Noise Reduction in LED-Based Photoacoustic Imaging
Abstract
1. Introduction
2. Theory and Method
2.1. Conventional Coded Excitation and PUM Theory
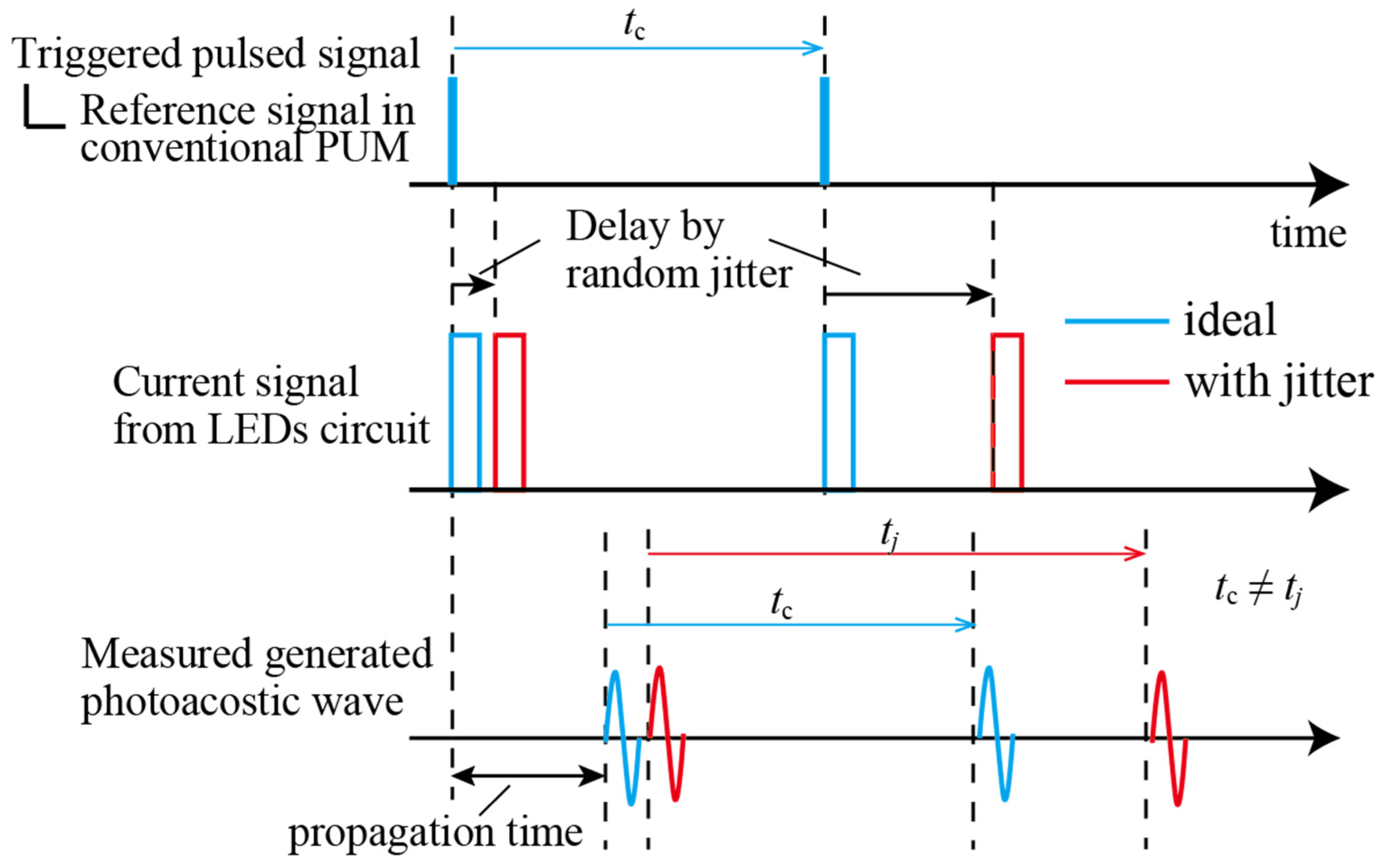
2.2. Proposed New Decoding Algorithm of Jitter-Compensated PUM
2.3. Theoretical Value of SNR Improvement by Jitter-Compensated PUM
3. Experiment
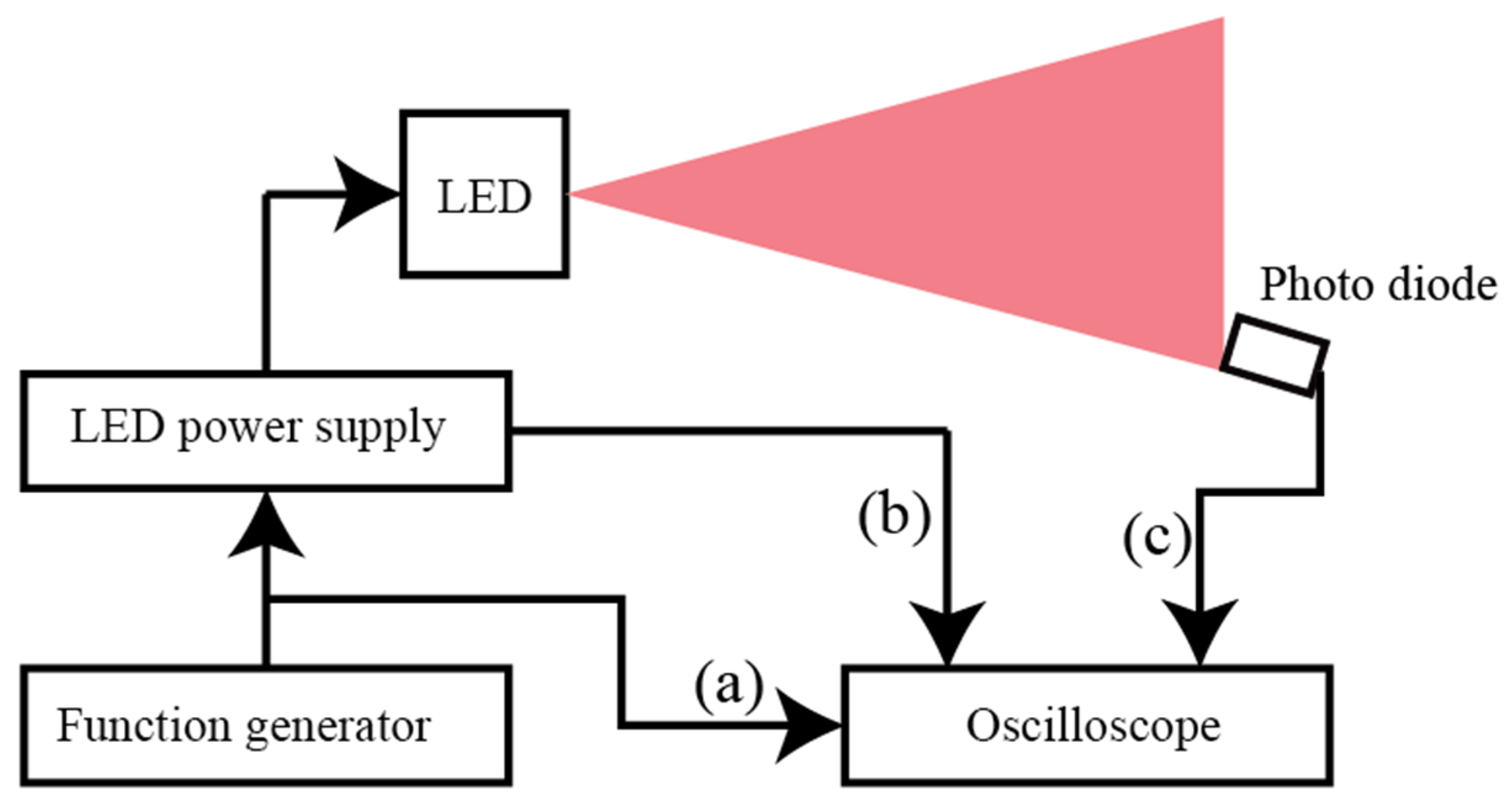
3.1. Experimental Setup
3.2. Experimental Conditions
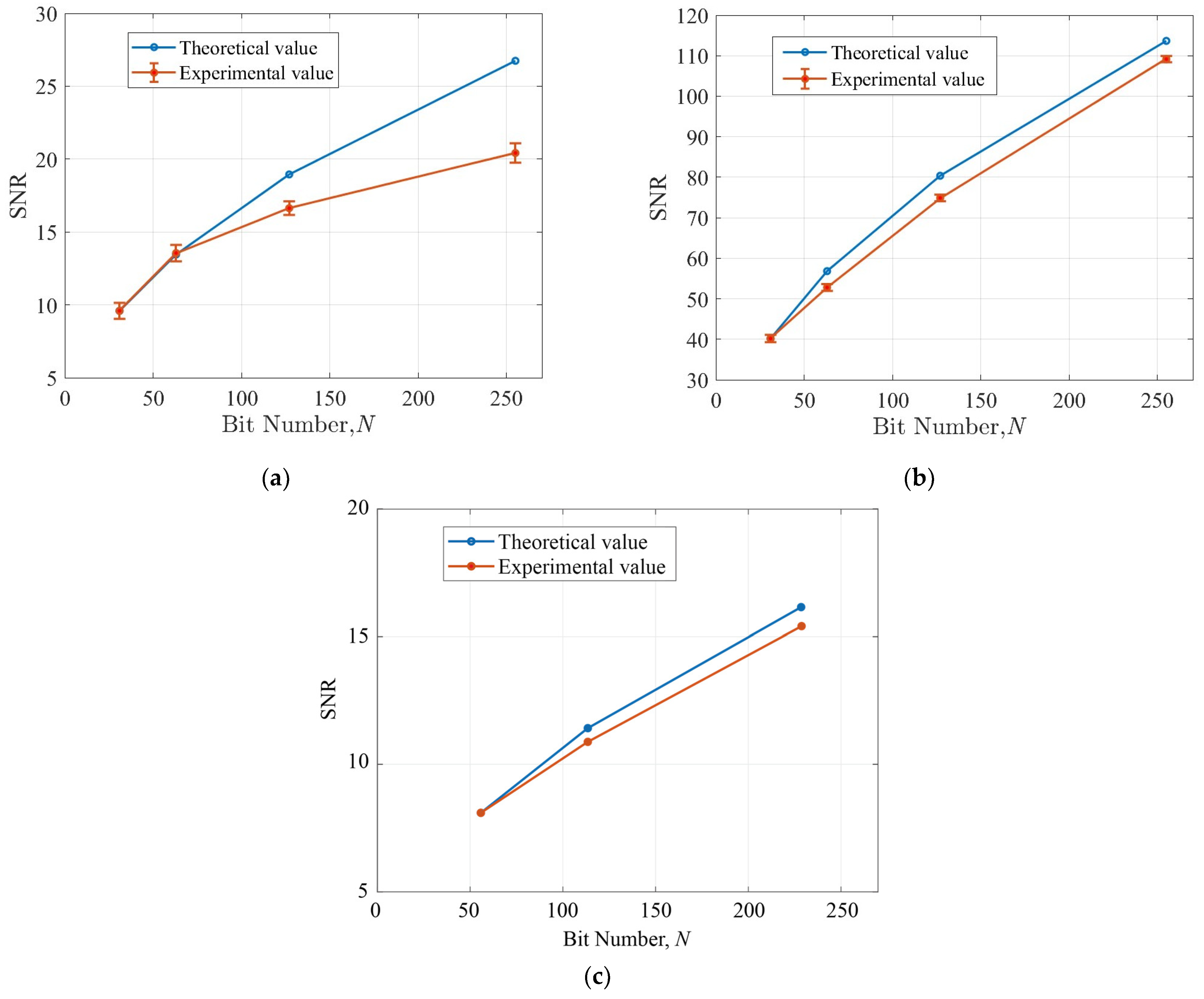
4. Result and Discussion
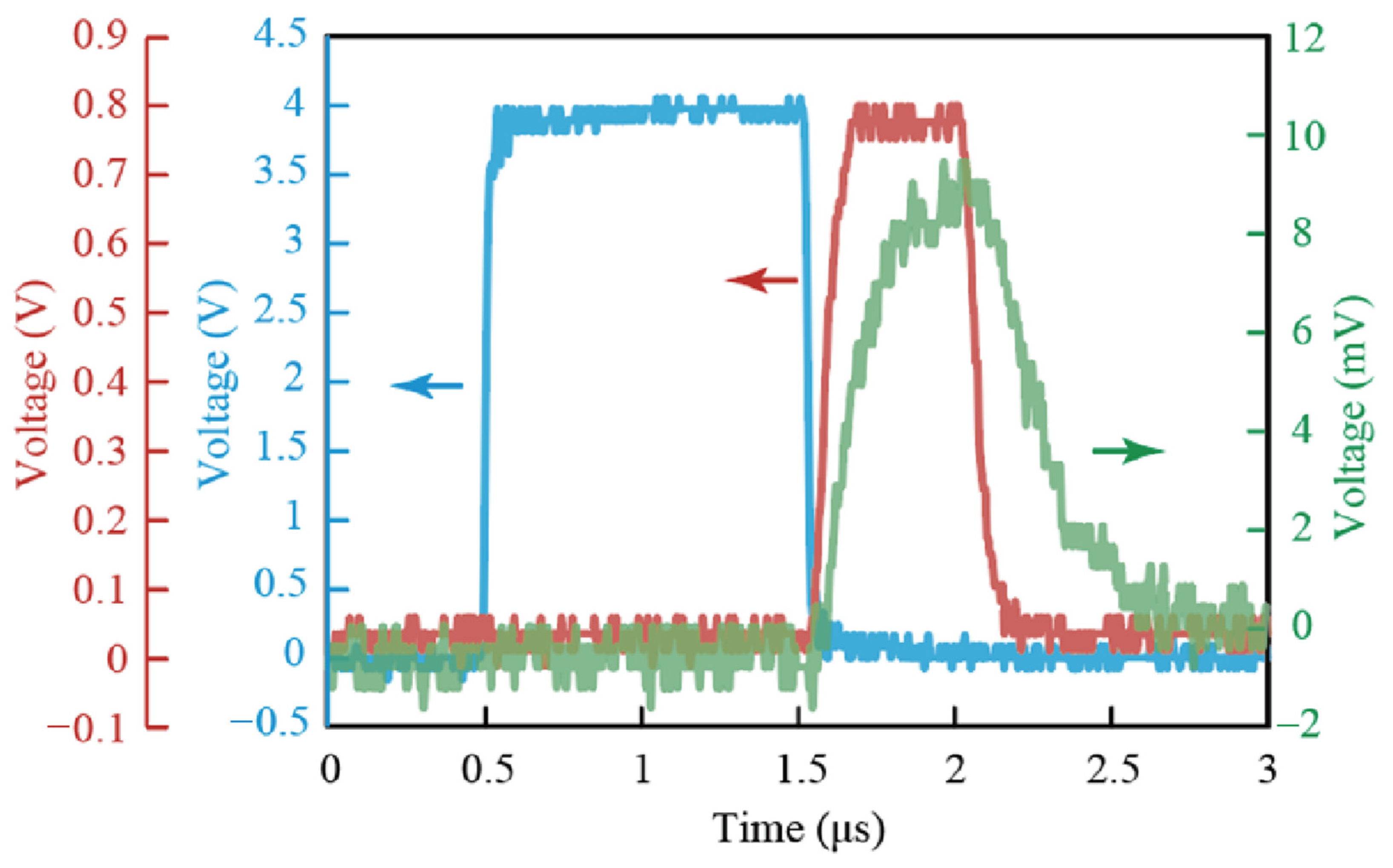
4.1. Evaluation of Time Delay of a Jitter in Circuit and Light Emission
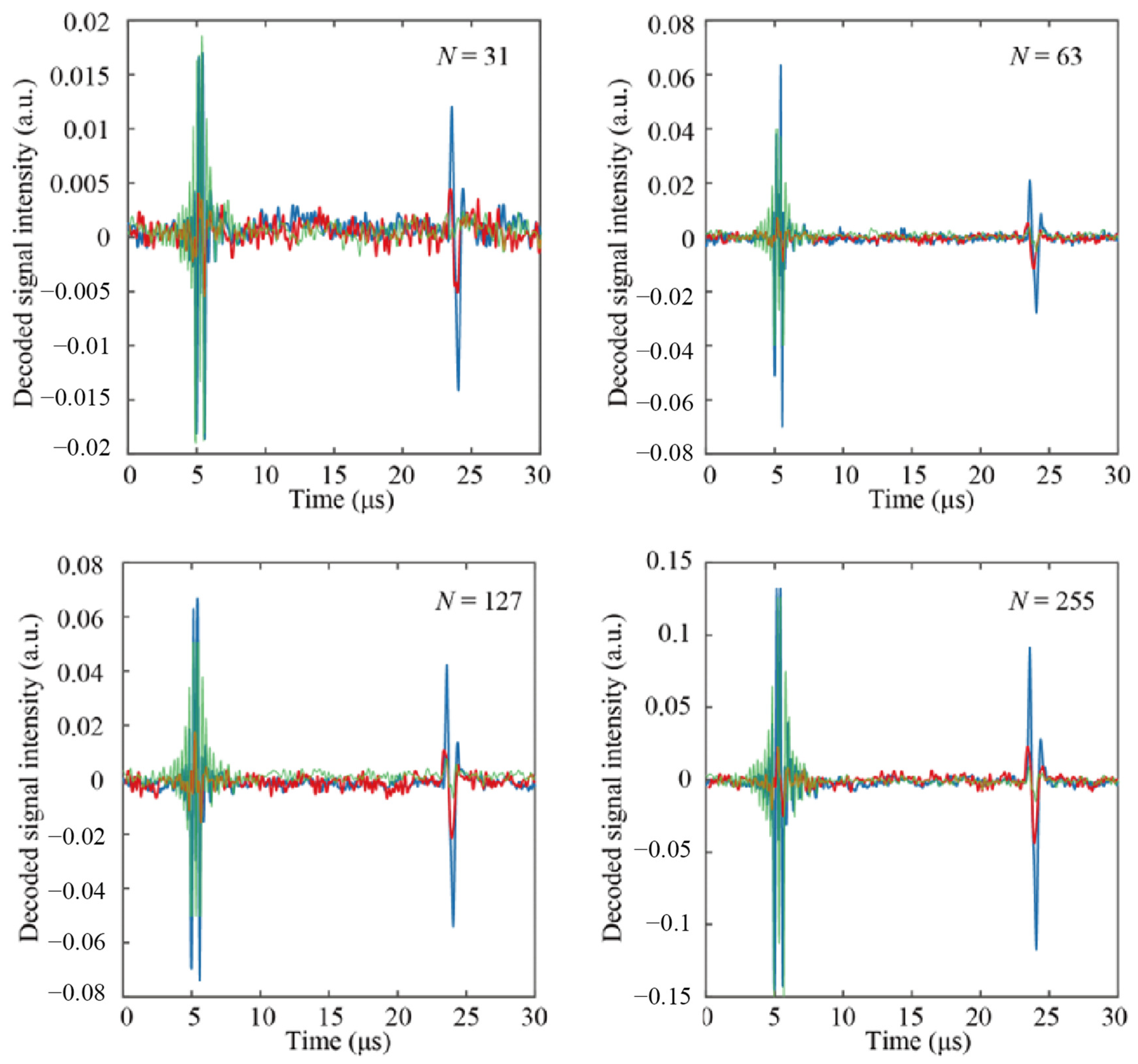
4.2. Evaluation of the Proposed Jitter-Compensated PUM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Oraevsky, A.A.; Savateeva, E.V.; Solomatin, S.V.; Karabutov, A.A.; Andreev, V.G.; Gatalica, Z.; Khamapirad, T.; Henrichs, P.M. Optoacoustic imaging of blood for visualization and diagnostics of breast cancer. Biomed. Optoacoust. III 2002, 4618, 81–94. [Google Scholar]
- Kim, C.; Song, K.H.; Gao, F.; Wang, L.V. Sentinel lymph nodes and lymphatic vessels: Noninvasive dual-modality in vivo mapping by using indocyanine green in rats—Volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology 2010, 255, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Oladipupo, S.; Hu, S.; Kovalski, J.; Yao, J.; Santeford, A.; Sohn, R.E.; Shohet, R.; Maslov, K.; Wang, L.V.; Arbeit, J.M. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc. Natl. Acad. Sci. USA 2011, 108, 13264–13269. [Google Scholar] [CrossRef]
- Oladipupo, S.S.; Hu, S.; Santeford, A.C.; Yao, J.; Kovalski, J.R.; Shohet, R.V.; Maslov, K.; Wang, L.V.; Arbeit, J.M. Conditional HIF-1 induction produces multistage neovascularization with stage-specific sensitivity to VEGFR inhibitors and myeloid cell independence. Blood J. Am. Soc. Hematol. 2011, 117, 4142–4153. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Yuan, J.; Jo, J.; Gandikota, G.; Demirci, H.; Agano, T.; Sato, N.; Shigeta, Y.; Wang, X. Light emitting diodes based photoacoustic imaging and potential clinical applications. Sci. Rep. 2018, 8, 9885. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lu, X.; Dong, X.; Yuan, J.; Fabiilli, M.L.; Wang, X. LED-based photoacoustic imaging for monitoring angiogenesis in fibrin scaffolds. Tissue Eng. Part C Methods 2019, 25, 523–531. [Google Scholar] [CrossRef]
- Zhang, E.Z.; Povazay, B.; Laufer, J.; Alex, A.; Hofer, B.; Pedley, B.; Glittenberg, C.; Treeby, B.; Cox, B.; Beard, P.; et al. Multimodal photoacoustic and optical coherence tomography scanner using an all optical detection scheme for 3D morphological skin imaging. Biomed. Opt. Express 2011, 2, 2202–2215. [Google Scholar] [CrossRef]
