Agreement on Anterior Chamber Depth Measurement between Three Commercially Available Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. ARK510A Autorefractor–Keratometer
2.3. Canon TX-10 Tonometer
2.4. VX-120 Multi-Diagnostic Platform
2.5. Lenstar LS900 Ocular Biometer
2.6. EchoScan US-800 Ocular Biometer
2.7. Statistical Analysis
3. Results
3.1. Differences in the ACD Values between Devices
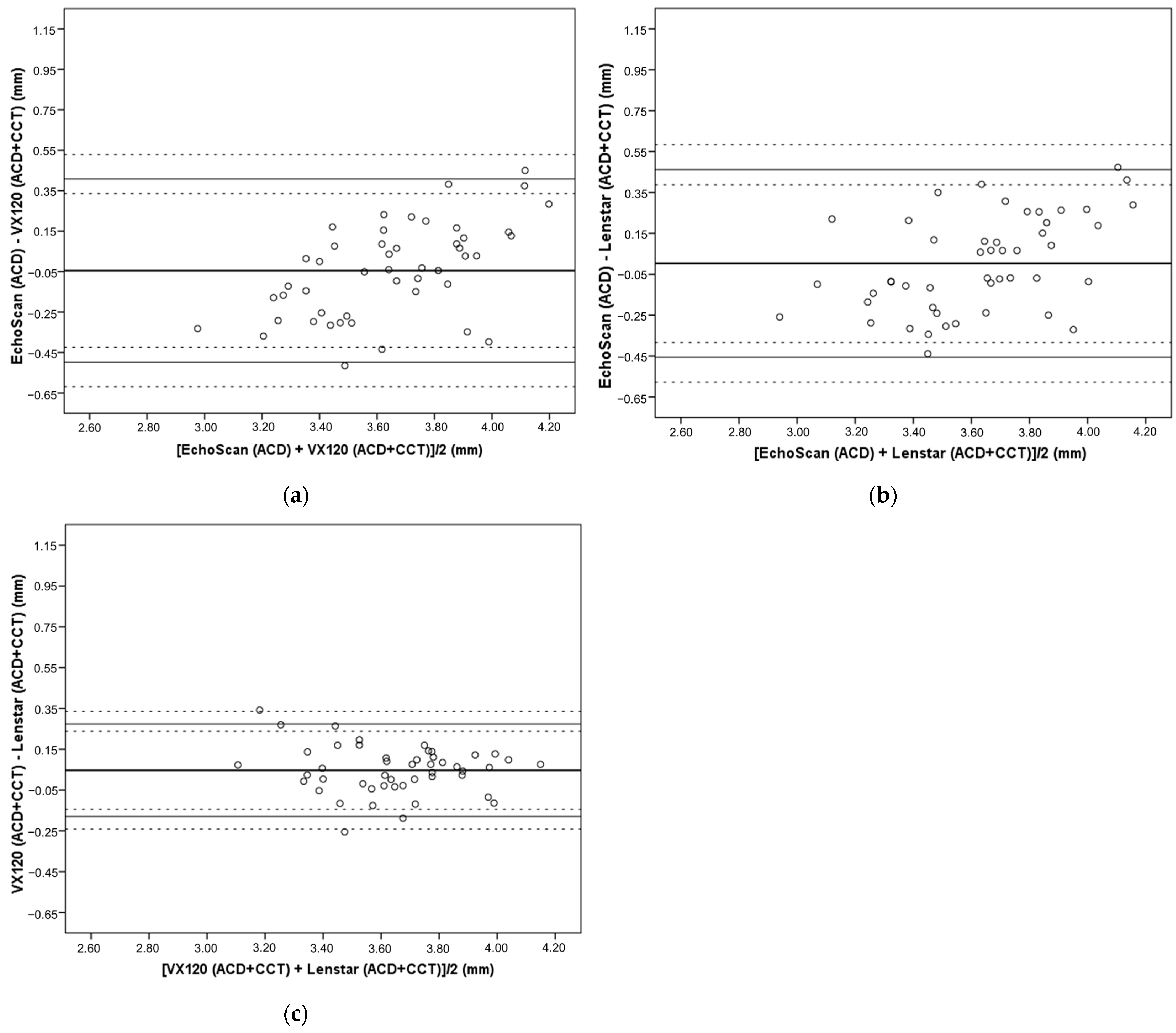
3.2. Differences in ACD Compensated by CCT Values between Devices
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, T.K.; Ryu, I.H.; Kim, J.K.; Lee, I.S.; Kim, H.K. A deep learning approach for detection of shallow anterior chamber depth based on the hidden features of fundus photographs. Comput. Methods Programs Biomed. 2022, 219, 106735. [Google Scholar] [CrossRef]
- Hoffer, K.J.; Savini, G. Update on Intraocular Lens Power Calculation Study Protocols: The Better Way to Design and Report Clinical Trials. Ophthalmology 2021, 128, e115–e120. [Google Scholar] [CrossRef]
- Savur, S.; Kaup, S.; Dinesh, A.; Shivalli, S.; Kondal, D. Can ultrasonic biometric indices with optimal cut-offs be a potential screening tool for primary angle closure disease? A case-control study. Eye 2022, 37, 1284–1289. [Google Scholar] [CrossRef]
- Upasna; Singh, V.K.; Rana, J.; Kumar, S.; Singh, A.; Singh, K. An evaluation of intraoperative and postoperative outcomes of phacoemulsification surgery in eyes with shallow anterior chamber. Indian J. Ophthalmol. 2021, 69, 1346–1347. [Google Scholar] [CrossRef]
- Dabasia, P.L.; Edgar, D.F.; Murdoch, I.E.; Lawrenson, J.G. Noncontact Screening Methods for the Detection of Narrow Anterior Chamber Angles. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3929–3935. [Google Scholar] [CrossRef][Green Version]
- Beyoglu, A.; Karakucuk, Y.; Comez, A. Evaluation of the effect of fasting on intraocular pressure, anterior segment parameters and density of crystalline lens and cornea. Int. Ophthalmol. 2020, 40, 2987–2994. [Google Scholar] [CrossRef]
- Pakuliene, G.; Zimarinas, K.; Nedzelskiene, I.; Siesky, B.; Kuzmiene, L.; Harris, A.; Januleviciene, I. Anterior segment optical coherence tomography imaging and ocular biometry in cataract patients with open angle glaucoma comorbidity. BMC Ophthalmol. 2021, 21, 127. [Google Scholar] [CrossRef]
- Aktas, S.; Aktas, H.; Tetikoglu, M.; Sagdık, H.M.; Özcura, F.J.M. Refractive results using a new optical biometry device: Comparison with ultrasound biometry data. Medicine 2015, 94, e2169. [Google Scholar] [CrossRef]
