Optical Coherence Tomography and Clinicopathological Correlation for Understanding the Pathogenic, Clinical, and Prognostic Implications in Age-Related Macular Degeneration
Abstract
1. Introduction
2. Clinicopathological Correlations with OCT Features in Non-Neovascular AMD
2.1. Crystalline Deposits
2.2. Calcified Structures
2.3. Outer Retinal Tubulations
2.4. Hyperreflective Foci
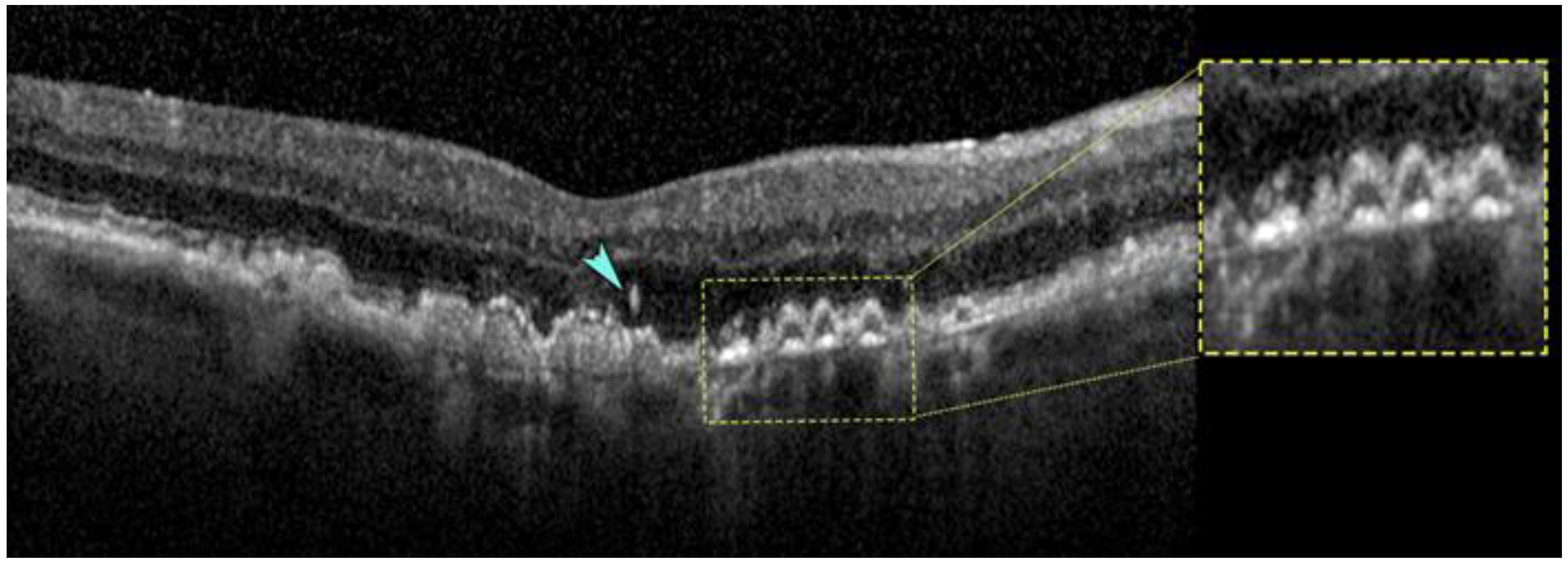
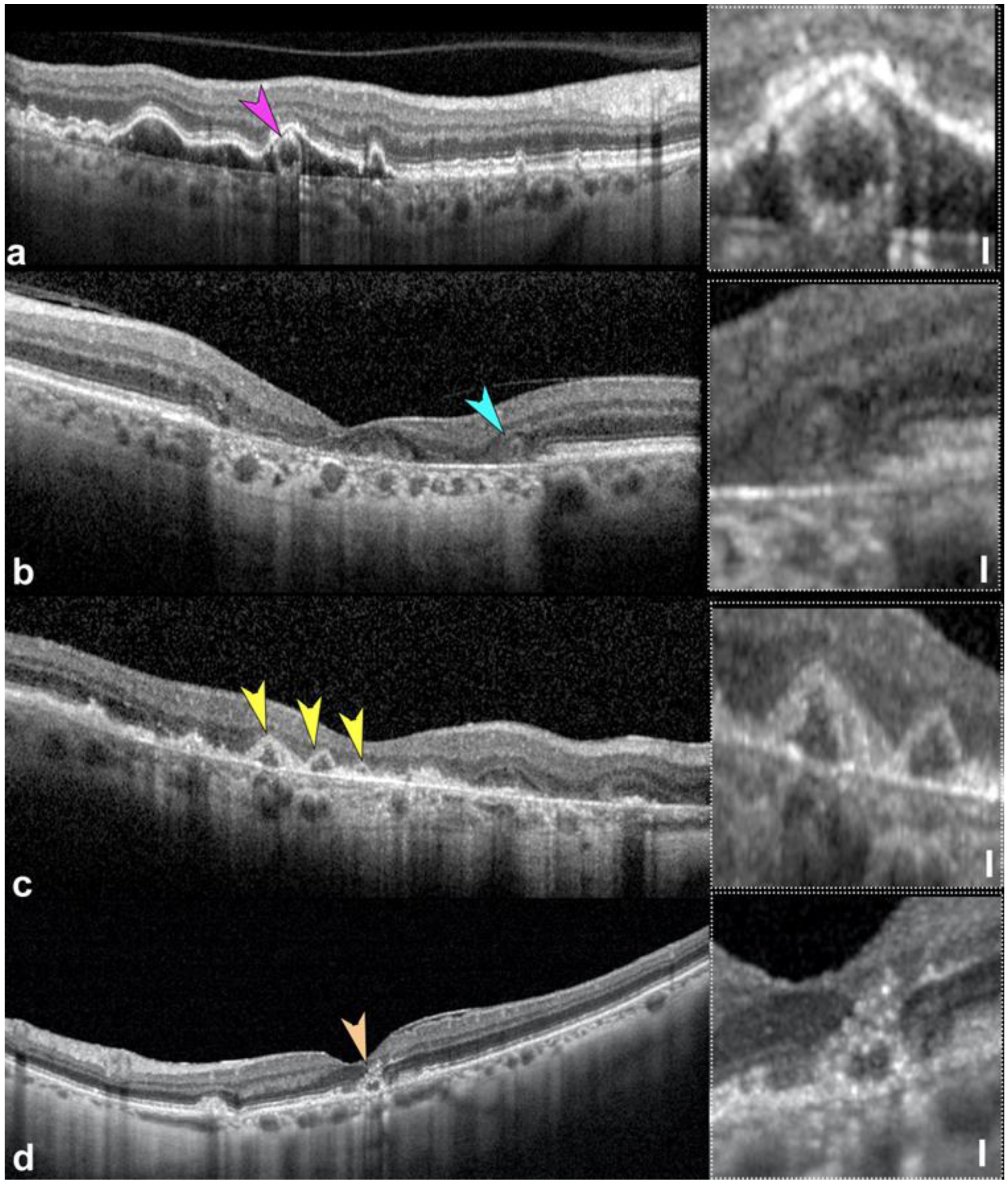
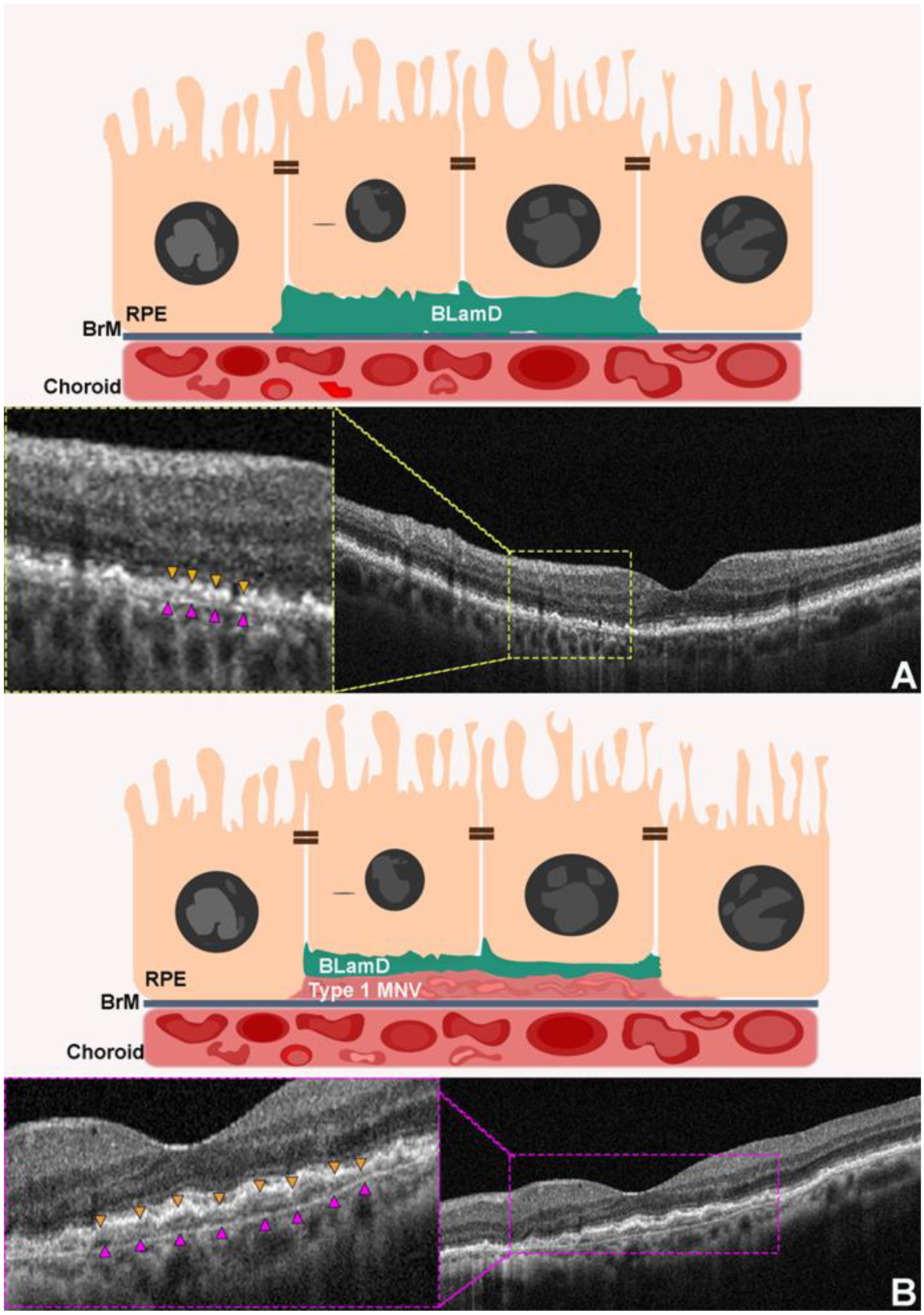
2.5. Thin Double Layer Sign Revealing Thick Basal Laminar Deposits
2.6. Choroidal Caverns or Choroidal Lipid Globules
3. Clinicopathological Correlations with OCT Features in Neovascular AMD
3.1. Crystalline Deposits
3.2. Subretinal Lipid Globules
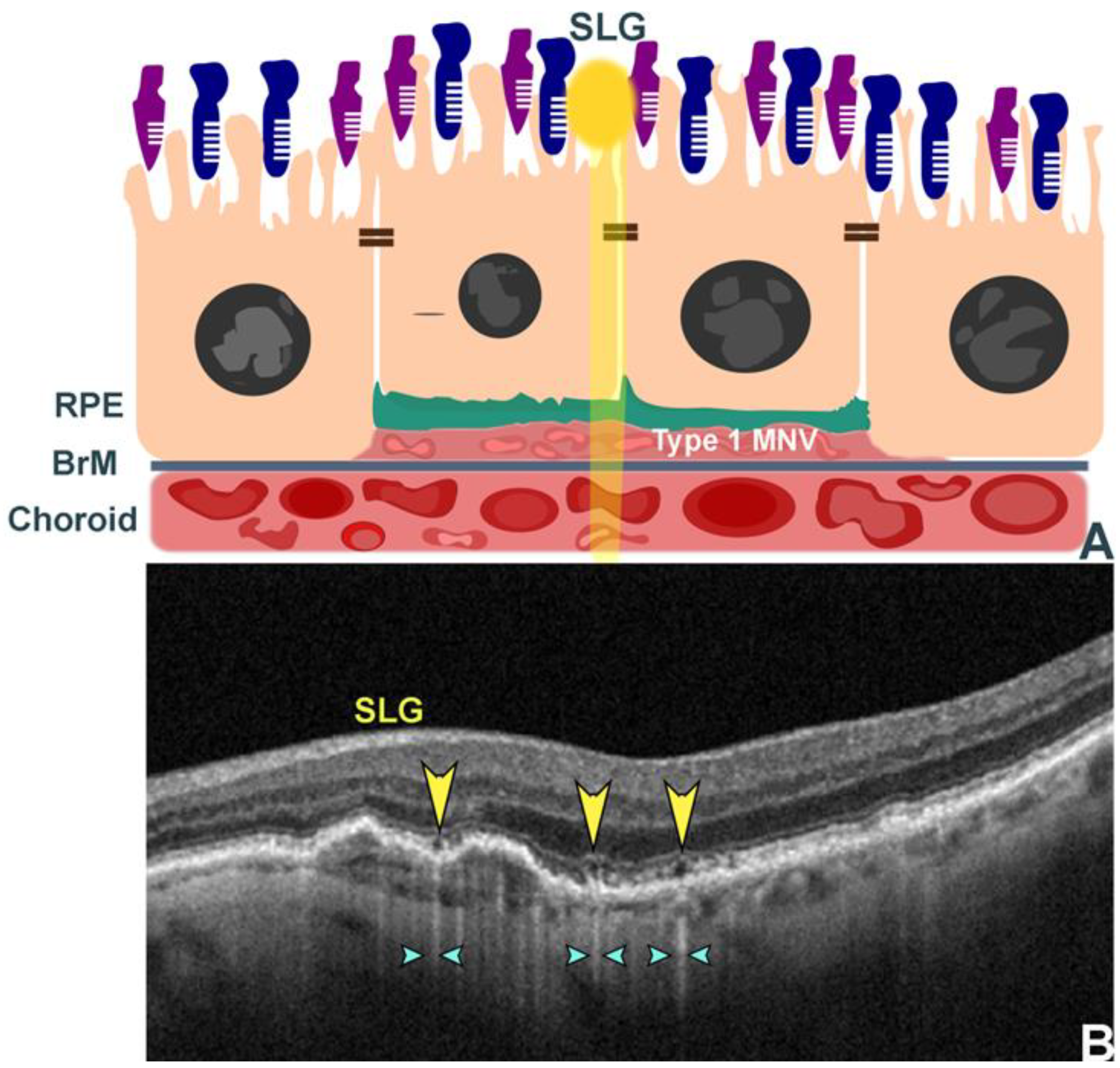
3.3. Double Layer Sign
3.4. Hyperreflective Foci in Macular Neovascularization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drexler, W.; Fujimoto, J.G. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 2008, 27, 45–88. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F.; International Nomenclature for Optical Coherence Tomography Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: The IN*OCT consensus. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Leong, B.C.S.; Fragiotta, S.; Kaden, T.R.; Freund, K.B.; Zweifel, S.; Engelbert, M. OCT En Face Analysis of the Posterior Vitreous Reveals Topographic Relationships among Premacular Bursa, Prevascular Fissures, and Cisterns. Ophthalmol. Retina 2020, 4, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.; Spaide, R.F. A Pilot Study of Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Normal Eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Lasave, A.F.; Arias, J.D.; Serrano, M.A.; Arevalo, F.A. Clinical applications of optical coherence tomography in the posterior pole: The 2011 Jose Manuel Espino Lecture—Part II. Clin. Ophthalmol. 2013, 7, 2181–2206. [Google Scholar] [CrossRef]
- Guymer, R.; Wu, Z. Age-related macular degeneration (AMD): More than meets the eye. The role of multimodal imaging in today’s management of AMD-A review. Clin. Exp. Ophthalmol. 2020, 48, 983–995. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Investigators, I.S.; Chakravarthy, U.; Harding, S.P.; Rogers, C.A.; Downes, S.M.; Lotery, A.J.; Wordsworth, S.; Reeves, B.C. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology 2012, 119, 1399–1411. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Chakravarthy, U.; Freund, K.B.; Guymer, R.H.; Holz, F.G.; Liakopoulos, S.; Mones, J.M.; Rosenfeld, P.J.; Sadda, S.R.; Sarraf, D.; et al. Imaging Features Associated with Progression to Geographic Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 5. Ophthalmol. Retina 2021, 5, 855–867. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Ciulla, T.A.; Ciardella, A.P.; Devin, F.; Dugel, P.U.; Eandi, C.M.; Masonson, H.; Mones, J.; Pearlman, J.A.; Quaranta-El Maftouhi, M.; et al. Dual Antagonism of PDGF and VEGF in Neovascular Age-Related Macular Degeneration: A Phase IIb, Multicenter, Randomized Controlled Trial. Ophthalmology 2017, 124, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Kal, M.; Brzdęk, M.; Winiarczyk, M.; Mackiewicz, J.; Kozieł, D.; Odrobina, D.; Zarębska-Michaluk, D. Retinal thickness in patients with elevated D-dimer and interleukin-6 levels as a result of SARS-CoV-2 infection. Med. Stud./Stud. Med. 2023, 39, 342–351. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, J.P.; Jang, H.; Kim, J.; Kang, S.H.; Kim, J.S.; Lee, J.; Jung, Y.H.; Na, D.L.; Seo, S.W. Optical coherence tomography angiography as a potential screening tool for cerebral small vessel diseases. Alzheimer’s Res. Ther. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Anegondi, N.; Gao, S.S.; Steffen, V.; Spaide, R.F.; Sadda, S.R.; Holz, F.G.; Rabe, C.; Honigberg, L.; Newton, E.M.; Cluceru, J.; et al. Deep Learning to Predict Geographic Atrophy Area and Growth Rate from Multi-modal Imaging. Ophthalmol. Retina 2022, 7, 243–252. [Google Scholar] [CrossRef]