- Favazza, C.P.; Jassim, O.; Cornelius, L.A.; Wang, L.V. In vivo photoacoustic microscopy of human cutaneous microvasculature and a nevus. J. Biomed. Opt. 2011, 16, 016015. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.V. Neurovascular photoacoustic tomography. Front. Neuroenergetics 2010, 2, 1560. [Google Scholar] [CrossRef]
- Hu, S.; Yan, P.; Maslov, K.; Lee, J.M.; Wang, L.V. Intravital imaging of amyloid plaques in a transgenic mouse model using optical-resolution photoacoustic microscopy. Opt. Lett. 2009, 34, 3899–3901. [Google Scholar] [CrossRef]
- Feng, T.; Perosky, J.E.; Kozloff, K.M.; Xu, G.; Cheng, Q.; Du, S.; Yuan, J.; Deng, C.X.; Wang, X. Characterization of bone microstructure using photoacoustic spectrum analysis. Opt. Express 2015, 23, 25217–25224. [Google Scholar] [CrossRef]
- Kaiplavil, S.; Mandelis, A.; Wang, X.; Feng, T. Photothermal tomography for the functional and structural evaluation, and early mineral loss monitoring in bones. Biomed. Opt. Express 2014, 5, 2488–2502. [Google Scholar] [CrossRef]
- Feng, T.; Kozloff, K.M.; Tian, C.; Perosky, J.E.; Hsiao, Y.S.; Du, S.; Yuan, J.; Deng, C.X.; Wang, X. Bone assessment via thermal photo-acoustic measurements. Opt. Lett. 2015, 40, 1721–1724. [Google Scholar] [CrossRef]
