Abstract
The species Hancornia speciosa (mangabeira) has varied potential. The bark has astringent properties, latex is used for tuberculosis, ulcers, herpes, dermatoses and warts, leaf tea is used for menstrual cramps and the root decoction to treat dislocations and hypertension. The aim of this work was to analyze the chemical composition of the fixed oil and antibacterial activities alone or in association with aminoglycosides against standard and MDR bacteria using broth microdilution assays. In the analysis of the oil by GC/MS, a high content of unsaturated fatty acids (73.46%) was identified in relation to saturated fatty acids (26.15%). Palmitic acid (22.49%) and elaidic acid (69.50%) were the main fatty acids identified. The antibacterial test results showed a more significant oil activity against Staphylococcus aureus SA–ATCC 6538 (MIC = 512 μg/mL). For other strains including standards and multidrug resistant bacteria, the oil presented MIC ≥ 1024 μg/mL. In association with antibiotics, the oil was able to improve antibacterial activity against bacterial strains. A synergic effect was observed for S. aureus SA–10, with MIC reduction of amikacin and gentamicin by 50.00 and 60.00%, respectively. The most significant association was found for Escherichia coli EC–06, with a reduction in MIC of 81.25%. The results indicate that seed oil of H. speciosa has the potential to act on bacterial resistance to aminoglycoside antibiotics.
1. Introduction
The increase in bacteria resistant to several antibiotic classes has challenged the search for new antibacterial agents [1]. The main mechanisms of resistance involve: target mutation; destruction or overproduction of drugs by enzymes and reduction of intracellular drug concentrations [2]. Due to resistance, toxicity is one of the problems for antibiotics of the aminoglycoside class, and it is associated with high doses or chronic treatment, leading to ototoxicity and/or nephrotoxicity [3].
Aminoglycosides are used to treat various bacterial infections and have a mechanism in protein synthesis by preventing proper reading of mRNA by the prokaryotic 30S ribosome [3]. Due to their broad bactericidal spectrum, bacterial resistance to aminoglycosides has become a problem in recent decades [4].
In this sense, in addition to the direct antibacterial activity, natural products can, in association with aminoglycoside antibiotics, change their effects, either improving or decreasing their activity in a way that has been a viable alternative to the issue of resistance [5,6].
Multiple mechanisms may be involved in the inhibition of microbial growth by natural products. Of particular importance, nonpolar components of natural products, including several fatty acids from fixed oils, have acted on cell membrane permeability and increased the penetration of antibiotics [6]. In addition, they can also interfere with the enzyme systems of bacteria that are integrated into the cell membrane, be they energy production mechanisms or efflux systems [2,7].
This strategy is called “synergistic multi-target” and is based on the use of natural products in an approach using mono or multi-treatment associated with antibiotics for different targets of the microorganism collaborating in a way as synergistic agonist [3,8].
The species Harconia speciosa (mangabeira) is a fruit tree of the Apocynacea family that has a wide occurrence in open vegetation regions, such as savannas, sandy tablelands, plateaus and caatingas [9,10]. The fruit has significant commercial value because, in addition to being consumed in natura, it is processed to obtain frozen pulp, bottled juice, ice cream, jam, liqueur and jelly, syrup, wine and vinegar [11].
On the other hand, the use of mangabeira in folk medicine is reported. For example, the bark has astringent properties, the latex is used against tuberculosis, ulcers, herpes, dermatoses and warts, the leaf tea is used for menstrual cramps and the root decoction, to treat dislocations and hypertension [12]. However, evaluations of the chemical composition with attention to the bioactive compounds and functional activities of mangabeira are still scarce, which reinforces the need for studies to increasingly understand the potential of this species.
Due to the problems caused by bacteria and the rapid emergence of resistance, this work aimed to analyze the chemical composition of the fixed oil of H. speciosa seed and its antibacterial potential and modulate the antibiotic activity of aminoglycosides against standard and MDR bacterial strains.
2. Materials and Methods
2.1. Plant Material
Fruits of Harconia speciosa (mangaba) were obtained in an area of Chapada do Araripe (Sítio Mane Coco), Crato Municipality, Ceará, Brazil. The collected fruits were sorted and sanitized in chlorinated water at 200 ppm for 15 min and then rinsed in running water.
