Rapid Determination of Rivaroxaban by Using Terahertz Metamaterial Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. THz Spectroscopy
2.2.1. Experimental Equipment
2.2.2. Rivaroxaban Qualitative Measurement
2.2.3. Rivaroxaban Quantitative Measurement
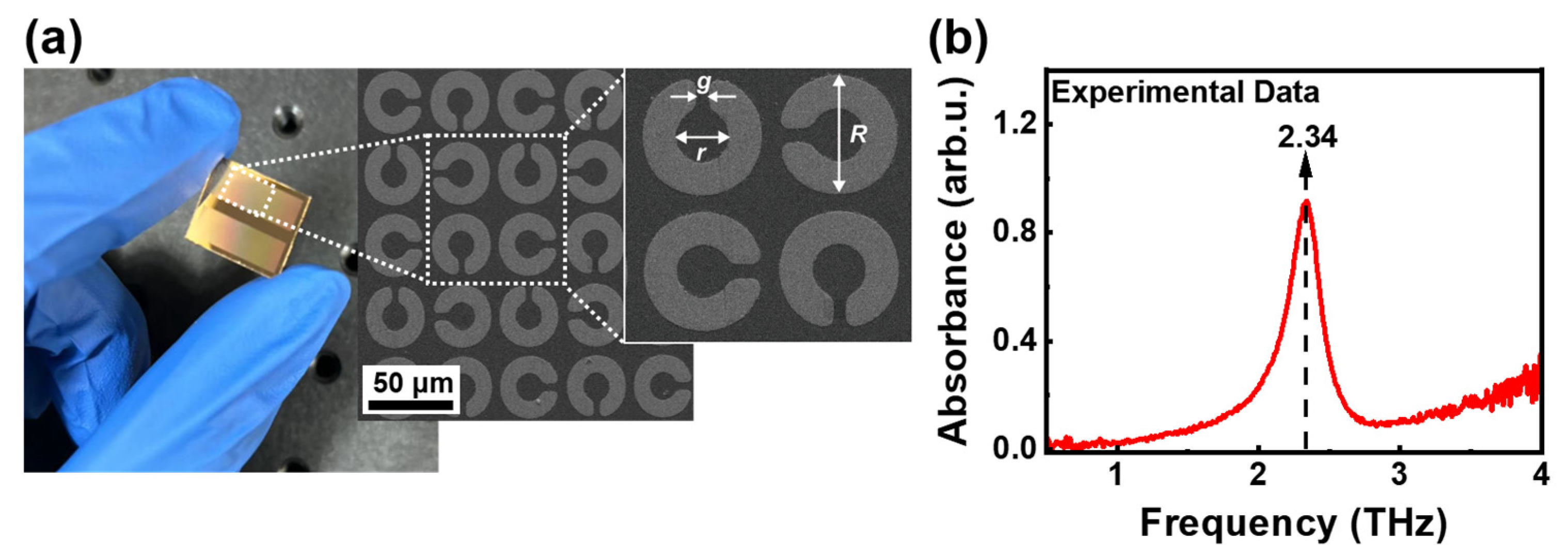
2.3. Manufacturing and Microscopic Imaging for THz Metamaterial Biosensor
2.4. Data Processing and Analysis
3. Results and Discussion
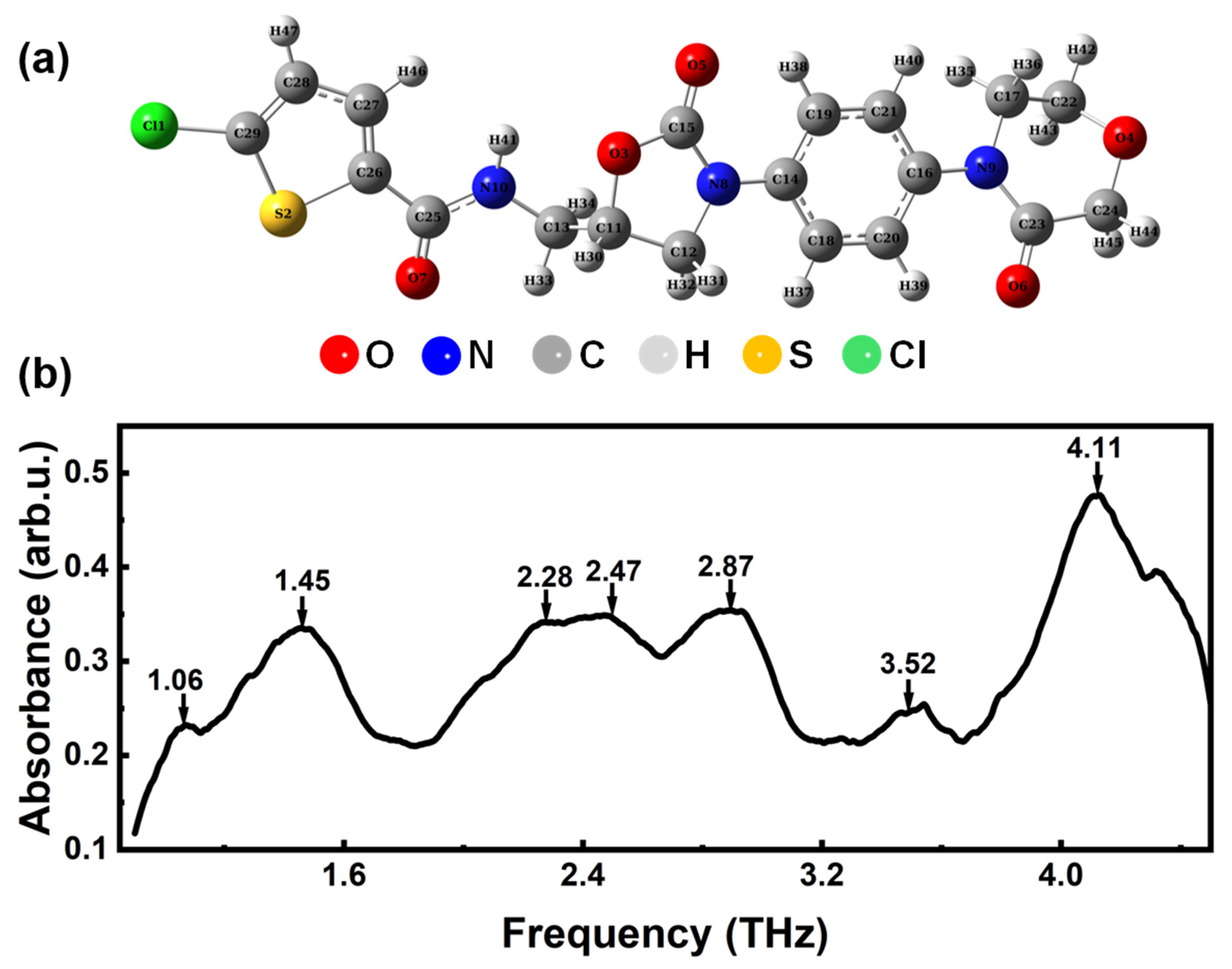
3.1. Analysis of THz Absorption Spectrum of Rivaroxaban
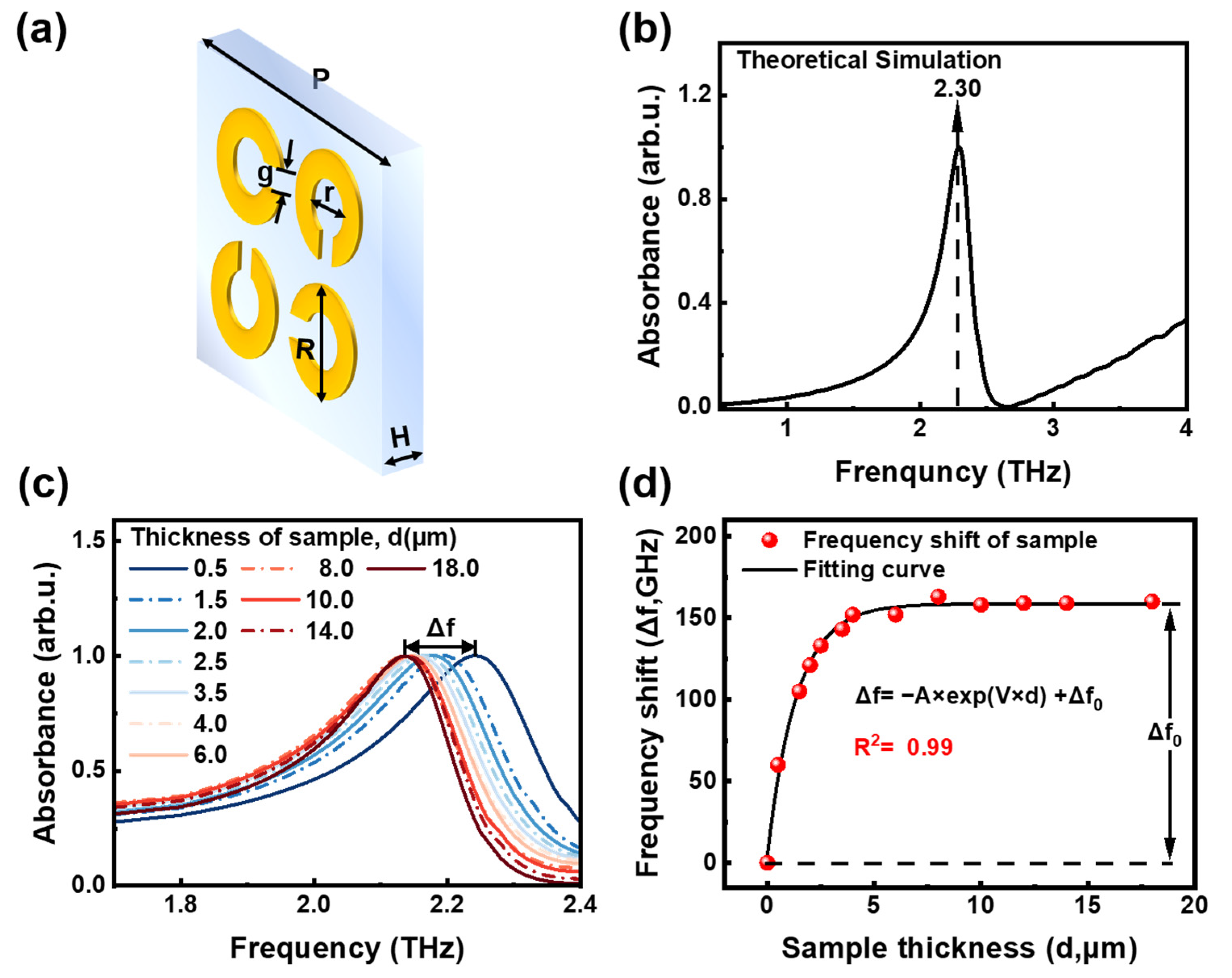
3.2. Design and Simulation of THz Metamaterial Biosensor
3.3. Quantitative Detection of Rivaroxaban Solutions Based on THz Metamaterial Biosensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.-M.; Liu, J.-Y.; Sun, X.-D.; Zhang, M.; Liu, X.-G.; Chen, X.-L. Rivaroxaban improves hidden blood loss, blood transfusion rate and reduces swelling of the knee joint in knee osteoarthritis patients after total knee replacement. Medicine 2018, 97, e12630. [Google Scholar] [CrossRef] [PubMed]
- Mueck, W.; Schwers, S.; Stampfuss, J. Rivaroxaban and other novel oral anticoagulants: Pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring. Thromb. J. 2013, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Perzborn, E.; Roehrig, S.; Straub, A.; Kubitza, D.; Misselwitz, F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat. Rev. Drug Discov. 2011, 10, 61–75. [Google Scholar] [CrossRef]
- Becker, R.C. Factor Xa inhibitors: Critical considerations for clinical development and testing. J. Thromb. Thrombolysis 2021, 52, 397–402. [Google Scholar] [CrossRef]
- Jeong, J.-S.; Ha, E.-S.; Park, H.; Lee, S.-K.; Kim, J.-S.; Kim, M.-S. Measurement and correlation of solubility of rivaroxaban in dichloromethane and primary alcohol binary solvent mixtures at different temperatures. J. Mol. Liq. 2022, 357, 119064. [Google Scholar] [CrossRef]
- Kuuskne, M.; Dankoff, J. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism: Are we ready? Can. J. Emerg. Med. 2014, 16, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Mladentsev, D.Y.; Kuznetsova, E.N.; Skvortsova, M.N.; Dashkin, R.R. Review on Synthetic Approaches toward Rivaroxaban (Xarelto), an Anticoagulant Drug. Org. Process Res. Dev. 2022, 26, 2311–2329. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.P.; Peng, Y.; Wu, F.; Cao, J.M.; Chen, X.H.; Wu, W.W.; Yang, H.N.; Xing, M.M.; Zhu, Y.M.; et al. Quantitative analysis of direct oral anticoagulant rivaroxaban by terahertz spectroscopy. Analyst 2020, 145, 3909–3915. [Google Scholar] [CrossRef]
