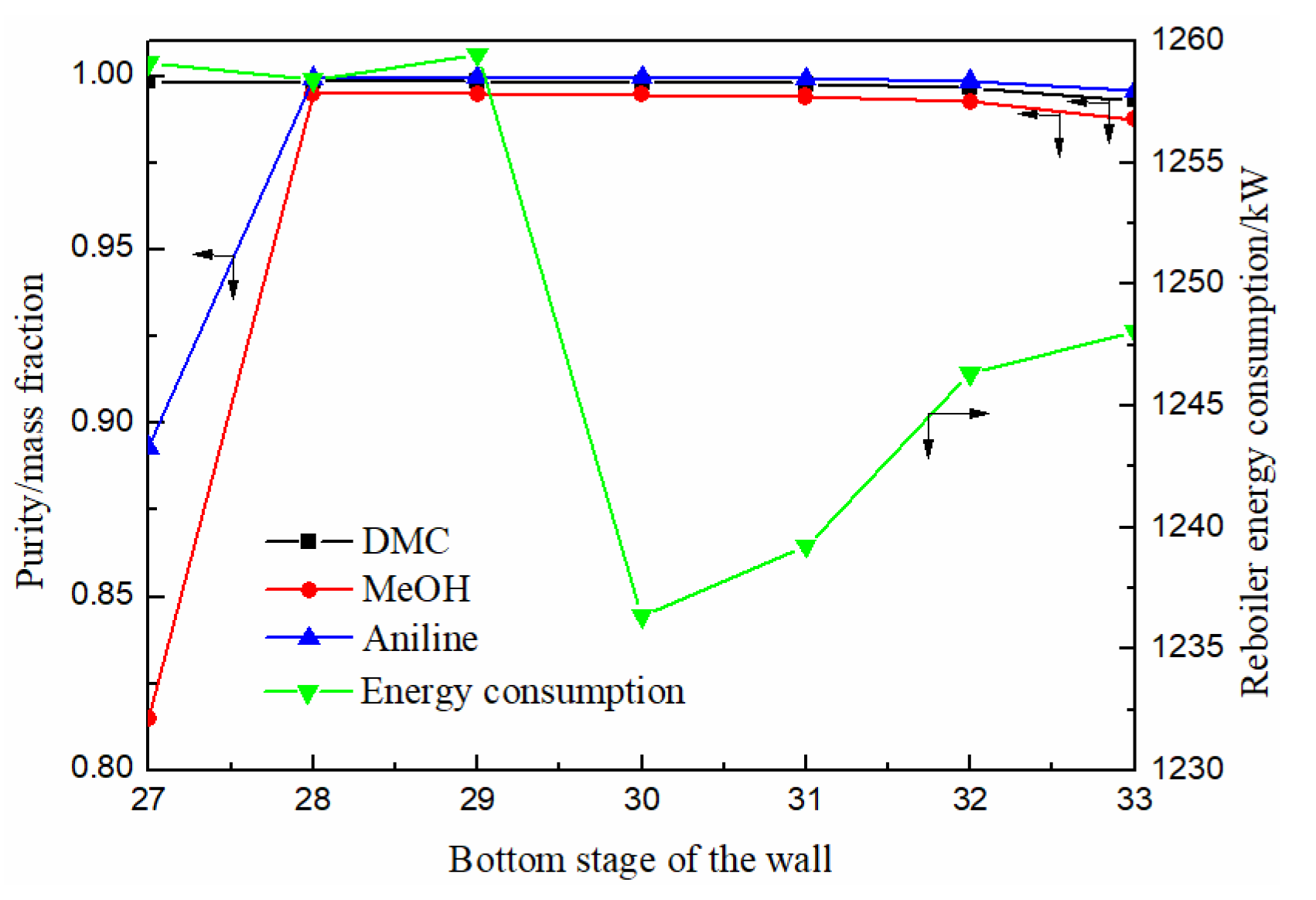
3.1.2. Effect of Bottom Stage of the Partition
The biggest difference between EDWC and CEP is the existence of partition in EDWC to achieve extractive distillation and extraction agent recovery in one column. So, the position of the partition is very important. The partition plate is a tray extending upward from the middle of the main column, which divides the column into five different sections. The number of plates in the extractive distillation section (III) and the side-line distillation section (IV) can be the same or different. Therefore, the best position of the partition needs to consider the position of the bottom of the partition, the position of raw material feeding and the number of attached columns, which will be analyzed one by one.
As can be seen from Figure 6, the purity of DMC decreased slowly with the downward movement of the bottom position of the partition, while methanol and solvent aniline both increased first and then decreased slowly. When the bottom position of the partition was between 27–30 stages, the purity of DMC reached above 0.998, which can meet the separation requirements. The energy consumption decreased first and then increased with the position of the bottom of the partition moving down. When the bottom of the position was located at the 30th stage, the energy consumption of the reboiler reached the lowest. Therefore, the optimal position of the partition was determined as the 30th stage.
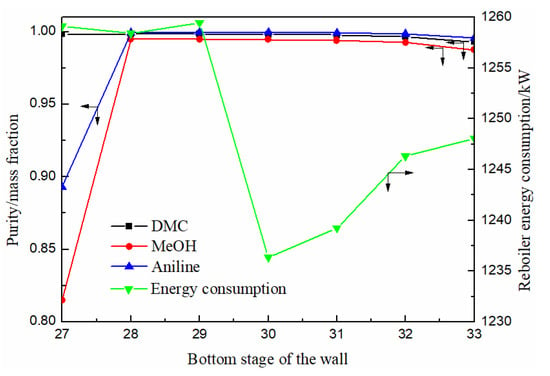
Figure 6.
Effect of bottom stage of the partition on product purity and heat duty of reboiler.