- Wang, X.; Feng, T.; Cao, M.; Perosky, J.E.; Kozloff, K.; Cheng, Q.; Yuan, J. Photoacoustic measurement of bone health: A study for clinical feasibility. In Proceedings of the 2016 IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016. [Google Scholar]
- Garcia-Uribe, A.; Erpelding, T.N.; Krumholz, A.; Ke, H.; Maslov, K.; Appleton, C.; Margenthaler, J.A.; Wang, L.V. Dual-modality photoacoustic and ultrasound imaging system for noninvasive sentinel lymph node detection in patients with breast cancer. Sci. Rep. 2015, 5, 15748. [Google Scholar] [CrossRef]
- Song, K.H.; Wang, L.V. Deep reflection-mode photoacoustic imaging of biological tissue. J. Biomed. Opt. 2007, 12, 060503. [Google Scholar] [CrossRef]
- Hariri, A.; Lemaster, J.; Wang, J.; Jeevarathinam, A.S.; Chao, D.L.; Jokerst, J.V. The characterization of an economic and portable LED-based photoacoustic imaging system to facilitate molecular imaging. Photoacoustics 2018, 9, 10–20. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Dantuma, M.; Joseph, F.K.; Manohar, S.; Steenbergen, W. Breast imaging using an LED-based photoacoustic and ultrasound imaging system: A proof-of-concept study. In Photons Plus Ultrasound: Imaging Sensing 2021; SPIE: Bellingham, WA, USA, 2021; Volume 11642, pp. 32–38. [Google Scholar]