- Yong, K.-L.; Gong, T.; Nongpiur, M.E.; How, A.C.; Lee, H.K.; Cheng, L.; Perera, S.A.; Aung, T.J.O. Myopia in Asian subjects with primary angle closure: Implications for glaucoma trends in East Asia. Ophthalmology 2014, 121, 1566–1571. [Google Scholar] [CrossRef]
- Findl, O.; Kriechbaum, K.; Sacu, S.; Kiss, B.; Polak, K.; Nepp, J.; Schild, G.; Rainer, G.; Maca, S.; Petternel, V.; et al. Influence of operator experience on the performance of ultrasound biometry compared to optical biometry before cataract surgery. J. Cataract Refract. Surg. 2003, 29, 1950–1955. [Google Scholar] [CrossRef]
- Cione, F.; De Bernardo, M.; Rosa, N. Comment on: Evaluation of intraoperative aphakic eye axial length measurements using swept-source OCT. J. Cataract Refract. Surg. 2022, 48, 1226–1227. [Google Scholar] [CrossRef]
- Kanclerz, P.; Khoramnia, R.; Wang, X. Current Developments in Corneal Topography and Tomography. Diagnostics 2021, 11, 1466. [Google Scholar] [CrossRef]
- Chen, Y.A.; Hirnschall, N.; Findl, O. Evaluation of 2 new optical biometry devices and comparison with the current gold standard biometer. J. Cataract Refract. Surg. 2011, 37, 513–517. [Google Scholar] [CrossRef]
- Vinciguerra, R.; Ambrosio, R., Jr.; Elsheikh, A.; Hafezi, F.; Yong Kang, D.S.; Kermani, O.; Koh, S.; Lu, N.; Padmanabhan, P.; Roberts, C.J.; et al. Detection of postlaser vision correction ectasia with a new combined biomechanical index. J. Cataract Refract. Surg. 2021, 47, 1314–1318. [Google Scholar] [CrossRef]
- De Bernardo, M.; Cione, F.; Capasso, L.; Coppola, A.; Rosa, N. A formula to improve the reliability of optical axial length measurement in IOL power calculation. Sci. Rep. 2022, 12, 18845. [Google Scholar] [CrossRef]
- Cheng, S.M.; Zhang, J.S.; Shao, X.; Wu, Z.T.; Li, T.T.; Wang, P.; Lin, J.H.; Yu, A.Y. Repeatability of a new swept-source optical coherence tomographer and agreement with other three optical biometers. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2022, 260, 2271–2281. [Google Scholar] [CrossRef]
- Kurian, M.; Negalur, N.; Das, S.; Puttaiah, N.K.; Haria, D.; J, T.S.; Thakkar, M.M. Biometry with a new swept-source optical coherence tomography biometer: Repeatability and agreement with an optical low-coherence reflectometry device. J. Cataract Refract. Surg. 2016, 42, 577–581. [Google Scholar] [CrossRef]
- Kanclerz, P.; Hoffer, K.J.; Rozema, J.J.; Przewlocka, K.; Savini, G. Repeatability and reproducibility of optical biometry implemented in a new optical coherence tomographer and comparison with a optical low-coherence reflectometer. J. Cataract Refract. Surg. 2019, 45, 1619–1624. [Google Scholar] [CrossRef]