The exsiccate (#12037) of the species is deposited in the Herbarium Caririense Dárdano Andrade Lima (HCDAL) of the Universidade Regional do Cariri (URCA).
2.2. Extraction of Fixed Oil
The collected fruits were manually pulped to obtain the seeds. The seeds were dried in an oven for 24 h at 60 °C and then ground in an industrial blender. The fixed oil was obtained by the continuous method using Soxhlet extractor from 30 g of seeds. The solvent used was hexane at a temperature of 60 °C for 3 h. Then, the extractive solution was subjected to distillation of the solvent in a rotary evaporator under reduced pressure and controlled temperature (±40 °C). The process was carried out in triplicate, and the average yield obtained for the fixed oil was 10.12%.
2.3. Obtaining Methyl Esters and Fatty Acid Analysis
Fatty acids were determined indirectly from fatty acid esters and glycerol present in the oil. Therefore, oil (0.2 g) was subjected to a saponification reaction with a solution of potassium hydroxide in methanol. The process was refluxed for 30 min. With appropriate treatment and pH adjustment, the free fatty acids were then methylated by acid catalysis to produce the respective methyl esters [13].
The methyl esters were analyzed using a Shimadzu GC–MS QP2010 series, provided by Shimadzu Scientific Instruments Inc. (Columbia, MD, USA), with fused silica capillary column SH-Rtx-5 (30 m × 0.25 mm I.D.; 0.25 m film thickness) and the following temperature program: 80–180 °C at 4 °C/min, then to 246 °C at 6.6 °C/min, closing with 10 min at 280 °C, 3.4 °C, totaling analysis time of 30 min.
He was used as the carrier gas, flow rate of 1.5 mL/min, split mode (1:100), and injection port was set at 220 °C. The quadrupole MS operating parameters: interface temperature (280 °C) and ion source (200 °C); electron impact ionization at 70 eV; scan mass range of 40–350 m/z with sampling rate of 1.0 scan/s. Injection volume: 1 µL of 500 ppm solution prepared with dichloromethane. The constituents were identified by computational search using digital libraries of mass spectral data (NIST 08) and by comparing their authentic mass spectra. The Kovats retention index was obtained by injecting a mixture of C8-C40 linear hydrocarbons under the same conditions as the samples. The identity of the compounds was confirmed by comparing their retention indices and mass spectra with those taken from the literature [14].
2.4. Antibacterial Analysis
2.4.1. Bacterial Material
Standard and multidrug-resistant strains of bacteria were used in the analyses. The standard strains were: Bacillus cereus BC-ATCC 33018, Staphylococcus aureus SA-ATCC 6538, Klebsiella pneumoniae KP-ATCC 10031, Escherichia coli EC-ATCC 25922, Proteus vulgaris PV-ATCC 13315 and Shigella flexneri SF-ATCC 12022. The multidrug-resistant strains were: E. coli EC-06 and S. aureus SA-10 (resistance profile in Pereira et al. [3]). The strains were maintained in blood agar base (Laboratory Difco Ltd., Curitiba, PR, Brazil) and cultured at 37 °C for 24 h in Heart Infusion Agar (HIA, Difco. Laboratories Ltd., Curitiba, PR, Brazil).
2.4.2. Drugs
The aminoglycoside antibiotics amikacin and gentamicin were the drugs used (Sigma Co., St. Louis, MO, USA). The drugs were solubilized to a concentration of 5000 μL/mL and diluted in sterile water.
2.4.3. Minimum Inhibitory Concentration Test—MIC
MIC of the fixed oil was obtained according to Pereira et al. [3]. Eppendorf flasks were prepared by adding 100 μL of the inoculum and 900 μL of the BHI (10%) liquid culture medium. Ninety-six-well plates were used and filled with 100 μL of this solution in numerical order, achieving 105 CFU/mL as the final concentration of the inoculums. The last well was used to check microbial growth (negative control). The solution utilized in the tests was prepared at a concentration of 10 mg/mL, dissolved in DMSO and then diluted with distilled water to obtain a concentration of 1024 mg/mL. The assay was performed in triplicate with a serial microdilution of 100 μL of the fixed oil with the concentration ranging from 1024 to 1.0 μg/mL. The plates were incubated in an oven for 24 h at 37 °C. The MIC value was verified by adding 20 μL of resazurin to each well and reading after 1 h. Microbial growth was indicated with the color change from blue to red and the absence of growth by the permanence of the blue color.
