The First Case of Human Hepatic Fasciolosis Presented as Hepatic Pseudotumor Histopathologically Diagnosed in Romania—A Case Report
Abstract
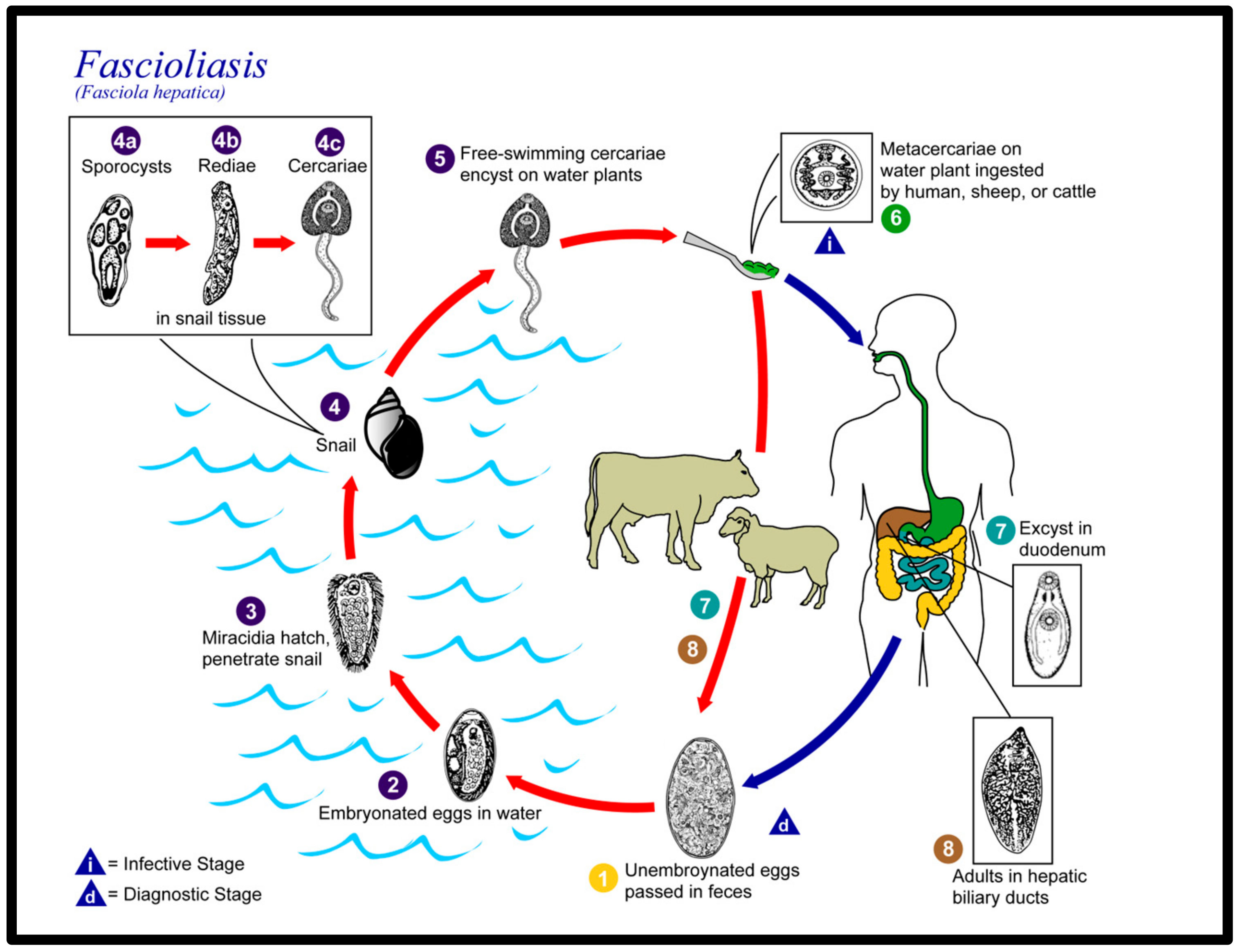
:1. Introduction
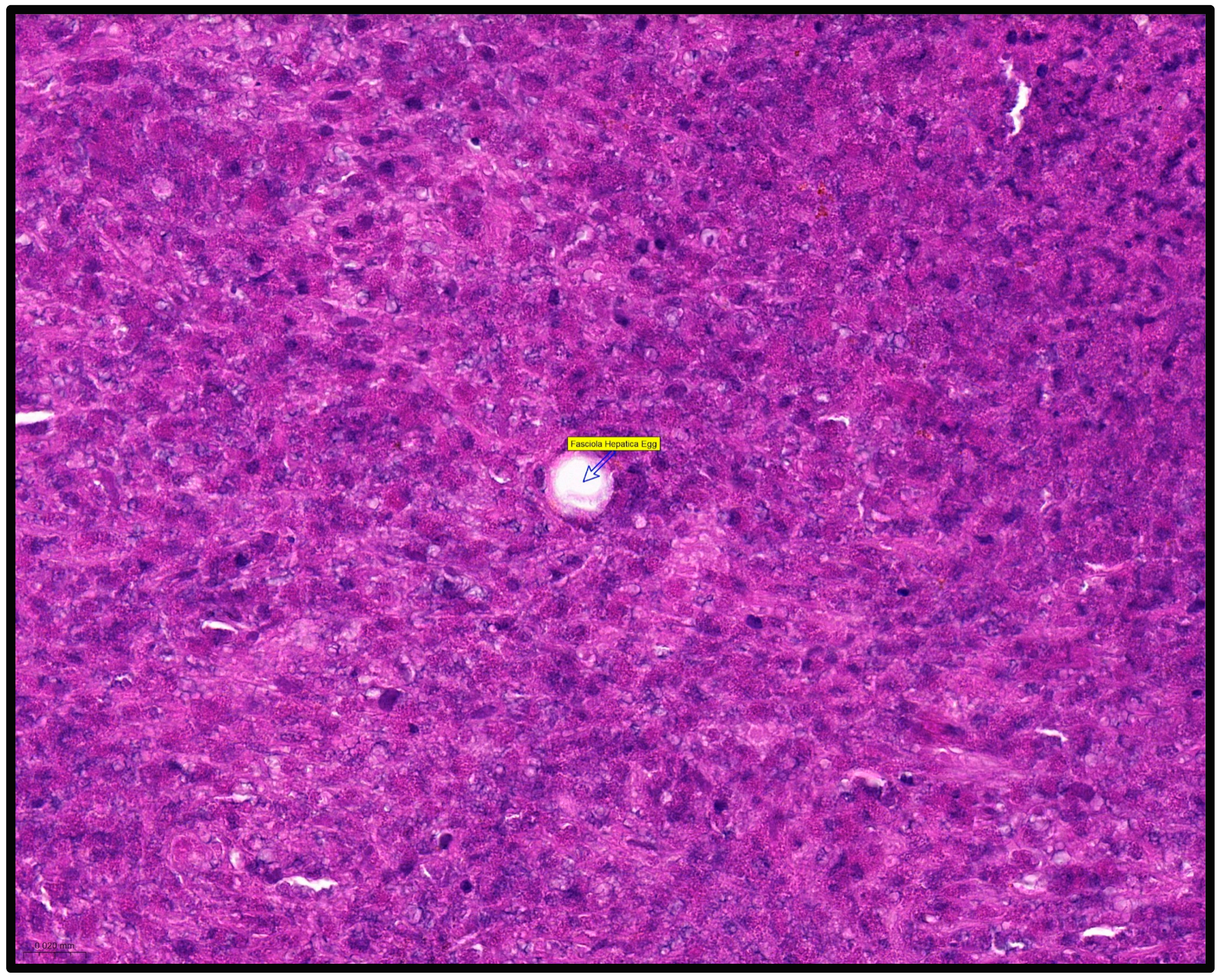
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fürst, T.; Duthaler, U.; Sripa, B.; Utzinger, J.; Keiser, J. Trematode infections: Liver and lung flukes. Infect Dis. Clin. N. Am. 2012, 26, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, S.; Calderini, P.; Dall’oglio, L.; Paola, D.A.; Maurizio, D.A.; Federico, S.; Cancrini, G. Parasitological and Molecular Observations on a Little Family Outbreak of Human Fasciolosis Diagnosed in Italy. Sci. World J. 2014, 2014, 417159. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Diagnosis of human fascioliasis by stool and blood techniques: Update for the present global scenario. Parasitology 2014, 141, 1918–1946. [Google Scholar] [CrossRef]
- Qureshi, A.W.; Zeb, A.; Mansoor, A.; Hayat, A.; Mas-Coma, S. Fasciola hepatica infection in children actively detected in a survey in rural areas of Mardan district, Khyber Pakhtunkhawa province, northern Pakistan. Parasitol. Int. 2018, 69, 39–46. [Google Scholar] [CrossRef]
- Sah, R.; Khadka, S.; Khadka, M.; Gurubacharya, D.; Sherchand, J.B.; Parajuli, K.; Shah, N.P.; Kattel, H.P.; Pokharel, B.M.; Rijal, B. Human fascioliasis by Fasciola hepatica: The first case report in Nepal. BMC Res. Notes 2017, 10, 439. [Google Scholar] [CrossRef]