- Hariri, A.; Hosseinzadeh, M.; Noei, S.; Nasiriavanaki, M.R. Photoacoustic signal enhancement: Towards utilization of very low-cost laser diodes in photoacoustic imaging. In Photons Plus Ultrasound: Imaging and Sensing 2017; SPIE: Bellingham, WA, USA, 2017; Volume 10064, pp. 822–826. [Google Scholar]
- Hariri, A.; Fatima, A.; Mohammadian, N.; Mahmoodkalayeh, S.; Ansari, M.A.; Bely, N.; Avanaki, M.R. Development of low-cost photoacoustic imaging systems using very low-energy pulsed laser diodes. J. Biomed. Opt. 2017, 22, 075001. [Google Scholar] [CrossRef]
- Shi, M.; Vercauteren, T.; Xia, W. Spatiotemporal singular value decomposition for denoising in photoacoustic imaging with a low-energy excitation light source. Biomed. Opt. Express 2022, 13, 6416–6430. [Google Scholar] [CrossRef]
- Sun, M.; Feng, N.; Shen, Y.I.; Shen, X.; Li, J. Photoacoustic signals denoising based on empirical mode decomposition and energy-window method. Adv. Adapt. Data Anal. 2012, 4, 1250004. [Google Scholar] [CrossRef]
- Ermilov, S.A.; Khamapirad, T.; Conjusteau, A.; Leonard, M.H.; Lacewell, R.; Mehta, K.; Miller, T.; Oraevsky, A.A. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt. 2009, 14, 024007. [Google Scholar] [CrossRef]