2.4.4. Antibiotic Activity Modifying Effect
Fixed oil was evaluated as a modifier of antibiotic activity according to Pereira et al. [3]. Eppendorf flasks were prepared using 1162 μL of BHI (10%) and 150 μL of the inoculum for a concentration of 105 CFU/mL and 128 μg/mL of fixed oil at subinhibitory concentration (MIC/8). Microdilution was performed in numerical order with 100 μL of antibiotic up to the last well. The assay was performed in triplicate using 1350 μL of BHI (10%) and 150 μL of bacterial suspension as controls. The plates were incubated in an oven at 37 °C for 24 h, and reading was performed with the addition of resazurin.
2.5. Statistical Analysis
The results of microbiological analyses performed in triplicate were submitted to statistical analysis using the statistical program GraphPad Prism 5.0. The values of the geometric means were analyzed by the one-way ANOVA test, and the Bonferroni post hoc test was performed (where p < 0.01 was considered significant).
3. Results and Discussion
3.1. Fatty Acid Profile
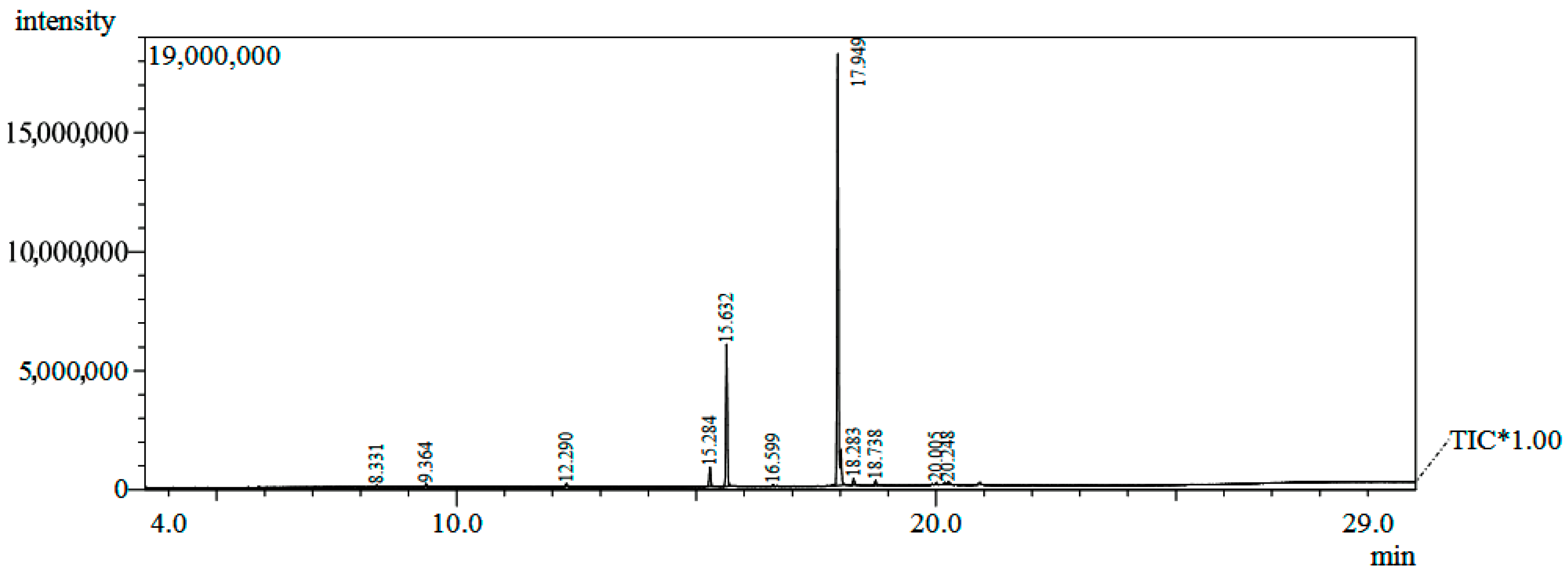
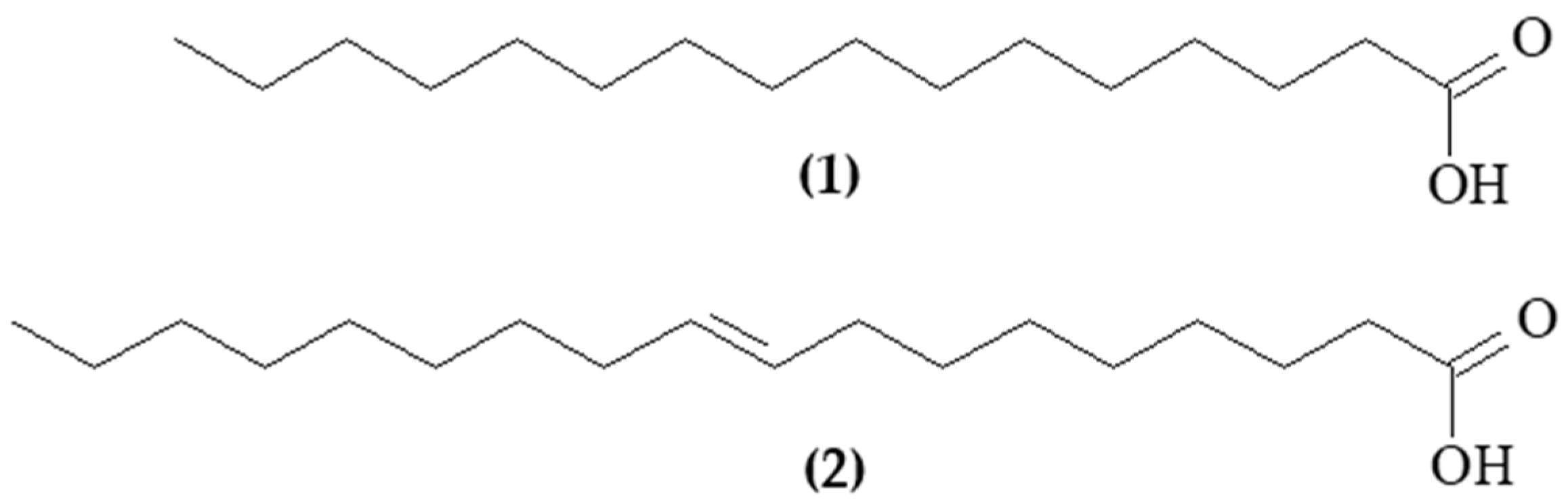
The analysis of the chemical composition of the oil by GC/MS allowed the identification of 99.61% of the fatty acids (Table 1 and Figure 1). The fixed oil presented a profile of chain fatty acids (C9 to C20) and higher content of monounsaturated (73.46%) in relation to saturated (26.15%). In bio-oils produced from mangaba seeds, fatty acids with C9 and C11 chains were found [15]. The main constituents of the oil were palmitic acid (C16:0, 22.49%), a saturated fatty acid, and elaidic acid (C18:1, 69.50%), a monounsaturated fatty acid (Figure 2).

Table 1.
Fatty acids in the fixed oil of Hancornia speciosa seed identified by gas chromatography/mass spectrometry (GC/MS), after obtaining corresponding methyl esters.
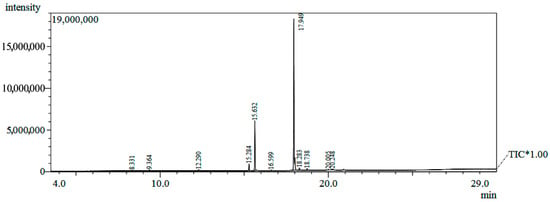
Figure 1.
Chromatogram of the GC/MS analysis of Hancornia speciosa seed fixed oil. TIC* - total ion chromatograms.
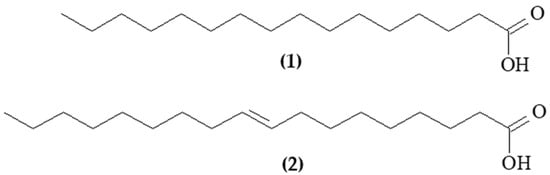
Figure 2.
