Evaluation of Fluorescence Contrast for the Differentiation of Ex Vivo Tissue Slides from Collagen-Related Degenerative Skin Diseases
Abstract
:1. Introduction
2. Materials and Methods
Data Processing for Relative Quantitative Assessment
- Scanning the image;
- Transformation from RGB to HSV for each pixel;
- Calculation of the histograms by counting the pixels with the same values for hue, saturation, and brightness.
3. Results
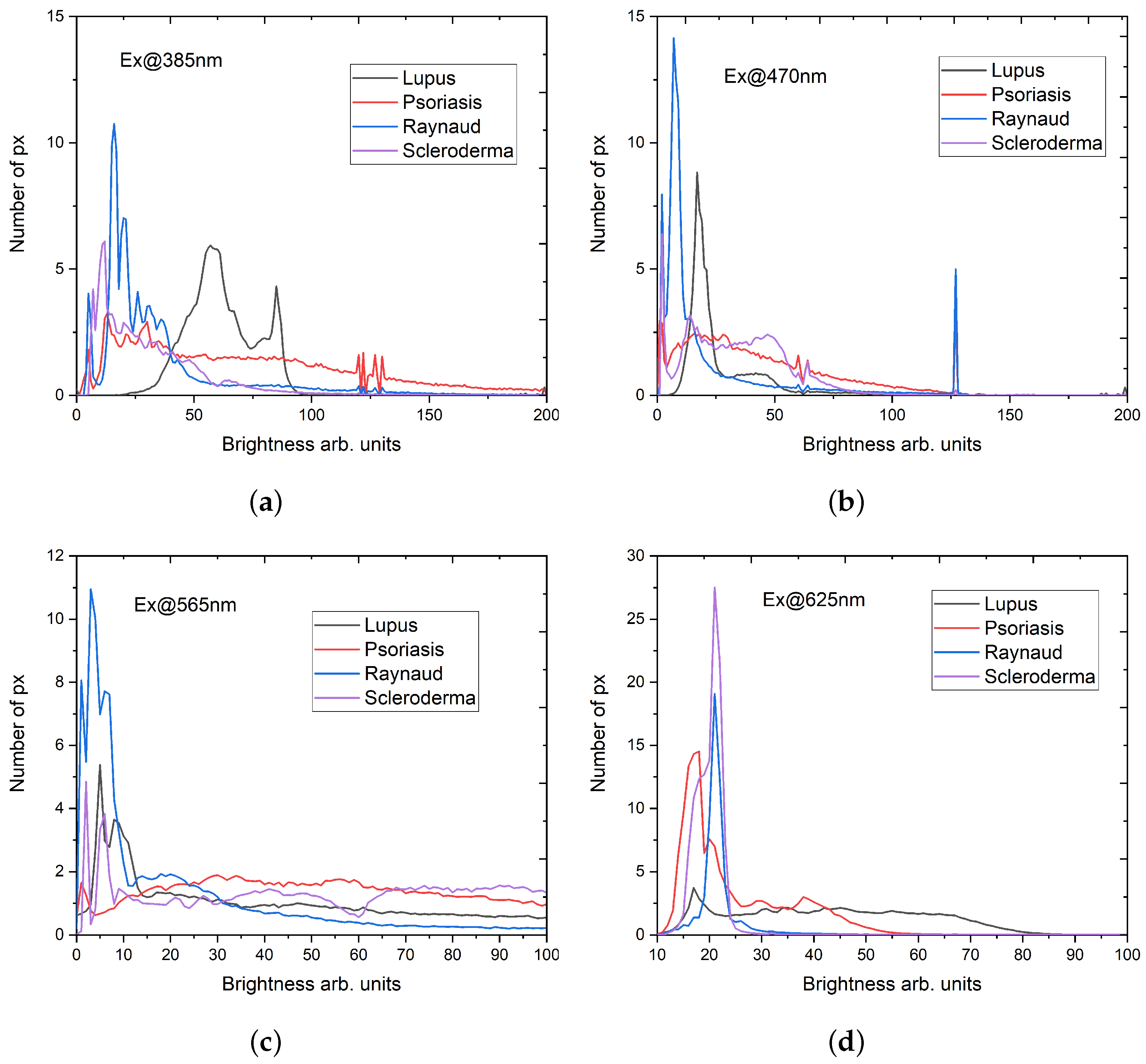
3.1. Relative Quantitative Assessment
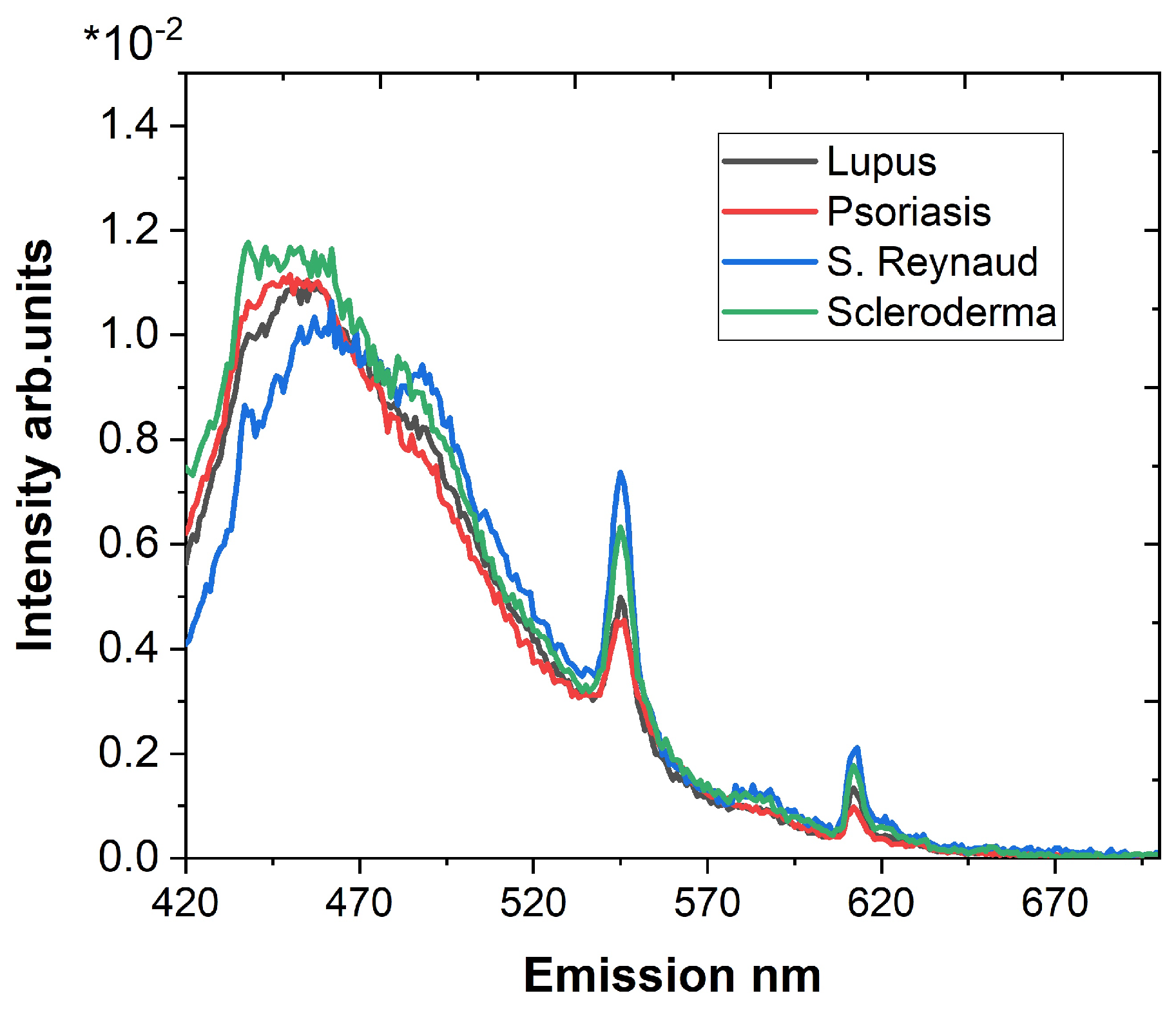
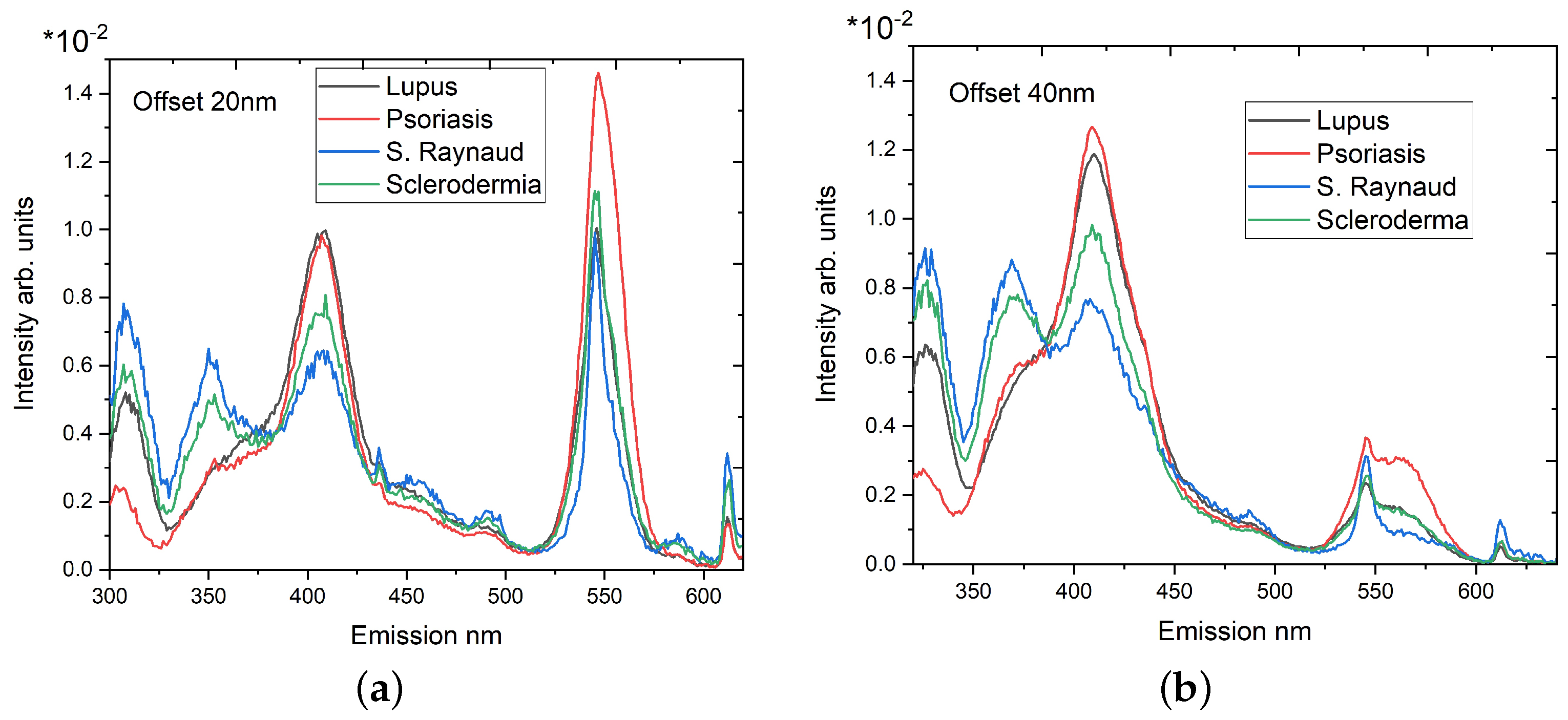
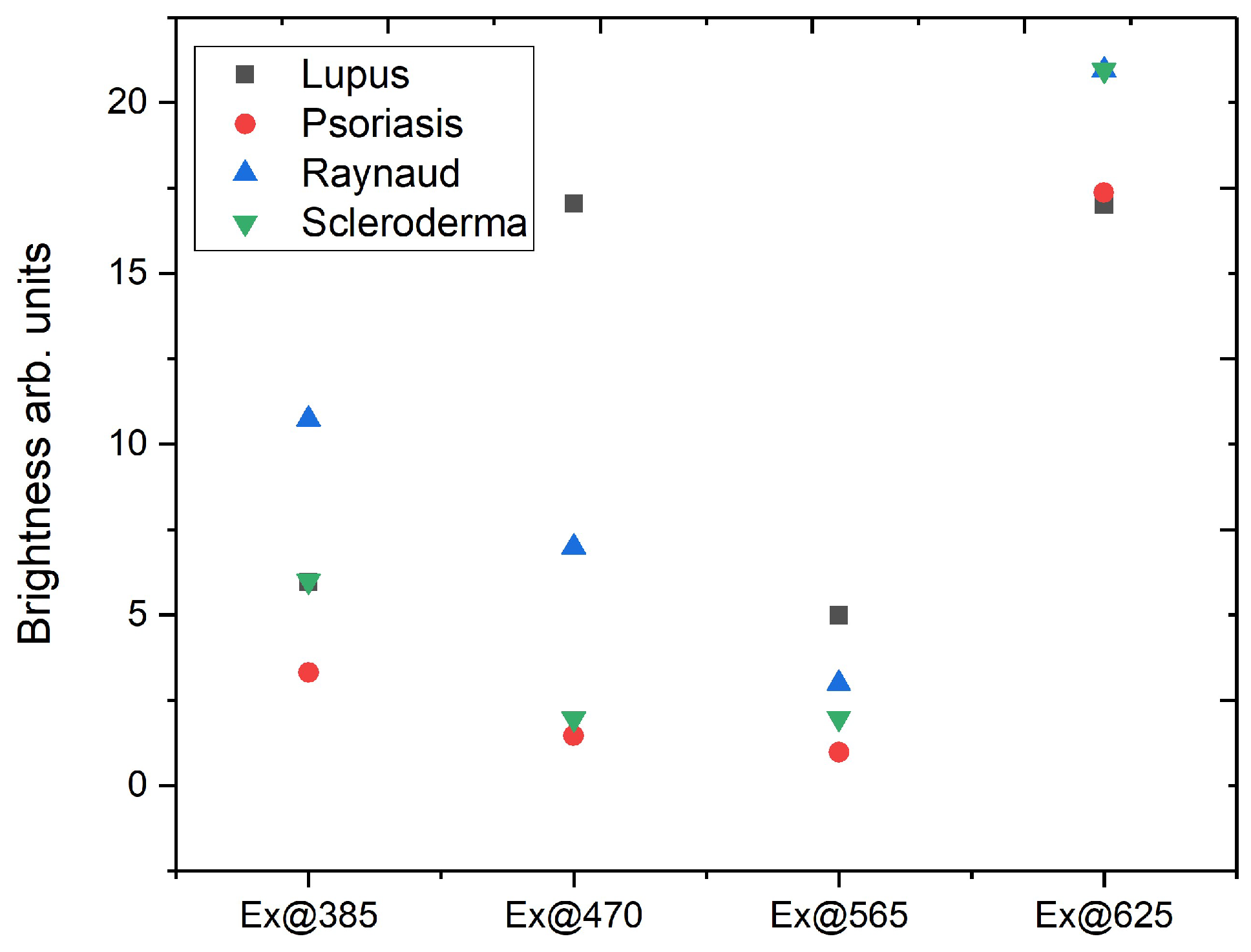
3.2. Synchronous Fluorescence Spectroscopy
- Maximum number of fluorescence maxima are obtained in a single scan;
- Offsets lower than 20 nm could include elastic scattering of the excitation light;
- Longer wavelength offsets exhibit a worse spectral resolution;
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RGB | Red, green, and blue |
| HSV | Hue, saturation, and value |
| H | Hue |
| S | Saturation |
| V | Value/brightness |
| SFS | Synchronous fluorescence spectroscopy |
References
- He, Z.; Wang, P.; Ye, X. Novel endoscopic optical diagnostic technologies in medical trial research: Recent advancements and future prospects. BioMed Eng. OnLine 2021, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Ilisanu, M.A.; Moldoveanu, F.; Moldoveanu, A. Multispectral Imaging for Skin Diseases Assessment—State of the Art and Perspectives. Sensors 2023, 23, 3888. [Google Scholar] [CrossRef]
- Pellacani, G.; Scope, A.; Gonzalez, S.; Guitera, P.; Farnetani, F.; Malvehy, J.; Witkowski, A.; De Carvalho, N.; Lupi, O.; Longo, C. Reflectance confocal microscopy made easy: The 4 must-know key features for the diagnosis of melanoma and nonmelanoma skin cancers. J. Am. Acad. Dermatol. 2019, 81, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Yélamos, O.; Manubens, E.; Jain, M.; Chavez-Bourgeois, M.; Pulijal, S.V.; Dusza, S.W.; Marchetti, M.A.; Barreiro, A.; Marino, M.L.; Malvehy, J.; et al. Improvement of diagnostic confidence and management of equivocal skin lesions by integration of reflectance confocal microscopy in daily practice: Prospective study in 2 referral skin cancer centers. J. Am. Acad. Dermatol. 2020, 83, 1057–1063. [Google Scholar] [CrossRef]