- Passi, S.F.; Thompson, A.C.; Gupta, P.K. Comparison of agreement and efficiency of a swept source-optical coherence tomography device and an optical low-coherence reflectometry device for biometry measurements during cataract evaluation. Clin. Ophthalmol. 2018, 12, 2245–2251. [Google Scholar] [CrossRef]
- Arriola-Villalobos, P.; Almendral-Gomez, J.; Garzon, N.; Ruiz-Medrano, J.; Fernandez-Perez, C.; Martinez-de-la-Casa, J.M.; Diaz-Valle, D. Agreement and clinical comparison between a new swept-source optical coherence tomography-based optical biometer and an optical low-coherence reflectometry biometer. Eye 2017, 31, 437–442. [Google Scholar] [CrossRef]
- van der Heijde, G.L.; Meinema, A.J.; Vlaming, M.S. Digital A-scan ultrasonography used to measure ocular distances. Am. J. Ophthalmol. 1977, 83, 276–277. [Google Scholar] [CrossRef]
- Mattern, A.I.; Neller, K.; Devenijn, M.; Schwahn, H.; Langenbucher, A.; Seitz, B.; Kaymak, H. A Comparison of Optical Biometers Used in Children for Myopia Control. Klin. Monatsblatter Fur Augenheilkd. 2023, 240, 1306–1313. [Google Scholar] [CrossRef]
- Yagmur Kanra, A.; Uslu, H. Evaluation of ocular biometric parameters in keratoconic eyes relative to healthy myopic eyes. Eur. J. Ophthalmol. 2021, 32, 798–805. [Google Scholar] [CrossRef]
- Noya-Padin, V.; Nores-Palmas, N.; Garcia-Queiruga, J.; Giraldez, M.J.; Pena Verdeal, H.; Yebra-Pimentel, E. Associations between Ocular Biometry, Refractive Error, and Body Characteristics. Photonics 2024, 11, 165. [Google Scholar] [CrossRef]
- Cruysberg, L.P.; Doors, M.; Verbakel, F.; Berendschot, T.T.; De Brabander, J.; Nuijts, R.M. Evaluation of the Lenstar LS 900 non-contact biometer. Br. J. Ophthalmol. 2010, 94, 106–110. [Google Scholar] [CrossRef]
- Guler, E.; Kulak, A.E.; Totan, Y.; Yuvarlak, A.; Hepsen, I.F. Comparison of a new optical biometry with an optical low-coherence reflectometry for ocular biometry. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2016, 39, 336–341. [Google Scholar] [CrossRef]
- Pinero, D.P.; Cabezos, I.; Lopez-Navarro, A.; de Fez, D.; Caballero, M.T.; Camps, V.J. Intrasession repeatability of ocular anatomical measurements obtained with a multidiagnostic device in healthy eyes. BMC Ophthalmol. 2017, 17, 193. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Zhai, C.B.; Ma, D.L. Comparison of corneal curvature parameters obtained from two different instruments-Pentacam and VX120. Int. J. Ophthalmol. 2019, 12, 1311–1316. [Google Scholar] [CrossRef]
