Copper Sulfide Small Nanoparticles as Efficient Contrast Agent for Photoacoustic Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Materials
2.3. Procedure
2.4. UV-Visible Spectroscopy
2.5. Photoacoustic Spectroscopy
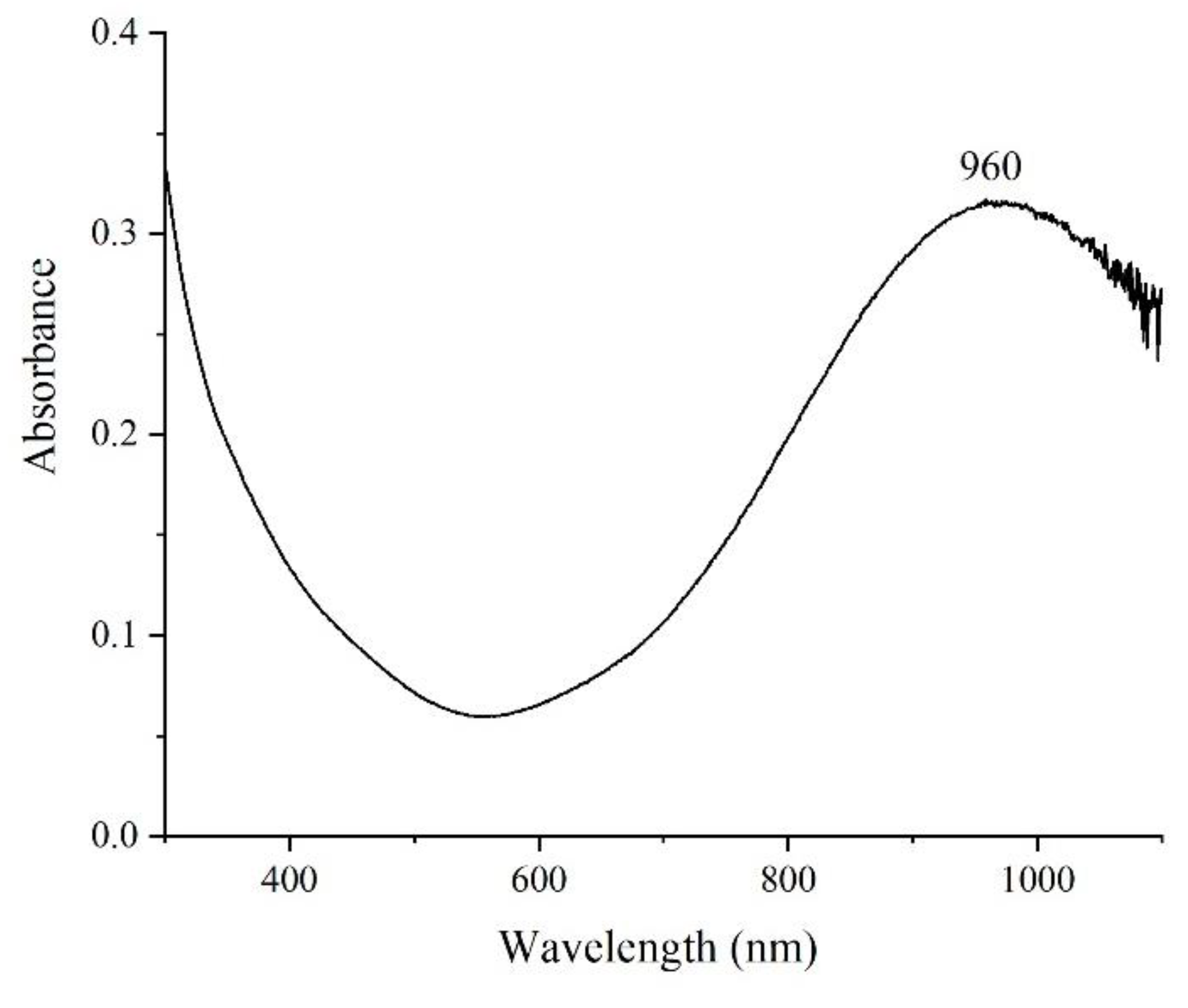
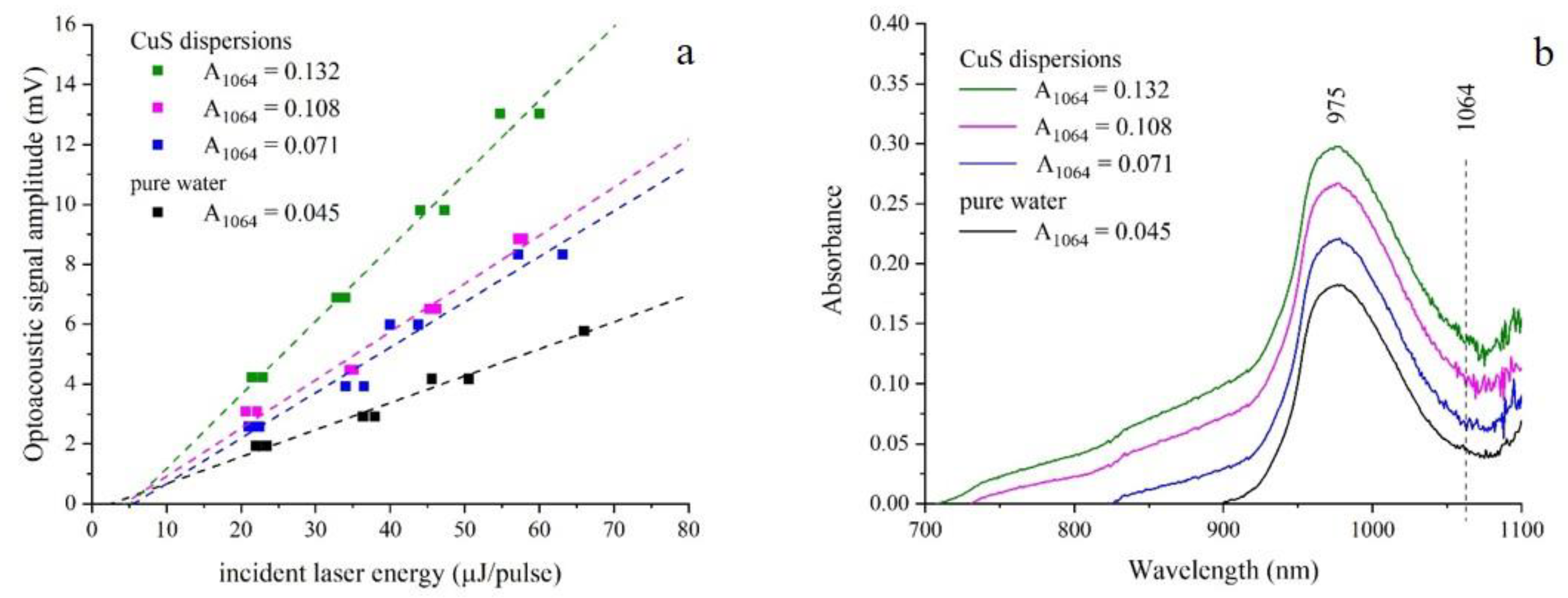
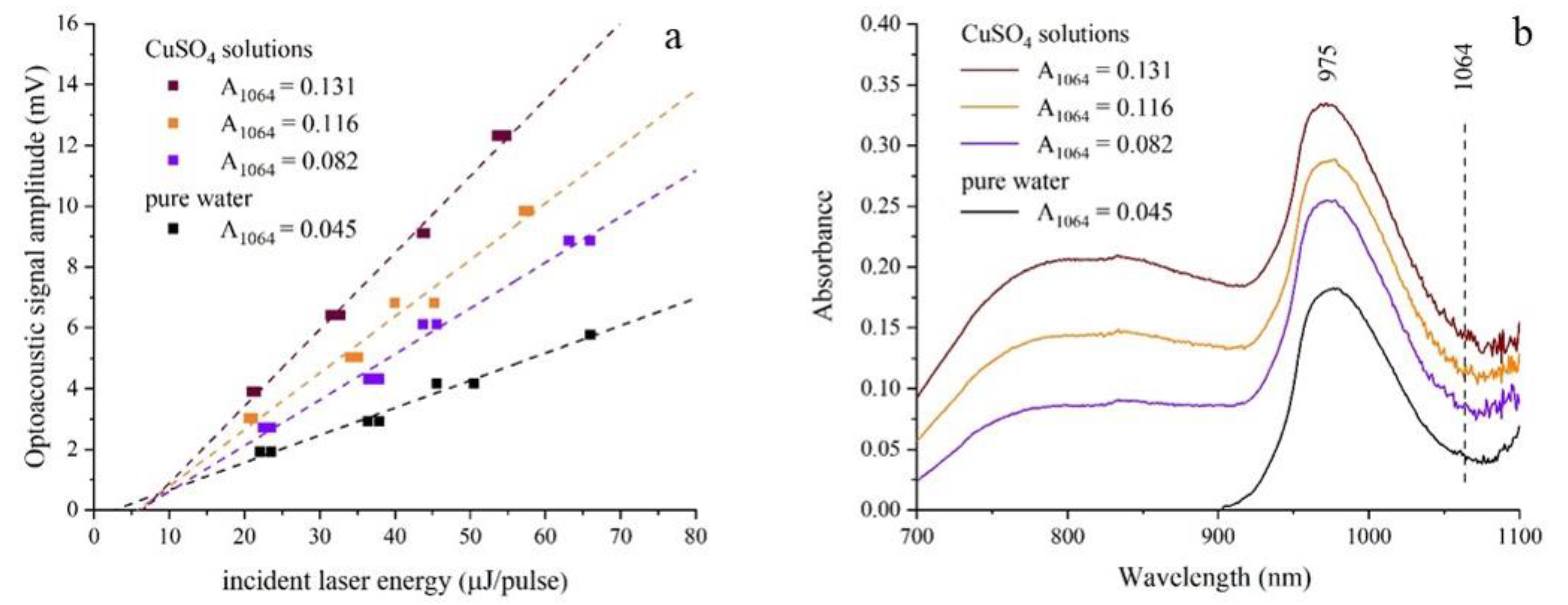
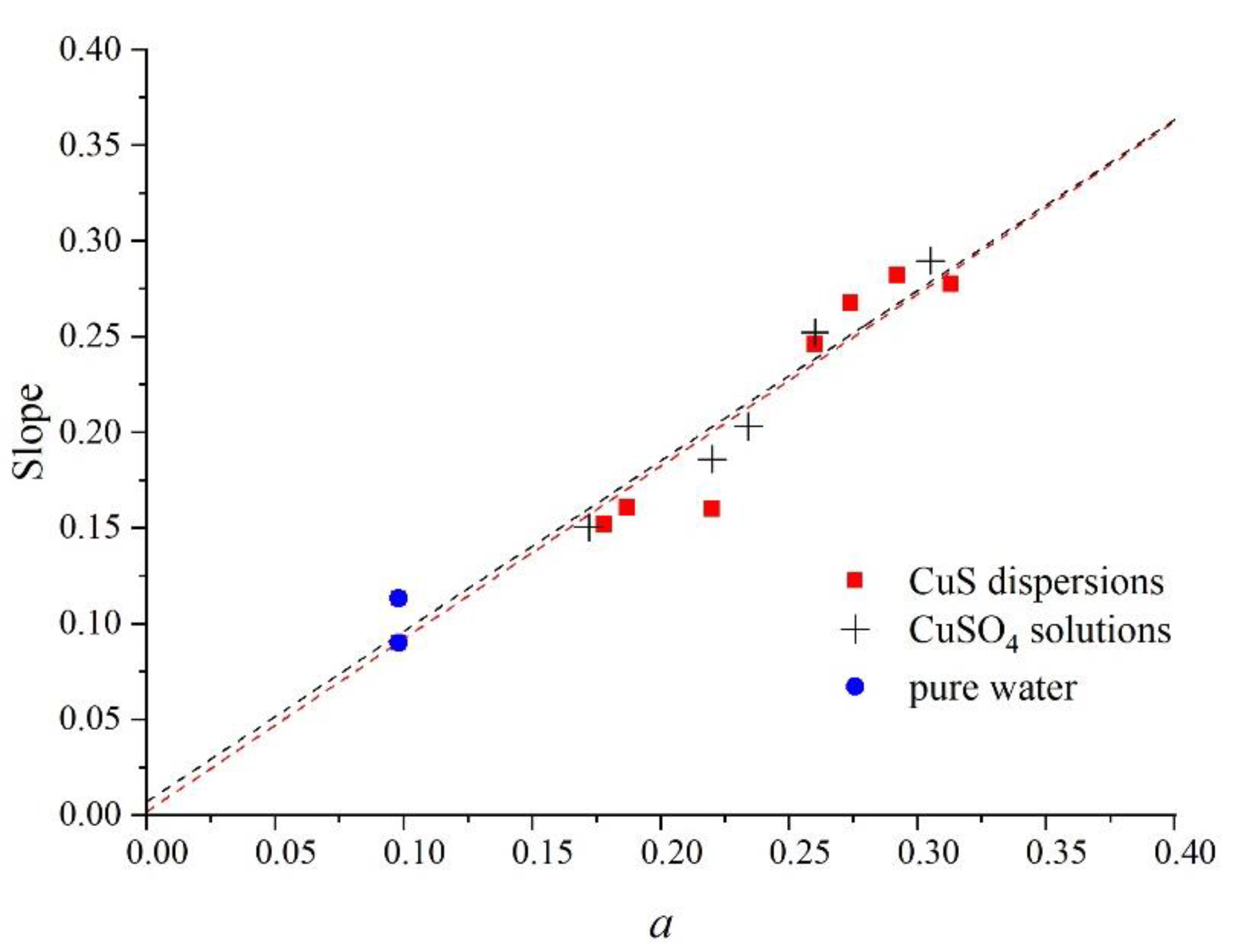
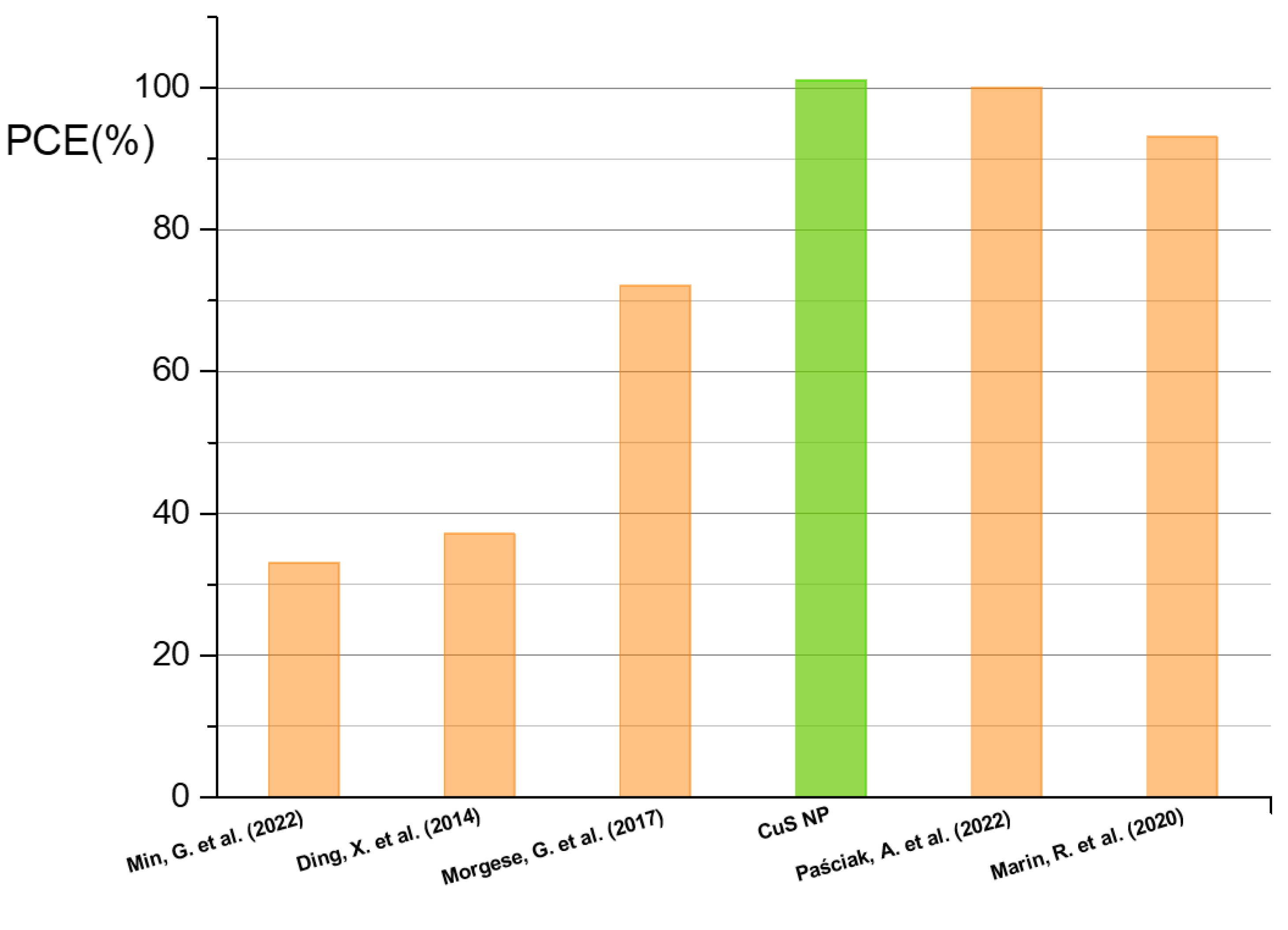
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Laramie, M.D.; Smith, M.K.; Marmarchi, F.; McNally, L.R.; Henary, M. Small Molecule Optoacoustic Contrast Agents: An Unexplored Avenue for Enhancing In Vivo Imaging. Molecules 2018, 23, 2766. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, Y.; Liu, B. Nanoparticles as contrast agents for photoacoustic brain imaging. Aggregate 2021, 2, 4–19. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Mantri, Y.; Jokerst, J.V. Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14, 9408–9422. [Google Scholar] [CrossRef] [PubMed]
- Steinbrueck, A.; Karges, J. Metal Complexes and Nanoparticles for Photoacoustic Imaging. Chembiochem 2023, e202300079. [Google Scholar] [CrossRef] [PubMed]