- Borsari, S.; Pampena, R.; Lallas, A.; Kyrgidis, A.; Moscarella, E.; Benati, E.; Raucci, M.; Pellacani, G.; Zalaudek, I.; Argenziano, G.; et al. Clinical Indications for Use of Reflectance Confocal Microscopy for Skin Cancer Diagnosis. JAMA Dermatol. 2016, 152, 1093–1098. [Google Scholar] [CrossRef]
- Braghiroli, N.; Sugerik, S.; Rodrigues de Freitas, L.; Oliviero, M.; Rabinovitz, H. The skin through reflectance confocal microscopy—Historical background, technical principles, and its correlation with histopathology. An. Bras. Dermatol. 2022, 97, 697–703. [Google Scholar] [CrossRef]
- Yaroslavsky, A.N.; Salomatina, E.V.; Neel, V.; Anderson, R.R.; Flotte, T.J. Fluorescence polarization of tetracycline derivatives as a technique for mapping nonmelanoma skin cancers. J. Biomed. Opt. 2007, 12, 014005. [Google Scholar] [CrossRef]
- Ortega, S.; Halicek, M.; Fabelo, H.; Camacho, R.; Plaza, M.D.L.L.; Godtliebsen, F.; Callicó, G.M.; Fei, B. Hyperspectral Imaging for the Detection of Glioblastoma Tumor Cells in H&E Slides Using Convolutional Neural Networks. Sensors 2020, 20, 1911. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, H.; Chang, J.; Zeng, N.; Liu, S.; Li, M.; Ma, H. Differentiating characteristic microstructural features of cancerous tissues using Mueller matrix microscope. Micron 2015, 79, 8–15. [Google Scholar] [CrossRef]
- Sieryi, O.; Ushenko, Y.; Ushenko, V.; Dubolazov, O.; Syvokorovskaya, A.V.; Vanchulyak, O.; Ushenko, A.G.; Gorsky, M.; Tomka, Y.; Bykov, A.; et al. Optical anisotropy composition of benign and malignant prostate tissues revealed by Mueller-matrix imaging. Biomed. Opt. Express 2022, 13, 6019–6034. [Google Scholar] [CrossRef]
- Sanborn, W.R.; Heuck, C.C.; El Aouad, R.; Storch, W.B. Fluorescence Microscopy for Disease Diagnosis and Environmental Monitoring; World Health Organization: Geneva, Switzerland, 2005; pp. 19–23. [Google Scholar]