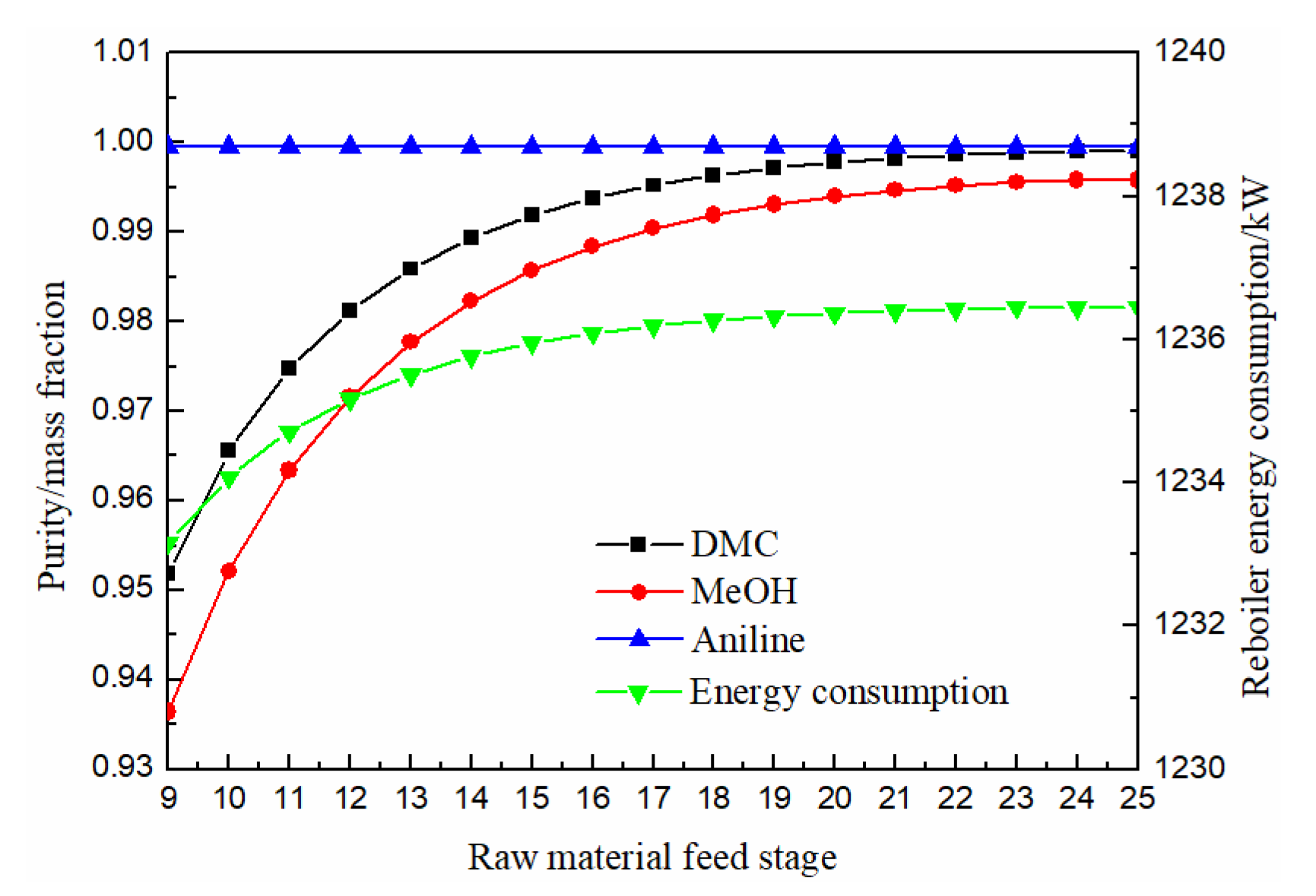
3.1.3. Effect of Raw Material Feed Stage
As can be seen from Figure 7, both methanol and DMC increased first and then slowly stabilized with the downward movement of the feeding position of raw materials. When the feeding position of raw materials was greater than 21 stage, DMC purity reached above 0.998, and solvent aniline was almost not affected by the feeding position of raw materials. The energy consumption column increased first and then slowly stabilized with the downward movement of the feeding position. Therefore, the optimal feeding position of raw material was finally determined as the 21st stage.
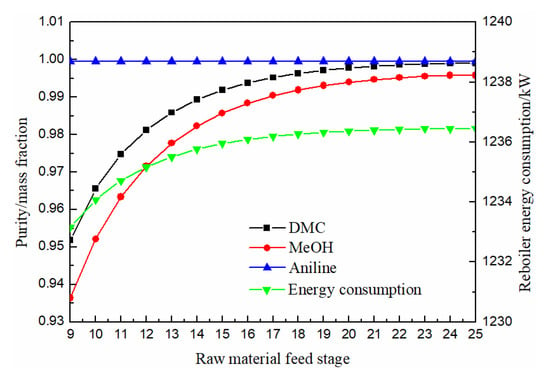
Figure 7.
Effect of raw material feed stage on products purity and heat duty of reboiler.
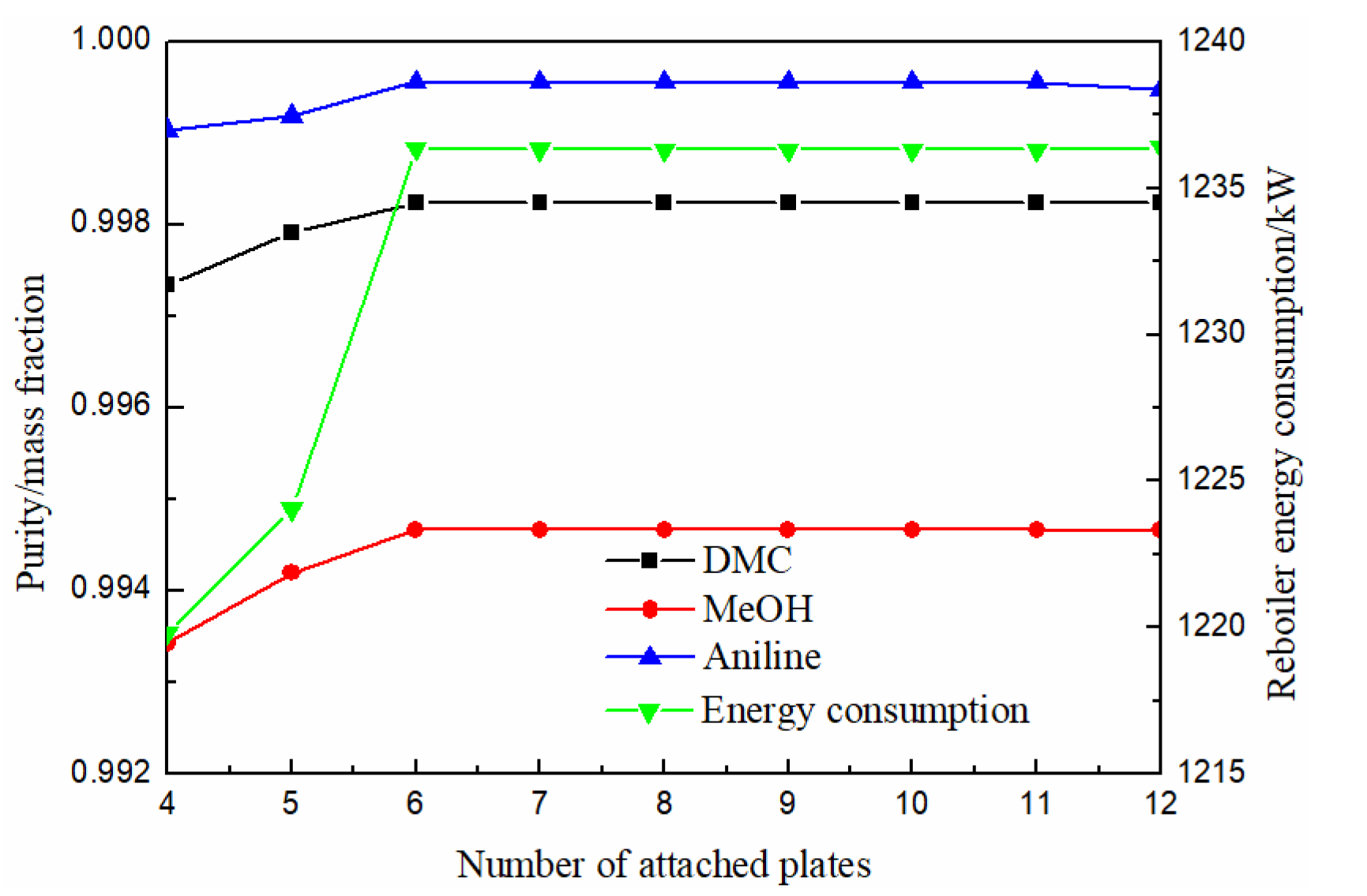
3.1.4. Effect of Number of Attached Plates
It can be seen from the above analysis that the number of theoretical plates of the attached column needs to be determined in order to accurately determine the location of the partition.
