Complementary Metal Oxide Semiconductor-Based Optical Detection System for Fluidic Cellular Medium pH Quantification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optical Wavelength Selection
2.2. Photodiode Specification
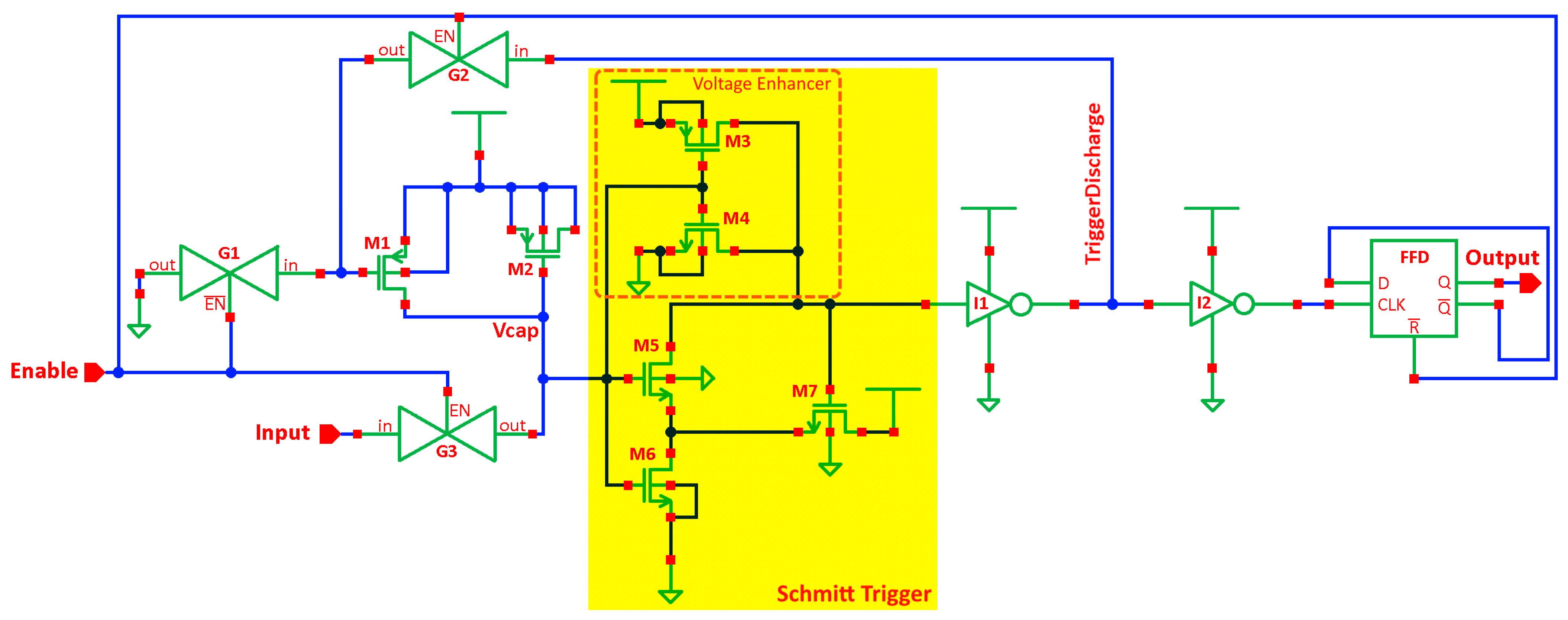
2.3. Signal Readout Electronics Design
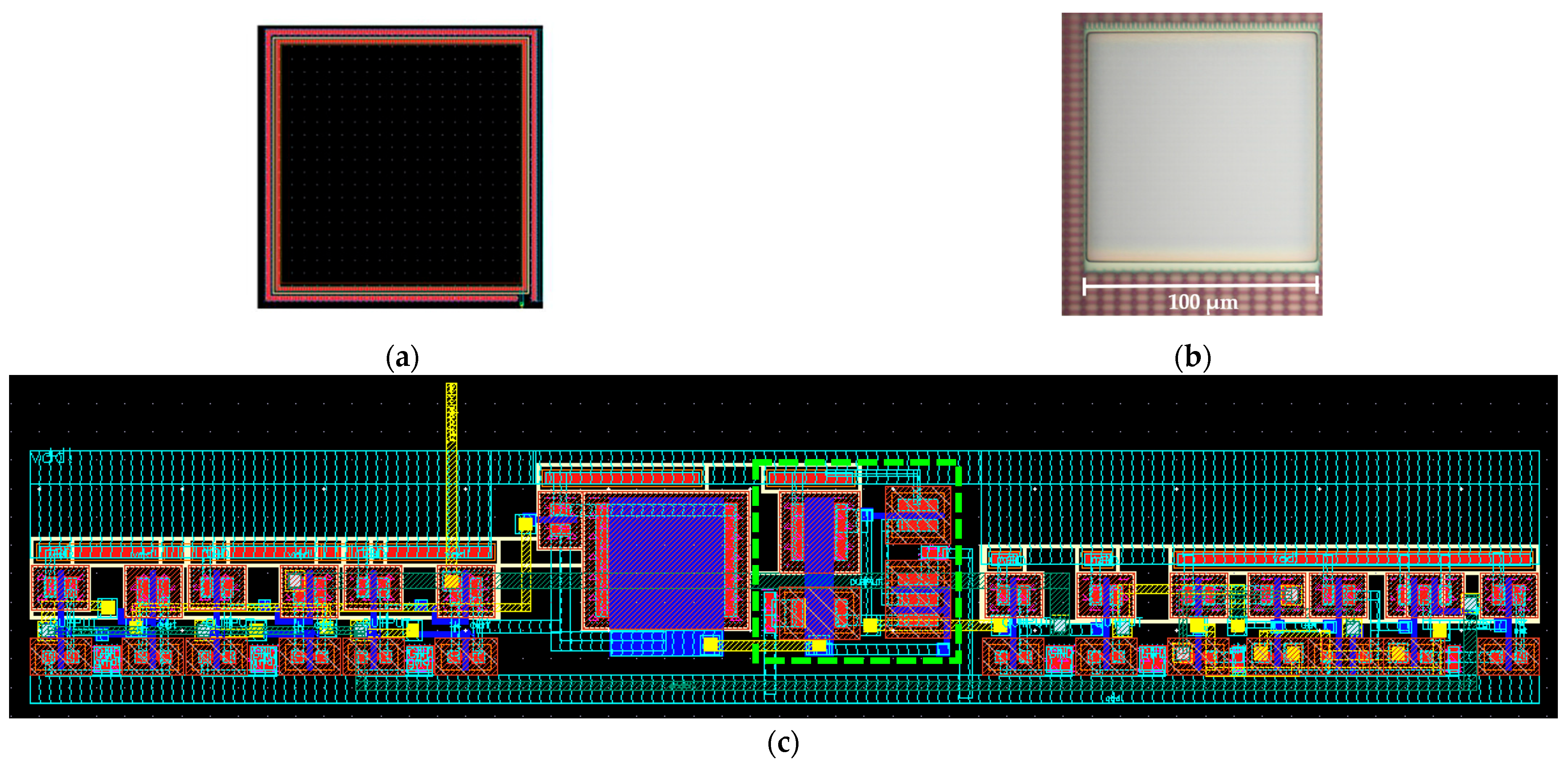
2.4. Optical Detection System Fabrication
2.5. Organ-on-a-Chip Microfluidic System Integration
3. Results and Discussion
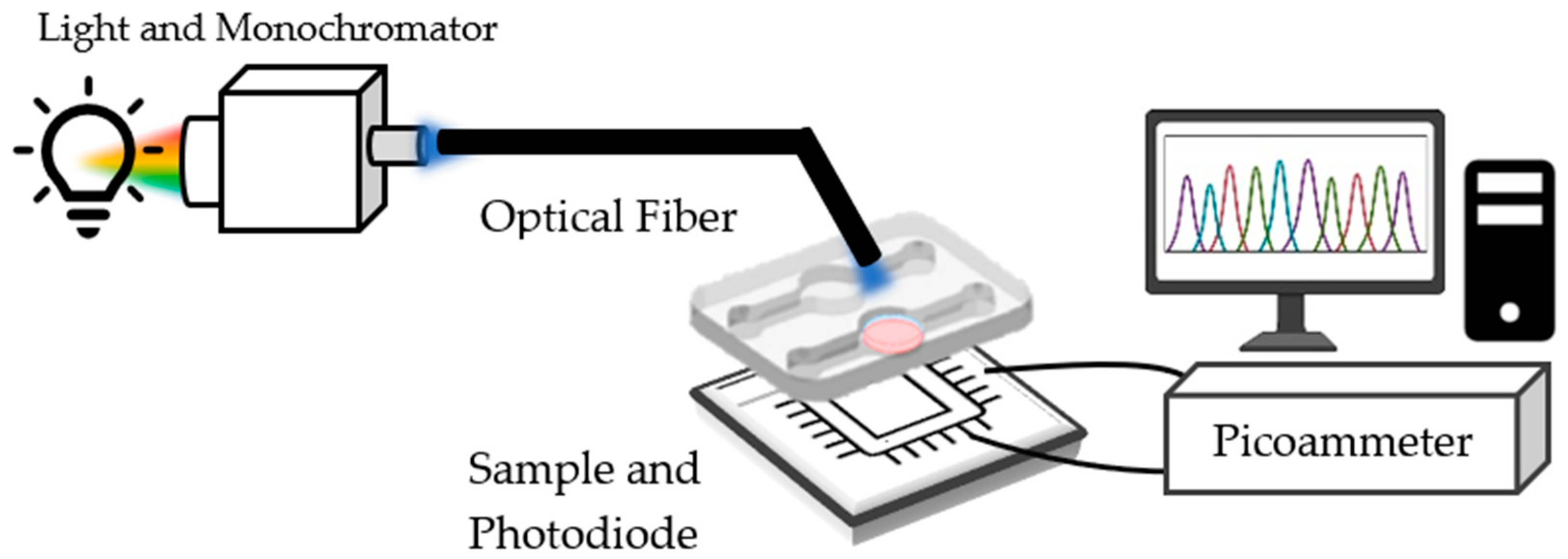
3.1. Measurement Setup and Sample Preparation
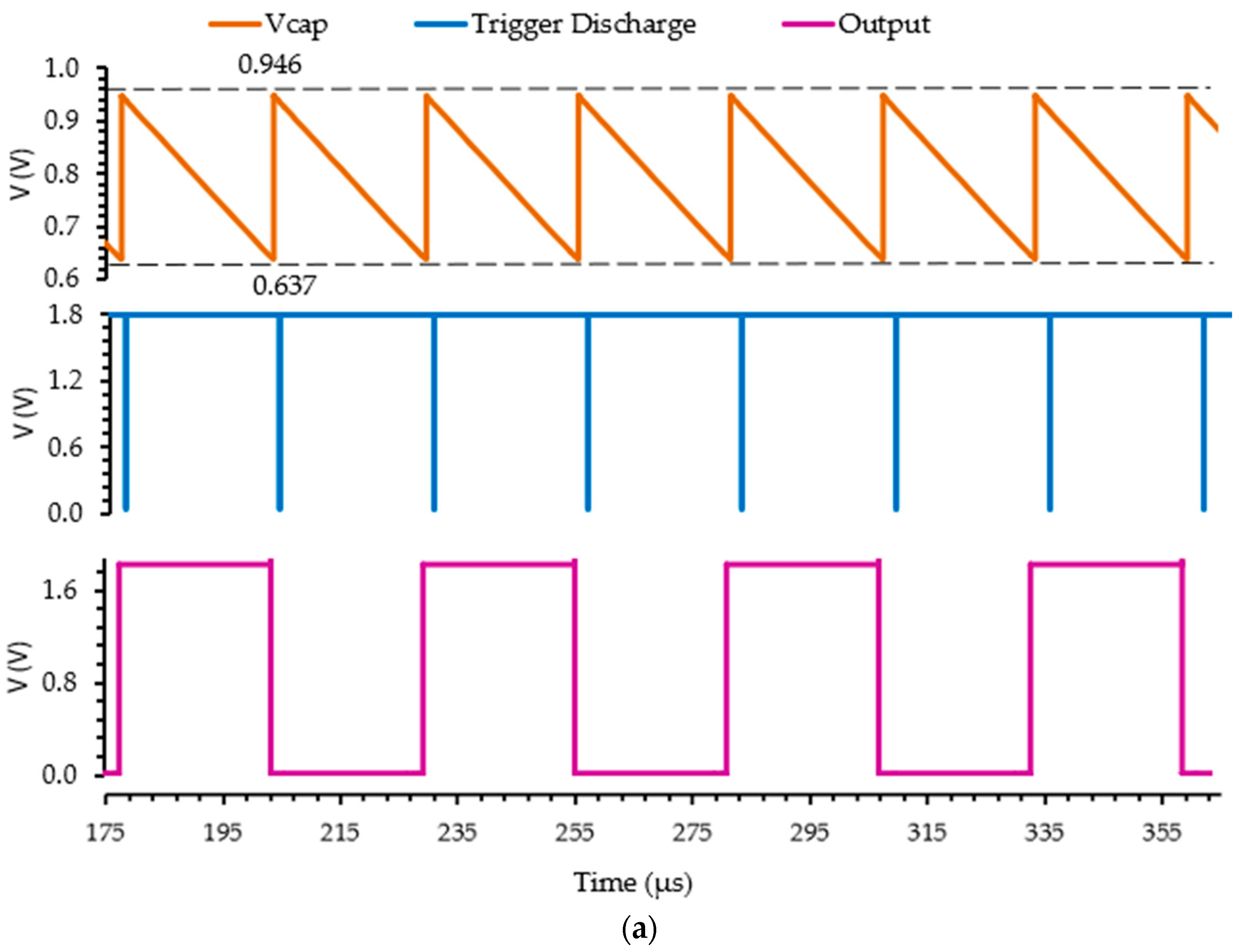
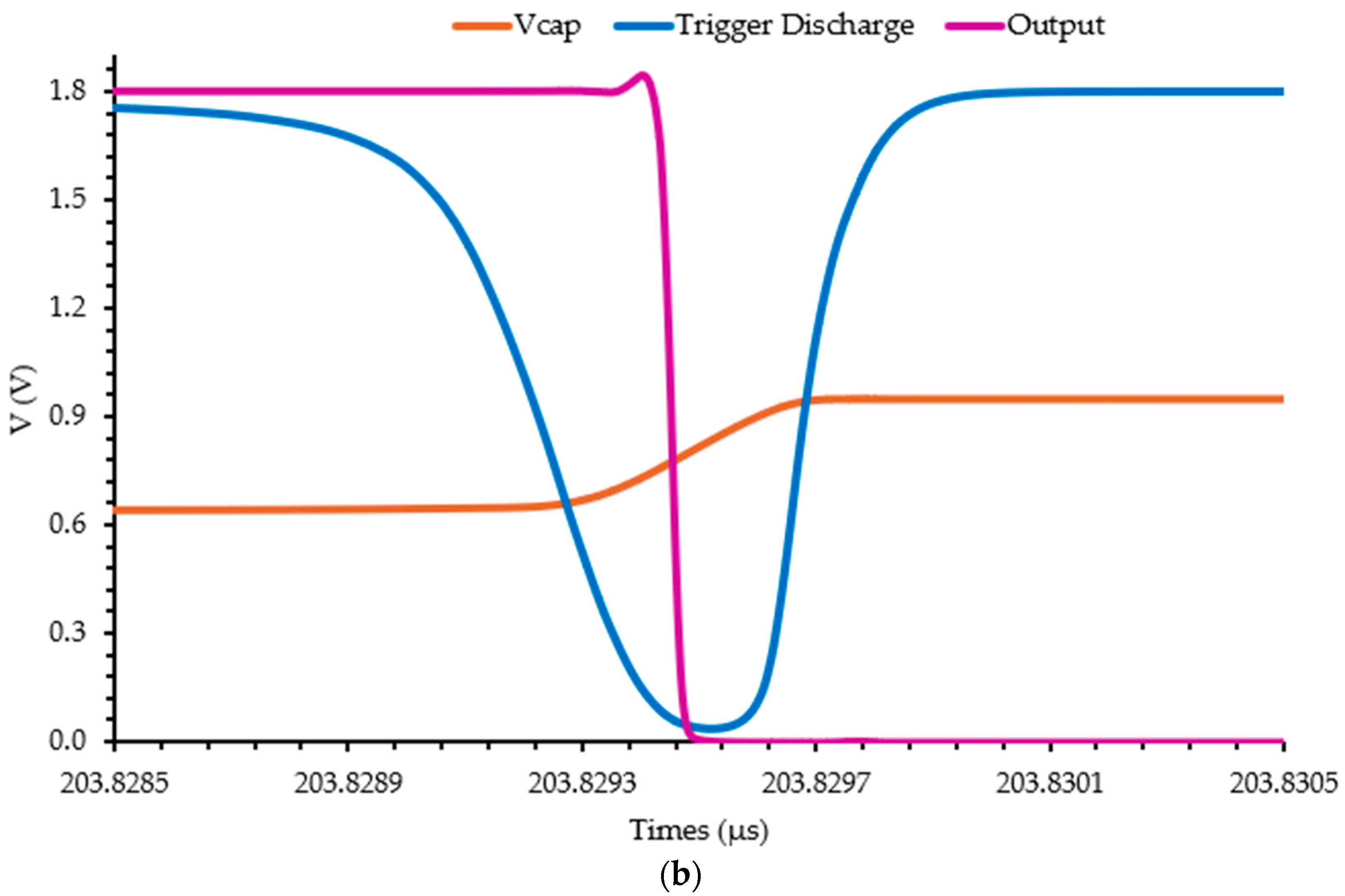
3.2. Readout Electronics Characterization
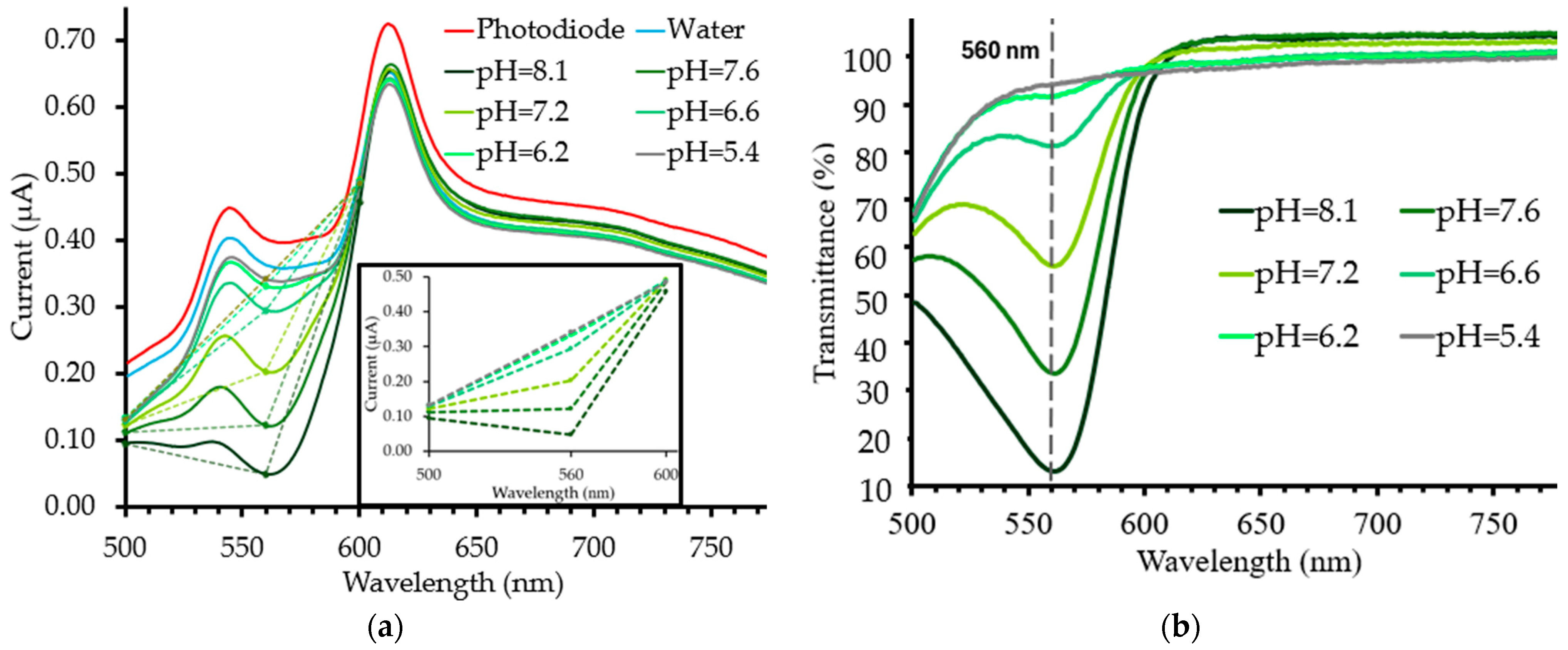
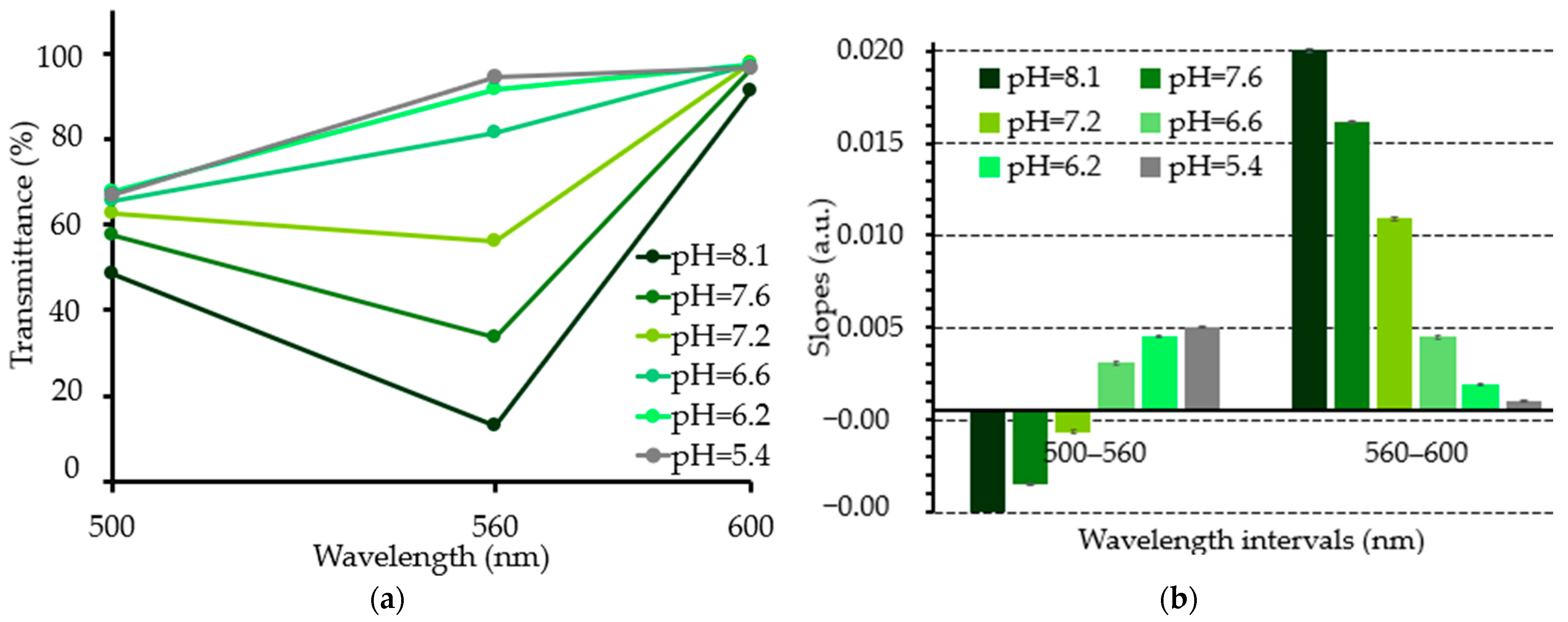
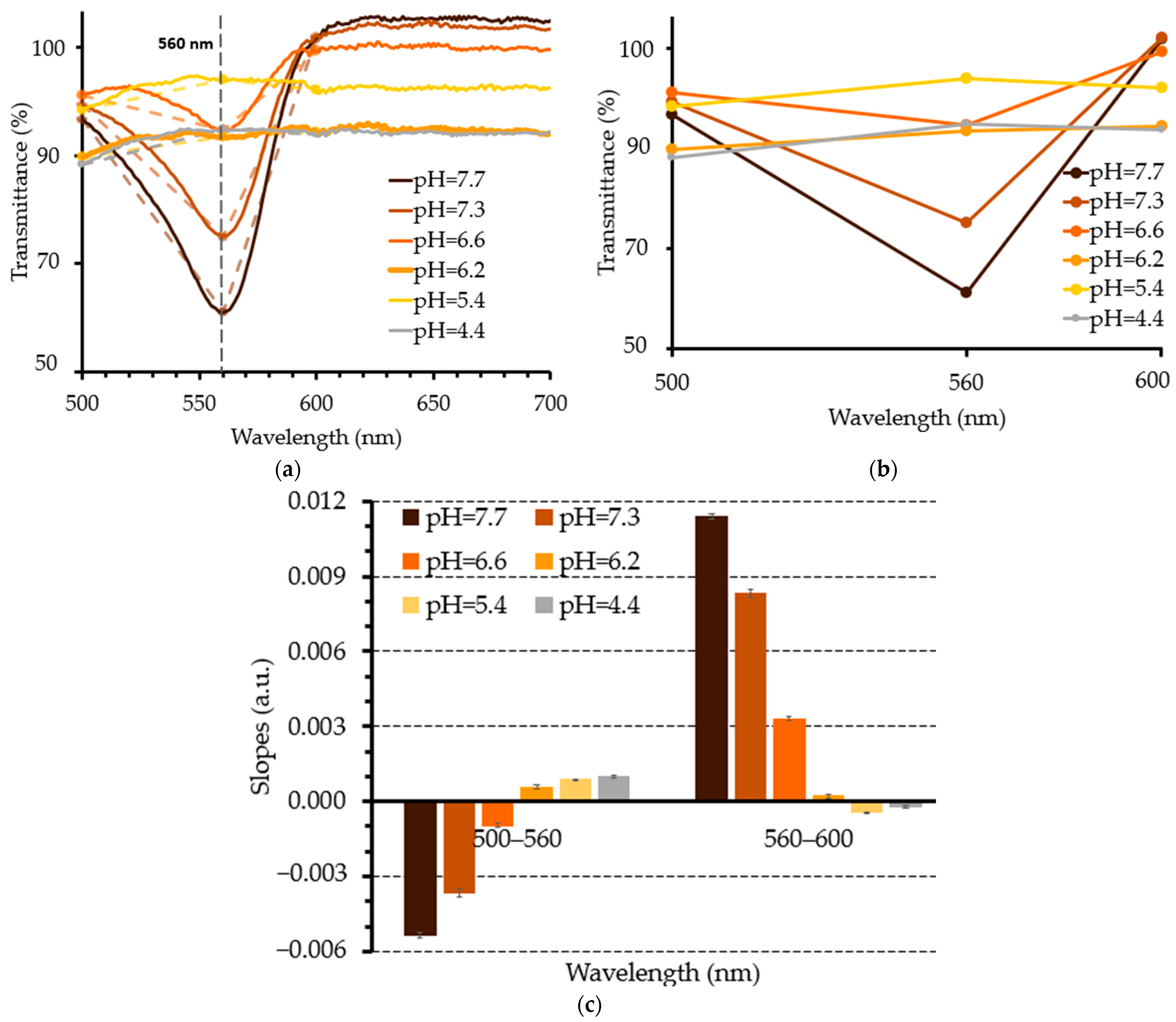
3.3. Experimental pH Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danku, A.E.; Dulf, E.H.; Braicu, C.; Jurj, A.; Berindan-Neagoe, I. Organ-On-A-Chip: A Survey of Technical Results and Problems. Front. Bioeng. Biotechnol. 2022, 10, 840674. [Google Scholar] [CrossRef] [PubMed]