- Shi, R.B.; Qiu, J.; Maida, V. Towards algorithm-enabled home wound monitoring with smartphone photography: A hue-saturation-value colour space thresholding technique for wound content tracking. Int. Wound J. 2019, 16, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.A.; Satrya, G.B.; Usman, M.R.; Shin, S.Y. Detection of small colon bleeding in wireless capsule endoscopy videos. Comput. Med Imaging Graph. 2016, 54, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Chaudhuri, S.S. Polyps Segmentation using Fuzzy Thresholding in HSV Color Space. In Proceedings of the 2020 IEEE-HYDCON, Hyderabad, India, 11–12 September 2020; IEEE: Piscataway Township, NJ, USA, 2020; pp. 1–5. [Google Scholar]
- Satrya, G.B.; Ramatryana, I.N.A.; Shin, S.Y. Compressive Sensing of Medical Images Based on HSV Color Space. Sensors 2023, 23, 2616. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J. Synchronized excitation of fluorescence emission spectra. Nat. Phys. Sci. 1971, 231, 64–65. [Google Scholar] [CrossRef]
- Vo-Dinh, T. Multicomponent analysis by synchronous luminescence spectrometry. Anal. Chem. 1978, 50, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Tarai, M.; Mishra, A.K. Unconventional steady-state fluorescence spectroscopy as an analytical technique for analyses of complex-multifluorophoric mixtures. TrAC Trends Anal. Chem. 2017, 97, 216–243. [Google Scholar] [CrossRef]
- Borisova, E.; Gisbrecht, A.; Genova-Hristova, T.; Troyanova, P.; Pavlova, E.; Penkov, N.; Bratchenko, I.; Zakharov, V.; Lihachova, I.; Kuzmina, I.; et al. Multispectral autoflourescence detection of skin neoplasia using steady-state techniques. In Proceedings of the 20th International Conference and School on Quantum Electronics: Laser Physics and Applications, Nessebar, Bulgaria, 17–21 September 2018; p. 1104704. [Google Scholar]