- Hassan, E.; Motwani, J. Real world experience of efficacy and safety of rivaroxaban in paediatric venous thromboembolism. Thromb. Res. 2023, 221, 92–96. [Google Scholar] [CrossRef]
- Tomić, N.; Anđić, V.; Ćurlik, D.; Čeko, J.; Tanović Avdić, A.; Mehić, M.; Šukalo, A.; Glamočlija, U. Therapy adherence, safety and efficacy of rivaroxaban in prevention of venous thromboembolism in patients with hip or knee endoprosthesis. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 1435–1440. [Google Scholar] [CrossRef]
- Siegmund, H.U.; Burghaus, R.; Kubitza, D.; Coboeken, K. Contribution of rivaroxaban to the international normalized ratio when switching to warfarin for anticoagulation as determined by simulation studies. Br. J. Clin. Pharmacol. 2015, 79, 959–966. [Google Scholar] [CrossRef]
- Li, Y.; Du, L.-p.; Chen, Y.-x.; Mei, D. Pharmacokinetics and clinical monitoring of rivaroxaban. Clin. Medicat. J. 2018, 16, 80–85. [Google Scholar]
- Pengo, V.; Crippa, L.; Falanga, A.; Finazzi, G.; Marongiu, F.; Palareti, G.; Poli, D.; Testa, S.; Tiraferri, E.; Tosetto, A.; et al. Questions and answers on the use of dabigatran and perpectives on the use of other new oral anticoagulants in patients with atrial fibrillation A consensus document of the Italian Federation of Thrombosis Centers (FCSA). Thromb. Haemost. 2011, 106, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jang, S.; Lee, Y.J.; Park, N.; Cho, Y.U.; Park, C.J. The rivaroxaban-adjusted normalized ratio: Use of the prothrombin time to monitor the therapeutic effect of rivaroxaban. Br. J. Biomed. Sci. 2019, 76, 122–128. [Google Scholar] [CrossRef]
- Tripodi, A. Which test to use to measure the anticoagulant effect of rivaroxaban: The prothrombin time test. J. Thromb. Haemost. 2013, 11, 576–578. [Google Scholar] [CrossRef]