As can be seen from Figure 8, the purity of methanol, DMC and solvent aniline all increased first and then slowly stabilized with the increase of theoretical stage number. When the theoretical stage number was more than 6, the purity of DMC reached above 0.998. The energy consumption increased firstly and then tended to be stable with the increase of theoretical stage. In order to facilitate processing, the number of theoretical stages on the left and right sides of the baffle stage was the same. Based on the above, it can be seen that the number of stages in the extraction section (III) was 9, so the optimal number of theoretical stages with attached columns was finally determined to be 9.
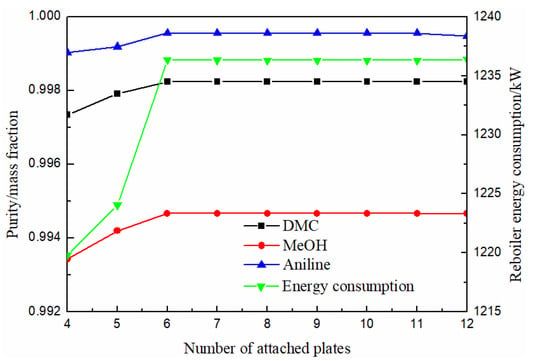
Figure 8.
Effect of numbers of attached plate on products purity and heat duty of reboiler.
3.1.5. Effect of Distribution Ratio of the Vapor Phase
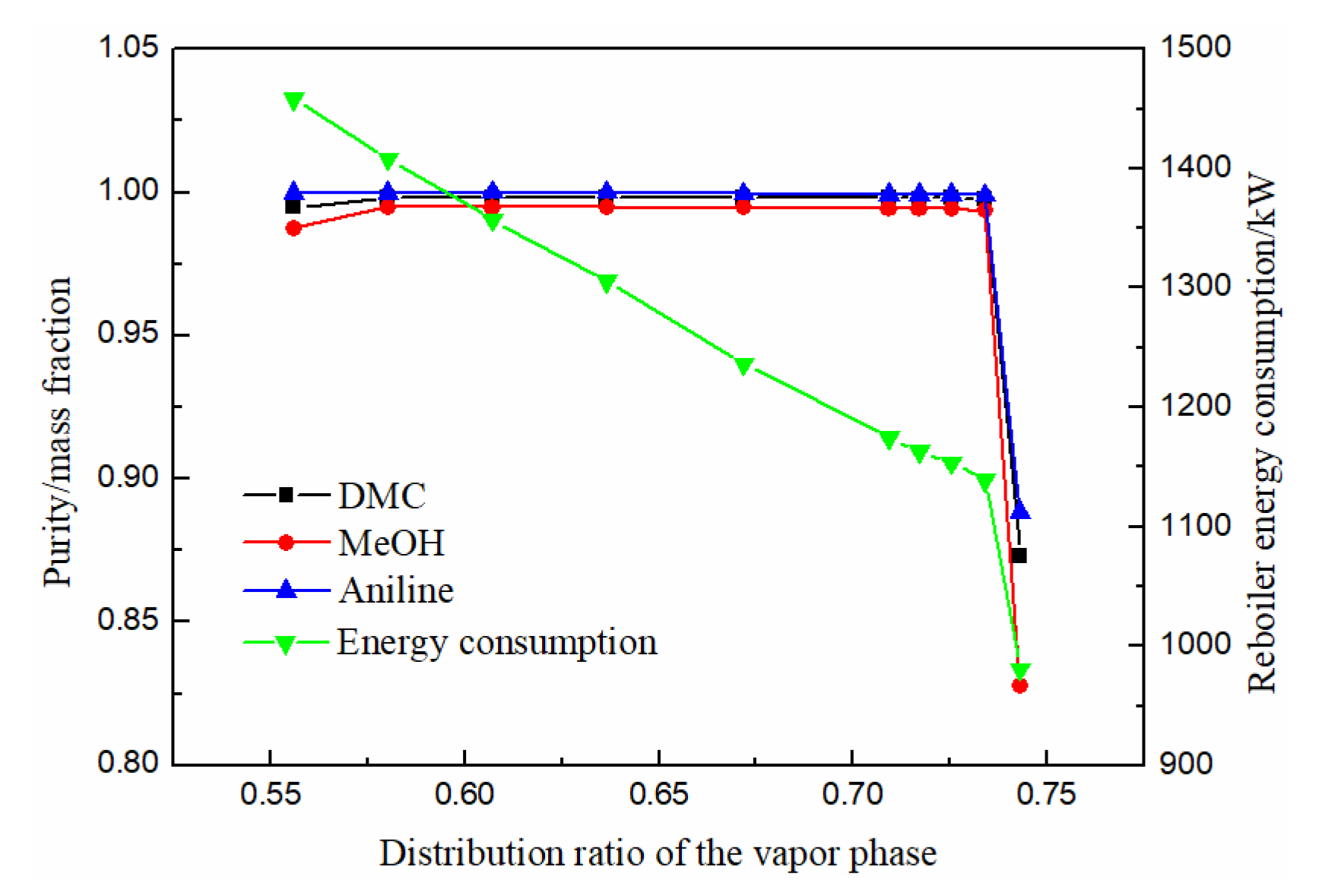
There is only one reboiler at the bottom of the main column, and the rising steam provided by the reboiler is distributed to the extraction section (III) and the side-line distillation section (IV). Whether the distribution is reasonable will affect the vapor–liquid equilibrium relationship between the two sides of the partition, thus affecting the separation effect and the energy consumption of the reboiler. Therefore, its distribution needs to be considered. The gas phase distribution ratio is defined as the ratio of the rising steam in the extractive section (III) to the total rising steam.
As can be seen from Figure 9, the purity of methanol, DMC and solvent aniline all increased at first, then slowly stabilized, and finally decreased rapidly with the increase of the gas phase distribution ratio. When the gas phase distribution ratio was between 0.58 and 0.67, the purity of DMC reached above 0.998. The energy consumption decreased rapidly with the increase in the gas phase distribution ratio. Therefore, the optimal gas phase distribution ratio was 0.67.
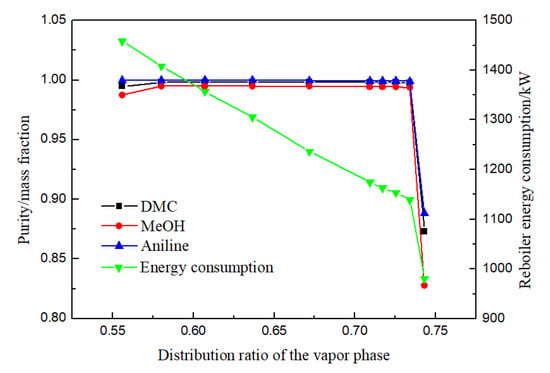
Figure 9.
Effect of distribution ratio of the vapor phase on product purity and heat duty of reboiler.