- Borisova, E.; Zhelyazkova, A.; Keremedchiev, M.; Penkov, N.; Semyachkina-Glushkovskaya, O.; Avramov, L. Endogenous synchronous fluorescence spectroscopy (SFS) of basal cell carcinoma-initial study. Opt. Spectrosc. 2016, 120, 38–44. [Google Scholar] [CrossRef]
- Genova, T.; Borisova, E.; Penkov, N.; Vladimirov, B.; Zhelyazkova, A.; Avramov, L. Excitation—Emission matrices and synchronous fluorescence spectroscopy for cancer diagnostics in gastrointestinal tract. Quantum Electron. 2016, 46, 510. [Google Scholar] [CrossRef]
- Genova, T.; Borisova, E.; Zhelyazkova, A.; Penkov, N.; Vladimirov, B.; Terziev, I.; Semyachkina-Glushkovskaya, O.; Avramov, L. Colorectal cancer stage evaluation using synchronous fluorescence spectroscopy technique. Opt. Quant. Electron. 2016, 48, 378. [Google Scholar] [CrossRef]
- Gibbs, S.L.; Genega, E.; Salemi, J.; Kianzad, V.; Goodwill, H.L.; Xie, Y.; Oketokoun, R.; Khurd, P.; Kamen, A.; Frangioni, J.V. Near-Infrared Fluorescent Digital Pathology for the Automation of Disease Diagnosis and Biomarker Assessment. Mol. Imaging 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Smith, A.R. Color gamut transform pairs. SIGGRAPH Comput. Graph. 1978, 12, 12–19. [Google Scholar] [CrossRef]
- Rangayyan, R.M.; Acha, B.; Serrano, C. Color Image Processing with Biomedical Applications; SPIE Press: Bellingham, WA, USA, 2011; pp. 12–15. [Google Scholar]
- Zheng, W.; Lau, W.; Cheng, C.; Chee Soo, K.; Olivo, M. Optical excitationemission wavelengths for autofluorescence diagnosis of bladder tumors. Int. J. Cancer 2003, 104, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, A.; Graf, A.; Wenzel, U.; Princz, S.; Mantz, H.; Hessling, M. Development of a highly sensitive spectral camera for cartilage monitoring using fluorescence spectroscopy. J. Sens. Sens. Syst. 2015, 4, 289–294. [Google Scholar] [CrossRef]
- Smirnova, O.D.; Rogatkin, D.A.; Litvinova, K.S. Collagen as In Vivo Quantitative Fluorescent Biomarkers of Abnormal Tissue Changes. J. Innov. Opt. Health Sci. 2012, 5, 1250010. [Google Scholar] [CrossRef]