- Gellini, C.; Feis, A. Optothermal properties of plasmonic inorganic nanoparticles for photoacoustic applications. Photoacoustics 2021, 23, 100281. [Google Scholar] [CrossRef]
- Lyu, Y.; Li, J.; Pu, K. Second Near-Infrared Absorbing Agents for Photoacoustic Imaging and Photothermal Therapy. Small Methods 2019, 3, 1900553. [Google Scholar] [CrossRef]
- Skripka, A.; Mendez-Gonzalez, D.; Marin, R.; Ximendes, E.; del Rosal, B.; Jaque, D.; Rodríguez-Sevilla, P. Near infrared bioimaging and biosensing with semiconductor and rare-earth nanoparticles: Recent developments in multifunctional nanomaterials. Nanoscale Adv. 2021, 3, 6310–6329. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Zhang, X.; Sui, J.; Jin, L.; Lin, L.; Fu, Q.; Lin, H.; Song, J. NIR-II Functional Materials for Photoacoustic Theranostics. Bioconjugate Chem. 2022, 33, 67–86. [Google Scholar] [CrossRef]
- Paściak, A.; Pilch-Wróbel, A.; Marciniak, Ł.; Schuck, P.J.; Bednarkiewicz, A. Standardization of Methodology of Light-to-Heat Conversion Efficiency Determination for Colloidal Nanoheaters. ACS Appl. Mater. Interfaces 2021, 13, 44556–44567. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Su, M.; Wang, Z.; Zhang, J. Second Near-Infrared Plasmonic Nanomaterials for Photoacoustic Imaging and Photothermal Therapy. Small 2023, e2300539. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Jang, Y.; Kim, M.; Kim, H. Types/Applications of Photoacoustic Contrast Agents: A Review. Photonics 2021, 8, 287. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, L.; Yin, B.; Meng, H.; Yin, X.; Huan, S.; Song, G.; Zhang, X.-B. Chemical Design of Activatable Photoacoustic Probes for Precise Biomedical Applications. Chem. Rev. 2022, 122, 6850–6918. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Dou, T.; Ma, L.; Ma, J. Biomedical Photoacoustic Imaging for Molecular Detection and Disease Diagnosis: “Always-On” and “Turn-On” Probes. Adv. Sci. 2022, 9, 2202384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Swartchick, C.B.; Chan, J. Targeted contrast agents and activatable probes for photoacoustic imaging of cancer. Chem. Soc. Rev. 2022, 51, 829–868. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.; Huang, M.; Lu, W.; Song, S.; Melancon, M.P.; Tian, M.; Liang, D.; Li, C. A Chelator-Free Multifunctional [64Cu]CuS Nanoparticle Platform for Simultaneous Micro-PET/CT Imaging and Photothermal Ablation Therapy. J. Am. Chem. Soc. 2010, 132, 15351–15358. [Google Scholar] [CrossRef]
- Comin, A.; Manna, L. New materials for tunable plasmonic colloidal nanocrystals. Chem. Soc. Rev. 2014, 43, 3957–3975. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Heibel, G.E. Time-resolved photothermal and photoacoustic methods applied to photoinduced processes in solution. Chem. Rev. 1992, 92, 1381–1410. [Google Scholar] [CrossRef]
- Marin, R.; Skripka, A.; Besteiro, L.V.; Benayas, A.; Wang, Z.; Govorov, A.O.; Canton, P.; Vetrone, F. Highly Efficient Copper Sulfide-Based Near-Infrared Photothermal Agents: Exploring the Limits of Macroscopic Heat Conversion. Small 2018, 14, e1803282. [Google Scholar] [CrossRef] [PubMed]
- Feis, A.; Gellini, C.; Salvi, P.R.; Becucci, M. Photoacoustic excitation profiles of gold nanoparticles. Photoacoustics 2014, 2, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef]
- Cole, J.R.; Mirin, N.A.; Knight, M.W.; Goodrich, G.P.; Halas, N.J. Photothermal Efficiencies of Nanoshells and Nanorods for Clinical Therapeutic Applications. J. Phys. Chem. C 2009, 113, 12090–12094. [Google Scholar] [CrossRef]
- Jiang, K.; Smith, D.A.; Pinchuk, A. Size-Dependent Photothermal Conversion Efficiencies of Plasmonically Heated Gold Nanoparticles. J. Phys. Chem. C 2013, 117, 27073–27080. [Google Scholar] [CrossRef]