- Outa, J.O.; Sattmann, H.; Köhsler, M.; Walochnik, J.; Jirsa, F. Diversity of digenean trematode larvae in snails from Lake Victoria, Kenya: First reports and bioindicative aspects. Acta Trop. 2020, 206, 105437. [Google Scholar] [CrossRef]
- Carolus, H.; Muzarabani, K.C.; Hammoud, C.; Schols, R.; Volckaert, F.A.; Barson, M.; Huyse, T. A cascade of biological invasions and parasite spillback in man-made Lake Kariba. Sci. Total Environ. 2018, 659, 1283–1292. [Google Scholar] [CrossRef]
- Carmona, C.; Tort, J. Fasciolosis in South America: Epidemiology and control challenges. J. Helminthol. 2016, 91, 99–109. [Google Scholar] [CrossRef]
- Parkinson, M.; O’neill, S.M.; Dalton, J.P. Controlling fasciolosis in the Bolivian Altiplano. Trends Parasitol. 2007, 23, 238–239. [Google Scholar] [CrossRef]
- Sierra, R.M.Y.; Agramunt, V.H.; Cuervo, P.; Mas-Coma, S. Human fascioliasis in Argentina: Retrospective overview, critical analysis and baseline for future research. Parasites Vectors 2011, 4, 104. [Google Scholar] [CrossRef]
- Hotez, P.J.; Savioli, L.; Fenwick, A. Neglected Tropical Diseases of the Middle East and North Africa: Review of Their Prevalence, Distribution, and Opportunities for Control. PLoS Neglected Trop. Dis. 2012, 6, e1475. [Google Scholar] [CrossRef]
- Temido, H.; Oliveira-Santos, M.; Parente, F.; Santos, L. Fascioliasis—A rare cause of hepatic nodules. BMJ Case Rep. 2017, 2017, 220363. [Google Scholar] [CrossRef]
- Remacha, M.A.; Goñi, M.P.; Espinel, J. Obstructive jaundice of a parasitic etiology. Rev. Esp. Enferm. Dig. 2019, 111, 165–166. [Google Scholar] [CrossRef]
- Otranto, D.; Eberhard, M.L. Zoonotic helminths affecting the human eye. Parasites Vectors 2011, 4, 41. [Google Scholar] [CrossRef]