- Nagarajan, U.; Christopher, J.G.; Jonnalagadda, R.R.; Chandrasekaran, B.; Balachandran, U.N. Studies on the chemico-biological characteristics of bilirubin binding with collagen. Mater. Sci. Eng. C 2013, 33, 4965–4971. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Long, F.; Tang, F.; Jing, Y.; Wang, X.; Yao, L.; Ma, J.; Fei, Y.; Chen, L.; Wang, G.; et al. Autofluorescence Imaging and Spectroscopy of Human Lung Cancer. Appl. Sci. 2017, 7, 32. [Google Scholar] [CrossRef]
- Bolboacă, S.; Jantschi, L. Amino acids sequence analysis on collagen. Bull. USAMV-CN 2007, 63–64, 311–316. [Google Scholar]
- Martin Tornero, E.; Sierra-Tadeo, F.; Durán-Merás, I.; Espinosa-Mansilla, A. Phenylalanine Photoinduced Fluorescence and Characterization of the Photoproducts by LC-MS. J. Fluoresc. 2019, 29, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- König, K.; Meyer, H.; Schneckenburger, H.; Schneckenburger, H.; Rück, A. The study of endogenous porphyrins in human skin and their potential for photodynamic therapy by laser induced fluorescence spectroscopy. Laser Med. Sci. 1993, 8, 127–132. [Google Scholar] [CrossRef]
- Kollias, N.; Gillies, R.; Moran, M.; Kochevar, I.E.; Anderson, R.R. Endogenous Skin Fluorescence In Vivo on Human Skin. J. Invest. Dermatol. 1998, 111, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Muir, R.; Forbes, S.; Birch, D.J.S.; Vyshemirsky, V.; Rolinski, O.J. Collagen Glycation Detected by Its Intrinsic Fluorescence. J. Phys. Chem. B. 2021, 125, 11058–11066. [Google Scholar] [CrossRef] [PubMed]
- Gnanatheepam, E.; Kanniyappan, U.; Dornadula, K.; Prakasarao, A.; Singaravelu, G. Synchronous Luminescence Spectroscopy as a Tool in the Discrimination and Characterization of Oral Cancer Tissue. J. Fluoresc. 2019, 29, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, G.; Gnanatheepam, E.; Prakasarao, A. Synchronous luminescence spectroscopy of tryptophan in head and neck cancer. In Biophotonics, Tryptophan and Disease; Sordillo, L.A., Sordillo, P.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 147–155. [Google Scholar]
- Alfano, R.R.; Yang, Y. Stokes shift emission spectroscopy of human tissue and key biomolecules. IEEE J. Sel. Top Quantum Electron 2003, 9, 148–153. [Google Scholar] [CrossRef]
