Highly Luminescent Rb-Doped Cs4PbBr6 Nanocrystals in Borogermanate Glass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Spectral Characterisation
2.3. Structural Characterisation
3. Results
3.1. XRF Studies
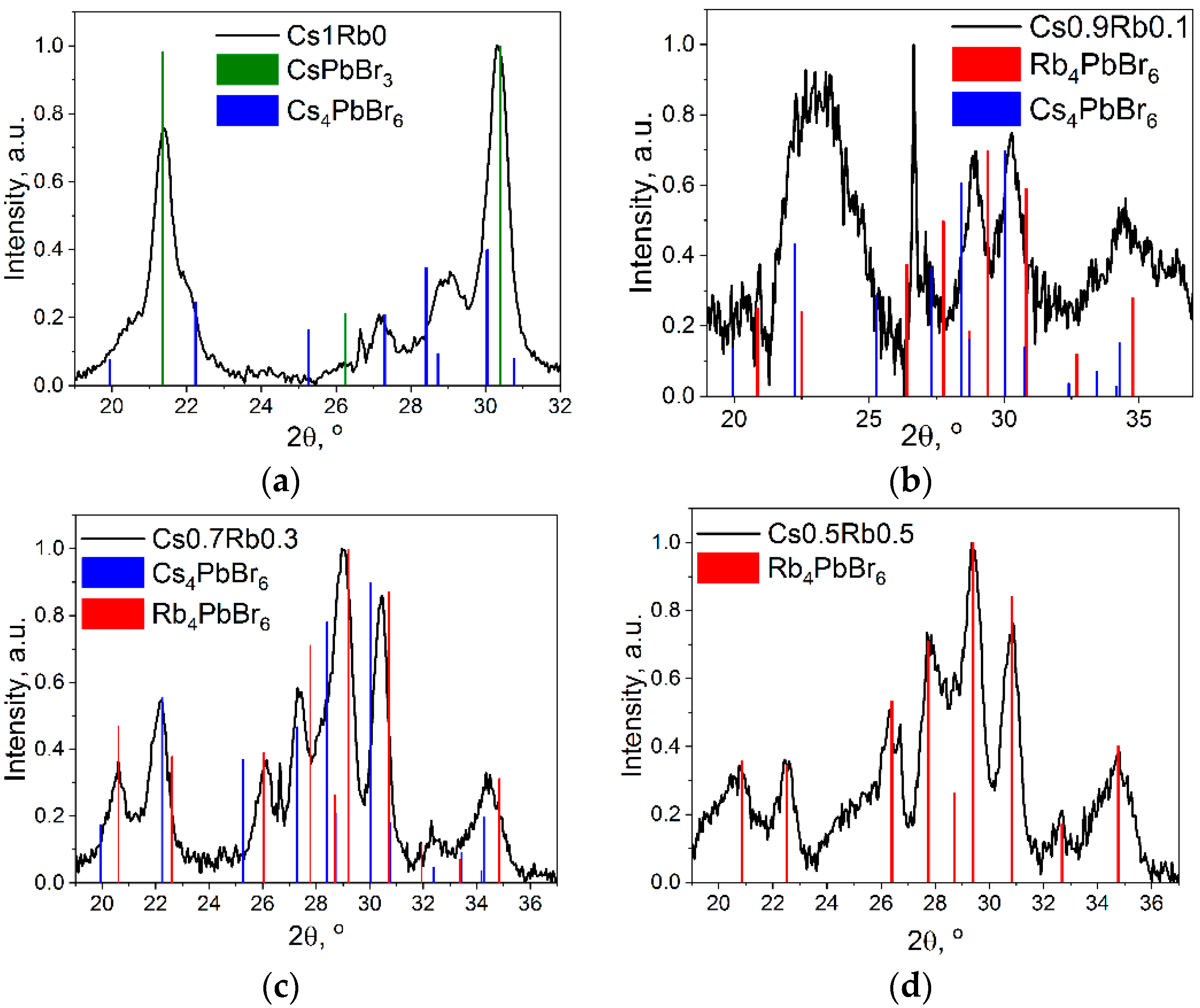
3.2. Structural Properties
3.3. Spectral Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pathak, S.; Sakai, N.; Wisnivesky Rocca Rivarola, F.; Stranks, S.D.; Liu, J.; Eperon, G.E.; Ducati, C.; Wojciechowski, K.; Griffiths, J.T.; Haghighirad, A.A.; et al. Perovskite Crystals for Tunable White Light Emission. Chem. Mater. 2015, 27, 8066–8075. [Google Scholar] [CrossRef]
- Schreuder, M.A.; Xiao, K.; Ivanov, I.N.; Weiss, S.M.; Rosenthal, S.J. White Light-Emitting Diodes Based on Ultrasmall CdSe Nanocrystal Electroluminescence. Nano Lett. 2010, 10, 573–576. [Google Scholar] [CrossRef]
- Kirstein, E.; Kopteva, N.E.; Yakovlev, D.R.; Zhukov, E.A.; Kolobkova, E.V.; Kuznetsova, M.S.; Belykh, V.V.; Yugova, I.A.; Glazov, M.M.; Bayer, M.; et al. Mode Locking of Hole Spin Coherences in CsPb(Cl, Br)3 Perovskite Nanocrystals. Nat. Commun. 2023, 14, 699. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, S.; Chen, X.; Li, J.; Mao, Q.; Li, X.; Zhong, J. CsPbX3 (X = Br, I) Perovskite Quantum Dot Embedded Low-Melting Phosphosilicate Glasses: Controllable Crystallization, Thermal Stability and Tunable Emissions. J. Mater. Chem. C 2018, 6, 6832–6839. [Google Scholar] [CrossRef]
- Xiang, X.; Lin, H.; Xu, J.; Cheng, Y.; Wang, C.; Zhang, L.; Wang, Y. CsPb(Br,I)3 Embedded Glass: Fabrication, Tunable Luminescence, Improved Stability and Wide-Color Gamut LCD Application. Chem. Eng. J. 2019, 378, 122255. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, C.; Huang, S.; Li, Z.; Kong, L.; Jin, L.; Wang, J.; Wu, K.; Li, L. Postsynthesis Phase Transformation for CsPbBr3/Rb4PbBr6 Core/Shell Nanocrystals with Exceptional Photostability. ACS Appl. Mater. Interfaces 2018, 10, 23303–23310. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, W.; Pan, G.; Liu, Y.; Yang, M.; Hua, S.; Chen, X.; Peng, H.; Song, H. Enhancing the Exciton Emission of CsPbCl3 Perovskite Quantum Dots by Incorporation of Rb+ Ions. Mater. Res. Bull. 2019, 112, 142–146. [Google Scholar] [CrossRef]
- Linaburg, M.R.; McClure, E.T.; Majher, J.D.; Woodward, P.M. Cs1−XRbxPbCl3 and Cs1−XRbxPbBr3 Solid Solutions: Understanding Octahedral Tilting in Lead Halide Perovskites. Chem. Mater. 2017, 29, 3507–3514. [Google Scholar] [CrossRef]