- Lalor, R.; Cwiklinski, K.; Calvani, N.E.D.; Dorey, A.; Hamon, S.; Corrales, J.L.; Dalton, J.P.; Verissimo, C.D.M. Pathogenicity and virulence of the liver flukes Fasciola hepatica and Fasciola gigantica that cause the zoonosis Fasciolosis. Virulence 2021, 12, 2839–2867. [Google Scholar] [CrossRef]
- McManus, D.P. Recent Progress in the Development of Liver Fluke and Blood Fluke Vaccines. Vaccines 2020, 8, 553. [Google Scholar] [CrossRef]
- Fried, B.; Abruzzi, A. Food-borne trematode infections of humans in the United States of America. Parasitol. Res. 2010, 106, 1263–1280. [Google Scholar] [CrossRef]
- Micic, D.; Oto, A.; Charlton, M.R.; Benoit, J.-L.; Siegler, M. Hiding in the Water. N. Engl. J. Med. 2020, 382, 1844–1849. [Google Scholar] [CrossRef]
- Kain, D.; Mukkala, A.N.; Boggild, A.K. Prolonged antibiotic use leading to Clostridium difficile colitis in an ill returned traveller with acute fascioliasis. J. Travel Med. 2018, 25, 1. [Google Scholar] [CrossRef]
- Kwok, J.; Buxbaum, J.L. Liver fluke. N. Engl. J. Med. 2019, 381, e34. [Google Scholar] [CrossRef]
- Weisenberg, S.A.; Perlada, D.E. Case report: Domestically acquired fascioliasis in northern california. Am. J. Trop. Med. Hyg. 2013, 89, 588–591. [Google Scholar] [CrossRef]
- Caravedo, M.A.; Cabada, M.M. Human Fascioliasis: Current Epidemiological Status and Strategies for Diagnosis, Treatment, and Control. Res. Rep. Trop. Med. 2020, 11, 149–158. [Google Scholar] [CrossRef]
- Centers of Disease Control and Prevention. Available online: https://phil.cdc.gov/Details.aspx?pid=3392 (accessed on 16 May 2023).
