Dissociative Ionization of the CHBr2Cl Molecule in 800 nm and 400 nm Femtosecond Laser Fields
Abstract
:1. Introduction
2. Materials and Methods
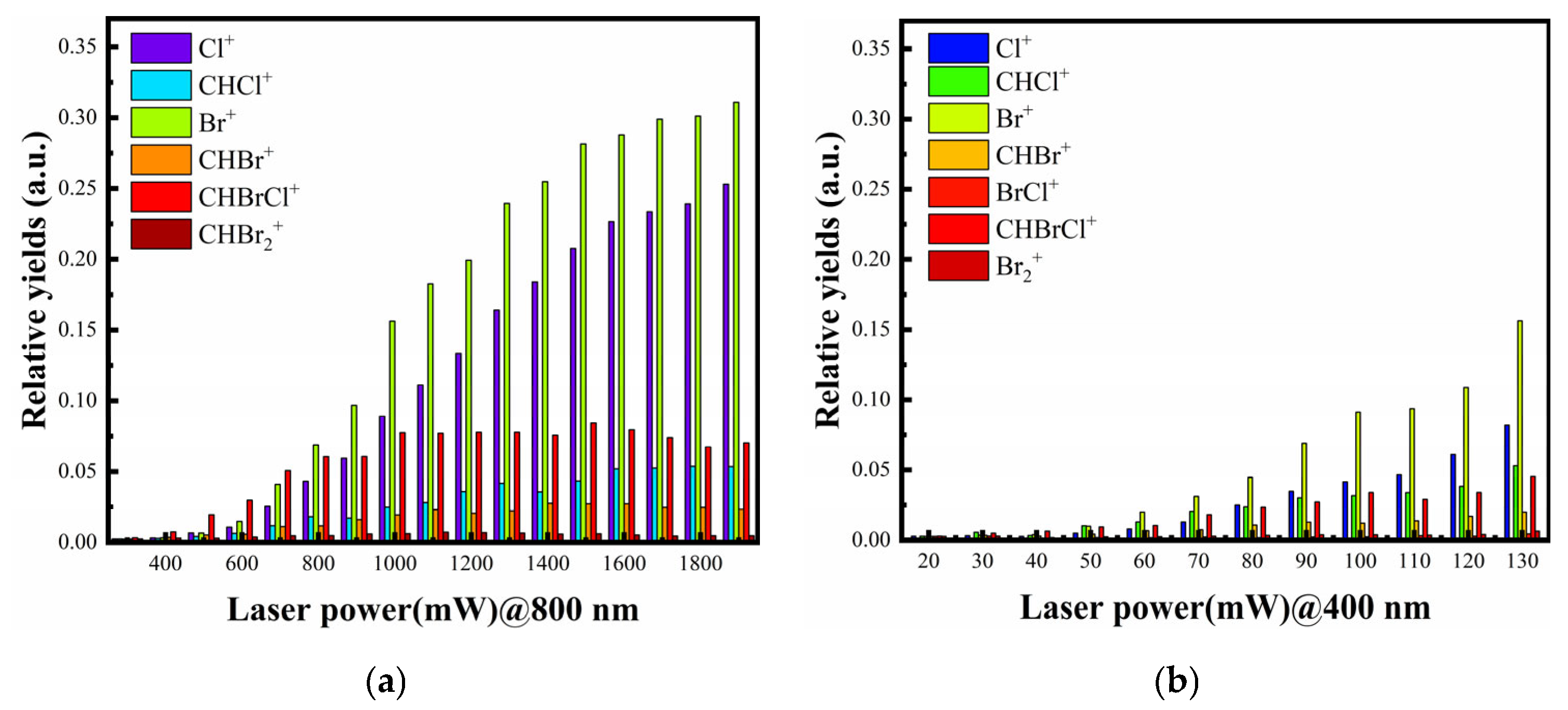
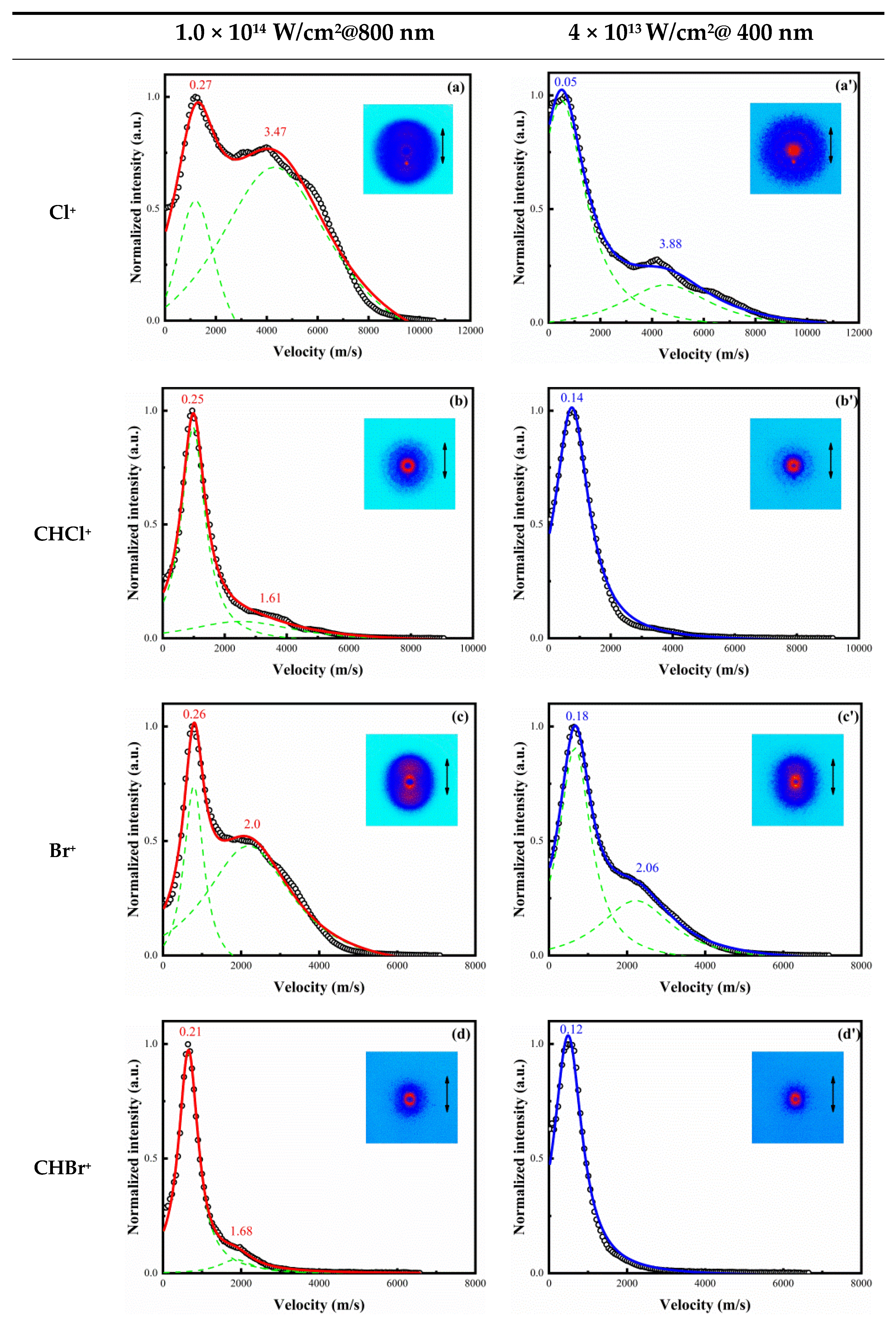
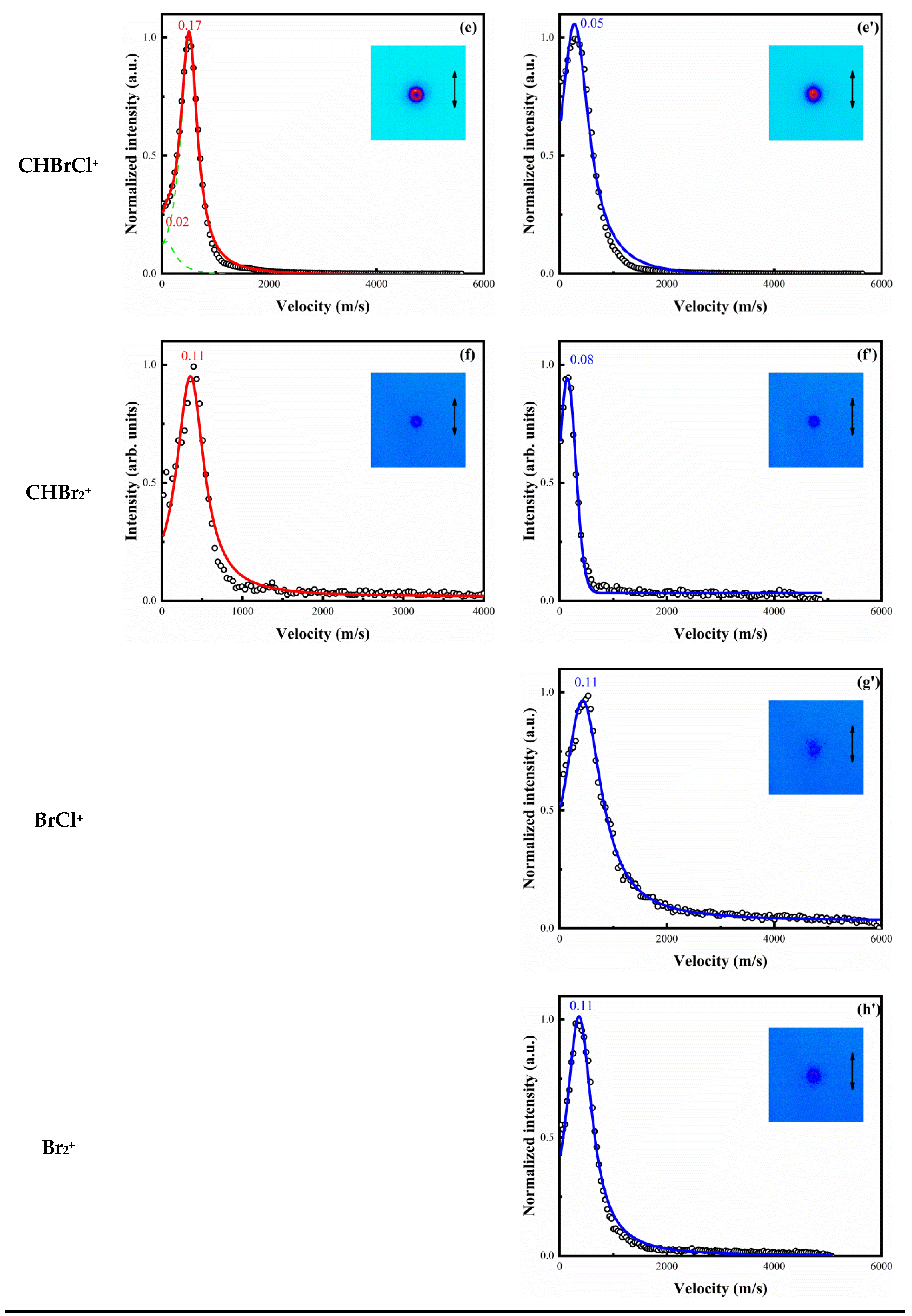
3. Results
4. Discussion
4.1. Dissociation of Single-Charged Parent Ion CHBr2Cl+ in 800 and 400 nm Femtosecond Laser Fields
4.2. The Special Dissociation Channels for CHBr2Cl in 400 nm Laser Fields
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hertel, I.; Radloff, W. Ultrafast dynamics in isolated molecules and molecular clusters. Rep. Prog. Phys. 2006, 69, 1897. [Google Scholar] [CrossRef]
- Brinks, D.; Hildner, R.; Van Dijk, E.M.; Stefani, F.D.; Nieder, J.B.; Hernando, J.; van Hulst, N.F. Ultrafast dynamics of single molecules. Chem. Soc. Rev. 2014, 43, 2476–2491. [Google Scholar] [CrossRef]
- Cornaggia, C. Electronic dynamics of charge resonance enhanced ionization probed by laser-induced alignment in C2H2. J. Phys. B At. Mol. Opt. Phys. 2016, 49, 19LT01. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Zhang, J.; Yang, Y.; Deng, L.; Jia, T.; Wang, Z.; Sun, Z. Observation of hydrogen migration in cyclohexane under an intense femtosecond laser field. J. Phys. Chem. A 2015, 119, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Guo, C. Selective charge asymmetric distribution in heteronuclear diatomic molecules in strong laser fields. Phys. Rev. A 2015, 92, 013402. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, C.; Liu, X.; Deng, Y.; Gong, Q.; Song, D.; Su, H. Double ionization of nitrogen from multiple orbitals. J. Phys. Chem. A 2010, 114, 6751–6756. [Google Scholar] [CrossRef] [PubMed]
- Hatherly, P.; Stankiewicz, M.; Codling, K.; Frasinski, L.J.; Cross, G.M. The multielectron dissociative ionization of molecular iodine in intense laser fields. J. Phys. B At. Mol. Opt. Phys. 1994, 27, 2993. [Google Scholar] [CrossRef]
- McKenna, J.; Suresh, M.; Srigengan, B.; Williams, I.D.; Bryan, W.A.; English, E.M.L.; Stebbings, S.L.; Newell, W.R.; Turcu, I.C.E.; Smith, J.M.; et al. Ultrafast ionization study of N2 in intense linearly and circularly polarized laser fields. Phys. Rev. A 2006, 73, 043401. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Yang, Y. Multi-ionization of the Cl2 molecule in the near-infrared femtosecond laser field. RSC Adv. 2020, 10, 332–337. [Google Scholar] [CrossRef]