- Paściak, A.; Marin, R.; Abiven, L.; Pilch-Wróbel, A.; Misiak, M.; Xu, W.; Prorok, K.; Bezkrovnyi, O.; Marciniak, Ł.; Chanéac, C.; et al. Quantitative Comparison of the Light-to-Heat Conversion Efficiency in Nanomaterials Suitable for Photothermal Therapy. ACS Appl. Mater. Interfaces 2022, 14, 33555–33566. [Google Scholar] [CrossRef] [PubMed]
- Morgese, G.; Dolcet, P.; Feis, A.; Gellini, C.; Gialanella, S.; Speghini, A.; Badocco, D.; Pastore, P.; Casarin, M.; Gross, S. Room-Temperature Crystallization of CuS Nanostructures for Photothermal Applications through a Nanoreactor Approach. Eur. J. Inorg. Chem. 2017, 2017, 2745–2754. [Google Scholar] [CrossRef]
- Ding, X.; Liow, C.H.; Zhang, M.; Huang, R.; Li, C.; Shen, H.; Liu, M.; Zou, Y.; Gao, N.; Zhang, Z.; et al. Surface Plasmon Resonance Enhanced Light Absorption and Photothermal Therapy in the Second Near-Infrared Window. J. Am. Chem. Soc. 2014, 136, 15684–15693. [Google Scholar] [CrossRef]
- Min, G.; Hong, F.; Shi, C.; Zhao, Q.; Lin, N.; Liu, X.-Y. Biomimetic synthesis of 2D ultra-small copper sulfide nanoflakes based on reconfiguration of the keratin secondary structure for cancer theranostics in the NIR-II region. J. Mater. Chem. B 2022, 10, 3152–3161. [Google Scholar] [CrossRef]