- Feitor, J.F.; Brazaca, L.C.; Lima, A.M.; Ferreira, V.G.; Kassab, G.; Bagnato, V.S.; Carrilho, E.; Cardoso, D.R. Organ-on-a-Chip for Drug Screening: A Bright Future for Sustainability? A Critical Review. ACS Biomater. Sci. Eng. 2023, 9, 2220–2234. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chen, Y.-Q.; Luo, G.-A.; Zhang, M.; Zhang, H.-Y.; Wang, Y.-R.; Hu, P. Organs-on-chips and Its Applications. Chin. J. Anal. Chem. 2016, 44, 533–541. [Google Scholar] [CrossRef]
- Clarke, G.A.; Hartse, B.X.; Niaraki Asli, A.E.; Taghavimehr, M.; Hashemi, N.; Abbasi Shirsavar, M.; Montazami, R.; Alimoradi, N.; Nasirian, V.; Ouedraogo, L.J.; et al. Advancement of Sensor Integrated Organ-on-Chip Devices. Sensors 2021, 21, 1367. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Lin, J.-L.; Wang, J.; Cui, Z.; Cui, Z. Development of high throughput optical sensor array for on-line pH monitoring in micro-scale cell culture environment. Biomed. Microdevices 2009, 11, 265–273. [Google Scholar] [CrossRef]
- Magnusson, E.B.; Halldorsson, S.; Fleming, R.M.T.; Leosson, K. Real-time optical pH measurement in a standard microfluidic cell culture system. Biomed. Opt. Express 2013, 4, 1749. [Google Scholar] [CrossRef]