- Balaskas, K.; Glinton, S.; Keenan, T.D.L.; Faes, L.; Liefers, B.; Zhang, G.; Pontikos, N.; Struyven, R.; Wagner, S.K.; McKeown, A.; et al. Prediction of visual function from automatically quantified optical coherence tomography biomarkers in patients with geographic atrophy using machine learning. Sci. Rep. 2022, 12, 15565. [Google Scholar] [CrossRef]
- Banerjee, I.; de Sisternes, L.; Hallak, J.A.; Leng, T.; Osborne, A.; Rosenfeld, P.J.; Gregori, G.; Durbin, M.; Rubin, D. Prediction of age-related macular degeneration disease using a sequential deep learning approach on longitudinal SD-OCT imaging biomarkers. Sci. Rep. 2020, 10, 15434. [Google Scholar] [CrossRef]
- Fragiotta, S.; Grassi, F.; Abdolrahimzadeh, S. Implementing Predictive Models in Artificial Intelligence through OCT Biomarkers for Age-Related Macular Degeneration. Photonics 2023, 10, 149. [Google Scholar] [CrossRef]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of atrophy report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef]
- Sadda, S.R.; Abdelfattah, N.S.; Lei, J.; Shi, Y.; Marion, K.M.; Morgenthien, E.; Gune, S.; Balasubramanian, S. Spectral-Domain OCT Analysis of Risk Factors for Macular Atrophy Development in the HARBOR Study for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1360–1370. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Lei, J.; Balasubramanian, S.; Abdelfattah, N.S.; Nittala, M.G.; Sadda, S.R. Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Nassisi, M.; Lei, J.; Abdelfattah, N.S.; Karamat, A.; Balasubramanian, S.; Fan, W.; Uji, A.; Marion, K.M.; Baker, K.; Huang, X.; et al. OCT Risk Factors for Development of Late Age-Related Macular Degeneration in the Fellow Eyes of Patients Enrolled in the HARBOR Study. Ophthalmology 2019, 126, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Corvi, F.; Tiosano, L.; Corradetti, G.; Nittala, M.G.; Lindenberg, S.; Alagorie, A.R.; McLaughlin, J.A.; Lee, T.K.; Sadda, S.R. Choriocapillaris Flow Deficit as a risk factor for progression of Age-Related Macular Degeneration. Retina 2020, 41, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, K.; Yu, H.J.; Wakatsuki, Y.; Marion, K.M.; Wykoff, C.C.; Sadda, S.R. OCT Risk Factors for Development of Atrophy in Eyes with Intermediate Age-Related Macular Degeneration. Ophthalmol. Retina 2023, 7, 253–260. [Google Scholar] [CrossRef]
- Menean, M.; Apuzzo, A.; Introini, U.; Bandello, F.; Cicinelli, M.V. Morphometric Risk Factors for Drusenoid Pigment Epithelium Detachment Collapse and Retinal Pigment Epithelium Atrophy Expansion. Investig. Ophthalmol. Vis. Sci. 2023, 64, 38. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Klimscha, S.; Sadeghipour, A.; Hu, X.; Gerendas, B.S.; Osborne, A.; Bogunovic, H. Prediction of Individual Disease Conversion in Early AMD Using Artificial Intelligence. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3199–3208. [Google Scholar] [CrossRef]
- Waldstein, S.M.; Vogl, W.D.; Bogunovic, H.; Sadeghipour, A.; Riedl, S.; Schmidt-Erfurth, U. Characterization of Drusen and Hyperreflective Foci as Biomarkers for Disease Progression in Age-Related Macular Degeneration Using Artificial Intelligence in Optical Coherence Tomography. JAMA Ophthalmol. 2020, 138, 740–747. [Google Scholar] [CrossRef]
- Sleiman, K.; Veerappan, M.; Winter, K.P.; McCall, M.N.; Yiu, G.; Farsiu, S.; Chew, E.Y.; Clemons, T.; Toth, C.A. Optical Coherence Tomography Predictors of Risk for Progression to Non-Neovascular Atrophic Age-Related Macular Degeneration. Ophthalmology 2017, 124, 1764–1777. [Google Scholar] [CrossRef]
- Vogl, W.D.; Bogunovic, H.; Waldstein, S.M.; Riedl, S.; Schmidt-Erfurth, U. Spatio-temporal alterations in retinal and choroidal layers in the progression of age-related macular degeneration (AMD) in optical coherence tomography. Sci. Rep. 2021, 11, 5743. [Google Scholar] [CrossRef]
- Dolz-Marco, R.; Balaratnasingam, C.; Messinger, J.D.; Li, M.; Ferrara, D.; Freund, K.B.; Curcio, C.A. The border of macular atrophy in age-related macular degeneration: A clinicopathologic correlation. Am. J. Ophthalmol. 2018, 193, 166–177. [Google Scholar] [CrossRef]