- Ibuki, T.; Hiraya, A.; Shobatake, K. Vacuum ultraviolet absorption spectra and photodissociative excitation of CHBr2Cl and CHBrCl2. J. Chem. Phys. 1992, 96, 8793–8798. [Google Scholar] [CrossRef]
- Zheng, X.; Lee, C.W.; Li, Y.-L.; Fang, W.-H.; Phillips, D.L. Transient resonance Raman spectroscopy and density functional theory investigation of iso-CHBr2Cl and iso-CCl3Br photoproducts produced following ultraviolet excitation of CHBr2Cl and CCl3Br. J. Chem. Phys. 2001, 114, 8347–8356. [Google Scholar] [CrossRef]
- Yang, S.-X.; Hou, G.-Y.; Dai, J.-H.; Chang, C.-H.; Chang, B.-C. Spectroscopic Investigation of the Multiphoton Photolysis Reactions of Bromomethanes (CHBr3, CHBr2Cl, CHBrCl2, and CH2Br2) at Near-Ultraviolet Wavelengths. J. Phys. Chem. A 2010, 114, 4785–4790. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, B.; Kasai, T.; Lin, K.-C. Probing BrCl from photo-dissociation of CH2BrCl and CHBr2Cl at 248 nm using cavity ring-down spectroscopy. Phys. Chem. Chem. Phys. 2021, 23, 6098–6106. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Li, Z.; Sun, H.; Zhang, S.; Sun, Z. Channel-resolved multiorbital double ionization of molecular Cl2 in an intense femtosecond laser field. Phys. Rev. A 2018, 98, 043402. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Li, Z.; Sun, Z. Dissociative ionization of CH2Br2 in 800 and 400 nm femtosecond laser fields. Chem. Phys. Lett. 2017, 685, 151–156. [Google Scholar] [CrossRef]
- Guo, C.; Li, M.; Nibarger, J.P.; Gibson, G.N. Single and double ionization of diatomic molecules in strong laser fields. Phys. Rev. A 1998, 58, R4271–R4274. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Pople, J.A.; Head-Gordon, M.; Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 1987, 87, 5968–5975. [Google Scholar] [CrossRef]
- Truhlar, D.G. Basis-set extrapolation. Chem. Phys. Lett. 1998, 294, 45–48. [Google Scholar] [CrossRef]
- Brandhorst, K.; Grunenberg, J. Efficient computation of compliance matrices in redundant internal coordinates from Cartesian Hessians for nonstationary points. J. Chem. Phys. 2010, 132, 184101. [Google Scholar] [CrossRef]


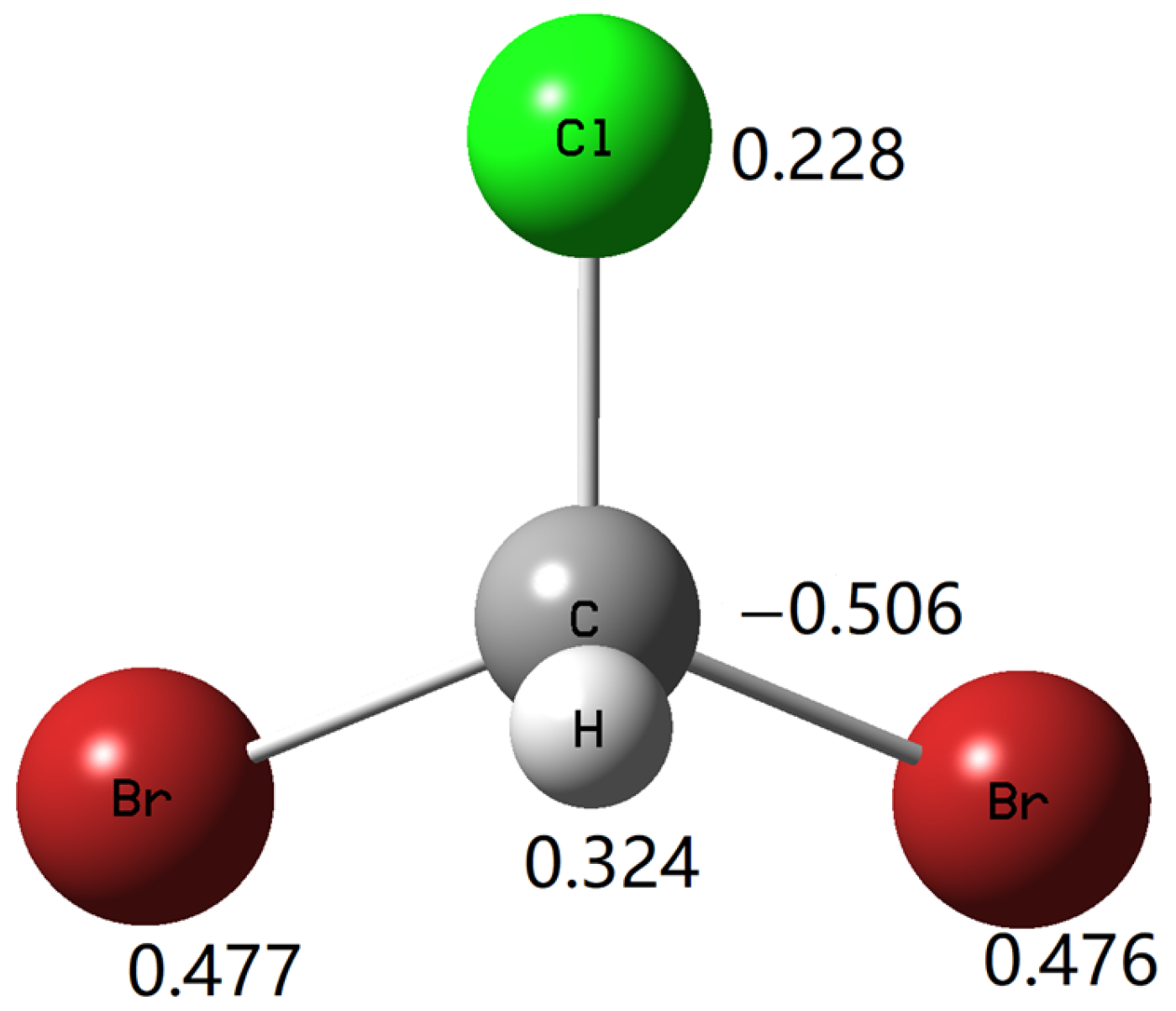