- Zhang, C.; Maslov, K.; Yao, J.; Wang, L.V. In vivo photoacoustic microscopy with 7.6-µm axial resolution using a commercial 125-MHz ultrasonic transducer. J. Biomed. Opt. 2012, 17, 116016. [Google Scholar] [CrossRef]
- Mienkina, M.P.; Friedrich, C.S.; Gerhardt, N.C.; Beckmann, M.F.; Schiffner, M.F.; Hofmann, M.R.; Schmitz, G. Multispectral photoacoustic coded excitation imaging using unipolar orthogonal Golay codes. Opt. Express 2010, 18, 9076–9087. [Google Scholar] [CrossRef]
- Zhang, H.; Kondo, K.; Yamakawa, M.; Shiina, T. Simultaneous multispectral coded excitation using Gold codes for photoacoustic imaging. Jpn. J. Appl. Phys. 2012, 51, 07GF03. [Google Scholar] [CrossRef]
- Zhang, H.K.; Kondo, K.; Yamakawa, M.; Shiina, T. Coded excitation using periodic and unipolar M-sequences for photoacoustic imaging and flow measurement. Opt. Express 2016, 24, 17–29. [Google Scholar] [CrossRef]
- Paul, A.; Mallidi, S. U-Net enhanced real-time LED-based photoacoustic imaging. J. Biophotonics 2024, 17, e202300465. [Google Scholar] [CrossRef]
- Anas, E.M.A.; Zhang, H.K.; Kang, J.; Boctor, E. Enabling fast and high quality LED photoacoustic imaging: A recurrent neural networks based approach. Biomed. Opt. Express 2018, 9, 3852–3866. [Google Scholar] [CrossRef]
- Balestrieri, E.; Picariello, F.; Rapuano, S.; Tudosa, I. Review on jitter terminology and definitions. Measurement 2019, 145, 264–273. [Google Scholar] [CrossRef]