- Liu, Q.; Grant, G.; Vo-Dinh, T. Investigation of synchronous fluorescence method in multicomponent analysis in tissue. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 927–940. [Google Scholar]
- Devi, S.; Mozumder, M.; Ghosh, N.; Pradhan, A. Extraction of masked fluorescence peaks through synchronous fluorescence spectroscopy. In Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues X; SPIE Press: Bellingham, WA, USA, 2012; Volume 8225, p. 822517. [Google Scholar]
- Karas, A.; Holmannova, D.; Borsky, P.; Fiala, Z.; Andrys, C.; Hamakova, K.; Svadlakova, T.; Palicka, V.; Krejsek, J.; Rehacek, V.; et al. Significantly Altered Serum Levels of NAD, AGE, RAGE, CRP, and Elastin as Potential Biomarkers of Psoriasis and Aging—A Case-Control Study. Biomedicines 2022, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.; Thivolet, J.; Perrot, H. Ultrastructural study of the cutaneous elastic fibres in lupus erythematosus. Br. J. Dermatol. 1972, 87, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.; Park, S.; Im, M.; Seo, Y.; Lee, J.; Lee, Y. Eruptive anetoderma in a patient with systemic lupus erythematosus. Ann. Dermatol. 2014, 26, 621–623. [Google Scholar] [CrossRef]
- Quaglino, D., Jr.; Bergamini, G.; Boraldi, F.; Manzini, E.; Davidson, J.; Pasquali, R. Connective tissue in skin biopsies from patients suffering systemic sclerosis. J. Submi.-Crosc. Cytol. Pathol. 1996, 28, 287–296. [Google Scholar]
- Chatterjee, S.; Mark, M.E.; Wooley, P.H.; Lawrence, W.D.; Mayes, M.D. Increased dermal elastic fibers in the tight skin mouse. Clin Exp Rheumatol. 2004, 22, 617–620. [Google Scholar] [PubMed]
- Koivukangas, V.; Kallionen, M.; Karvonen, J.; Autio-Harmainen, H.; Risteli, J.; Risteli, L.; Oikarinen, A. Increased Collagen Synth. Psoriasis Vivo. Arch. Dermatol. Res. 1995, 287, 171–175. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, Y.; Li, D.; Qu, J.Y. Autofluorescence of epithelial tissue: Single-photon versus two-photon excitation. J. Biomed. Opt. 2008, 13, 054010. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Kim, J.W.; Park, H.S.; Jang, S.J.; Choi, J.C. Calcinosis cutis of the fingertip associated with Raynaud’s phenomenon. J. Dermatol. 2006, 33, 884–886. [Google Scholar] [CrossRef]