- Ju, S.; Mao, C.; Liu, Y.; Zhu, Y.; Xu, Z.; Yang, K.; Guo, T.; Hu, H.; Li, F. Inhibiting Phase Separation of Perovskite Quantum Dots for Achieving Stable Blue Light-Emitting Diodes. Org. Electron. 2023, 113, 106718. [Google Scholar] [CrossRef]
- Wu, H.; Yang, Y.; Zhou, D.; Li, K.; Yu, J.; Han, J.; Li, Z.; Long, Z.; Ma, J.; Qiu, J. Rb+ Cations Enable the Change of Luminescence Properties in Perovskite (RbXCs1−xPbBr3) Quantum Dots. Nanoscale 2018, 10, 3429–3437. [Google Scholar] [CrossRef]
- Xiao, J.W.; Liang, Y.; Zhang, S.; Zhao, Y.; Li, Y.; Chen, Q. Stabilizing RbPbBr3 Perovskite Nanocrystals through Cs+ Substitution. Chem. A Eur. J. 2019, 25, 2597–2603. [Google Scholar] [CrossRef]
- Shao, G.; Liu, S.; Ding, L.; Zhang, Z.; Xiang, W.; Liang, X. KxCs1−xPbBr3 NCs Glasses Possessing Super Optical Properties and Stability for White Light Emitting Diodes. Chem. Eng. J. 2019, 375, 122031. [Google Scholar] [CrossRef]
- Lin, Y.H.; Qiu, Z.H.; Wang, S.H.; Zhang, X.H.; Wu, S.F. All-Inorganic RbxCs1−xPbBrI2 Perovskite Nanocrystals with Wavelength-Tunable Properties for Red Light-Emitting. Inorg. Chem. Commun. 2019, 103, 47–52. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, R.; Jin, M.; Zhang, Z.; Yu, Y.; Xiang, W.; Liang, X. Rb+-Doped CsPbBr3 Quantum Dots with Multi-Color Stabilized in Borosilicate Glass via Crystallization. J. Eur. Ceram. Soc. 2020, 40, 94–102. [Google Scholar] [CrossRef]
- Lu, X.; Lin, H.; Xu, J.; Lin, S.; Cheng, Y.; Wang, Y. K+ -Doping-Induced Highly Efficient Red Emission in CsPb(Br,I)3 Quantum Dot Glass toward Rec. 2020 Displays. Opt. Lett. 2022, 47, 1431–1434. [Google Scholar] [CrossRef]
- Nikl, M.; Nitsch, K.; Polak, K. Photoluminescence of RbPb2Cl5. Phys. Status Solidi 1991, 166, 511–518. [Google Scholar] [CrossRef]
- Nikl, M.; Nitsch, K.; Velicka, I.; Hybler, J.; Polak, K.; Fabian, T. Photoluminescence of KPb2Cl5. Phys. Status Solidi 1991, 168, K37–K42. [Google Scholar] [CrossRef]
- Nikl, M.; Mihokova, E.; Nitsch, K. Photoluminescence & Decay Kinetics of Cs4PbCl6 Single Crystals. Solid State Commun. 1992, 84, 1089–1092. [Google Scholar] [CrossRef]
- Nikl, M.; Mihokova, E.; Nitsch, K.; Somma, F.; Giampaolo, C.; Pazzi, G.; Fabeni, P.; Zazubovich, S. Photoluminescence of Cs4PbBr6 Crystals and Thin Films. Chem. Phys. Lett. 1999, 306, 280–284. [Google Scholar] [CrossRef]
- de Gruijter, W.C. Luminescence of Lead Chloride and Lead Bromide Single Crystals: I. The Excitation and Emission Spectra. J. Solid State Chem. 1973, 6, 151–162. [Google Scholar] [CrossRef]