Structural representation of the main components of Hancornia speciosa seed fixed oil. (1) Palmitic acid and (2) elaidic acid.
These major fatty acids are common compounds in fixed vegetable oils and have relevant biological activities [4,6,16]. Normally, fixed oils have unsaturated fatty acids (C10 to C24), with some oils commonly containing monounsaturated fatty acids [3,7,17].
3.2. Antibacterial and Antibiotic Modifying Activity
The results of the antibacterial activity assay for the fixed oil are shown in Table 2. In this analysis, a more significant level of antibacterial activity of the oil was observed against S. aureus SA-ATCC 6538 (MIC = 512 μg/mL). In the other bacterial strains, including standard and multidrug-resistant bacteria, the fixed oil showed MIC ≥ 1024 μg/mL.

Table 2.
Results for the minimum inhibitory concentration—MIC of the fixed oil of the seed of Hancornia speciosa.
The lower MIC seen for the bacterium S. aureus SA-ATCC 6538 corroborates other works described in the literature on the antibacterial activity of fixed oils against Gram-positive strains. This is probably due to the difference in cell wall and membrane structure that makes Gram-positives more sensitive to the components of fixed oils [4,5]. The nature of the Gram-negative cell membrane restricts the uptake of molecules and their movement into cells through porin channels [6,7].
Even though the present study is a pioneer in the application of fixed oil from mangaba seed (H. speciosa) for microbiological purposes, several oils have been used for this purpose, such as “pequi” (Caryocar coriaceum), “buriti” (Mauritia flexuosa) and “babassu” (Orbignya speciosa), which showed antibacterial activity against several strains of standard and multidrug-resistant bacteria [4,6,7,16,17].
Table 3 presents the MIC of antibiotics and the synergistic effects of fixed oil in association with aminoglycoside antibiotics. The fixed oil showed weak antibacterial activity, but showed a relevant synergistic effect in association with the antibiotics. The MIC of antibiotics against bacteria were in the range of 512 to 64 μg/mL and were reduced in association with the fixed oil.

Table 3.
Results for the minimum inhibitory concentration—MICs (µg/mL) of aminoglycoside antibiotics in the absence and presence of Hancornia speciosa fixed seed oil.
A synergistic effect was observed for S. aureus SA-10, with a reduction in the MIC of amikacin and gentamicin by 50.00 and 60.00%, respectively. The most significant association was found for E. coli EC-06, with an MIC reduction of 81.25%. Interference in the antibiotic activity of antibiotics varied according to the type of antibiotic in association with the fixed oil and the type of bacterial strain.
The use of fixed oils that alter the activity of aminoglycoside antibiotics, similar to what was observed in this study, has been demonstrated and may be a viable alternative in clinical terms against bacterial resistance. Fixed oils from “pequi”, “buriti” and “babassu” altered the activity of gentamicin, amikacin, kanamycin and neomycin against standard and multidrug-resistant strains of S. aureus and E. coli [3,6,7,16].
The antibacterial and antibiotic modifying activity attributed to the fixed oil may be associated with the fatty acids present in its composition, since some fatty acids have already demonstrated the ability to inhibit bacterial growth and improve antibiotic activity [18]. This property of fatty acids has been partly associated with the detergent action against the amphipathic structure of the bacterial plasma membrane [4]. As a result, it increases the permeability of the membrane to antibiotics and reduces the minimum concentration necessary for its effect on the bacteria [19].
Several mechanisms of action may be involved in the inhibition of microbial growth by fatty acids. However, it is reported that the association of antibiotics with long-chain fatty acids, such as palmitic acid and elaidic acid, potentiated antibiotic activity due to alteration in membrane permeability [6,20]. This change in permeability creates spaces that alter energy metabolic processes essential for the bacterial cell, such as the electron transport chain and oxidative phosphorylation, and can also hinder nutrient absorption, inhibit enzyme activity and promote toxic peroxidation [4,21].