- Ferrer-Blasco, T.; Esteve-Taboada, J.J.; Domínguez-Vicent, A.; Aloy, M.A.; Adsuara, J.E.; Mimica, P.; Montés-Micó, R. Effect of contact lenses on ocular biometric measurements based on swept-source optical coherence tomography. Arq. Bras. Oftalmol. 2019, 82, 129–135. [Google Scholar] [CrossRef]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. A cross-sectional study of non-modifiable and modifiable risk factors of dry eye disease states. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2023, 46, 101800. [Google Scholar] [CrossRef]
- Achiron, A.; Yavnieli, R.; Olshaker, H.; Levinger, E.; Tuuminen, R.; Livny, E.; Elbaz, U.; Bahar, I.; Nahum, Y. Validation of the multi-metric D-index change in the assessment of keratoconus progression. Int. Ophthalmol. 2022, 42, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, G.Z.; Munteanu, Z.V.I.; Roiu, G.; Daina, C.M.; Daina, L.G.; Coroi, M.C.; Domnariu, C.; Neculoiu, D.C.; Cotovanu, A.S.; Badau, D. Detection of Intraocular Hypertension during Opportunity Screening (Check-Up Medical Inspections). J. Pers. Med. 2022, 12, 777. [Google Scholar] [CrossRef] [PubMed]
- Prum, B.E., Jr.; Lim, M.C.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Gedde, S.J.; Herndon, L.W., Jr.; Rosenberg, L.F.; Williams, R.D. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern((R)) Guidelines. Ophthalmology 2016, 123, P112–P151. [Google Scholar] [CrossRef] [PubMed]
- Prum, B.E., Jr.; Rosenberg, L.F.; Gedde, S.J.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Herndon, L.W., Jr.; Lim, M.C.; Williams, R.D. Primary Open-Angle Glaucoma Preferred Practice Pattern((R)) Guidelines. Ophthalmology 2016, 123, P41–P111. [Google Scholar] [CrossRef]
- Sihota, R.; Kamble, N.; Sharma, A.K.; Bhari, A.; Gupta, A.; Midha, N.; Selvan, H.; Dada, T.; Gupta, V.; Pandey, R.M. V‘an Herick Plus’: A modified grading scheme for the assessment of peripheral anterior chamber depth and angle. Br. J. Ophthalmol. 2019, 103, 960–965. [Google Scholar] [CrossRef]
- Armstrong, R.A. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. 2013, 33, 7–14. [Google Scholar] [CrossRef]
- Xiong, S.; Lv, M.; Zou, H.; Zhu, J.; Lu, L.; Zhang, B.; Deng, J.; Yao, C.; He, X.; Xu, X. Comparison of Refractive Measures of Three Autorefractors in Children and Adolescents. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2017, 94, 894–902. [Google Scholar] [CrossRef]
- Garcia-Resua, C.; Pena-Verdeal, H.; Minones, M.; Giraldez, M.J.; Yebra-Pimentel, E. Reliability of the non-contact tono-pachymeter Tonopachy NT-530P in healthy eyes. Clin. Exp. Optom. J. Aust. Optom. Assoc. 2013, 96, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.S.; Peter, M.; Blum, M.; Haigis, W.; Sekundo, W.; Schutze, J.; Buehren, T. Repeatability and agreement in optical biometry of a new swept-source optical coherence tomography-based biometer versus partial coherence interferometry and optical low-coherence reflectometry. J. Cataract Refract. Surg. 2016, 42, 76–83. [Google Scholar] [CrossRef]