- Messinger, J.D.; Brinkmann, M.; Kimble, J.A.; Berlin, A.; Freund, K.B.; Grossman, G.H.; Ach, T.; Curcio, C.A. Ex Vivo OCT-Based Multimodal Imaging of Human Donor Eyes for Research into Age-Related Macular Degeneration. J. Vis. Exp. 2023, 10–3791. [Google Scholar] [CrossRef] [PubMed]
- Zanzottera, E.C.; Messinger, J.D.; Ach, T.; Smith, R.T.; Freund, K.B.; Curcio, C.A. The project MACULA retinal pigment epithelium grading system for histology and optical coherence tomography in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Green, W.R.; Key, S.N., 3rd. Senile macular degeneration: A histopathologic study. Trans. Am. Ophthalmol. Soc. 1977, 75, 180–254. [Google Scholar]
- Li, M.; Dolz-Marco, R.; Huisingh, C.; Messinger, J.D.; Feist, R.M.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Clinicopathologic Correlation of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Retina 2019, 39, 802–816. [Google Scholar] [CrossRef]
- Pang, C.E.; Messinger, J.D.; Zanzottera, E.C.; Freund, K.B.; Curcio, C.A. The onion sign in neovascular age-related macular degeneration represents cholesterol crystals. Ophthalmology 2015, 122, 2316–2326. [Google Scholar] [CrossRef]
- Fragiotta, S.; Fernandez-Avellaneda, P.; Breazzano, M.P.; Curcio, C.A.; Leong, B.C.S.; Kato, K.; Yannuzzi, L.A.; Freund, K.B. The Fate and Prognostic Implications of Hyperreflective Crystalline Deposits in Nonneovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3100–3109. [Google Scholar] [CrossRef]
- Querques, G.; Georges, A.; Ben Moussa, N.; Sterkers, M.; Souied, E.H. Appearance of regressing drusen on optical coherence tomography in age-related macular degeneration. Ophthalmology 2014, 121, 173–179. [Google Scholar] [CrossRef]
- Fragiotta, S.; Fernandez-Avellaneda, P.; Breazzano, M.P.; Yannuzzi, L.A.; Curcio, C.A.; Freund, K.B. Linear and planar reflection artifacts on swept-source and spectral-domain optical coherence tomography due to hyperreflective crystalline deposits. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 491–501. [Google Scholar] [CrossRef]
- Fragiotta, S.; Abdolrahimzadeh, S.; Dolz-Marco, R.; Sakurada, Y.; Gal-Or, O.; Scuderi, G. Significance of Hyperreflective Foci as an Optical Coherence Tomography Biomarker in Retinal Diseases: Characterization and Clinical Implications. J. Ophthalmol. 2021, 2021, 6096017. [Google Scholar] [CrossRef]
- Sarks, S.H. Ageing and degeneration in the macular region: A clinico-pathological study. Br. J. Ophthalmol. 1976, 60, 324–341. [Google Scholar] [CrossRef]
- Bonnet, C.; Querques, G.; Zerbib, J.; Oubraham, H.; Garavito, R.B.; Puche, N.; Souied, E.H. Hyperreflective pyramidal structures on optical coherence tomography in geographic atrophy areas. Retina 2014, 34, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Laiginhas, R.; Shen, M.; Shi, Y.; Li, J.; Trivizki, O.; Waheed, N.K.; Gregori, G.; Rosenfeld, P.J. Multimodal Imaging and En Face OCT Detection of Calcified Drusen in Eyes with Age-Related Macular Degeneration. Ophthalmol. Sci. 2022, 2, 100162. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Curcio, C.A.; Mullins, R.F.; Spaide, R.F. Refractile drusen: Clinical imaging and candidate histology. Retina 2015, 35, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Oishi, A.; Thiele, S.; Nadal, J.; Oishi, M.; Fleckenstein, M.; Schmid, M.; Holz, F.G.; Schmitz-Valckenberg, S. Prevalence, natural course, and prognostic role of refractile drusen in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2198–2206. [Google Scholar] [CrossRef]
- Mentes, J.; Karaca, I.; Sermet, F. Multimodal imaging characteristics of quiescent type 1 neovascularization in an eye with angioid streaks. Am. J. Ophthalmol. Case Rep. 2018, 10, 132–136. [Google Scholar] [CrossRef]
- Tan, A.C.S.; Pilgrim, M.G.; Fearn, S.; Bertazzo, S.; Tsolaki, E.; Morrell, A.P.; Li, M.; Messinger, J.D.; Dolz-Marco, R.; Lei, J.; et al. Calcified nodules in retinal drusen are associated with disease progression in age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaat4544. [Google Scholar] [CrossRef]