- Samama, M.M. Which test to use to measure the anticoagulant effect of rivaroxaban: The anti-factor Xa assay. J. Thromb. Haemost. 2013, 11, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Von Horn, H.; Rasmusson, A.; Söderblom, L.; Malmström, R.E.; Antovic, J. Using a low-molecular weight heparin-calibrated anti-factor Xa assay to assess the concentration of apixaban and rivaroxaban. Int. J. Lab. Hematol. 2022, 44, 163–167. [Google Scholar] [CrossRef]
- Turpie, A.G.G.; Kreutz, R.; Llau, J.; Norrving, B.; Haas, S. Management consensus guidance for the use of rivaroxaban—An oral, direct factor Xa inhibitor. Thromb. Haemost. 2012, 108, 876–886. [Google Scholar] [CrossRef]
- Barrett, Y.C.; Wang, Z.Q.; Frost, C.; Shenker, A. Clinical laboratory measurement of direct factor Xa inhibitors: Anti-Xa assay is preferable to prothrombin time assay. Thromb. Haemost. 2010, 104, 1263–1271. [Google Scholar] [CrossRef]
- Mueck, W.; Lensing, A.W.A.; Agnelli, G.; Decousus, H.; Prandoni, P.; Misselwitz, F. Rivaroxaban Population Pharmacokinetic Analyses in Patients Treated for Acute Deep-Vein Thrombosis and Exposure Simulations in Patients with Atrial Fibrillation Treated for Stroke Prevention. Clin. Pharmacokinet. 2011, 50, 675–686. [Google Scholar] [CrossRef]
- Testa, S.; Tripodi, A.; Legnani, C.; Pengo, V.; Abbate, R.; Dellanoce, C.; Carraro, P.; Salomone, L.; Paniccia, R.; Paoletti, O.; et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: Results observed in four anticoagulation clinics. Thromb. Res. 2016, 137, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Samama, M.M.; Contant, G.; Spiro, T.E.; Perzborn, E.; Guinet, C.; Gourmelin, Y.; Le Flem, L.; Rohde, G.; Martinoli, J.L.; Rivaroxaban Anti Factor Xa, C. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb. Haemost. 2012, 107, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Bardy, G.; Fischer, F.; Appert, A.; Baldin, B.; Stève, M.; Spreux, A.; Lavrut, T.; Drici, M.D. Is anti-factor Xa chromogenic assay for Rivaroxaban appropriate in clinical practice? Advantages and comparative drawbacks. Thromb. Res. Int. J. Vasc. Obs. Hemorrhage Hemost. 2015, 136, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Derogis, P.B.M.; Sanches, L.R.; de Aranda, V.F.; Colombini, M.P.; Mangueira, C.L.P.; Katz, M.; Faulhaber, A.C.L.; Mendes, C.E.A.; Ferreira, C.E.D.; França, C.N.; et al. Determination of rivaroxaban in patient’s plasma samples by anti-Xa chromogenic test associated to High Performance Liquid Chromatography tandem Mass Spectrometry (HPLC-MS/MS). PLoS ONE 2017, 12, e0171272. [Google Scholar] [CrossRef]