3.1.6. Effect of Solvent Ratio
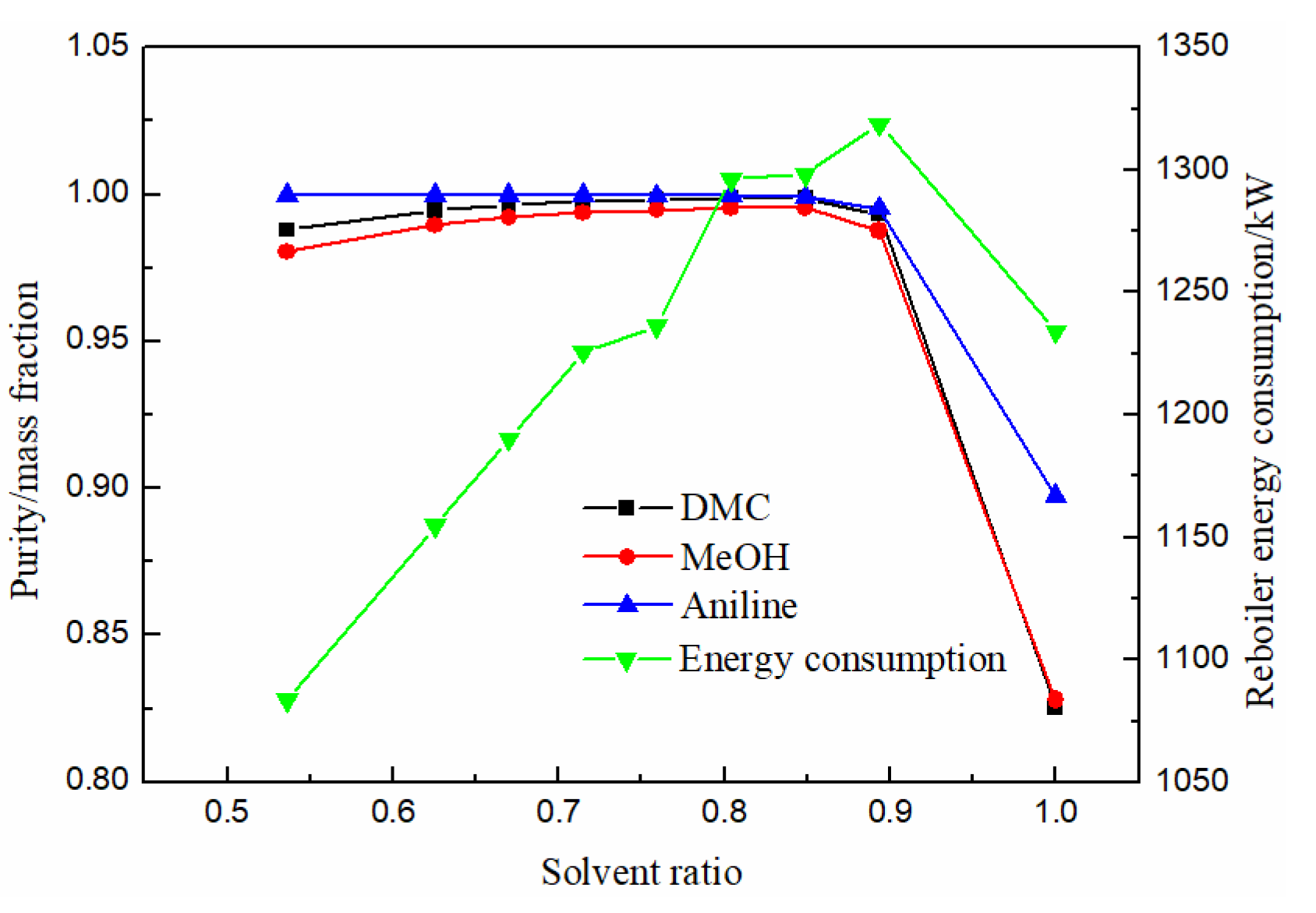
The influence of solvent ratio on product separation effect and energy consumption of reboiler is shown in Figure 10. Methanol, DMC and solvent aniline all increased first with the increase of solvent ratio. When the solvent ratio was 0.76, DMC purity reached the maximum of 0.9982 and then began to decrease. The energy consumption increased gradually and then decreased with the increase in solvent ratio. Therefore, the optimal solvent ratio was 0.76.
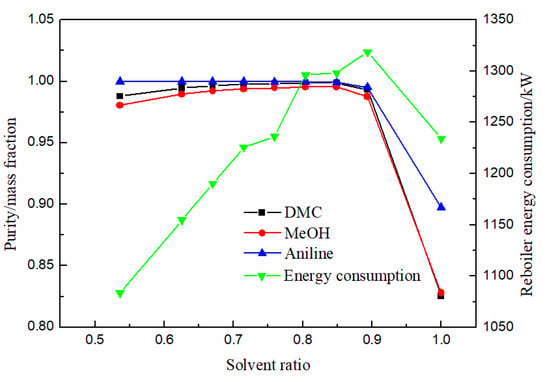
Figure 10.
Effect of solvent ratio on product purity and heat duty of reboiler.