- Fragiotta, S.; Parravano, M.; Sacconi, R.; Costanzo, E.; De Geronimo, D.; Prascina, F.; Capuano, V.; Souied, E.H.; Han, I.C.; Mullins, R.; et al. Sub-retinal pigment epithelium tubules in non-neovascular age-related macular degeneration. Sci. Rep. 2022, 12, 15198. [Google Scholar] [CrossRef]
- Ooto, S.; Vongkulsiri, S.; Sato, T.; Suzuki, M.; Curcio, C.A.; Spaide, R.F. Outer retinal corrugations in age-related macular degeneration. JAMA Ophthalmol. 2014, 132, 806–813. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Charbel Issa, P.; Helb, H.M.; Schmitz-Valckenberg, S.; Finger, R.P.; Scholl, H.P.; Loeffler, K.U.; Holz, F.G. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4137–4144. [Google Scholar] [CrossRef]
- Zweifel, S.A.; Engelbert, M.; Laud, K.; Margolis, R.; Spaide, R.F.; Freund, K.B. Outer retinal tubulation: A novel optical coherence tomography finding. Arch. Ophthalmol. 2009, 127, 1596–1602. [Google Scholar] [CrossRef]
- Curcio, C.A.; Medeiros, N.E.; Millican, C.L. Photoreceptor loss in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1236–1249. [Google Scholar] [PubMed]
- Litts, K.M.; Messinger, J.D.; Dellatorre, K.; Yannuzzi, L.A.; Freund, K.B.; Curcio, C.A. Clinicopathological correlation of outer retinal tubulation in age-related macular degeneration. JAMA Ophthalmol. 2015, 133, 609–612. [Google Scholar] [CrossRef]
- Litts, K.M.; Wang, X.; Clark, M.E.; Owsley, C.; Freund, K.B.; Curcio, C.A.; Zhang, Y. Exploring Photoreceptor Reflectivity through Multimodal Imaging of Outer Retinal Tubulation in Advanced Age-Related Macular Degeneration. Retina 2017, 37, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Schaal, K.B.; Freund, K.B.; Litts, K.M.; Zhang, Y.; Messinger, J.D.; Curcio, C.A. Outer Retinal Tubulation in Advanced Age-Related Macular Degeneration: Optical Coherence Tomographic Findings Correspond to Histology. Retina 2015, 35, 1339–1350. [Google Scholar] [CrossRef]
- Dolz-Marco, R.; Litts, K.M.; Tan, A.C.S.; Freund, K.B.; Curcio, C.A. The evolution of outer retinal tubulation, a neurodegeneration and gliosis prominent in macular diseases. Ophthalmology 2017, 124, 1353–1367. [Google Scholar] [CrossRef]
- Jung, J.J.; Freund, K.B. Long-term follow-up of outer retinal tubulation documented by eye-tracked and en face spectral-domain optical coherence tomography. Arch. Ophthalmol. 2012, 130, 1618–1619. [Google Scholar] [CrossRef]
- Wolff, B.; Matet, A.; Vasseur, V.; Sahel, J.A.; Mauget-Faysse, M. En Face OCT Imaging for the Diagnosis of Outer Retinal Tubulations in Age-Related Macular Degeneration. J. Ophthalmol. 2012, 2012, 542417. [Google Scholar] [CrossRef]
- Litts, K.M.; Ach, T.; Hammack, K.M.; Sloan, K.R.; Zhang, Y.; Freund, K.B.; Curcio, C.A. Quantitative Analysis of Outer Retinal Tubulation in Age-Related Macular Degeneration From Spectral-Domain Optical Coherence Tomography and Histology. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2647–2656. [Google Scholar] [CrossRef]
- Litts, K.M.; Messinger, J.D.; Freund, K.B.; Zhang, Y.; Curcio, C.A. Inner Segment Remodeling and Mitochondrial Translocation in Cone Photoreceptors in Age-Related Macular Degeneration With Outer Retinal Tubulation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2243–2253. [Google Scholar] [CrossRef]
- Curcio, C.A.; Zanzottera, E.C.; Ach, T.; Balaratnasingam, C.; Freund, K.B. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO211–BIO226. [Google Scholar] [CrossRef]
- Vujosevic, S.; Bini, S.; Torresin, T.; Berton, M.; Midena, G.; Parrozzani, R.; Martini, F.; Pucci, P.; Daniele, A.R.; Cavarzeran, F.; et al. Hyperreflective retinal spots in normal and diabetic eyes: B-Scan and En Face Spectral Domain Optical Coherence Tomography Evaluation. Retina 2017, 37, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Coscas, G.; De Benedetto, U.; Coscas, F.; Li Calzi, C.I.; Vismara, S.; Roudot-Thoraval, F.; Bandello, F.; Souied, E. Hyperreflective dots: A new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica 2013, 229, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dolz-Marco, R.; Messinger, J.D.; Wang, L.; Feist, R.M.; Girkin, C.A.; Gattoussi, S.; Ferrara, D.; Curcio, C.A.; Freund, K.B. Clinicopathologic Correlation of Anti-Vascular Endothelial Growth Factor-Treated Type 3 Neovascularization in Age-Related Macular Degeneration. Ophthalmology 2018, 125, 276–287. [Google Scholar] [CrossRef]