4. Conclusions
This work was the first report on the potentiation of the action of antibiotics by the fixed oil of the seed of H. speciosa; the data obtained demonstrate a modulating potential of the oil against the aminoglycosides in addition to antibacterial activity against the standard strain S. aureus.
The chemical characterization by GC/MS showed a composition, mainly of fatty acids, commonly found in other fixed vegetable oils that have relevant biological activities, and highlighted elaidic acid as the major monounsaturated fatty acid. Thus, future investigations are necessary to verify if the compounds present in the oil of the seed of H. speciosa, in the isolated form, promote the antibacterial action and modulatory potential observed in the tests with the fixed oil, directing possibilities for new applications of this potential by the pharmaceutical industries and demonstrating the importance of further studies on the species H. speciosa.
Author Contributions
Conceptualization, E.O.d.S. and J.G.M.C.; methodology, M.d.S.C. and C.d.F.A.N.; validation, C.D.M.O.-T. and J.C.A.P.; formal analysis, I.R.A.d.M.; investigation, M.d.S.C., C.D.M.O.-T. and C.d.F.A.N.; writing—review and editing, E.O.d.S., J.G.M.C. and H.D.M.C.; supervision, E.O.d.S. and H.D.M.C.; project administration, E.O.d.S. and I.R.A.d.M.; funding acquisition, E.O.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP)—Finance Code BPI and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Financiadora de Estudos e Projetos—Brasil (FINEP).
Data Availability Statement
Data supporting reported results can be found with the correspondence authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Freitas, P.R.; Araújo, A.C.J.; Santos Barbosa, C.R.; Muniz, D.F.; Silva, A.C.A.; Rocha, J.E.; Morais Oliveira-Tintino, C.D.; Ribeiro-Filho, J.; Silva, L.E.; Confortin, C.; et al. GC-MS-FID and potentiation of the antibiotic activity of the essential oil of Baccharis reticulata (Ruiz & Pav.) Pers. and α-pinene. Ind. Crops Prod. 2020, 145, 112106. [Google Scholar] [CrossRef]
- Tintino, S.R.; Oliveira-Tintino, C.D.M.; Campina, F.F.; Costa, M.S.; Cruz, R.P.; Pereira, R.L.S.; Andrade, J.C.; Sousa, E.O.; Siqueira-Junior, J.P.; Coutinho, H.D.M. Cholesterol and ergosterol affect the activity of Staphylococcus aureus antibiotic efflux pumps. Microb. Pathog. 2017, 104, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Pereira, Y.F.; Costa, M.D.S.; Tintino, S.R.; Rocha, J.E.; Rodrigues, F.F.G.; Feitosa, M.K.S.B.; Menezes, I.R.A.; Coutinho, H.D.M.; Costa, J.G.M.; Sousa, E.O. Modulation of the antibiotic activity by the Mauritia flexuosa (buriti) fixed oil against methicillin-resistant Staphylococcus aureus (MRSA) and other multidrug-resistant (MDR) bacterial strains. Pathogens 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.B.; Sousa, E.O.; Silva, J.M.L.; Coutinho, H.D.; Costa, J.G. Chemical composition and antibacterial activity of fixed oils of Mauritia flexuosa and Orbignya speciosa associated with aminoglycosides. Eur. J. Integr. Med. 2018, 1, 84–89. [Google Scholar] [CrossRef]