- Marin, R.; Lifante, J.; Besteiro, L.V.; Wang, Z.; Govorov, A.O.; Rivero, F.; Alfonso, F.; Sanz-Rodríguez, F.; Jaque, D. Plasmonic Copper Sulfide Nanoparticles Enable Dark Contrast in Optical Coherence Tomography. Adv. Healthc. Mater. 2020, 9, e1901627. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gellini, C.; Ricci, M.; Feis, A. Copper Sulfide Small Nanoparticles as Efficient Contrast Agent for Photoacoustic Imaging. Photonics 2023, 10, 772. https://doi.org/10.3390/photonics10070772
Gellini C, Ricci M, Feis A. Copper Sulfide Small Nanoparticles as Efficient Contrast Agent for Photoacoustic Imaging. Photonics. 2023; 10(7):772. https://doi.org/10.3390/photonics10070772
Chicago/Turabian StyleGellini, Cristina, Marilena Ricci, and Alessandro Feis. 2023. "Copper Sulfide Small Nanoparticles as Efficient Contrast Agent for Photoacoustic Imaging" Photonics 10, no. 7: 772. https://doi.org/10.3390/photonics10070772
APA StyleGellini, C., Ricci, M., & Feis, A. (2023). Copper Sulfide Small Nanoparticles as Efficient Contrast Agent for Photoacoustic Imaging. Photonics, 10(7), 772. https://doi.org/10.3390/photonics10070772




