Speckle-Reduced Optical Coherence Tomography Using a Tunable Quasi-Supercontinuum Source
Abstract
:1. Introduction
2. Principle and Experiment
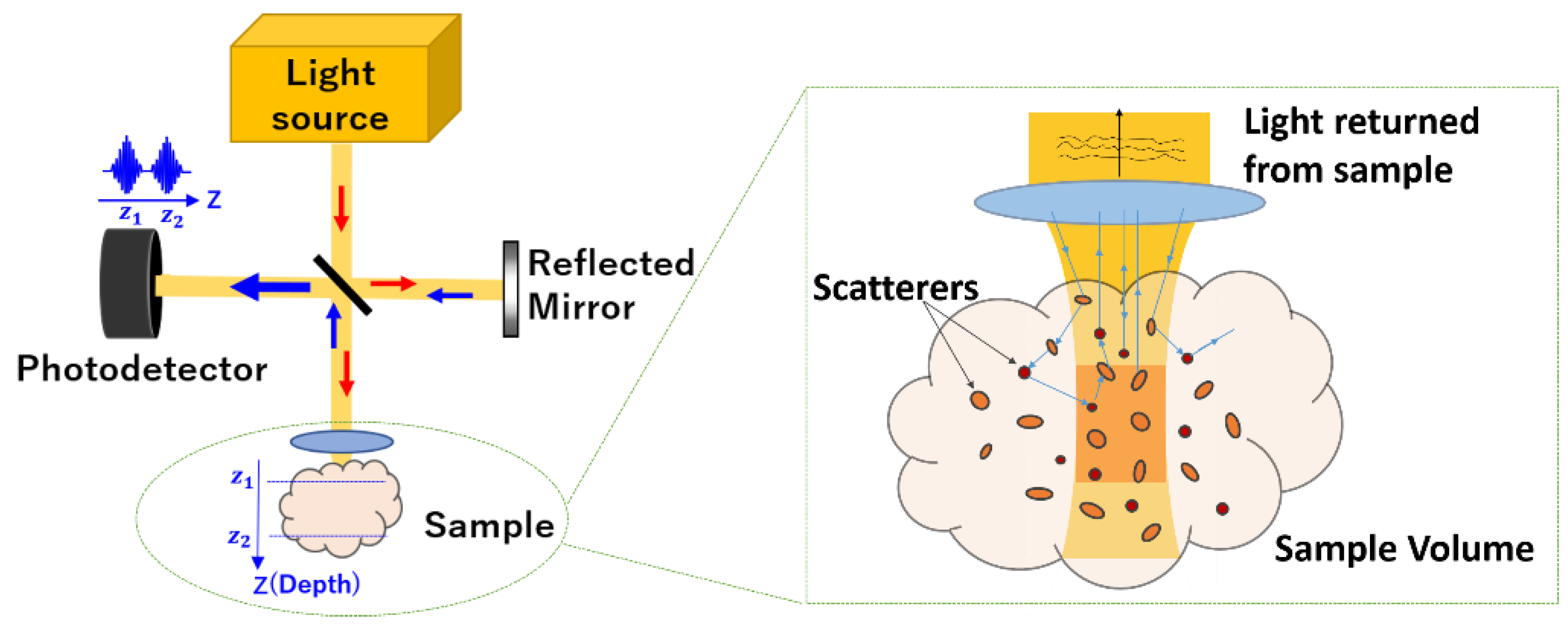
2.1. Principle
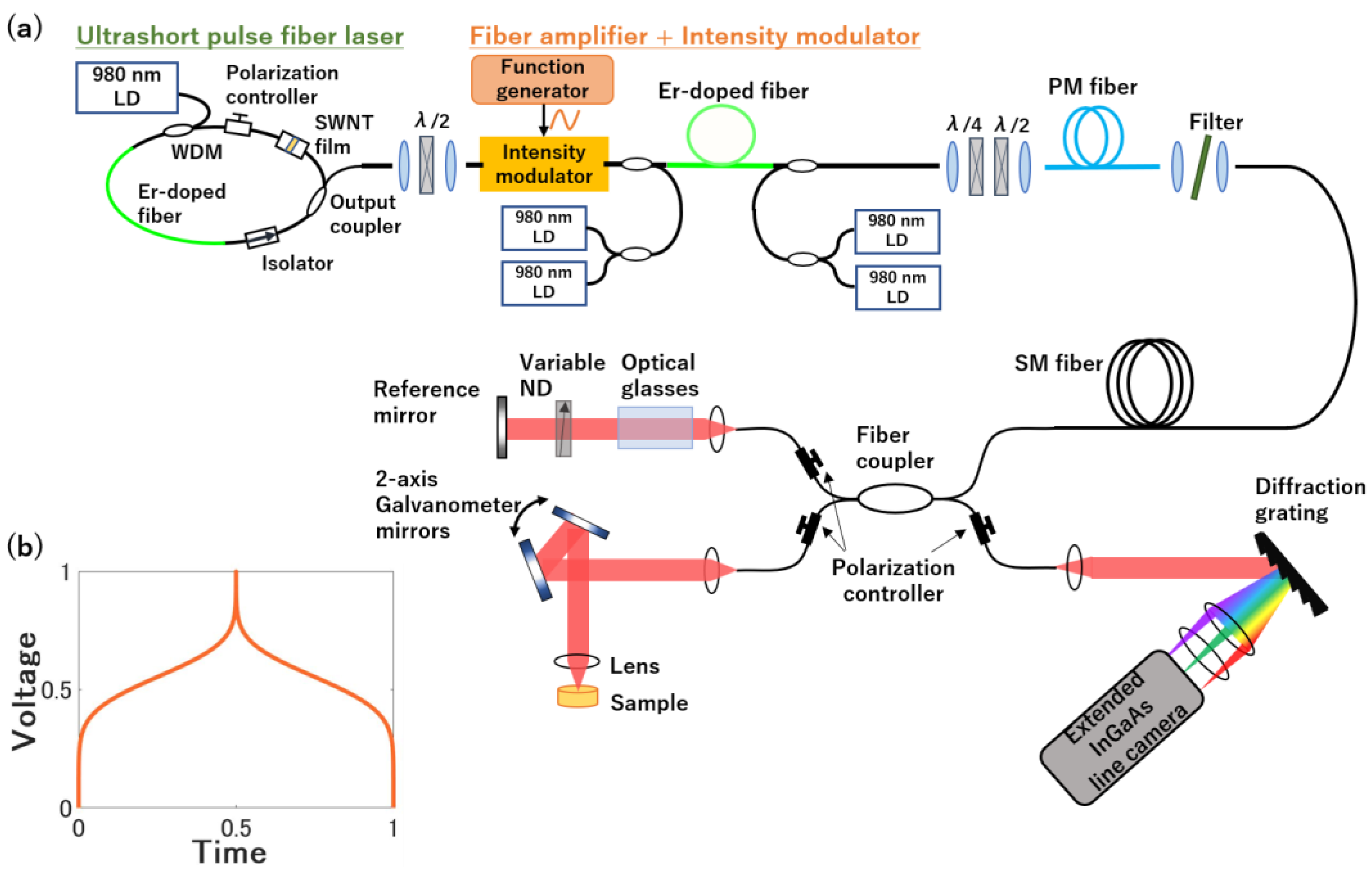
2.2. Experimental Setup
3. Results
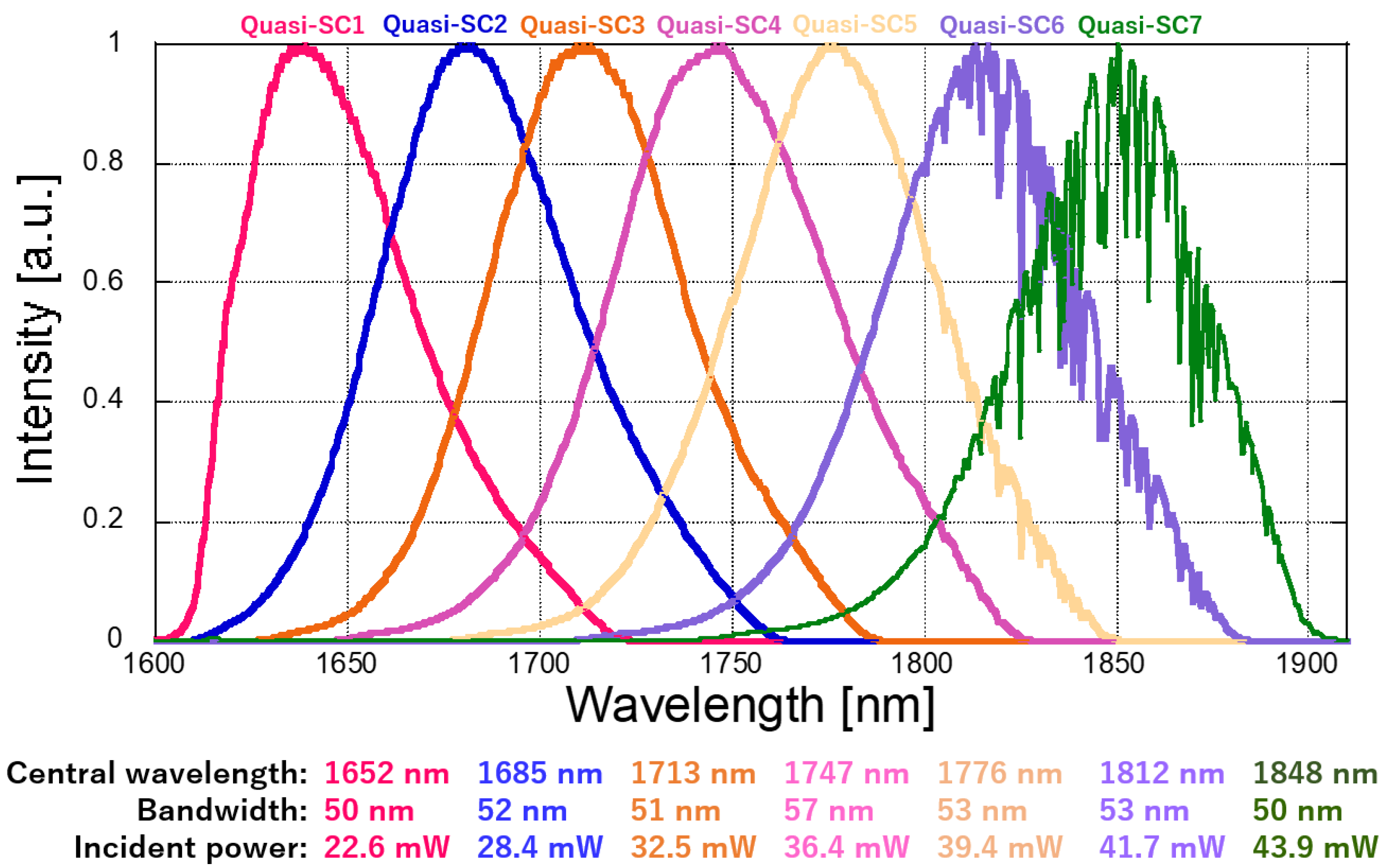
3.1. Tunable Quasi-SC Output
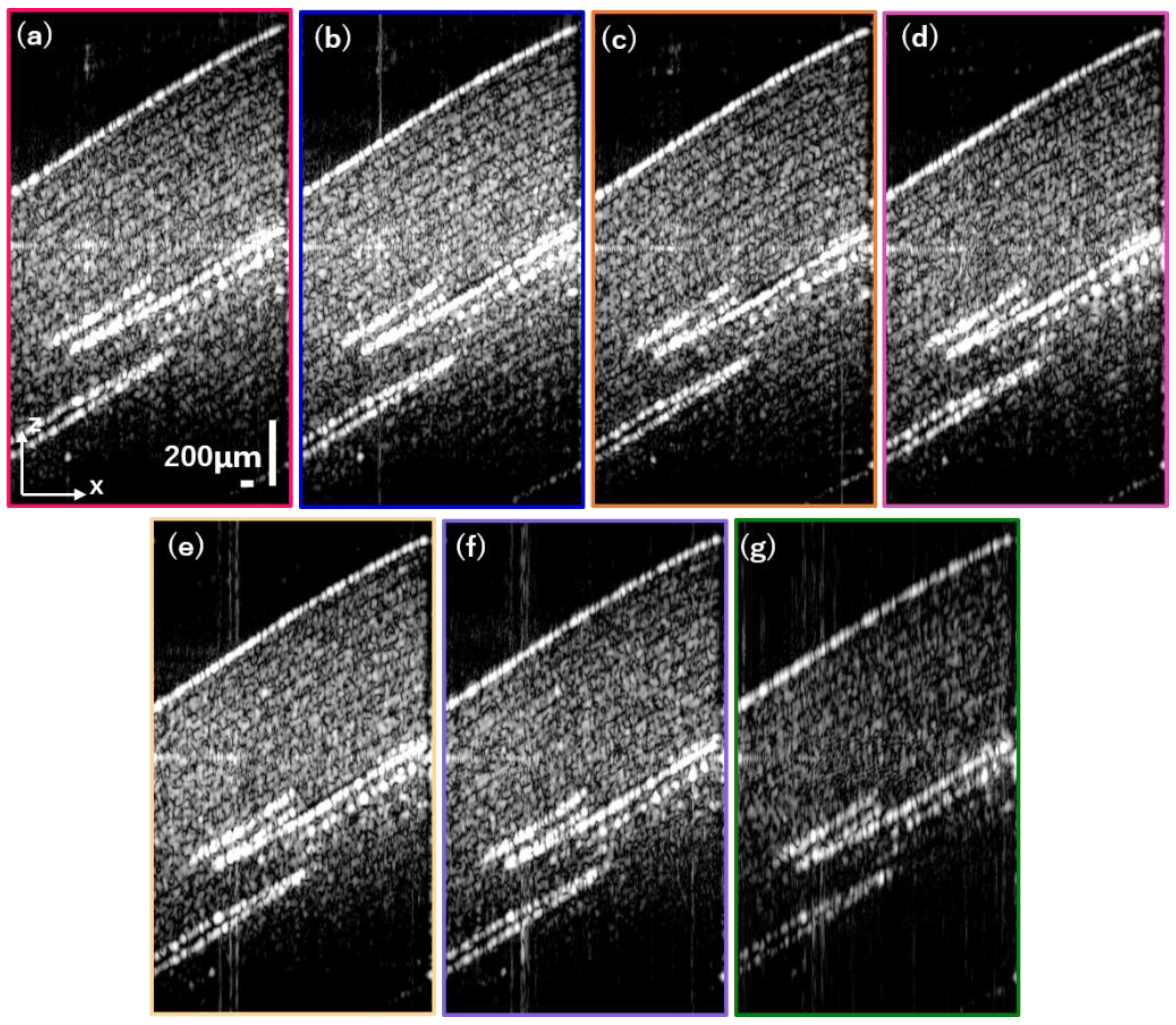
3.2. Tape Stacks
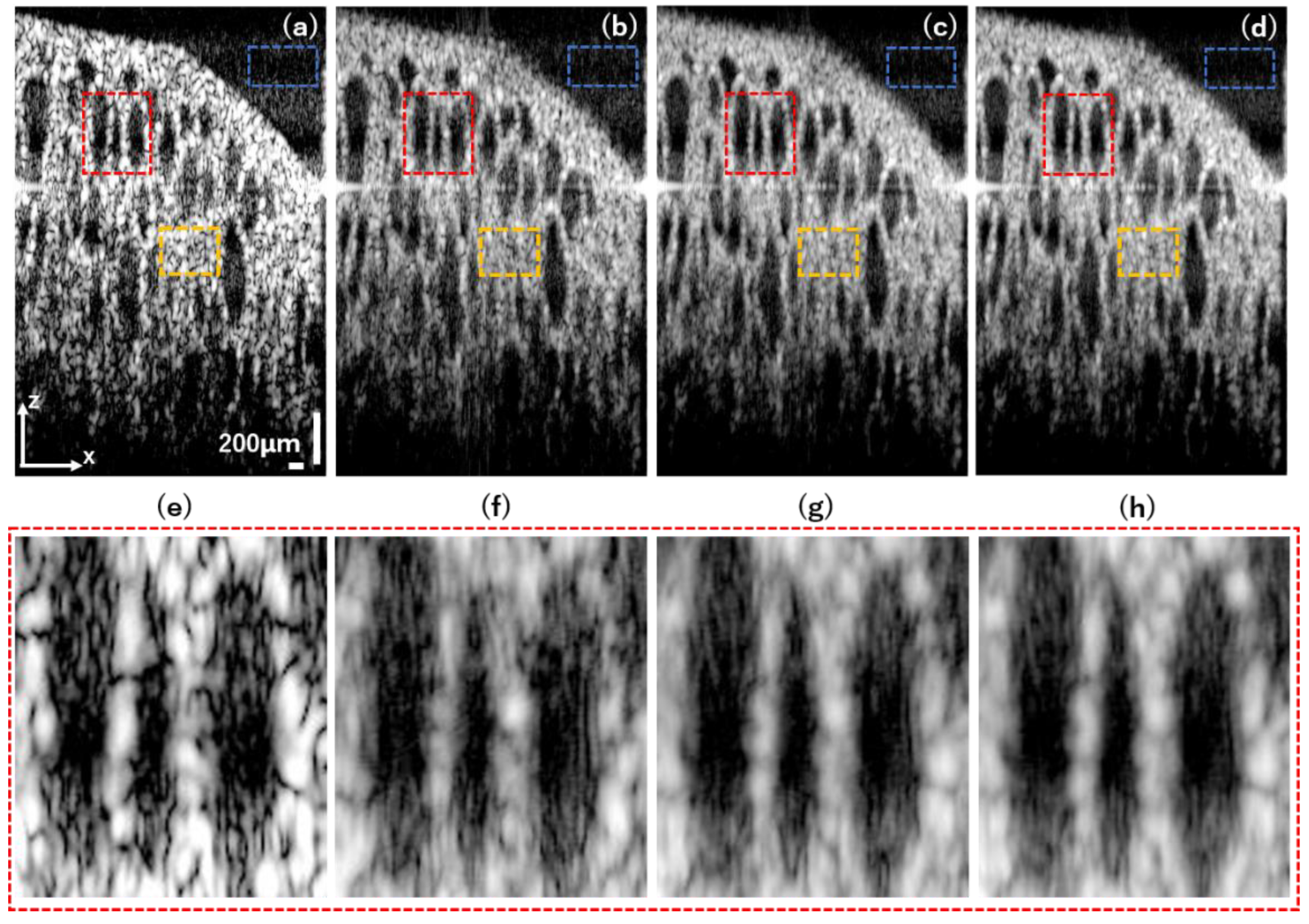
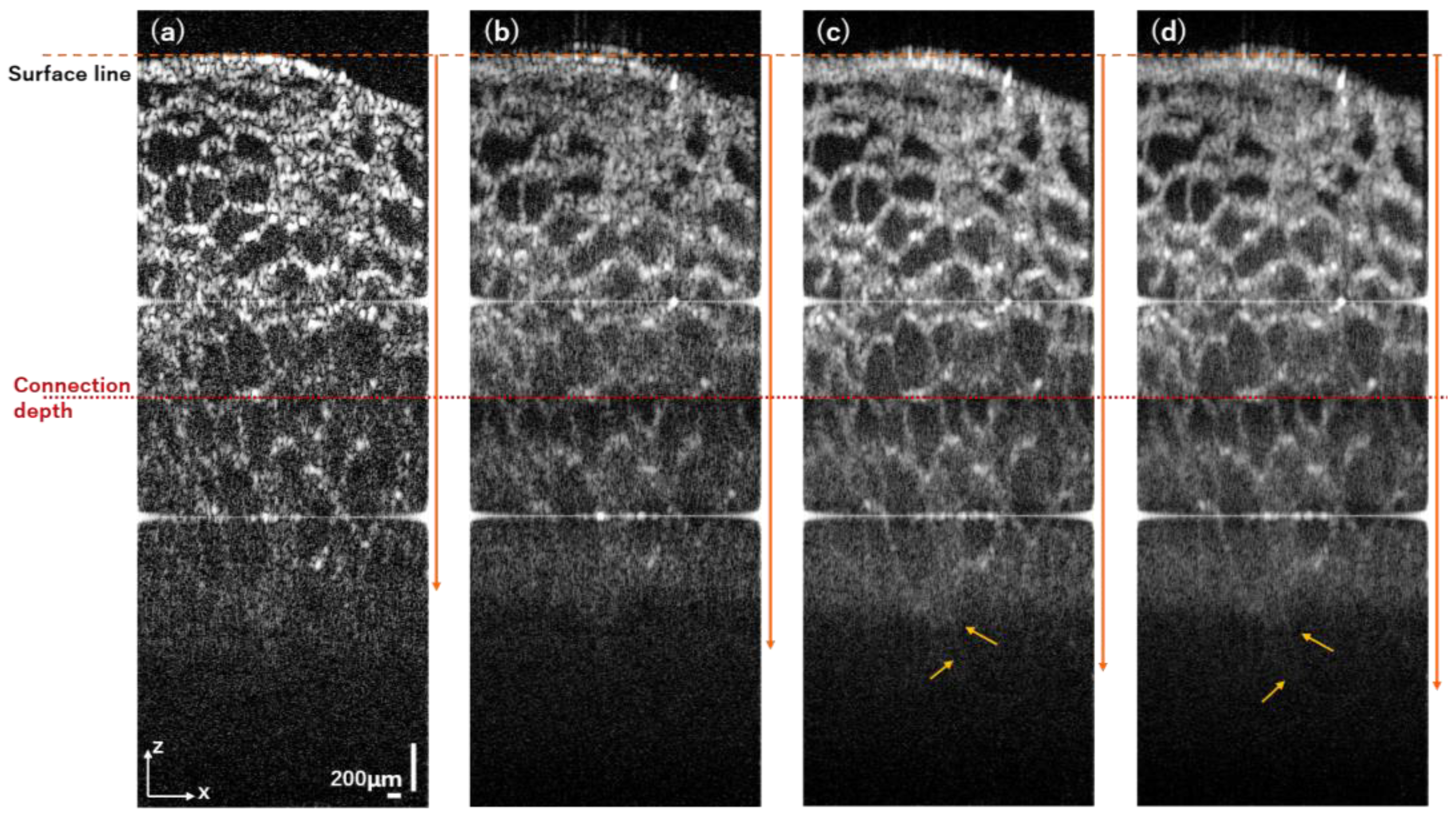
3.3. Pig Thyroid Gland
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, D.; Swanson, E.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M. Optical coherence tomography (OCT): A review. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1205–1215. [Google Scholar] [CrossRef]
- Drexler, W.; Fujimoto, J.G. Optical Coherence Tomography: Technology and Applications, 2nd ed.; Springer: Berlin, Germany, 2015; pp. 22–35. [Google Scholar]
- Goodman, J.W. Speckle Phenomena in Optics: Theory and Applications, 2nd ed.; Society of Photo Optical: Washington, DC, USA, 2010; pp. 11–20. [Google Scholar]