- Sales, D.L.; Oliveira, O.P.; Cabral, M.E.; Dias, D.Q.; Kerntopf, M.R.; Coutinho, H.D.M.; Costa, J.G.M.; Freitas, F.R.; Ferreira, F.S.; Alves, R.R.; et al. Chemical identification and evaluation of the antimicrobial activity of fixed oil extracted from Rhinella jimi. Pharm. Biol. 2015, 53, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.F.; Costa, M.d.S.; Tintino, S.R.; Rodrigues, F.F.G.; Nobre, C.B.; Coutinho, H.D.M.; Costa, J.G.M.; Menezes, I.R.A.; Sousa, E.O. Antibiotic activity potentiation and physicochemical characterization of the fixed Orbignya speciosa almond oil against MDR Staphylococcus aureus and other bacteria. Antibiotics 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.F.G.; Feitosa, M.K.S.B.; Costa, M.S.; Tintino, S.R.; Rodrigues, F.F.G.; Menezes, I.R.A.; Coutinho, H.D.M.; Costa, J.G.M.; Sousa, E.O. Characterization, antibacterial activity and antibiotic modifying action of the Caryocar coriaceum Wittm. pulp and almond fixed oil. Nat. Prod. Res. 2019, 34, 3239–3243. [Google Scholar] [CrossRef] [PubMed]
- Lucena, B.F.F.; Tintino, S.R.; Figueredo, F.G.; Oliveira, C.D.M.; Aguiar, J.J.S.; Cardoso, E.N.; Aquino, P.E.A.; Andrade, J.C.; Coutinho, H.D.M.; Matias, E.F.F. Evaluation of antibacterial activity of aminoglycosides and modulating the essential oil of Cymbopogon citratus (DC.) Stapf. Acta Biol. Colomb. 2015, 20, 39–45. [Google Scholar] [CrossRef]
- Torres-Rêgo, M.; Furtado, A.A.; Bitencourt, M.A.O.; Lima, M.C.J.S.; Andrade, R.C.L.C.; Azevedo, E.P.; Soares, T.C.; Tomaz, J.C.; Lopes, N.P.; Silva-Júnior, A.A. Anti-Inflammatory activity of aqueous extract and bioactive compounds identified from the fruits of Hancornia speciosa Gomes (Apocynaceae). BMC Complementary Altern. Med. 2016, 16, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.L.C.; Oliveira, E.N.A.; Almeida, E.C.; Silva, L.N.; Santos, Y.M.G.; Luna, L.C. Sensory study of alcoholic beverages mangaba (Hancornia speciosa Gomes). Braz. J. Food Technol. 2020, 23, e2019208. [Google Scholar] [CrossRef]
- Silva, T.G.M.; Paulo, R.V.C.; Nascimento, A.N.; Sousa, E.O. Elaboration and characterization of ake cnriched with pulp flour from Hancornia speciosa (Mangaba). Rev. Bras. Agrotecnol. 2021, 11, 118–123. [Google Scholar] [CrossRef]
- Vieira, M.C.; Souza, E.R.B.; Paula, M.S.P.; Naves, R.V.; Silva, G.D. Mangabeira fruits (Hancornia speciosa Gomez): A promising fruit of brazil. Sci. Elec. Arch. 2017, 10, 45–55. [Google Scholar] [CrossRef]
- Hartman, L. Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract. 1973, 22, 475–476. [Google Scholar] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2012. [Google Scholar]
- Santos, R.M.; Santos, A.O.; Sussuchi, E.M.; Nascimento, J.S.; Lima, Á.S.; Freitas, L.S. Pyrolysis of mangaba seed: Production and characterization of bio-oil. Bioresour. Technol. 2015, 196, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.G.M.; Brito, S.A.; Nascimento, E.M.M.; Botelho, M.A.; Rodrigues, F.F.G.; Fabíola, F.G.; Coutinho, H.D.M. Antibacterial properties of pequi pulp oil (Caryocar coriaceum–WITTM.). Int. J. Food Prop. 2011, 14, 411–416. [Google Scholar] [CrossRef]
- Saraiva, R.A.; Matias, E.F.F.; Coutinho, H.D.M.; Costa, J.G.M.; Souza, H.H.F.; Fernandes, C.N.; Rocha, J.B.T.; Menezes, I.R.A. Synergistic action between Caryocar coriaceum Wittm. fixed oil with aminoglycosides in vitro. Eur. J. Lipid Sci. Technol. 2011, 113, 967–972. [Google Scholar] [CrossRef]
- Kim, S.A.; Rhee, M.S. Highly enhanced bactericidal effects of medium chain fatty acids (caprylic, capric, and lauric acid) combined with edible plant essential oils (carvacrol, eugenol, β-resorcylic acid, trans-cinnamaldehyde, thymol, and vanillin) against Escherichia coli O157: H7. Food Control. 2016, 60, 447–454. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Zhanel, G.G.; Schweizer, F. Design, synthesis, and antibacterial activities of neomycin-lipid conjugates: Polycationic lipids with potent gram-positive activity. J. Med. Chem. 2008, 51, 6160–6164. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological Potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).