- Freshney, R.I. Culture of Animal Cells; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Gonçalves, I.M.; Carvalho, V.; Rodrigues, R.O.; Pinho, D.; Teixeira, S.F.C.F.; Moita, A.; Hori, T.; Kaji, H.; Lima, R.; Minas, G. Organ-on-a-Chip Platforms for Drug Screening and Delivery in Tumor Cells: A Systematic Review. Cancers 2022, 14, 935. [Google Scholar] [CrossRef]
- Tawade, P.; Mastrangeli, M. Integrated Electrochemical and Optical Biosensing in Organs-on-Chip. ChemBioChem 2024, 25, e202300560. [Google Scholar] [CrossRef]
- Kilic, T.; Navaee, F.; Stradolini, F.; Renaud, P.; Carrara, S. Organs-on-chip monitoring: Sensors and other strategies. Microphysiol. Syst. 2018, 1, 1–32. [Google Scholar] [CrossRef]
- Chen, H.; Luo, Z.; Lin, X.; Zhu, Y.; Zhao, Y. Sensors-integrated organ-on-a-chip for biomedical applications. Nano Res. 2023, 16, 10072–10099. [Google Scholar] [CrossRef]
- Pinto, V.C.; Araújo, C.F.; Sousa, P.J.; Gonçalves, L.M.; Minas, G. A low-cost lab-on-a-chip device for marine pH quantification by colorimetry. Sens. Actuators B Chem. 2019, 290, 285–292. [Google Scholar] [CrossRef]
- Saetchnikov, A.V.; Tcherniavskaia, E.A.; Saetchnikov, V.A.; Ostendorf, A. Two-photon polymerization of optical microresonators for precise pH sensing. arXiv 2024, arXiv:2403.15117. [Google Scholar] [CrossRef]