3.1.7. Effect of Reflux Ratio
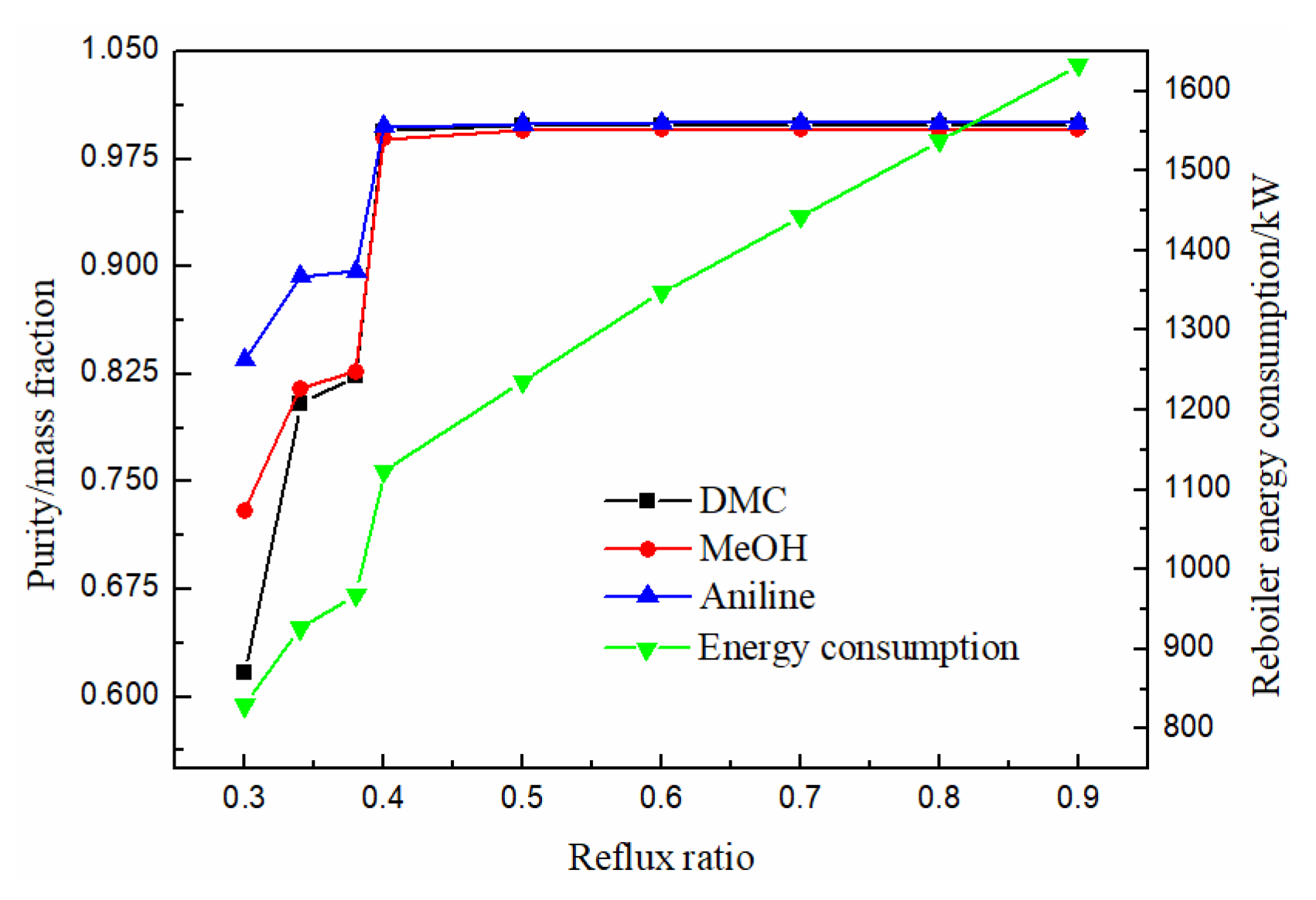
The reflux ratio is an important parameter in the process of a distillation column. Generally, the higher the reflux ratio is, the better the separation effect will be. However, the higher the energy consumption will be, so a reasonable reflux ratio should be selected. As can be seen from Figure 11, the purity of methanol, DMC and solvent aniline all increased with the increase of reflux ratio, and almost remained unchanged when the reflux ratio was greater than 0.5, while the energy consumption of the reboiler at the bottom of the column gradually increased with the increase of reflux ratio. Therefore, the optimal reflux ratio was 0.5.
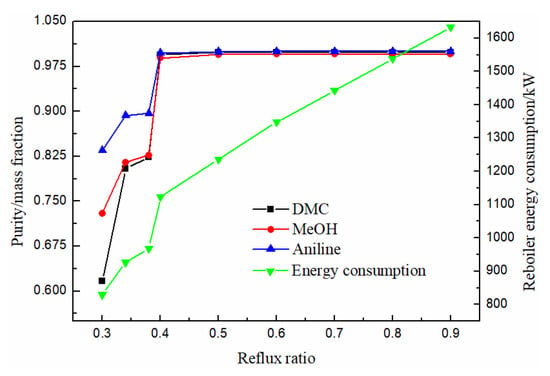
Figure 11.
Effect of reflux ratio on product purity and heat duty of reboiler.
3.2. Profile Analysis
By analyzing the composition distribution and temperature distribution in the column, the profile analysis of CEP and EDWC was carried out to describe the function of each zone of the EDWC and analyse the coupling principle of the extractive dividing wall column.
3.2.1. Composition Distribution Analysis
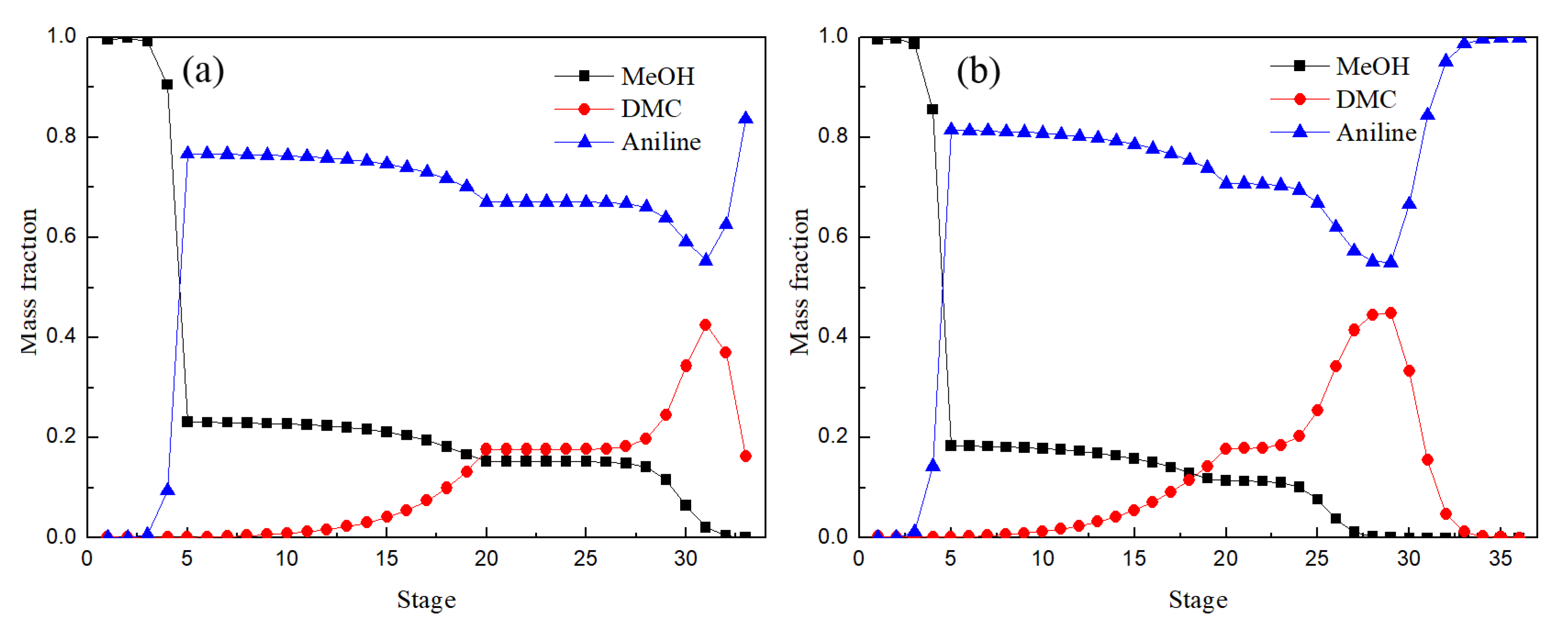
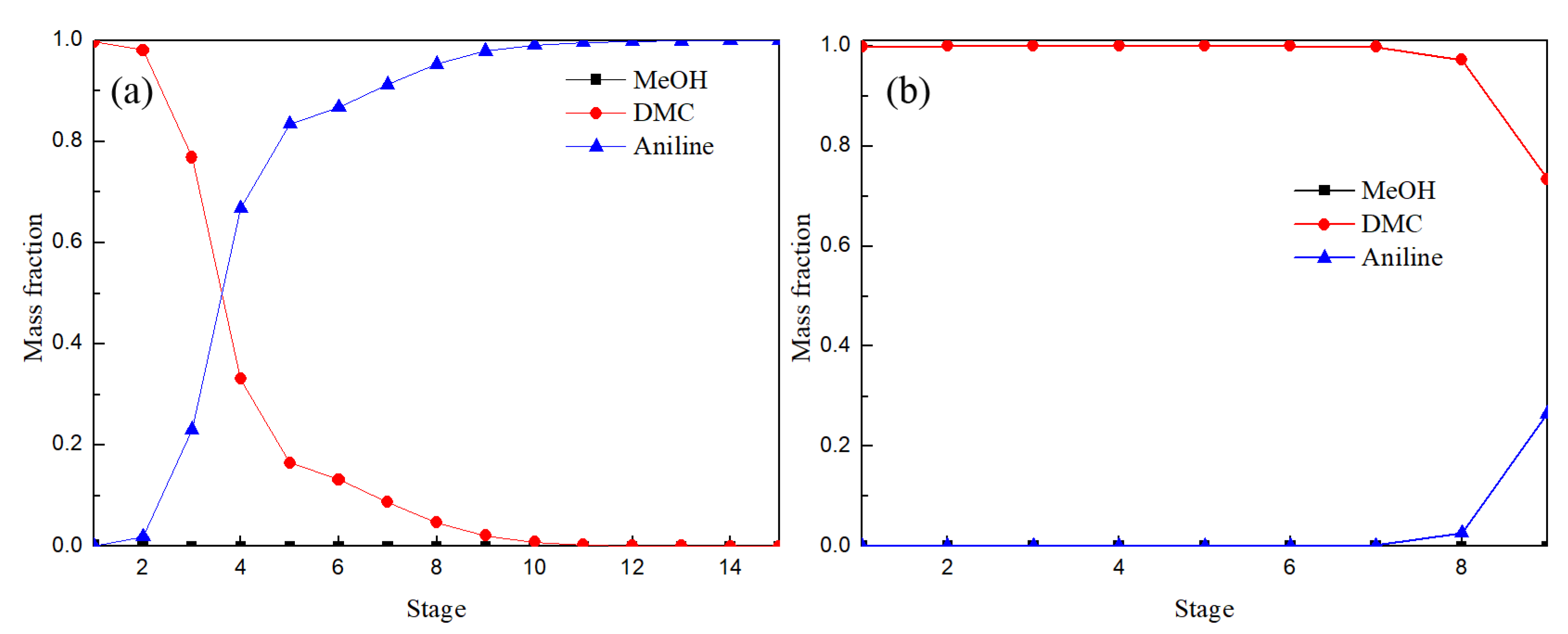
Figure 12 shows the distribution of liquid phase components in the EDC and main column of EDWC. The liquid phase composition distribution trend was basically the same, especially between the 2–5 theoretical stages, where the concentration of DMC from bottom to top reduced rapidly, while the concentration of methanol rose rapidly. These results illustrated that the azeotropic point of methanol and DMC was destroyed due to the addition of solvent aniline for both the CEP and EDWC and the relative volatility of methanol with respect to DMC increased.
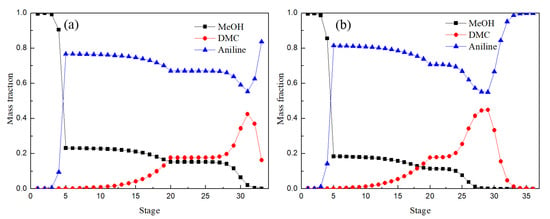
Figure 12.
Liquid composition profiles of EDC (a) and main column of EDWC (b).