- Christenbury, J.G.; Folgar, F.A.; O’Connell, R.V.; Chiu, S.J.; Farsiu, S.; Toth, C.A. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology 2013, 120, 1038–1045. [Google Scholar] [CrossRef]
- Leuschen, J.N.; Schuman, S.G.; Winter, K.P.; McCall, M.N.; Wong, W.T.; Chew, E.Y.; Hwang, T.; Srivastava, S.; Sarin, N.; Clemons, T.; et al. Spectral-domain optical coherence tomography characteristics of intermediate age-related macular degeneration. Ophthalmology 2013, 120, 140–150. [Google Scholar] [CrossRef]
- Nassisi, M.; Fan, W.; Shi, Y.; Lei, J.; Borrelli, E.; Ip, M.; Sadda, S.R. Quantity of Intraretinal Hyperreflective Foci in Patients With Intermediate Age-Related Macular Degeneration Correlates With 1-Year Progression. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3431–3439. [Google Scholar] [CrossRef]
- Zanzottera, E.C.; Ach, T.; Huisingh, C.; Messinger, J.D.; Spaide, R.F.; Curcio, C.A. Visualizing Retinal Pigment Epithelium Phenotypes in the Transition to Geographic Atrophy in Age-Related Macular Degeneration. Retina 2016, 36 (Suppl. S1), S12–S25. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Bogunovic, H.; Grechenig, C.; Bui, P.; Fabianska, M.; Waldstein, S.; Reiter, G.S. Role of Deep Learning-Quantified Hyperreflective Foci for the Prediction of Geographic Atrophy Progression. Am. J. Ophthalmol. 2020, 216, 257–270. [Google Scholar] [CrossRef]
- Vogl, W.D.; Riedl, S.; Mai, J.; Reiter, G.S.; Lachinov, D.; Bogunović, H.; Schmidt-Erfurth, U. Predicting Topographic Disease Progression and Treatment Response of Pegcetacoplan in Geographic Atrophy Quantified by Deep Learning. Ophthalmol. Retina 2022, 7, 4–13. [Google Scholar] [CrossRef]
- Sato, T.; Kishi, S.; Watanabe, G.; Matsumoto, H.; Mukai, R. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina 2007, 27, 589–594. [Google Scholar] [CrossRef]
- Sura, A.A.; Chen, L.; Messinger, J.D.; Swain, T.A.; McGwin, G., Jr.; Freund, K.B.; Curcio, C.A. Measuring the Contributions of Basal Laminar Deposit and Bruch’s Membrane in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 19. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Chen, L.; Messinger, J.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Double-layer sign in neovascular age-related macular degeneration—Do we treat? Acta Ophthalmol. 2022, 100, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Sarks, S.; Cherepanoff, S.; Killingsworth, M.; Sarks, J. Relationship of Basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Takahashi, H.; Ploner, S.B.; Karbole, W.; Abu-Qamar, O.; Yaghy, A.; Marmalidou, A.; Kaiser, S.; Hwang, Y.; Lin, J.; et al. Topographic Measurement of the Subretinal Pigment Epithelium Space in Normal Aging and Age-Related Macular Degeneration Using High-Resolution OCT. Investig. Ophthalmol. Vis. Sci. 2024, 65, 18. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Martens, C.; Kosanetzky, S.; Brinkmann, C.K.; Hageman, G.S.; Holz, F.G. Fundus autofluorescence and spectral-domain optical coherence tomography characteristics in a rapidly progressing form of geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3761–3766. [Google Scholar] [CrossRef]
- Fragiotta, S.; Parravano, M.; Sacconi, R.; Costanzo, E.; Viggiano, P.; Prascina, F.; Capuano, V.; Souied, E.H.; Querques, G. A Common Finding in Foveal-Sparing Extensive Macular Atrophy with Pseudodrusen Implicates Basal Laminar Deposits. Retina 2022, 42, 1319–1329. [Google Scholar] [CrossRef]
- Capuano, V.; Miere, A.; Querques, L.; Sacconi, R.; Carnevali, A.; Amoroso, F.; Bandello, F.; Souied, E.H.; Querques, G. Treatment-Naive Quiescent Choroidal Neovascularization in Geographic Atrophy Secondary to Nonexudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2017, 182, 45–55. [Google Scholar] [CrossRef]