- Schmitt, K.; Sivasankar, V.S.; Jacobs, P.W.M. Emission and Decay Time Studies on Pb2+ Centers in KBr, RbBr, and RbCl. J. Lumin. 1982, 27, 313–326. [Google Scholar] [CrossRef]
- Polak, K.; Nikl, M.; Mihokova, E. Decay Kinetics of the Slow Component of Pb2+ Emission in KX (X = Cl, Br, I) Crystals. J. Lumin. 1992, 54, 189–196. [Google Scholar] [CrossRef]
- Nitsch, K.; Cihlář, A.; Dušek, M.; Hamplová, V.; Nikl, M.; Rodová, M.; Ryšavá, N. Growth and Characterization of Crystals of Incongruently Melting Ternary Alkali Lead Chlorides. Phys. Status Solidi 1993, 135, 565–571. [Google Scholar] [CrossRef]
- Nikl, M.; Mihokova, E.; Nitsch, K.; Polak, K.; Rodova, M.; Dusek, M.; Pazzi, G.P.; Fabeni, P.; Salvini, L.; Gurioli, M. Photoluminescence and Decay Kinetics of CsPbCl3 Single Crystals. Chem. Phys. Lett. 1994, 220, 14–18. [Google Scholar] [CrossRef]
- Nikl, M.; Nitsch, K.; Polák, K.; Mihókova, E.; Zazubovich, S.; Pazzi, G.P.; Fabeni, P.; Salvini, L.; Aceves, R.; Barbosa-Flores, M.; et al. Quantum Size Effect in the Excitonic Luminescence of CsPbX3-like Quantum Dots in CsX (X = Cl, Br) Single Crystal Host. J. Lumin. 1997, 72–74, 377–379. [Google Scholar] [CrossRef]
- Nikl, M.; Nitsch, K.; Polak, K.; Pazzi, G.P.; Fabeni, P.; Citrin, D.S.; Gurioli, M. Optical Properties of the Pb2+-Based Aggregated Phase in a CsCl Host Crystal: Quantum-Confinement Effects. Phys. Rev. B 1995, 51, 5192–5199. [Google Scholar] [CrossRef]
- Kondo, S.; Amaya, K.; Higuchi, S.; Saito, T.; Asada, H.; Ishikane, M. In Situ Optical Absorption and Reflection Spectroscopy of Doping CsCl Crystal with Pb2+ Ions. J. Phys. Condens. Matter 2001, 13, 11077–11085. [Google Scholar] [CrossRef]
- Aceves, R.; Babin, V.; Barboza Flores, M.; Fabeni, P.; Nikl, M.; Nitsch, K.; Pazzi, G.P.; Perez Salas, R.; Zazubovich, S. Luminescence of CsPbCl3-like Quantum Dots in CsCl: Pb Crystals. Phys. Status Solidi 2001, 225, 247–255. [Google Scholar] [CrossRef]
- Somma, F.; Aloe, P.; Lo Mastro, S.; Santucci, S.; Giampaolo, C.; Nikl, M.; Nitsch, K.; Fabeni, P.; Pazzi, G.P. Structural and Optical Properties of Ternary Cs–Pb–Cl Nanoaggregates in Thin Films. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2001, 19, 2237. [Google Scholar] [CrossRef]