- Tripathi, J.N.; Sharma, V.K.; Shrimali, H. A review on power supply induced jitter. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 9, 511–524. [Google Scholar] [CrossRef]
- Kolkman, R.G.; Steenbergen, W.; Van Leeuwen, T.G. In vivo photoacoustic imaging of blood vessels with a pulsed laser diode. Lasers Med. Sci. 2006, 21, 134–139. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kono, T.; Hashimoto, K.; Fukuda, K.; Rajagopalan, U.M.; Nakamura, K.; Yamada, J. Noise Reduction in LED-Based Photoacoustic Imaging. Photonics 2025, 12, 398. https://doi.org/10.3390/photonics12040398
Kono T, Hashimoto K, Fukuda K, Rajagopalan UM, Nakamura K, Yamada J. Noise Reduction in LED-Based Photoacoustic Imaging. Photonics. 2025; 12(4):398. https://doi.org/10.3390/photonics12040398
Chicago/Turabian StyleKono, Takahiro, Kazuma Hashimoto, Keisuke Fukuda, Uma Maheswari Rajagopalan, Kae Nakamura, and Jun Yamada. 2025. "Noise Reduction in LED-Based Photoacoustic Imaging" Photonics 12, no. 4: 398. https://doi.org/10.3390/photonics12040398
APA StyleKono, T., Hashimoto, K., Fukuda, K., Rajagopalan, U. M., Nakamura, K., & Yamada, J. (2025). Noise Reduction in LED-Based Photoacoustic Imaging. Photonics, 12(4), 398. https://doi.org/10.3390/photonics12040398