- Noya-Padin, V.; Garcia-Queiruga, J.; Iacubitchii, M.; Giraldez, M.J.; Yebra-Pimentel, E.; Pena-Verdeal, H. Lenstar LS900 vs EchoScan US-800: Comparison between optical and ultrasound biometry with and without contact lenses and its relationship with other biometric parameters. Expert Rev. Med. Devices 2023, 20, 681–690. [Google Scholar] [CrossRef]
- Noya-Padin, V.; Nores-Palmas, N.; Giraldez, M.J.; Yebra-Pimentel, E.; Pena-Verdeal, H. Comparison between Ocular Biometric Parameters and Intraocular Pressure with and without Contact Lenses. Eye Contact Lens 2023, 49, 464–470. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Davies, L.N.; Dunne, M.C.; Gilmartin, B. Statistical guidelines for clinical studies of human vision. Ophthalmic Physiol. Opt. 2011, 31, 123–136. [Google Scholar] [CrossRef]
- Armstrong, R.A. Recommendations for analysis of repeated-measures designs: Testing and correcting for sphericity and use of manova and mixed model analysis. Ophthalmic Physiol. Opt. 2017, 37, 585–593. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Eperjesi, F.; Gilmartin, B. The application of analysis of variance (ANOVA) to different experimental designs in optometry. Ophthalmic Physiol. Opt. 2002, 22, 248–256. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Carkeet, A. Exact parametric confidence intervals for Bland-Altman limits of agreement. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2015, 92, e71–e80. [Google Scholar] [CrossRef]
- Bian, L.; Li, W.; Qin, R.; Sun, Z.; Zhao, L.; Zhou, Y.; Liu, D.; Liu, Y.; Sun, T.; Qi, H. Ocular Biometry Features and Their Relationship with Anterior and Posterior Segment Lengths among a Myopia Population in Northern China. J. Clin. Med. 2024, 13, 1001. [Google Scholar] [CrossRef]
- Pereira, J.M.M.; Neves, A.; Alfaiate, P.; Santos, M.; Aragao, H.; Sousa, J.C. Lenstar(R) LS 900 vs Pentacam(R)-AXL: Comparative study of ocular biometric measurements and intraocular lens power calculation. Eur. J. Ophthalmol. 2018, 28, 645–651. [Google Scholar] [CrossRef]

| Mean | SD | Minimum | Maximum | p | |
|---|---|---|---|---|---|
| EchoScan (ACD) | 3.627 | 0.357 | 2.810 | 4.340 | <0.001 |
| VX-120 (ACD) | 3.137 | 0.236 | 2.640 | 3.670 | |
| Lenstar (ACD) | 3.088 | 0.256 | 2.410 | 3.600 |
| Mean Difference | SD | p | 95% LoA (95% CI) | |||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| EchoScan (ACD) | VX-120 (ACD) | 0.490 | 0.235 | <0.001 | 0.029 (−0.094 to 0.103) | 0.951 (0.877 to 1.074) |
| Lenstar (ACD) | 0.539 | 0.243 | <0.001 | 0.063 (−0.065 to 0.139) | 1.015 (0.939 to 1.143) | |