- Duan, M.; Zhong, X.; Zhao, X.; El-Agnaf, O.M.; Lee, Y.-K.; Bermak, A. An Optical and Temperature Assisted CMOS ISFET Sensor Array for Robust E. coli Detection. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef]
- Lee, S.; Ibey, B.L.; Coté, G.L.; Pishko, M.V. Measurement of pH and dissolved oxygen within cell culture media using a hydrogel microarray sensor. Sens. Actuators B Chem. 2008, 128, 388–398. [Google Scholar] [CrossRef]
- Yang, W.; Li, T.; Liao, S.; Zhou, J.; Huang, L. Organ-on-a-chip platforms integrated with biosensors for precise monitoring of the cells and cellular microenvironment. TrAC Trends Anal. Chem. 2024, 172, 117569. [Google Scholar] [CrossRef]
- Ferreira, G.M.; Silva, V.; Minas, G.; Catarino, S.O. Simulation Study of Vertical p–n Junction Photodiodes’ Optical Performance According to CMOS Technology. Appl. Sci. 2022, 12, 2580. [Google Scholar] [CrossRef]
- Chandrasekharan, H.K.; Wlodarczyk, K.L.; MacPherson, W.N.; Maroto-Valer, M.M. In-situ multicore fibre-based pH mapping through obstacles in integrated microfluidic devices. arXiv 2023, arXiv:2308.02967. [Google Scholar] [CrossRef]
- Cao, M.; Mahto, S.K.; Yadid-Pecht, O. Real-Time Optical pH Sensor With CMOS Contact Imaging and Microfluidics. IEEE Sens. J. 2016, 16, 3611–3619. [Google Scholar] [CrossRef]