- Fukuyama, H.; Huang, B.B.; BouGhanem, G.; Fawzi, A.A. The Fovea-Protective Impact of Double-Layer Sign in Eyes With Foveal-Sparing Geographic Atrophy and Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4. [Google Scholar] [CrossRef]
- Manafi, N.; Mahmoudi, A.; Emamverdi, M.; Corradetti, G.; Corona, S.T.; Wykoff, C.C.; Sadda, S.R. Topographic analysis of local OCT biomarkers which predict progression to atrophy in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 2083–2091. [Google Scholar] [CrossRef]
- Fragiotta, S.; Dysli, C.; Parravano, M.; Sacconi, R.; Fantaguzzi, F.; Servillo, A.; Severo, A.A.; Tombolini, B.; Costanzo, E.; De Geronimo, D.; et al. Phenotypic characterization of predictors for development and progression of geographic atrophy using optical coherence tomography. Retina 2024, 44, 1232–1241. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Chandra, S.; Sadda, S.; Teo, K.Y.C.; Thottarath, S.; de Cock, E.; Empeslidis, T.; Esmaeelpour, M. Predict and Protect: Evaluating the Double-Layer Sign in Age-Related Macular Degeneration. Ophthalmol. Ther. 2024, 13, 2511–2541. [Google Scholar] [CrossRef] [PubMed]
- Dolz-Marco, R.; Glover, J.P.; Gal-Or, O.; Litts, K.M.; Messinger, J.D.; Zhang, Y.; Cozzi, M.; Pellegrini, M.; Freund, K.B.; Staurenghi, G.; et al. Choroidal and Sub-Retinal Pigment Epithelium Caverns: Multimodal Imaging and Correspondence with Friedman Lipid Globules. Ophthalmology 2018, 125, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.; Smith, T.R. Clinical and pathological study of choroidal lipid globules. Arch. Ophthalmol. 1966, 75, 334–336. [Google Scholar] [CrossRef]
- Small, D.M. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis 1988, 8, 103–129. [Google Scholar] [CrossRef]
- Querques, G.; Costanzo, E.; Miere, A.; Capuano, V.; Souied, E.H. Choroidal Caverns: A Novel Optical Coherence Tomography Finding in Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2578–2582. [Google Scholar] [CrossRef]
- Sacconi, R.; Borrelli, E.; Marchese, A.; Gelormini, F.; Pennisi, F.; Cerutti, A.; Bandello, F.; Querques, G. Re: Dolz-Marco et al. Choroidal and sub-retinal pigment epithelium caverns: Multimodal imaging and correspondence with Friedman lipid globules (Ophthalmology. 2018;125:1287–1301). Ophthalmology 2019, 126, e53–e54. [Google Scholar] [CrossRef]
- Carnevali, A.; Sacconi, R.; Corbelli, E.; Querques, L.; Bandello, F.; Querques, G. Choroidal Caverns: A Previously Unreported Optical Coherence Tomography Finding in Best Vitelliform Dystrophy. Ophthalmic Surg. Lasers Imaging Retina 2018, 49, 284–287. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, Y.; Gu, C.; Wu, Y.; Liu, H.; Chang, Q.; Lei, B.; Wang, M. Characteristics and Classification of Choroidal Caverns in Patients with Various Retinal and Chorioretinal Diseases. J. Clin. Med. 2022, 11, 6994. [Google Scholar] [CrossRef]
- Metrangolo, C.; Donati, S.; Mazzola, M.; Fontanel, L.; Messina, W.; D’Alterio, G.; Rubino, M.; Radice, P.; Premi, E.; Azzolini, C. OCT Biomarkers in Neovascular Age-Related Macular Degeneration: A Narrative Review. J. Ophthalmol. 2021, 2021, 9994098. [Google Scholar] [CrossRef]
- Pederzolli, M.; Sacconi, R.; Battista, M.; Bandello, F.; Querques, G. Bilateral choroidal caverns in a child with pachychoroid and anxious personality. Am. J. Ophthalmol. Case Rep. 2022, 26, 101505. [Google Scholar] [CrossRef]
- Sakurada, Y.; Leong, B.C.S.; Parikh, R.; Fragiotta, S.; Freund, K.B. Association between Choroidal Caverns and Choroidal Vascular Hyperpermeability in Eyes with Pachychoroid Diseases. Retina 2018, 38, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Feng, N.; Hua, R. “Choroidal caverns” spectrum lesions. Eye 2021, 35, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Mukkamala, S.K.; Costa, R.A.; Fung, A.; Sarraf, D.; Gallego-Pinazo, R.; Freund, K.B. Optical coherence tomographic imaging of sub-retinal pigment epithelium lipid. Arch. Ophthalmol. 2012, 130, 1547–1553. [Google Scholar] [CrossRef]
- Li, M.; Dolz-Marco, R.; Messinger, J.D.; Sloan, K.R.; Ferrara, D.; Curcio, C.A.; Freund, K.B. Clinicopathologic Correlation of Aneurysmal Type 1 Neovascularization in Age-Related Macular Degeneration. Ophthalmol. Retina 2019, 3, 99–111. [Google Scholar] [CrossRef]