| Bond | Compliance Matrix Diagonal Element | Relaxed Force Constant (mdyn/Å) |
|---|---|---|
| C–H | 0.18 | 5.56 |
| C–Cl | 0.28 | 3.57 |
| C–Br(1) | 0.71 | 1.41 |
| C–Br(2) | 0.71 | 1.41 |
| Channel | Eappear (eV) | KER (eV) | Eavail (eV) | ||
|---|---|---|---|---|---|
| 800 nm | 400 nm | 800 nm | 400 nm | ||
| (1) | 17.58 | 0.27 | 0.05 | 1.02 | 1.02 |
| (2) | 11.32 | 0.11 | 0.08 | 1.08 | 1.08 |
| (3) | 15.67 | 0.26 | 0.18 | 1.38 | 2.93 |
| (4) | 10.77 | 0.17 | 0.05 | 0.08 | 1.63 |
| (5) | 15.24 | 0.21 | 0.12 | 0.26 | 0.26 |
| (6) | 14.74 | 0.25 | 0.14 | 0.76 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Li, Z. Dissociative Ionization of the CHBr2Cl Molecule in 800 nm and 400 nm Femtosecond Laser Fields. Photonics 2024, 11, 706. https://doi.org/10.3390/photonics11080706
Liu B, Li Z. Dissociative Ionization of the CHBr2Cl Molecule in 800 nm and 400 nm Femtosecond Laser Fields. Photonics. 2024; 11(8):706. https://doi.org/10.3390/photonics11080706
Chicago/Turabian StyleLiu, Botong, and Zhipeng Li. 2024. "Dissociative Ionization of the CHBr2Cl Molecule in 800 nm and 400 nm Femtosecond Laser Fields" Photonics 11, no. 8: 706. https://doi.org/10.3390/photonics11080706