- Guleva, D.; Balabanova, M.; Miteva, L.; Dourmishev, L. Histology of Skin Alterations in Lupus Erythematosus. Acta Medica Bulg. 2022, 49, 28–32. [Google Scholar] [CrossRef]
- Wu, Y.; Xi, P.; Qu, J.Y.; Cheung, T.H.; Yu, M.Y. Depth-resolved fluorescence spectroscopy reveals layered structure of tissue. Opt. Express 2004, 12, 3218–3223. [Google Scholar] [CrossRef]
- Sand, J.M.B.; Genovese, F.; Karsdal, M.A. Type IV Collagen. Biochemistry of Collagens, Laminins and Elastin; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 31–41. [Google Scholar]
- Balsini, P.; Weinzettl, P.; Samardzic, D.; Zila, N.; Buchberger, M.; Tschandl, P.; Wielscher, M.; Weninger, W.; Pfisterer, K. Stiffness-dependent LOX regulation via HIF-1 drives extracellular matrix modifications in psoriasis. bioRxiv 2024. preprint. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Kuroda, K.; Hazan, R.; Gordon, R.E.; Lebwohl, M.G.; Sapadin, A.N.; Unda, F.; Iehara, N.; Yamada, Y. Basement membrane alterations in psoriasis are accompanied by epidermal overexpression of MMP-2 and its inhibitor TIMP-2. J. Investig. Dermatol. 2000, 115, 771–777. [Google Scholar] [CrossRef]
- Ustinova, A.O.; Bratchenko, I.A.; Artemyev, D.N. Monte Carlo simulation of skin multispectral autofluorescence. J. Biomed. Photonics Eng. 2019, 5, 020306. [Google Scholar] [CrossRef]
- Deshmukh, S.; Dive, A.; Moharil, R.; Munde, P. Enigmatic insight into collagen. J. Oral Maxillofac. Pathol. 2016, 20, 276–283. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Saletnik, Ł.; Wesołowski, R. Fluorescent spectroscopy of collagen as a diagnostic tool in medicine. J. Med. Sci. 2022, 91, e584. [Google Scholar] [CrossRef]
- Raineri, D. The Role of Extracellular Matrix Proteins in Pathogenesis. Int. J. Mol. Sci. 2024, 25. [Google Scholar]