It is worth noting that the main column of EDWC realized the function of extractive distillation and extraction solvent recovery in a column because of the existence of the partition, where zone (IV) was to realize the separation of DMC, and zone (V) was to realize the separation of solvent aniline. So, the mass fraction of solvent aniline was close to 1 and the mass fraction of methanol and DMC was close to 0 in the EDWC. It is proved that EDWC can achieve the coupling of extraction and recovery.
Figure 13 shows the composition distribution of the liquid phase in ERC and the attached column of EDWC. By comparing Figure 13a,b, it was found that the liquid phase distribution of 1–3 theoretical stages in the ERC was consistent with that in the attached column of EDWC. The composition of the liquid phase distribution of 4–15 theoretical stages in ERC was similar to that of solvent recovery section V (30–36 theoretical stages) of EDWC as shown in Figure 12b, which fully indicates that EDWC has achieved the coupling of extraction and recovery. Zones IV and V in the EDWC are equivalent to the extractant recovery column in the CEP.
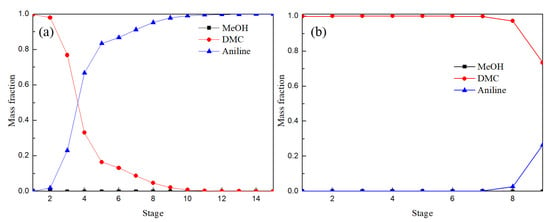
Figure 13.
Liquid composition profiles of ERC (a) and attached column of EDWC (b).
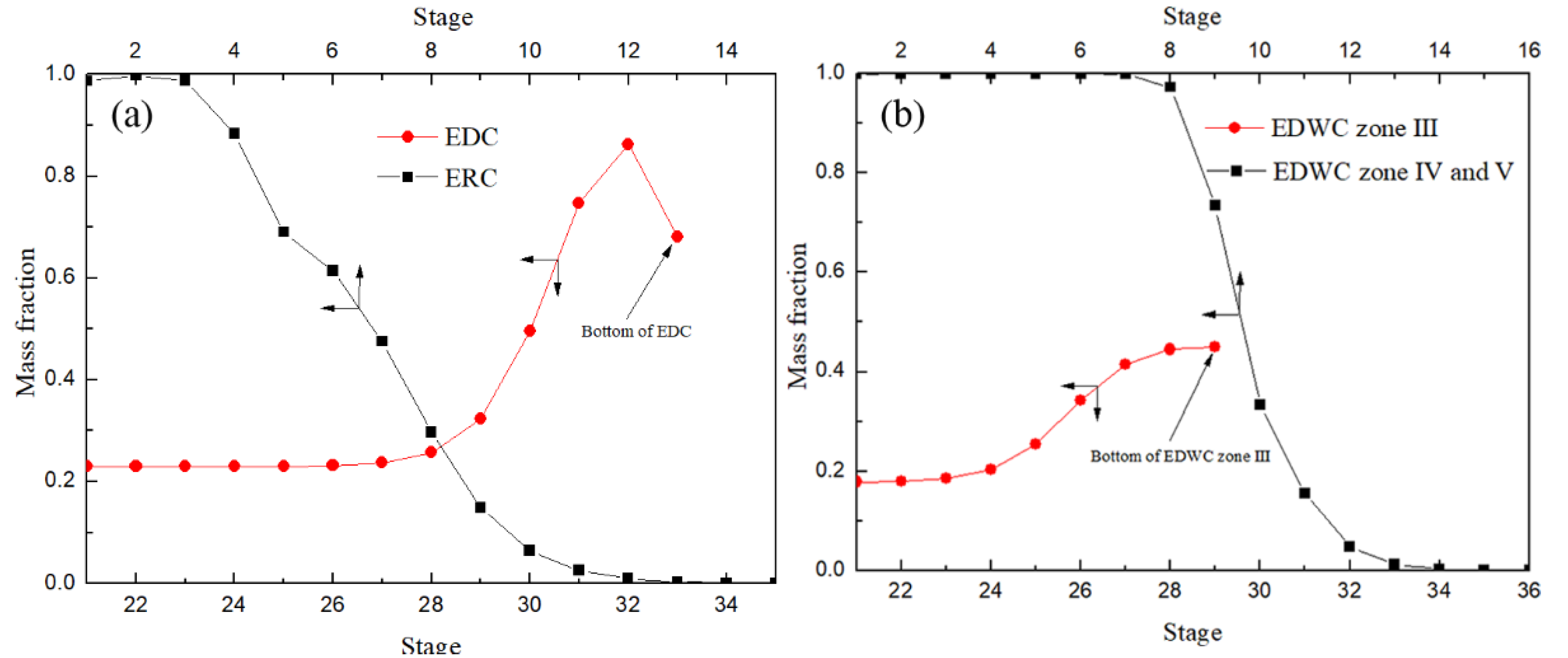
It is worth noting, as shown in Figure 14a, that the DMC mass fraction in CEP with the stage down first increased, and at the bottom of the 31st feed position reached the highest position, and then reduced rapidly, resulting in the DMC at a relatively low concentration to further separation. The results indicated that in CEP existed back-mixing. While the mass fraction of DMC reached the maximum at the bottom of the extraction section (III) of the main column and then entered the side-line distillation section (IV) and solvent recovery section (V) to further separate product DMC and solvent aniline, effectively avoiding back-mixing and improving the separation efficiency. Therefore, the EDWC is more energy efficient than the CEP.