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y.; et al. All Inorganic Halide Perovskites Nanosystem: Synthesis, Structural Features, Optical Properties and Optoelectronic Applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A.A.; Begum, R.; Pan, J.; Cho, N.; Mohammed, O.F.; Bakr, O.M. Pure Cs4PbBr6: Highly Luminescent Zero-Dimensional Perovskite Solids. ACS Energy Lett. 2016, 1, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Akkerman, Q.A.; Park, S.; Radicchi, E.; Nunzi, F.; Mosconi, E.; De Angelis, F.; Brescia, R.; Rastogi, P.; Prato, M.; Manna, L. Nearly Monodisperse Insulator Cs4PbX6 (X = Cl, Br, I) Nanocrystals, Their Mixed Halide Compositions, and Their Transformation into CsPbX3 Nanocrystals. Nano Lett. 2017, 17, 1924–1930. [Google Scholar] [CrossRef]
- De Weerd, C.; Lin, J.; Gomez, L.; Fujiwara, Y.; Suenaga, K.; Gregorkiewicz, T. Hybridization of Single Nanocrystals of Cs4PbBr6 and CsPbBr3. J. Phys. Chem. C 2017, 121, 19490–19496. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S.; Masaki, A.; Saito, T.; Asada, H. Fundamental Optical Absorption of CsPbI3 and Cs4PbI6. Solid State Commun. 2002, 124, 211–214. [Google Scholar] [CrossRef]
- Kondo, S.; Amaya, K.; Saito, T. Localized Optical Absorption in Cs4PbBr6. J. Phys. Condens. Matter 2002, 14, 2093–2099. [Google Scholar] [CrossRef]
- Körbel, S.; Marques, M.A.L.; Botti, S. Stability and Electronic Properties of New Inorganic Perovskites from High-Throughput: Ab Initio Calculations. J. Mater. Chem. C 2016, 4, 3157–3167. [Google Scholar] [CrossRef]
- Thumu, U.; Piotrowski, M.; Owens-Baird, B.; Kolen’ko, Y.V. Zero-Dimensional Cesium Lead Halide Perovskites: Phase Transformations, Hybrid Structures, and Applications. J. Solid State Chem. 2019, 271, 361–377. [Google Scholar] [CrossRef]
- Moller, C.K. Crystal Structure and Photoconductivity of Caesium Plumbohalides. Nature 1958, 182, 1436. [Google Scholar] [CrossRef]
- Velázquez, M.; Ferrier, A.; Péchev, S.; Gravereau, P.; Chaminade, J.P.; Portier, X.; Moncorgé, R. Growth and Characterization of Pure and Pr3+-Doped Cs4PbBr6 Crystals. J. Cryst. Growth 2008, 310, 5458–5463. [Google Scholar] [CrossRef]
- Xu, J.; Huang, W.; Li, P.; Onken, D.R.; Dun, C.; Guo, Y.; Ucer, K.B.; Lu, C.; Wang, H.; Geyer, S.M.; et al. Imbedded Nanocrystals of CsPbBr3 in Cs4PbBr6: Kinetics, Enhanced Oscillator Strength, and Application in Light-Emitting Diodes. Adv. Mater. 2017, 29, 1703703. [Google Scholar] [CrossRef]
- Rao, L.; Zhang, Q.; Sun, B.; Wen, M.; Zhang, J.; Yu, S.; Fu, T.; Niu, X. CsPbBr3/Cs4PbBr6 Heterostructure Solids with High Stability and Photoluminescence for White Light-Emitting Diodes. J. Alloys Compd. 2022, 919, 165857. [Google Scholar] [CrossRef]