| VX-120 (ACD) | EchoScan (ACD) | −0.490 | 0.235 | <0.001 | −0.951 (−1.074 to −0.877) | −0.029 (−0.103 to 0.094) |
| Lenstar (ACD) | 0.048 | 0.117 | 0.019 | −0.181 (−0.243 to −0.145) | 0.277 (0.241 to 0.339) | |
| Lenstar (ACD) | EchoScan (ACD) | −0.539 | 0.243 | <0.001 | −1.015 (−1.143 to −0.939) | −0.063 (−0.139 to 0.065) |
| VX-120 (ACD) | −0.048 | 0.117 | 0.019 | −0.277 (−0.339 to −0.241) | 0.181 (0.145 to 0.243) | |
| Mean | SD | Minimum | Maximum | p | |
|---|---|---|---|---|---|
| EchoScan (ACD) | 3.627 | 0.357 | 2.810 | 4.340 | 0.200 |
| VX-120 (ACD + CCT) | 3.672 | 0.233 | 3.142 | 4.187 | |
| Lenstar (ACD + CCT) | 3.624 | 0.249 | 3.010 | 4.111 |
| Mean Difference | SD | p | 95% LoA (95% CI) | |||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| EchoScan (ACD) | VX-120 (ACD + CCT) | −0.045 | 0.231 | 0.559 | −0.498 (−0.619 to −0.425) | 0.408 (0.335 to 0.529) |
| Lenstar (ACD + CCT) | 0.003 | 0.234 | 0.999 | −0.456 (−0.578 to −0.382) | 0.462 (0.388 to 0.584) | |
| VX-120 (ACD + CCT) | EchoScan (ACD) | 0.045 | 0.231 | 0.559 | −0.408 (−0.529 to −0.335) | 0.498 (0.425 to 0.619) |
| Lenstar (ACD + CCT) | 0.047 | 0.116 | 0.021 | −0.180 (−0.241 to −0.144) | 0.274 (0.238 to 0.335) | |
| Lenstar (ACD + CCT) | EchoScan (ACD) | −0.003 | 0.234 | 0.999 | −0.462 (−0.338 to −0.584) | 0.456 (0.382 to 0.578) |
| VX-120 (ACD + CCT) | −0.047 | 0.116 | 0.021 | −0.274 (−0.335 to −0.238) | 0.180 (0.144 to 0.241) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noya-Padin, V.; Pena-Verdeal, H.; Garcia-Queiruga, J.; Sampedro-Mariño, I.; Giraldez, M.J.; Yebra-Pimentel, E. Agreement on Anterior Chamber Depth Measurement between Three Commercially Available Devices. Photonics 2024, 11, 377. https://doi.org/10.3390/photonics11040377
Noya-Padin V, Pena-Verdeal H, Garcia-Queiruga J, Sampedro-Mariño I, Giraldez MJ, Yebra-Pimentel E. Agreement on Anterior Chamber Depth Measurement between Three Commercially Available Devices. Photonics. 2024; 11(4):377. https://doi.org/10.3390/photonics11040377
Chicago/Turabian StyleNoya-Padin, Veronica, Hugo Pena-Verdeal, Jacobo Garcia-Queiruga, Irene Sampedro-Mariño, Maria Jesus Giraldez, and Eva Yebra-Pimentel. 2024. "Agreement on Anterior Chamber Depth Measurement between Three Commercially Available Devices" Photonics 11, no. 4: 377. https://doi.org/10.3390/photonics11040377
APA StyleNoya-Padin, V., Pena-Verdeal, H., Garcia-Queiruga, J., Sampedro-Mariño, I., Giraldez, M. J., & Yebra-Pimentel, E. (2024). Agreement on Anterior Chamber Depth Measurement between Three Commercially Available Devices. Photonics, 11(4), 377. https://doi.org/10.3390/photonics11040377