- Tearney, G.J.; Brezinski, M.E. In Vivo Endoscopic Optical Biopsy with Optical Coherence Tomography. Science 1997, 276, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Podoleanu, A.G.; Rogers, J.A. Three dimensional OCT images from retina and skin. Opt. Express 2000, 7, 292–298. [Google Scholar] [CrossRef]
- Pierce, M.C.; Strasswimmer, J. Advances in optical coherence tomography imaging for dermatology. J. Investig. Dermatol. 2004, 123, 458–463. [Google Scholar] [CrossRef]
- Herz, P.R.; Chen, Y. Ultrahigh resolution optical biopsy with endoscopic optical coherence tomography. Opt. Express 2004, 12, 3532–3542. [Google Scholar] [CrossRef]
- Triesscheijn, M.; Baas, P. Non-invasive monitoring of morphologic changes after mTHPC mediated photodynamic therapy (PDT) in a mouse tumor model using optical coherence tomography (OCT). Cancer Res. 2005, 65, 1118. [Google Scholar]
- Freitas, A.Z.; Zezell, D.M. Imaging carious human dental tissue with optical coherence tomography. J. Appl. Phys. 2006, 99, 024906. [Google Scholar] [CrossRef]
- Li, X.; Yin, J. High-resolution coregistered intravascular imaging with integrated ultrasound and optical coherence tomography probe. Appl. Phys. Lett. 2010, 97, 133702. [Google Scholar] [CrossRef]