- Rao, L.; Sun, B.; Zhang, Q.; Wen, M.; Zhang, J.; Zhong, G.; Fu, T.; Niu, X.; Tang, Y. Highly Emissive Green CsPbBr3/Cs4PbBr6 Composites: Formation Kinetics, Excellent Heat, Light, and Polar Solvent Resistance, and Flexible Light-Emitting Application. Opt. Express 2022, 30, 45376–45392. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Du, K.; Gao, X.; Lu, Y.; Wen, D.; Yao, S.; Feng, J.; Zhang, H. One-Step Conversion of CsPbBr3 into Cs4PbBr6/CsPbBr3@Ta2O5 Core-Shell Microcrystals with Enhanced Stability and Photoluminescence. J. Mater. Chem. C 2021, 9, 1228–1234. [Google Scholar] [CrossRef]
- Babin, V.; Fabeni, P.; Nikl, M.; Nitsch, K.; Pazzi, G.P. Luminescent CsPbI3 and Cs4PbI6 Aggregates in Annealed CsI: Pb Crystals. Phys. Status Solidi 2001, 428, 419–428. [Google Scholar] [CrossRef]
- Almutlaq, J.; Yin, J.; Mohammed, O.F.; Bakr, O.M. The Benefit and Challenges of Zero-Dimensional Perovskites. J. Phys. Chem. Lett. 2018, 9, 4131–4138. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Liu, Q.; Nie, L.; Yao, G.; Zeng, F.; He, Y.; Xiang, W. Ultrastable Zero-Dimensional Cs4PbBr6 Perovskite Quantum Dot Glass. ACS Sustain. Chem. Eng. 2020, 8, 10646–10652. [Google Scholar] [CrossRef]
- Zhuang, W.; Liu, H.; Chen, Y.; Xu, W.; Gao, H.; Tian, Y.; Yao, D.; Zhang, H. Lead-Free Double Perovskite Rb+, Sb3+-Codoped Cs2NaInCl6 Nanocrystals with Highly Efficient and Tunable Photoluminescence. Ceram. Int. 2023, 49, 15761–15770. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Xu, J.; Yang, S.; Zhao, H.; Huang, H.; Liu, S.; Yao, J. All-Inorganic 0D/3D Cs4Pb(IBr)6/CsPbI3−xBrx mixed-Dimensional Perovskite Solar Cells with Enhanced Efficiency and Stability. J. Mater. Chem. C 2020, 8, 6977–6987. [Google Scholar] [CrossRef]
- Yang, B.; Zheng, F.; Mei, S.; Chen, Z.; Xie, Y.; Dai, H.; Wei, X.; Zhang, W.; Xie, F.; Ju, J.; et al. Component Regulation and Crystallization Mechanism of CsPbBr3/Cs4PbBr6 Perovskite Composite Quantum Dots-Embedded Borosilicate Glass for Light Emitting Application. Appl. Surf. Sci. 2020, 512, 145655. [Google Scholar] [CrossRef]
- He, Z.; Wang, Q.; Liang, X.; Yang, K.; Xiang, W. CsPbBr3/Cs4PbBr6 NCs Glass Prepared by a Composition Regulation Strategy for Amplification Spontaneous Emission and White Light Emitting Diode. Appl. Phys. Lett. 2021, 119, 161902. [Google Scholar] [CrossRef]
- Li, S.; Yao, G.; Liu, G.; Nie, L.; Li, C.; Lin, H.; Zeng, F.; Xiang, W. Low Lead Migration 0D Cs4PbBr6 Nanocrystal Glass with Super Stability as a New Member of the Luminous Family. J. Alloys Compd. 2022, 904, 164058. [Google Scholar] [CrossRef]