- Saba, R.; Korkmaz, M.; Inan, D.; Mamikoğlu, L.; Turhan, Ö.; Günseren, F.; Çevikol, C.; Kabaalioğlu, A. Human fascioliasis. Clin. Microbiol. Infect. 2004, 10, 385–387. [Google Scholar] [CrossRef]
- Torres, G.B.; Iwashita, A.T.; Vargas, C.M.; Luján, L.V.; Bianchi, H.A.; Casanova, R.T. Human fasciolasis and gastrointestinal compromise: Study of 277 patients in the Cayetano Heredia National Hospital (1970–2002). Rev. Gastroenterol. Peru 2004, 24, 143–157. [Google Scholar]
- Chang Wong, M.R.; Pinto Elera, J.O.A.; Guzman Rojas, P.; Terashima Iwashita, A.; Samalvides Cuba, F. Demographic and clinical aspects of hepatic fascioliasis between 2013–2010 in National Hospital Cayetano Heredia, Lima, Peru. Rev. Gastroenterol. Peru 2016, 36, 23–28. [Google Scholar]
- Krsak, M.; Patel, N.U.; Poeschla, E.M. Case Report: Hepatic Fascioliasis in a Young Afghani Woman with Severe Wheezing, High-Grade Peripheral Eosinophilia, and Liver Lesions: A Brief Literature Review. Am. J. Trop. Med. Hyg. 2019, 100, 588–590. [Google Scholar] [CrossRef]
- Aksoy, D.Y.; Kerimoğlu, U.; Oto, A.; Ergüven, S.; Arslan, S.; Unal, S.; Batman, F.; Bayraktar, Y. Fasciola hepatica infection: Clinical and computerized tomographic findings of ten patients. Turk. J. Gastroenterol. 2006, 17, 40–45. [Google Scholar]
- Choi Sy Kim, J.W.; Jang, J.C. Hepatobiliary Fascioliasis with Multiple Aneurysms and Active Bleeding: A Case Report. J. Korean Soc. Radiol. 2015, 72, 291–294. [Google Scholar] [CrossRef]
- Lim, J.H.; Mairiang, E.; Ahn, G.H. Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis. Abdom. Imaging 2008, 33, 157–165. [Google Scholar] [CrossRef]
- Taghipour, A.; Zaki, L.; Rostami, A.; Foroutan, M.; Ghaffarifar, F.; Fathi, A.; Abdoli, A. Highlights of human ectopic fascioliasis: A systematic review. Infect Dis. 2019, 51, 785–792. [Google Scholar] [CrossRef]
- Keiser, J.; Engels, D.; Büscher, G.; Utzinger, J. Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin. Investig. Drugs 2005, 14, 1513–1526. [Google Scholar] [CrossRef]
- Favennec, L.; Ortiz, J.J.; Gargala, G.; Chegne, N.L.; Ayoub, A.; Rossignol, J.F. Double-blind, randomized, placebo-controlled study of nitazoxanide in the treatment of fascioliasis in adults and children from northern Peru. Aliment. Pharmacol. Ther. 2003, 17, 265–270. [Google Scholar] [CrossRef]
- Castillo Contreras, O.B.; Velarde, O.F. Pseudotumor hepático en fasciolosis aguda. Acta Gastroenterol. Latinoam. 2013, 43, 53–58. [Google Scholar] [PubMed]
- Neghina, R.; Neghina, A.M.; Marincu, I.; Iacobiciu, I. Epidemiology and history of human parasitic diseases in Romania. Parasitol. Res. 2011, 108, 1333–1346. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birlutiu, V.; Birlutiu, R.-M. The First Case of Human Hepatic Fasciolosis Presented as Hepatic Pseudotumor Histopathologically Diagnosed in Romania—A Case Report. Healthcare 2023, 11, 1451. https://doi.org/10.3390/healthcare11101451
Birlutiu V, Birlutiu R-M. The First Case of Human Hepatic Fasciolosis Presented as Hepatic Pseudotumor Histopathologically Diagnosed in Romania—A Case Report. Healthcare. 2023; 11(10):1451. https://doi.org/10.3390/healthcare11101451
Chicago/Turabian StyleBirlutiu, Victoria, and Rares-Mircea Birlutiu. 2023. "The First Case of Human Hepatic Fasciolosis Presented as Hepatic Pseudotumor Histopathologically Diagnosed in Romania—A Case Report" Healthcare 11, no. 10: 1451. https://doi.org/10.3390/healthcare11101451
APA StyleBirlutiu, V., & Birlutiu, R.-M. (2023). The First Case of Human Hepatic Fasciolosis Presented as Hepatic Pseudotumor Histopathologically Diagnosed in Romania—A Case Report. Healthcare, 11(10), 1451. https://doi.org/10.3390/healthcare11101451