- Vakoc, B.J.; Dai, F. Cancer imaging by optical coherence tomography: Preclinical progress and clinical potential. Nat. Rev. Cancer 2012, 12, 363–368. [Google Scholar] [CrossRef]
- Choi, W.J.; Zhi, Z. In vivo OCT microangiography of rodent iris. Opt. Lett. 2014, 39, 2455–2458. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Xiang, S.H. Speckle in optical coherence tomography. J. Biomed. Opt. 1999, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Bashkansky, M.; Reintjes, J. Statistics and reduction of speckle in optical coherence tomography. Opt. Lett. 2000, 25, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Karamata, B.; Hassler, K. Speckle statistics in optical coherence tomography. J. Opt. Soc. Am. A 2005, 22, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Vakoc, B.J. Angle-resolved Optical Coherence Tomography with sequential angular selectivity for speckle reduction. Opt. Express 2007, 15, 6200–6209. [Google Scholar] [CrossRef] [PubMed]
- Říha, R.; Marques, M.J. Direct en-face, speckle-reduced images using angular-compounded Master–Slave optical coherence tomography. J. Opt. 2020, 22, 055302. [Google Scholar] [CrossRef]
- Pircher, M.; Götzinger, E. Speckle reduction in optical coherence tomography by frequency compounding. Biomed. Opt. Express 2003, 8, 565–569. [Google Scholar] [CrossRef]
- Magnain, C.; Wang, H. En face speckle reduction in optical coherence microscopy by frequency compounding. Opt. Lett. 2016, 41, 1925–1928. [Google Scholar] [CrossRef]
- Li, R.; Yin, H. Speckle reducing OCT using optical chopper. Opt. Express 2020, 28, 4021–4031. [Google Scholar] [CrossRef]
- Liba, O.; Lew, M.D. Speckle-modulating optical coherence tomography in living mice and humans. Nat. Commun. 2017, 8, 15845. [Google Scholar] [CrossRef]
- Adler, D.C.; Ko, T.H. Speckle reduction in optical coherence tomography images by use of a spatially adaptive wavelet filter. Opt. Lett. 2004, 29, 2878–2880. [Google Scholar] [CrossRef] [PubMed]
- Lippok, N.; Nielsen, P. Single-shot speckle reduction and dispersion compensation in optical coherence tomography by compounding fractional Fourier domains. Opt. Lett. 2013, 38, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Bilenca, A. Speckle reduction in optical coherence tomography images using digital filtering. J. Opt. Soc. Am. 