- Duan, Y.; Li, P.; Lu, Y.; Wang, X.; Xu, S.; Zhang, J. Erasable Cs4PbBr6 Quantum Dots Glass with Switchable Photoluminescence. Opt. Lett. 2021, 46, 3580–3583. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Zhang, Y.; Mohammed, O.F. Photoluminescence Origin of Zero-Dimensional Cs4PbBr6 Perovskite. ACS Energy Lett. 2020, 5, 87–99. [Google Scholar] [CrossRef]


| Components | Composition #2 | Composition #3 | Composition #4 | |||
|---|---|---|---|---|---|---|
| Batch | XRF | Batch | XRF | Batch | XRF | |
| Na2O | 6.65 | 3.67 | 6.65 | 3.33 | 6.65 | 5.11 |
| Cs2O | 5.59 | 11.12 | 3.74 | 6.9 | 1.87 | 3.58 |
| Rb2O | 1.86 | 1.52 | 3.71 | 2.8 | 5.57 | 4.18 |
| ZnO | 5.21 | 3.27 | 5.21 | 4.31 | 5.21 | 3.65 |
| B2O3 | 24.46 | 27.75 | 24.46 | 26.84 | 24.46 | 25.45 |
| GeO2 | 39.48 | 39.75 | 39.48 | 41.75 | 39.48 | 43.31 |
| TiO2 | 1.77 | 0.85 | 1.77 | 1.59 | 1.77 | 2.6 |
| PbO | 1.92 | 3.94 | 1.92 | 4.25 | 1.92 | 3.57 |
| K2O | 3.96 | 1.77 | 3.96 | 1.99 | 3.96 | 2.58 |
| Br | 7.92 | 4.71 | 7.92 | 4.21 | 7.92 | 3.85 |
| P2O5 | 1.18 | 1.66 | 1.18 | 2.02 | 1.18 | 2.12 |
| Cs/Rb ratio | 0.75/025 | 0.9/0.1 | 0.5/05 | 0.3/0.7 | 0.25/0.75 | 0.5/0.5 |
| Sample | A1 | τ1 (ns) | A2 | τ2 (ns) | τavg (ns) |
|---|---|---|---|---|---|
| Cs1Rb0 | 34 | 1.25 | 0.45 | 9.32 | 1.97 |
| Cs0.9Rb0.1 | 11.82 | 1.60 | 0.66 | 11.22 | 4.31 |
| Cs0.7Rb0.3 | 5.44 | 2.01 | 0.74 | 14.30 | 8.05 |
| Cs0.5Rb0.5 | 5.51 | 2.03 | 0.74 | 13.14 | 7.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiev, D.; Kharisova, R.; Babkina, A.; Zyryanova, K.; Kuzmenko, N.; Sgibnev, Y.; Shelaev, A.; Baryshev, A.V. Highly Luminescent Rb-Doped Cs4PbBr6 Nanocrystals in Borogermanate Glass. Photonics 2023, 10, 729. https://doi.org/10.3390/photonics10070729
Valiev D, Kharisova R, Babkina A, Zyryanova K, Kuzmenko N, Sgibnev Y, Shelaev A, Baryshev AV. Highly Luminescent Rb-Doped Cs4PbBr6 Nanocrystals in Borogermanate Glass. Photonics. 2023; 10(7):729. https://doi.org/10.3390/photonics10070729
Chicago/Turabian StyleValiev, Damir, Rufina Kharisova, Anastasiia Babkina, Ksenia Zyryanova, Natalia Kuzmenko, Yevgeniy Sgibnev, Artem Shelaev, and Alexander V. Baryshev. 2023. "Highly Luminescent Rb-Doped Cs4PbBr6 Nanocrystals in Borogermanate Glass" Photonics 10, no. 7: 729. https://doi.org/10.3390/photonics10070729
APA StyleValiev, D., Kharisova, R., Babkina, A., Zyryanova, K., Kuzmenko, N., Sgibnev, Y., Shelaev, A., & Baryshev, A. V. (2023). Highly Luminescent Rb-Doped Cs4PbBr6 Nanocrystals in Borogermanate Glass. Photonics, 10(7), 729. https://doi.org/10.3390/photonics10070729