- Lagoutte-Renosi, J.; Le Poupon, J.; Girard, A.; Montange, D.; Davani, S. A simple and fast HPLC-MS/MS method for simultaneous determination of direct oral anticoagulants apixaban, dabigatran, rivaroxaban in human plasma. J. Chromatogr. B 2018, 1100–1101, 43–49. [Google Scholar] [CrossRef]
- De Oliveira, A.C.; Davanço, M.G.; de Campos, D.R.; Sanches, P.H.G.; Cirino, J.P.G.; Carvalho, P.D.; Antônio, M.A.; Coelho, E.C.; Porcari, A.M. Sensitive LC-MS/MS method for quantification of rivaroxaban in plasma: Application to pharmacokinetic studies. Biomed. Chromatogr. 2021, 35, e5147. [Google Scholar] [CrossRef]
- Douxfils, J.; Tamigniau, A.; Chatelain, B.; Chatelain, C.; Wallemacq, P.; Dogné, J.M.; Mullier, F. Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb. Haemost. 2013, 110, 723–731. [Google Scholar] [CrossRef]
- Hu, R.; Li, H.; Luo, L.; Tan, Y.; Xiao, Y.; Yu, G.; Zhang, J.; Liao, X. Method for Detecting Rivaroxaban Related Substance Content, Involves Utilizing Octadecyl Silane Bonded Silica Gel as Stationary Phase, Buffered Saline Solution and Acetonitrile as Mobile Phase and UV Detector and Carrying out HPLC Process. Patent CN105651871-A, 8 June 2016. p. 11. [Google Scholar]
- Zhang, L.; Ding, H.; Lin, L.; Wang, Y.; Guo, X.; Tian, H. Transmission versus reflection spectroscopy for discrimination of human and nonhuman blood. Infrared Phys. Technol. 2019, 99, 1–4. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, C.J.; Zhu, Y.M.; Gu, M.; Zhuang, S.L. Terahertz spectroscopy in biomedical field: A review on signal-to-noise ratio improvement. Photonix 2020, 1, 12. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, L.; Wu, X.; Peng, Y. Review on near-field detection technology in the biomedical field. Adv. Photonics Nexus 2023, 2, 044002. [Google Scholar] [CrossRef]
- Wei, X.; Wu, X.; Lu, X.; Wang, J.; Li, J.; Yang, Y.; Chu, X.; Wang, Q.; Jin, Z.; Peng, Y. Far Infrared Spectrum of Antithrombotic Drug Xarelto. Chin. J. Lasers 2023, 50, 1507207. [Google Scholar] [CrossRef]