- Christakopoulos, C.; Pryds, A.; Larsen, M. Subretinal lamellar bodies in polypoidal choroidal vasculopathy. Acta Ophthalmol. 2013, 91, e248–e249. [Google Scholar] [CrossRef]
- Miere, A.; Sacconi, R.; Amoroso, F.; Capuano, V.; Jung, C.; Bandello, F.; Souied, E.H.; Querques, G. Sub-Retinal Pigment Epithelium Multilaminar Hyperreflectivity at the Onset of Type 3 Macular Neovascularization. Retina 2021, 41, 135–143. [Google Scholar] [CrossRef]
- Fernandez-Avellaneda, P.; Freund, K.B.; Wang, R.K.; He, Q.; Zhang, Q.; Fragiotta, S.; Xu, X.; Ledesma-Gil, G.; Sugiura, Y.; Breazzano, M.P.; et al. Multimodal Imaging Features and Clinical Relevance of Subretinal Lipid Globules. Am. J. Ophthalmol. 2020, 222, 112–125. [Google Scholar] [CrossRef]
- Fragiotta, S.; Parravano, M.; Costanzo, E.; De Geronimo, D.; Varano, M.; Fernandez-Avellaneda, P.; Freund, K.B. Subretinal Lipid Globules an Early Biomarker of Macular Neovascularization in Eyes with Intermediate Age-Related Macular Degeneration. Retina 2023, 43, 913–922. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Iafe, N.A.; Phasukkijwatana, N.; Sadda, S.R.; Sarraf, D. Biomarkers of Neovascular Activity in Age-Related Macular Degeneration Using Optical Coherence Tomography Angiography. Retina 2018, 38, 220–230. [Google Scholar] [CrossRef]
- Csincsik, L.; Muldrew, K.A.; Bettiol, A.; Wright, D.M.; Rosenfeld, P.J.; Waheed, N.K.; Empeslidis, T.; De Cock, E.; Yamaguchi, T.C.N.; Hogg, R.E.; et al. The Double Layer Sign Is Highly Predictive of Progression to Exudation in Age-Related Macular Degeneration. Ophthalmol. Retina 2024, 8, 234–245. [Google Scholar] [CrossRef]
- Shi, Y.; Motulsky, E.H.; Goldhardt, R.; Zohar, Y.; Thulliez, M.; Feuer, W.; Gregori, G.; Rosenfeld, P.J. Predictive Value of the OCT Double-Layer Sign for Identifying Subclinical Neovascularization in Age-Related Macular Degeneration. Ophthalmol. Retina 2019, 3, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tang, W.; Liu, W.; Chang, Q.; Xu, G. Clinical Features of Flat Irregular Pigment Epithelial Detachment Associated with Choroidal Neovascularization in Chronic Central Serous Chorioretinopathy. Retina 2021, 41, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Narita, C.; Wu, Z.; Rosenfeld, P.J.; Yang, J.; Lyu, C.; Caruso, E.; McGuinness, M.; Guymer, R.H. Structural OCT Signs Suggestive of Subclinical Nonexudative Macular Neovascularization in Eyes with Large Drusen. Ophthalmology 2020, 127, 637–647. [Google Scholar] [CrossRef]
- Sacconi, R.; Fragiotta, S.; Sarraf, D.; Sadda, S.R.; Freund, K.B.; Parravano, M.; Corradetti, G.; Cabral, D.; Capuano, V.; Miere, A.; et al. Towards a better understanding of non-exudative choroidal and macular neovascularization. Prog. Retin. Eye Res. 2022, 92, 101113. [Google Scholar] [CrossRef] [PubMed]
- Querques, G.; Sacconi, R.; Capuano, V.; Carnevali, A.; Colantuono, D.; Battista, M.; Borrelli, E.; Miere, A.; Parravano, M.; Costanzo, E.; et al. Treatment-naive quiescent macular neovascularization secondary to AMD: The 2019 Young Investigator Lecture of Macula Society. Eur. J. Ophthalmol. 2021, 31, 3164–3176. [Google Scholar] [CrossRef]
- Berlin, A.; Messinger, J.D.; Ramtohul, P.; Balaratnasingam, C.; Mendis, R.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Inflammatory cell activity in treated neovascular age-related macular degeneration: A Histologic Case Study. Retina 2023, 43, 1904–1913. [Google Scholar] [CrossRef]
- Cao, D.; Leong, B.; Messinger, J.D.; Kar, D.; Ach, T.; Yannuzzi, L.A.; Freund, K.B.; Curcio, C.A. Hyperreflective Foci, Optical Coherence Tomography Progression Indicators in Age-Related Macular Degeneration, Include Transdifferentiated Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2021, 62, 34. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Messinger, J.D.; Sloan, K.R.; Yannuzzi, L.A.; Freund, K.B.; Curcio, C.A. Histologic and Optical Coherence Tomographic Correlates in Drusenoid Pigment Epithelium Detachment in Age-Related Macular Degeneration. Ophthalmology 2017, 124, 644–656. [Google Scholar] [CrossRef]