2007, 24, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gao, J. Probability-based non-local means filter for speckle noise suppression in optical coherence tomography images. Opt. Express 2016, 41, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Cuartas-Vélez, C.; Restrepo, R. Volumetric non-local-means based speckle reduction for optical coherence tomography. Biomed. Opt. Express 2018, 9, 3354–3372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z. Denoising algorithm of OCT images via sparse representation based on noise estimation and global dictionary. Opt. Express 2022, 30, 5788–5802. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Zhang, H. Speckle noise reduction algorithm with total variation regularization in optical coherence tomography. Opt. Express 2015, 23, 24699–24712. [Google Scholar] [CrossRef]
- Chen, Y.; Yamanaka, M. Tunable quasi-supercontinuum generation in a 1.7 µm spectral band for spectral domain optical coherence tomography. Opt. Contin. 2023, 2, 1941–1949. [Google Scholar] [CrossRef]
- Nishizawa, N.; Kawagoe, H. Wavelength dependence of ultrahigh-resolution optical coherence tomography using supercontinuum for biological imaging. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 1–15. [Google Scholar] [CrossRef]
- Ishida, S.; Nishizawa, N. Quantitative comparison of contrast and imaging depth of ultrahigh-resolution optical coherence tomography images in 800-1700 nm wavelength region. Biomed. Opt. Express 2012, 3, 282–294. [Google Scholar] [CrossRef]
- Chen, Y.; Yamanaka, M.; Kitajima, S.; Nishizawa, N. Speckle Reduction by Frequency Compounding in 1.7 μm Optical Coherence Tomography using Tunable Quasi-Supercontinuum Laser Source. In Proceedings of the Conference on Lasers and Electro-Optics, JW3B.152, San Jose, CA, USA, 18 May 2022. [Google Scholar]
- Sumimura, K.; Ohta, T. Quasi-super-continuum generation using ultrahigh-speed wavelength-tunable soliton pulses. Opt. Lett. 2008, 33, 2892–2894. [Google Scholar] [CrossRef] [PubMed]
- Sumimura, K.; Ohta, T. Quasi-supercontinuum generation using 1.06 μm ultrashort-pulse laser system for ultrahigh-resolution optical-coherence tomography. Opt. Lett. 2010, 35, 3631–3633. [Google Scholar] [CrossRef] [PubMed]


| Quasi-SC1 | Quasi-SC2 | Quasi-SC3 | Quasi-SC4 | Quasi-SC5 | Quasi-SC6 | Quasi-SC7 | |