- Hu, S.Y.; Sun, C.; Wu, X.; Peng, Y. Polarization-Independent Terahertz Surface Plasmon Resonance Biosensor for Species Identification of Panax and Paeonia. Photonics 2023, 10, 250. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Zhang, Z.; Yang, Y.; Xiang, Y. Quantitative measurements of binary amino acids mixtures in yellow foxtail millet by terahertz time domain spectroscopy. Food Chem. 2016, 211, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.J.; Zhou, Z.H.; Liu, S.J.; Zuo, J.; Zhang, C.L. Analytical method for studying terahertz vibrations in different ginseng. In Proceedings of the International Conference on Optical Instruments and Technology-IRMMW-THz Technologies and Applications, Beijing, China, 26–28 October 2019. [Google Scholar]
- Ge, H.; Jiang, Y.; Lian, F.; Zhang, Y.; Xia, S. Quantitative determination of aflatoxin B1 concentration in acetonitrile by chemometric methods using terahertz spectroscopy. Food Chem. 2016, 209, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.Y.; Sonawane, D.D.; Erande, K.B.; Derle, D.V. Terahertz technology and its applications. Drug Invent. Today 2013, 5, 157–163. [Google Scholar] [CrossRef]
- Fu, X.J.; Liu, Y.J.; Chen, Q.; Fu, Y.; Cui, T.J. Applications of Terahertz Spectroscopy in the Detection and Recognition of Substances. Front. Phys. 2022, 10, 869537. [Google Scholar] [CrossRef]
- Lin, J.; Xue, Y.; Wang, W.; Sun, M.; Shi, S.; Zhang, S.; Shi, Y. Enhancing Multi-Spectral Fingerprint Sensing for Trace Explosive Molecules with All-Silicon Metasurfaces. Nanomaterials 2024, 14, 738. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Lin, J.; Wang, W.; Chai, Z.; Sun, M.; Shi, Y.; Zhang, Y. Metasurface-based sensor with terahertz molecular fingerprint enhancement in trace additives identification. J. Phys. D Appl. Phys. 2024, 57, 235104. [Google Scholar] [CrossRef]
- Gu, H.Y.; Shi, C.J.; Wu, X.; Peng, Y. Molecular methylation detection based on terahertz metamaterial technology. Analyst 2020, 145, 6705–6712. [Google Scholar] [CrossRef]
- Liang, L.; Wen, L.; Jiang, C.; Chen, Q. Research progress of terahertz sensor based on artificial microstructure. Infrared Laser Eng. 2019, 48, 12–28. [Google Scholar] [CrossRef]