- Sacconi, R.; Sarraf, D.; Garrity, S.; Freund, K.B.; Yannuzzi, L.A.; Gal-Or, O.; Souied, E.; Sieiro, A.; Corbelli, E.; Carnevali, A.; et al. Nascent Type 3 Neovascularization in Age-Related Macular Degeneration. Ophthalmol. Retina 2018, 2, 1097–1106. [Google Scholar] [CrossRef]
- Su, D.; Lin, S.; Phasukkijwatana, N.; Chen, X.; Tan, A.; Freund, K.B.; Sarraf, D. An Updated Staging System of Type 3 Neovascularization Using Spectral Domain Optical Coherence Tomography. Retina 2016, 36 (Suppl. S1), S40–S49. [Google Scholar] [CrossRef]
- Bousquet, E.; Santina, A.; Corradetti, G.; Sacconi, R.; Ramtohul, P.; Bijon, J.; Somisetty, S.; Voichanski, S.; Querques, G.; Sadda, S.; et al. From Drusen to Type 3 Macular Neovascularization. Retina 2024, 44, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Nagiel, A.; Sarraf, D.; Sadda, S.R.; Spaide, R.F.; Jung, J.J.; Bhavsar, K.V.; Ameri, H.; Querques, G.; Freund, K.B. Type 3 neovascularization: Evolution, association with pigment epithelial detachment, and treatment response as revealed by spectral domain optical coherence tomography. Retina 2015, 35, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Cabral, D.; Chen, L.; Messinger, J.D.; Balaratnasingam, C.; Mendis, R.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Histology of Type 3 Macular Neovascularization and Microvascular Anomalies in Treated Age-Related Macular Degeneration: A Case Study. Ophthalmol. Sci. 2023, 3, 100280. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Cabral, D.; Chen, L.; Messinger, J.D.; Balaratnasingam, C.; Mendis, R.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Correlation of Optical Coherence Tomography Angiography of Type 3 Macular Neovascularization With Corresponding Histology. JAMA Ophthalmol. 2022, 140, 628–633. [Google Scholar] [CrossRef]
- Kikushima, W.; Sakurada, Y.; Sugiyama, A.; Yoneyama, S.; Matsubara, M.; Fukuda, Y.; Shijo, T.; Kotoda, Y.; Fragiotta, S.; Kashiwagi, K. Characteristics of intermediate age-related macular degeneration with hyperreflective foci. Sci. Rep. 2022, 12, 18420. [Google Scholar] [CrossRef]
- Fragiotta, S.; Rossi, T.; Cutini, A.; Grenga, P.L.; Vingolo, E.M. Predictive factors for development of neovascular age-related macular degeneration: A Spectral-Domain Optical Coherence Tomography Study. Retina 2018, 38, 245–252. [Google Scholar] [CrossRef]
- Hu, X.; Waldstein, S.M.; Klimscha, S.; Sadeghipour, A.; Bogunovic, H.; Gerendas, B.S.; Osborne, A.; Schmidt-Erfurth, U. Morphological and Functional Characteristics at the Onset of Exudative Conversion in Age-Related Macular Degeneration. Retina 2020, 40, 1070–1078. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragiotta, S.; Di Pippo, M.; Fumi, D.; Ciancimino, C.; Abdolrahimzadeh, S. Optical Coherence Tomography and Clinicopathological Correlation for Understanding the Pathogenic, Clinical, and Prognostic Implications in Age-Related Macular Degeneration. Photonics 2025, 12, 237. https://doi.org/10.3390/photonics12030237
Fragiotta S, Di Pippo M, Fumi D, Ciancimino C, Abdolrahimzadeh S. Optical Coherence Tomography and Clinicopathological Correlation for Understanding the Pathogenic, Clinical, and Prognostic Implications in Age-Related Macular Degeneration. Photonics. 2025; 12(3):237. https://doi.org/10.3390/photonics12030237
Chicago/Turabian StyleFragiotta, Serena, Mariachiara Di Pippo, Daniele Fumi, Chiara Ciancimino, and Solmaz Abdolrahimzadeh. 2025. "Optical Coherence Tomography and Clinicopathological Correlation for Understanding the Pathogenic, Clinical, and Prognostic Implications in Age-Related Macular Degeneration" Photonics 12, no. 3: 237. https://doi.org/10.3390/photonics12030237
APA StyleFragiotta, S., Di Pippo, M., Fumi, D., Ciancimino, C., & Abdolrahimzadeh, S. (2025). Optical Coherence Tomography and Clinicopathological Correlation for Understanding the Pathogenic, Clinical, and Prognostic Implications in Age-Related Macular Degeneration. Photonics, 12(3), 237. https://doi.org/10.3390/photonics12030237