- Morgan, A.; Babu, D.; Reiz, B.; Whittal, R.; Suh, L.Y.K.; Siraki, A.G. Caution for the routine use of phenol red—It is more than just a pH indicator. Chem. Biol. Interact. 2019, 310, 108739. [Google Scholar] [CrossRef]
- Raffay, R.; Husin, N.; Omar, A.F. Spectrophotometry and colorimetry profiling of pure phenol red and cell culture medium on pH variation. Color. Technol. 2022, 138, 640–659. [Google Scholar] [CrossRef]
- Sharma, K.; Fizet, K.J.; Montgomery, K.R.; Smeltzer, N.A.; Sikorski, M.H.; Brown, K.G.; Beyke, B.J.; Burkhart, R.C.; Lynn, A.N.; Grandinetti, G. A simple colorimetric experiment using mammalian cell culture to study metabolism. Biochem. Mol. Biol. Educ. 2021, 49, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Gounella, R.; Ferreira, G.M.; Amorim, M.L.M.; Soares, J.N.; Carmo, J.P. A Review of Optical Sensors in CMOS. Electronics 2024, 13, 691. [Google Scholar] [CrossRef]
- Koklu, G.; Etienne-Cummings, R.; Leblebici, Y.; De Micheli, G.; Carrara, S. Characterization of standard CMOS compatible photodiodes and pixels for Lab-on-Chip devices. In Proceedings of the 2013 IEEE International Symposium on Circuits and Systems (ISCAS), Beijing, China, 19–23 May 2013; pp. 1075–1078. [Google Scholar] [CrossRef]