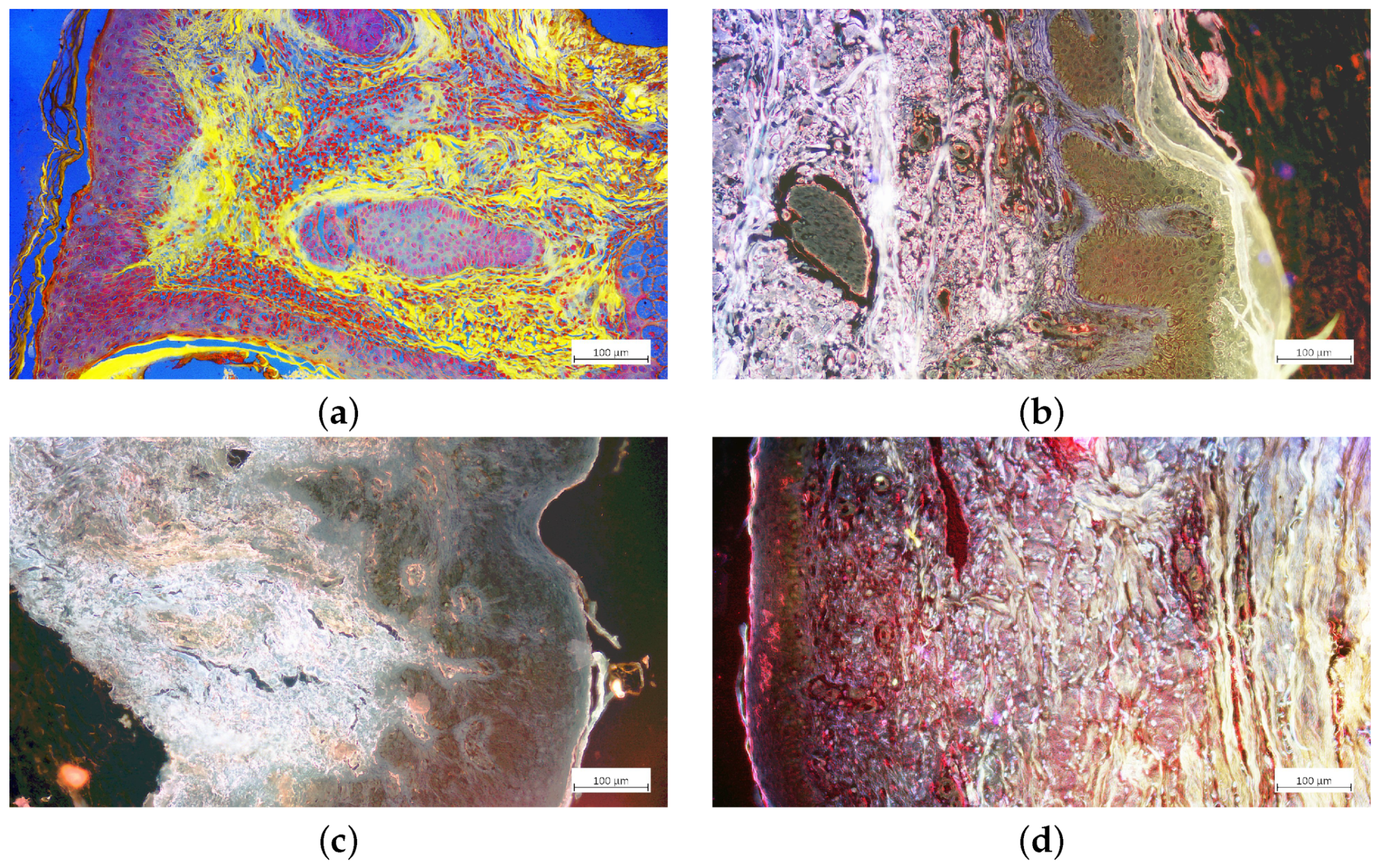

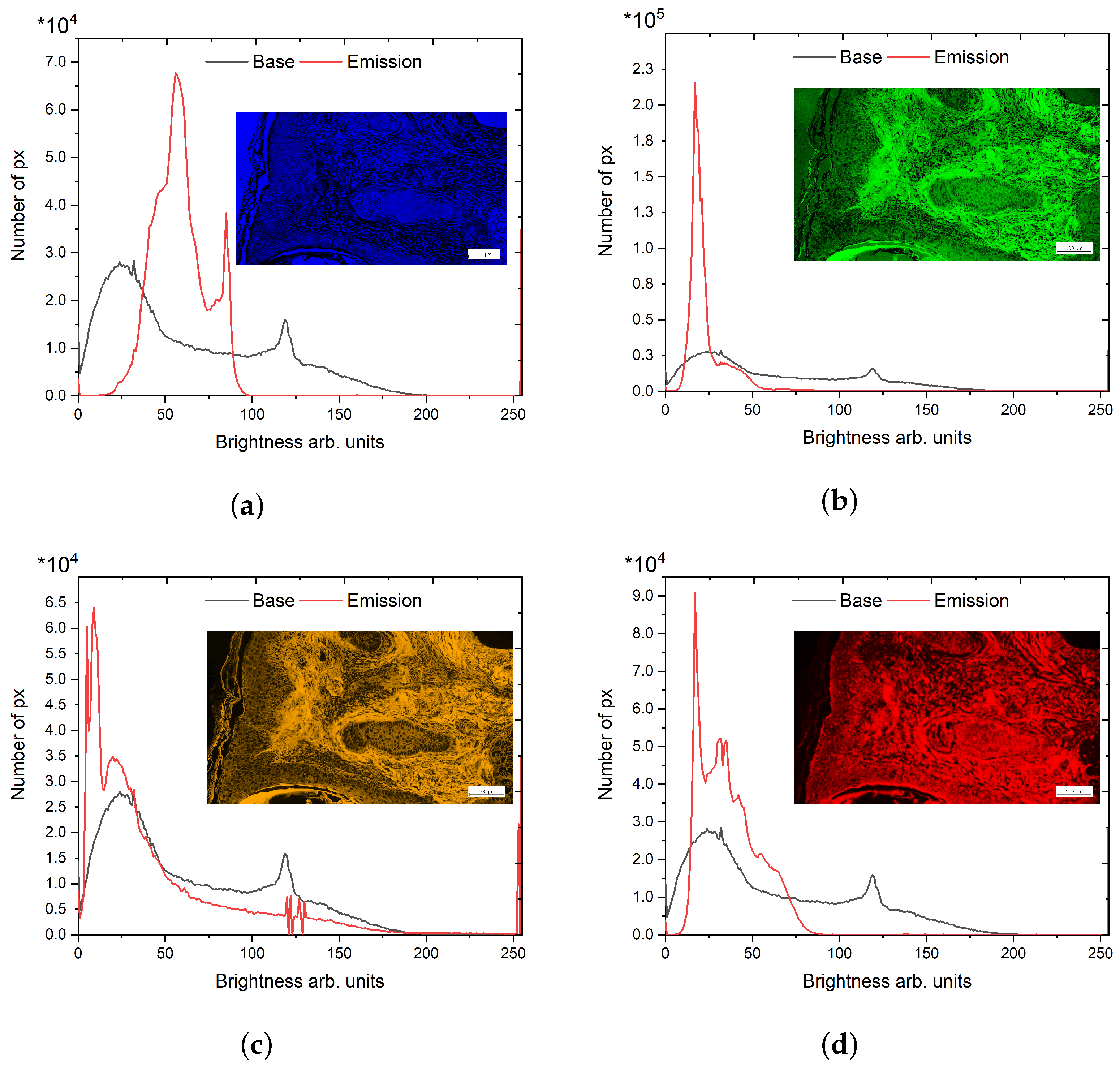

| Image Pseudo Color | R | G | B |
|---|---|---|---|
| Red | 659–759 nm; NS * | NS | NS |
| Yellow | 580–604 nm; 659–759 nm; NS | 580–604 nm; 659–759 nm; NS | NS |
| Green | NS | 499–529 nm; 580–604 nm; 659–759 nm; NS | NS |
| Blue | NS | NS | 410–440 nm; 499–529 nm; 580–604 nm; 659–759 nm; NS |
| Fluorophore | Excitation nm | Emission Maxima nm |
|---|---|---|
| Tyrosin | 275–290 | 305 |
| Elastin | 290, 325 | 340, 400 |
| Collagen | 325 | 400, 405 |
| Collagen cross-links, collagenase digestible | 360 | 440 |
| Collagen cross-links, pepsin digestible | 340 | 400 |
| Collagen–bilirubin complex [29] | 300 | 550 |
| Parameter | Syndrome of Raynaud | Scleroderma | Lupus | Psoriasis |
|---|---|---|---|---|
| Offset 20 nm | ||||
| 1.0073 ± 0.0376 | 1.5252 ± 0.0231 | 3.3883 ± 0.0566 | 3.1711 ± 0.0501 | |
| 1.1406 ± 0.0329 | 1.8825 ± 0.0604 | 1.9047 ± 0.0385 | 6.27 ± 0.2142 | |
| Offset 40 nm | ||||
| 0.8650 ± 0.0096 | 1.2676 ± 0.0078 | 2.3652 ± 0.0281 | 2.2982 ± 0.0102 | |
| 2.7789 ± 0.1163 | 1.1644 ± 0.0167 | 2.6984 ± 0.0564 | 0.7171 ± 0.0308 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genova, T.; Pavlova, P.; Zaharieva, L.; Troyanova, P.; Terziev, I. Evaluation of Fluorescence Contrast for the Differentiation of Ex Vivo Tissue Slides from Collagen-Related Degenerative Skin Diseases. Photonics 2024, 11, 687. https://doi.org/10.3390/photonics11080687
Genova T, Pavlova P, Zaharieva L, Troyanova P, Terziev I. Evaluation of Fluorescence Contrast for the Differentiation of Ex Vivo Tissue Slides from Collagen-Related Degenerative Skin Diseases. Photonics. 2024; 11(8):687. https://doi.org/10.3390/photonics11080687
Chicago/Turabian StyleGenova, Tsanislava, Petya Pavlova, Lidia Zaharieva, Petranka Troyanova, and Ivan Terziev. 2024. "Evaluation of Fluorescence Contrast for the Differentiation of Ex Vivo Tissue Slides from Collagen-Related Degenerative Skin Diseases" Photonics 11, no. 8: 687. https://doi.org/10.3390/photonics11080687