|---|---|---|---|---|---|---|---|
| Sensitivity (dB) | 97 | 97 | 99 | 96 | 98 | 95 | 88 |
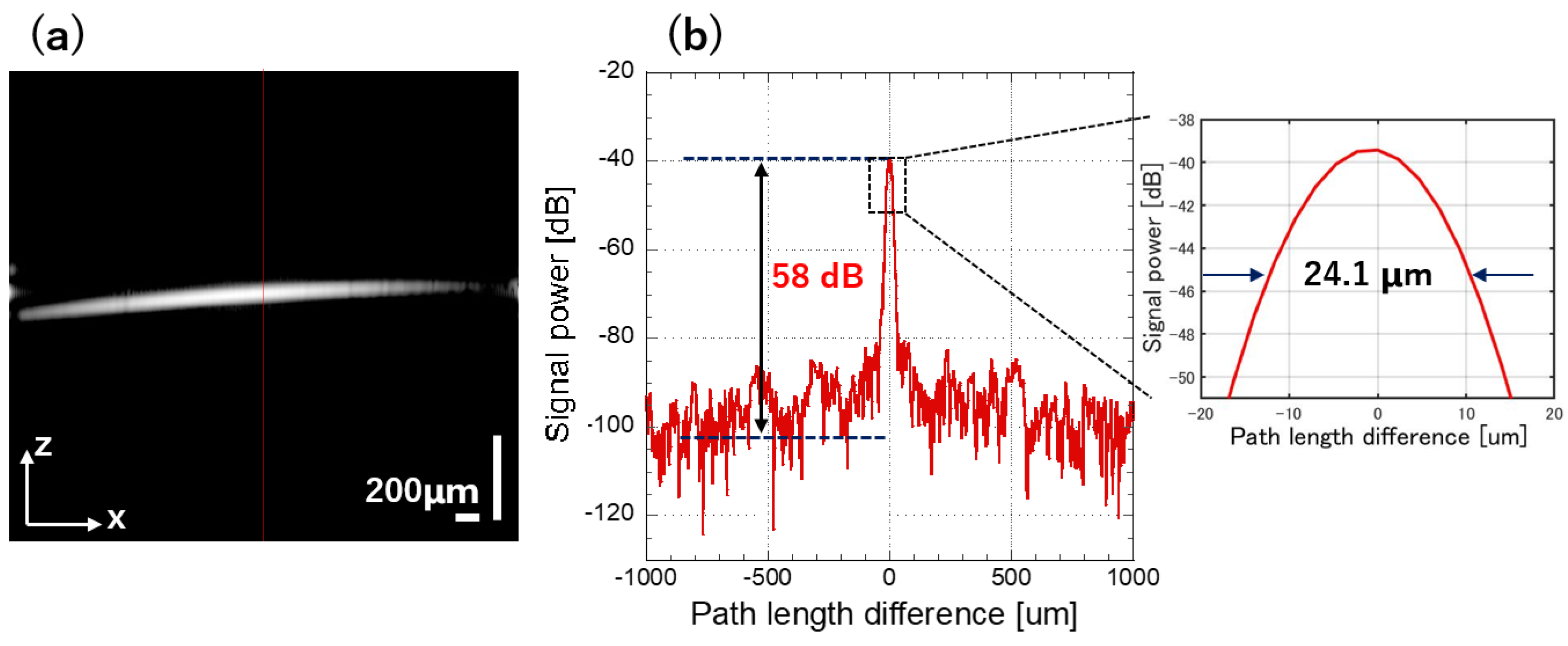
| Axial resolution (μm) (in air) /(in tissue) | 24.1/ 17.4 | 22.3/ 16.1 | 22.4/ 16.2 | 23.1/ 16.7 | 22.1/ 16.0 | 23.1/ 16.7 | 33.1/ 23.9 |
| Lateral resolution (μm) (theoretical value) /(measured value) | 30.2/ 31.9 | 30.8/ 32.3 | 31.4/ 32.6 | 32.0/ 33.1 | 32.5/ 34.2 | 33.2/ 36.2 | 33.8/ 41.7 |
| Quasi-SC1 | Quasi-SC2 | Quasi-SC3 | Quasi-SC4 | Quasi-SC5 | Quasi-SC6 | Quasi-SC7 | |
|---|---|---|---|---|---|---|---|
| Quasi-SC1 | 1 | ||||||
| Quasi-SC2 | 0.825 | 1 | |||||
| Quasi-SC3 | 0.804 | 0.823 | 1 | ||||
| Quasi-SC4 | 0.795 | 0.786 | 0.836 | 1 | |||
| Quasi-SC5 | 0.772 | 0.760 | 0.801 | 0.834 | 1 | ||
| Quasi-SC6 | 0.767 | 0.760 | 0.786 | 0.800 | 0.840 | 1 | |
| Quasi-SC7 | 0.673 | 0.664 | 0.697 | 0.706 | 0.705 | 0.751 | 1 |
| Initial Images Using Quasi-SCs | Speckle-Reduced Images | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quasi-SC1 | Quasi-SC2 | Quasi-SC3 | Quasi-SC4 | Quasi-SC5 | Quasi-SC6 | Quasi-SC7 | n = 3 | n = 5 | n = 7 | |
| SNR (dB) | 3.25 | 2.93 | 2.83 | 2.61 | 2.45 | 1.25 | 1.06 | 4.01 | 4.99 | 5.24 |