- Ferreira, G.M.; Baptista, V.; Silva, V.; Veiga, M.I.; Minas, G.; Catarino, S.O. CMOS spectrophotometric microsystem for malaria detection. IEEE Trans. Biomed. Eng. 2023, 70, 2318–2328. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.G.; Pimenta, S.; Minas, G. CMOS Integrated Photodetectors and Light-to-Frequency Converters for Spectrophotometric Measurements. IEEE Sens. J. 2017, 17, 3438–3445. [Google Scholar] [CrossRef]
- Jha, S.K.; Verma, P.; Taleja, M. Design of Low Power CMOS Based Schmitt Trigger in 180 nm Technology. In Proceedings of the 2019 4th International Conference on Internet of Things: Smart Innovation and Usages (IoT-SIU), Ghaziabad, India, 18–19 April 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Schroder, D.K. Negative bias temperature instability: What do we understand? Microelectron. Reliab. 2007, 47, 841–852. [Google Scholar] [CrossRef]
- Alam, M.A.; Mahapatra, S. A comprehensive model of PMOS NBTI degradation. Microelectron. Reliab. 2005, 45, 71–81. [Google Scholar] [CrossRef]
- Baker, R.J. CMOS: Circuit Design, Layout, and Simulation, 3rd ed.; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bucher, M.; Lallement, C.; Enz, C.; Théodoloz, F.; Krummenacher, F. The EPFL-EKV MOSFET Model Equations for Simulation; Technical Report; Eletronics Laboratory, Swiss Federal Institute of Technology: Zurich, Switzerland, 1997. [Google Scholar]
- Lu, T.T.; Li, Z.; Luo, C.; Xu, J.; Kong, W.; Guo, G. Characterization and Modeling of 0.18μm Bulk CMOS Technology at Sub-Kelvin Temperature. IEEE J. Electron Devices Soc. 2020, 8, 897–904. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Costa, M.S.; Baptista, V.; Ferreira, G.M.; Lima, D.; Minas, G.; Veiga, M.I.; Catarino, S.O. Multilayer Thin-Film Optical Filters for Reflectance-Based Malaria Diagnostics. Micromachines 2021, 12, 890. [Google Scholar] [CrossRef]
- Badugu, R.; Kostov, Y.; Rao, G.; Tolosa, L. Development and application of an excitation ratiometric optical pH sensor for bioprocess monitoring. Biotechnol. Prog. 2008, 24, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Lin, J. Recent development and applications of optical and fiber-optic pH sensors. TrAC Trends Anal. Chem. 2000, 19, 541–552. [Google Scholar] [CrossRef]