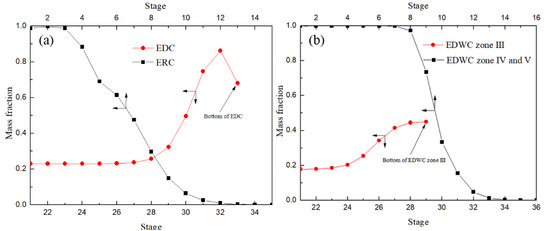
Figure 14.
DMC liquid concentration profiles of CEP (a) and EDWC (b).
3.2.2. Temperature Distribution Analysis
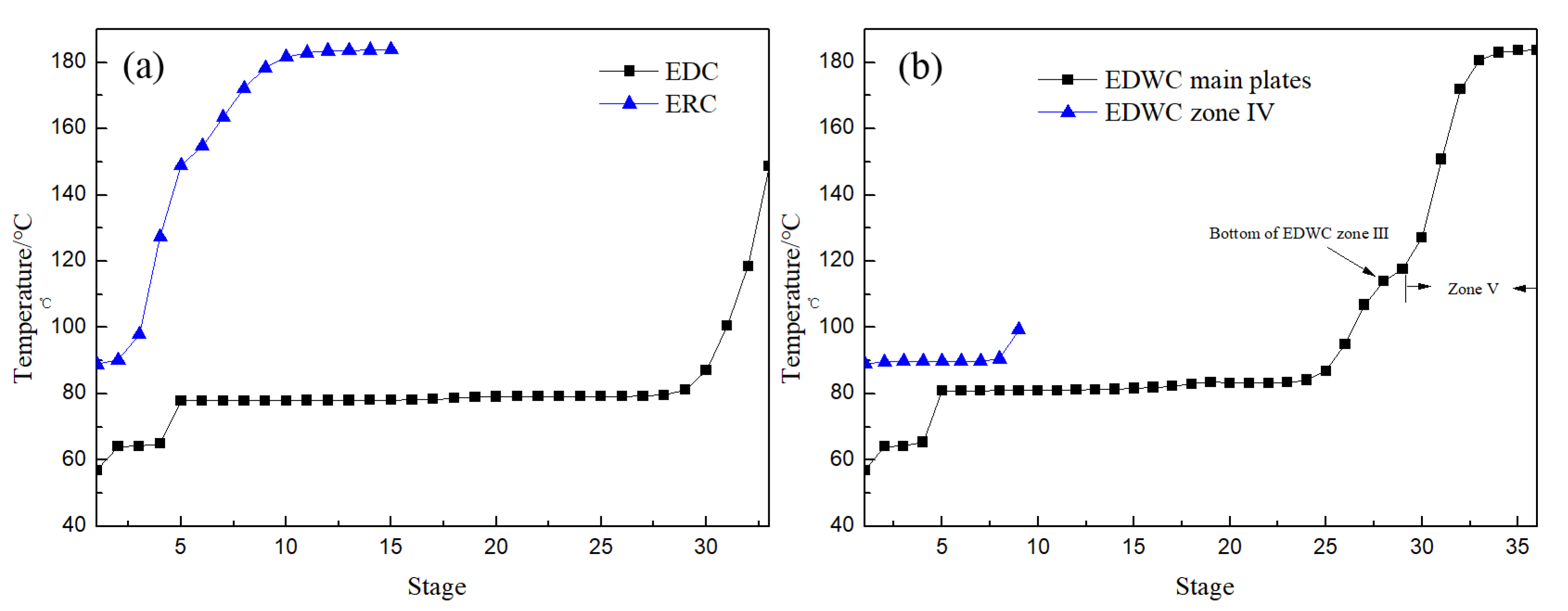
Figure 15a,b is the temperature distribution of CEP and EDWC, respectively. The temperature distribution trend of the EDC in the CEP was basically consistent with that of the main column I, II and III of the EDWC, indicating that zones I, II and III in the EDWC are equivalent to the EDC in the CEP. The other temperature distribution trend of the ERC was similar to the combined temperature distribution trend of the side-line distillation section (IV) and solvent recovery period (V), further proving that sections IV and V are equivalent to the ERC in the CEP.
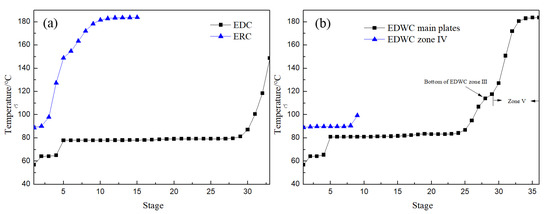
Figure 15.
Temperature profiles of CEP (a) and EDWC (b).
3.3. Comparison and Analysis of Simulation Results
According to the above sensitivity analysis and profile analysis, the optimal operating parameters of the corresponding column are shown in Table 2. It can be seen that the number of theoretical states of the EDWC was 36, which was lower than the sum of theoretical states of the two columns of CEP. At the same time, EDWC has only one reboiler column, which can save some equipment investment costs. Meanwhile, the energy consumption of the reboiler in the EDWC was 1236.32 kW, which was 16.09% less than that of the CEP. The energy consumption of the condenser in the EDWC was 1752.31 kW, which was 11.85% lower than that of the CEP.

Table 2.
Detailed operating parameters for CEP and EDWC.