| C | 18.53 | 20.64 | 21.50 | 20.43 | 19.48 | 5.51 | 4.67 | 21.11 | 30.74 | 41.90 |
| CNR (dB) | 3.11 | 2.89 | 2.80 | 2.59 | 2.30 | 0.80 | 0.62 | 3.96 | 4.99 | 5.24 |
| Imaging depth (mm) | 1.31 | 1.44 | 1.45 | 1.54 | 1.46 | 1.40 | 1.14 | 1.70 | 1.82 | 1.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yamanaka, M.; Nishizawa, N. Speckle-Reduced Optical Coherence Tomography Using a Tunable Quasi-Supercontinuum Source. Photonics 2023, 10, 1338. https://doi.org/10.3390/photonics10121338
Chen Y, Yamanaka M, Nishizawa N. Speckle-Reduced Optical Coherence Tomography Using a Tunable Quasi-Supercontinuum Source. Photonics. 2023; 10(12):1338. https://doi.org/10.3390/photonics10121338
Chicago/Turabian StyleChen, Ying, Masahito Yamanaka, and Norihiko Nishizawa. 2023. "Speckle-Reduced Optical Coherence Tomography Using a Tunable Quasi-Supercontinuum Source" Photonics 10, no. 12: 1338. https://doi.org/10.3390/photonics10121338
APA StyleChen, Y., Yamanaka, M., & Nishizawa, N. (2023). Speckle-Reduced Optical Coherence Tomography Using a Tunable Quasi-Supercontinuum Source. Photonics, 10(12), 1338. https://doi.org/10.3390/photonics10121338