- Marinenko, G.; Koch, W.F. A critical review of measurement practices for the determination of pH and acidity of atmospheric precipitation. Environ. Int. 1984, 10, 315–319. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.A.; Ferreira, G.M.; Sousa, P.J.; Sousa, P.C.; Catarino, S.O.; Minas, G. Complementary Metal Oxide Semiconductor-Based Optical Detection System for Fluidic Cellular Medium pH Quantification. Photonics 2024, 11, 1130. https://doi.org/10.3390/photonics11121130
Santos AA, Ferreira GM, Sousa PJ, Sousa PC, Catarino SO, Minas G. Complementary Metal Oxide Semiconductor-Based Optical Detection System for Fluidic Cellular Medium pH Quantification. Photonics. 2024; 11(12):1130. https://doi.org/10.3390/photonics11121130
Chicago/Turabian StyleSantos, André A., Gabriel M. Ferreira, Paulo J. Sousa, Patrícia C. Sousa, Susana O. Catarino, and Graça Minas. 2024. "Complementary Metal Oxide Semiconductor-Based Optical Detection System for Fluidic Cellular Medium pH Quantification" Photonics 11, no. 12: 1130. https://doi.org/10.3390/photonics11121130
APA StyleSantos, A. A., Ferreira, G. M., Sousa, P. J., Sousa, P. C., Catarino, S. O., & Minas, G. (2024). Complementary Metal Oxide Semiconductor-Based Optical Detection System for Fluidic Cellular Medium pH Quantification. Photonics, 11(12), 1130. https://doi.org/10.3390/photonics11121130