4. Conclusions
The conventional extractive distillation process and extractive dividing wall column of a methanol–DMC binary azeotrope system were compared and analyzed by Aspen Plus software. The optimal operating parameters of the EDWC were determined by univariate sensitivity analysis. At the same time, the coupling of extraction and recovery was proved by profile analysis. Finally, the number of stages in the EDWC was lower than the sum of the stage required in the CEP, and saved one reboiler, so a certain amount of equipment investment cost was saved. At the same time, the energy consumption of the reboiler and condenser in the EDWC can be saved by up to 16.09% and 11.85%, respectively. Therefore, it is feasible to separate methanol and DMC azeotrope in the EDWC and achieve good results of saving energy and reducing equipment investment.
Author Contributions
Conceptualization, M.Z. and J.W.; methodology, M.Z.; software, M.Z.; validation, M.Z. and J.W.; formal analysis, J.W.; investigation, J.W.; resources, M.Z.; data curation, M.Z.; writing—original draft preparation, J.W.; writing—review and editing, M.Z.; visualization, J.W.; supervision, M.Z.; project administration, M.Z.; funding acquisition, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Ningde Normal University] grant number [2018ZDK08].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We acknowledge the Foundation of Ningde Normal University (No. 2018ZDK08).
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| CEP | Conventional extractive process |
| DMC | Dimethyl carbonate |
| EDWC | Extractive dividing wall column |
| EDC | Extractive distillation column |
| ERC | Extraction agent recovery column |
| MeOH | Methanol |
References
- Li, X.; Ma, Q.; Wei, C.; Cai, W.; Chen, H.; Xing, R.; Song, P. Green and Simple Extraction of Arsenic Species from Rice Flour Using a Novel Ultrasound-Assisted Enzymatic Hydrolysis Method. Separations 2022, 9, 105. [Google Scholar] [CrossRef]
- Hsu, K.-Y.; Hsiao, Y.-C.; Chien, I.-L. Design and Control of Dimethyl CarbonateMethanol Separation via Extractive Distillation in the Dimethyl Carbonate Reactive-Distillation Process. Ind. Eng. Chem. Res. 2009, 49, 735–749. [Google Scholar] [CrossRef]
- Fang, Y.-J.; Xiao, W.-D. Experimental and modeling studies on a homogeneous reactive distillation system for dimethyl carbonate synthesis by transesterification. Sep. Purif. Technol. 2004, 34, 255–263. [Google Scholar] [CrossRef]
- Guo, C.; Wang, F.; Xing, J.; Cui, P. Thermodynamic and economic comparison of extractive distillation sequences for separating methanol/dimethyl carbonate/water azeotropic mixtures. Sep. Purif. Technol. 2022, 282, 120150–120160. [Google Scholar] [CrossRef]
- Yu, B.Y.; Chen, M.K.; Chien, I.L. Assessment on CO2 Utilization through Rigorous Simulation: Converting CO2 to Dimethyl Carbonate. Ind. Eng. Chem. Res. 2017, 57, 639–652. [Google Scholar] [CrossRef]
- Matsuda, H.; Takahara, H.; Fujino, S.; Constantinescu, D.; Kurihara, K.; Tochigi, K.; Ochi, K.; Gmehling, J. Selection of entrainers for the separation of the binary azeotropic system methanol+dimethyl carbonate by extractive distillation. Fluid Phase Equilibria 2011, 310, 166–181. [Google Scholar] [CrossRef]
- Glover, C.J.; Phillips, J.A.; Marchand, E.A.; Hiibel, S.R. Modeling and Life Cycle Assessment of a Membrane Bioreactor—Membrane Distillation Wastewater Treatment System for Potable Reuse. Separations 2022, 9, 151. [Google Scholar] [CrossRef]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Wei, H.M.; Wang, F.; Zhang, J.L.; Liao, B.; Zhao, N.; Xiao, F.K.; Wei, W.; Sun, Y.H. Design and Control of Dimethyl Carbonate–Methanol Separation via Pressure-Swing Distillation. Ind. Eng. Chem. Res. 2013, 52, 11463–11478. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Peng, J.Y.; Zhang, K. Design and control of dimethyl carbonate and methanol separation via heat-integrated pressure swing distillation. Chem. Eng. 2017, 45, 60–65. [Google Scholar]
- Lingna, L.; Tao, F.; Yurong, J. Simulation and economic evaluation of process for synthesizing dimethyl carbonate. Nat. Gas Chem. Ind. 2015, 40, 69–73. [Google Scholar]
- Dejanović, I.; Matijašević, L.; Olujić, Ž. Dividing wall column—A breakthrough towards sustainable distilling. Chem. Eng. Processing Process Intensif. 2010, 49, 559–580. [Google Scholar] [CrossRef]
- Guidat, R.; Vizza, A.; Jiang, Y. Advanced-Flow Reactor Technologies Makes Continuous-Flow Industrial Production Real. Spec. Chem. Mag. 2015, 35, 30–32. [Google Scholar]
- Lestak, F.; Collins, C. Advanced distillation saves energy & capital. Chem. Eng. 1997, 104, 72–76. [Google Scholar]
- Sander, S.; Flisch, C.; Geissler, E.; Schoenmakers, H.; Ryll, O.; Hasse, H. Methyl Acetate Hydrolysis in a Reactive Divided Wall Column. Chem. Eng. Res. Des. 2007, 85, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Ibarra-Sánchez, J.D.J.; Segovia-Hernández, J.G. Reducing energy consumption and CO2 emissions in extractive distillation. Chem. Eng. Res. Des. 2010, 88, 135–145. [Google Scholar] [CrossRef]
- Wang, S.-J.; Lee, C.-J.; Jang, S.-S.; Shieh, S.-S. Plant-wide design and control of acetic acid dehydration system via heterogeneous azeotropic distillation and divided wall distillation. J. Process Control 2008, 18, 45–60. [Google Scholar] [CrossRef]
- Shiyao, L.; Pingli, L.; Yajing, Z.; Yingdong, L.; Heying, C.; Enxia, S. Advances on applications of dividing wall column in extractive distillation technology. Mod. Chem. Ind. 2019, 39, 65–69. [Google Scholar]
- Xingwu, C.; Lanyi, S.; Qingsong, L.; Jinchuan, X. Simulation of Dividing-wall Extractive Distillation Column for the Separation of Methanol and Acetone. Sci. Online 2009, 1–5. [Google Scholar]
- Ke, P.; Benyuan, H.; Kefeng, W. Simulation study on separation of pentane mixture by extractive distillation dividing wall column. Mod. Chem. Ind. 2020, 40, 221–2255. [Google Scholar]
- Chengshuai, L.; Deqing, S.; Bowen, L.; Huilong, S. Simulation and optimization of separation process of tert-butanol ethanol and water mixture by extractive separation. Petrochem. Technol. Appl. 2020, 38, 108–112. [Google Scholar]
- Yang, S. Computer simulation and energy saving on separation of vinyl acetate-methanol by dividing wall extractive distillation column. Mod. Chem. Ind. 2020, 40, 250–253. [Google Scholar]
- Chengshuai, L.; Deqing, S.; Bowen, L.; Huilong, S. Simulation and optimization of acetonitrile-water separation with three equivalent models of extractive distillation dividing wall column. Nat. Gas Chem. Ind. 2021, 46, 94–99. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).