- Liu, B.W.; Peng, Y.; Hao, Y.F.; Zhu, Y.M.; Chang, S.J.; Zhuang, S.L. Ultra-wideband terahertz fingerprint enhancement sensing and inversion model supported by single-pixel reconfigurable graphene metasurface. Photonix 2024, 5, 10. [Google Scholar] [CrossRef]
- Singh, R.; Cao, W.; Al-Naib, I.; Cong, L.; Withayachumnankul, W.; Zhang, W. Ultrasensitive terahertz sensing with high-Q Fano resonances in metasurfaces. Appl. Phys. Lett. 2014, 105, 171101. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Cheng, S.; Zhang, H.; Yi, Z.; Sun, T.; Wu, P.; Zeng, Q.; Raza, R. Tunable metamaterial absorption device based on Fabry–Perot resonance as temperature and refractive index sensing. Opt. Lasers Eng. 2024, 181, 108368. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Ling, L.; Sheng, Z.; Cheng, S.; Yi, Z.; Wu, P.; Zeng, Q.; Tang, B.; Ahmad, S. The tunable absorber films of grating structure of AlCuFe quasicrystal with high Q and refractive index sensitivity. Surf. Interfaces 2024, 48, 104248. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Cheng, S.; Zhang, H.; Yang, W.; Yi, Z.; Zeng, Q.; Tang, B.; Ahmad, S.; Sun, T. Polarization independent tunable bandwidth absorber based on single-layer graphene. Diam. Relat. Mater. 2024, 142, 110793. [Google Scholar] [CrossRef]
- Kushwaha, A.S.; Kumar, A.; Kumar, R.; Srivastava, S.K. A study of surface plasmon resonance (SPR) based biosensor with improved sensitivity. Photonics Nanostruct.-Fundam. Appl. 2018, 31, 99–106. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Wu, J.; Wu, X.; Peng, Y. Rapid Determination of Rivaroxaban by Using Terahertz Metamaterial Biosensor. Photonics 2024, 11, 814. https://doi.org/10.3390/photonics11090814
Huang X, Wu J, Wu X, Peng Y. Rapid Determination of Rivaroxaban by Using Terahertz Metamaterial Biosensor. Photonics. 2024; 11(9):814. https://doi.org/10.3390/photonics11090814
Chicago/Turabian StyleHuang, Xinghao, Jing Wu, Xu Wu, and Yan Peng. 2024. "Rapid Determination of Rivaroxaban by Using Terahertz Metamaterial Biosensor" Photonics 11, no. 9: 814. https://doi.org/10.3390/photonics11090814
APA StyleHuang, X., Wu, J., Wu, X., & Peng, Y. (2024). Rapid Determination of Rivaroxaban by Using Terahertz Metamaterial Biosensor. Photonics, 11(9), 814. https://doi.org/10.3390/photonics11090814






