Microstructured Waveguide Sensors for Point-of-Care Health Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Samples
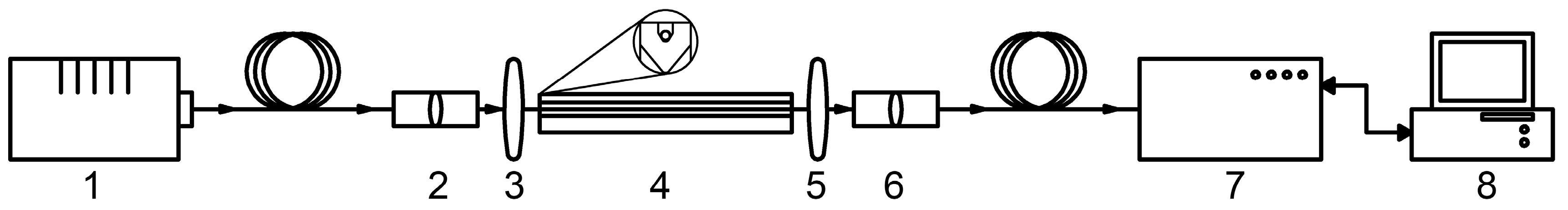
2.2. Optical Instrumentation
2.3. Data Processing
3. Results
3.1. Study of the Properties of the HC-MOW
3.2. Biochemical Analysis of Blood Serum
3.3. Spectral Analysis of Human Serum
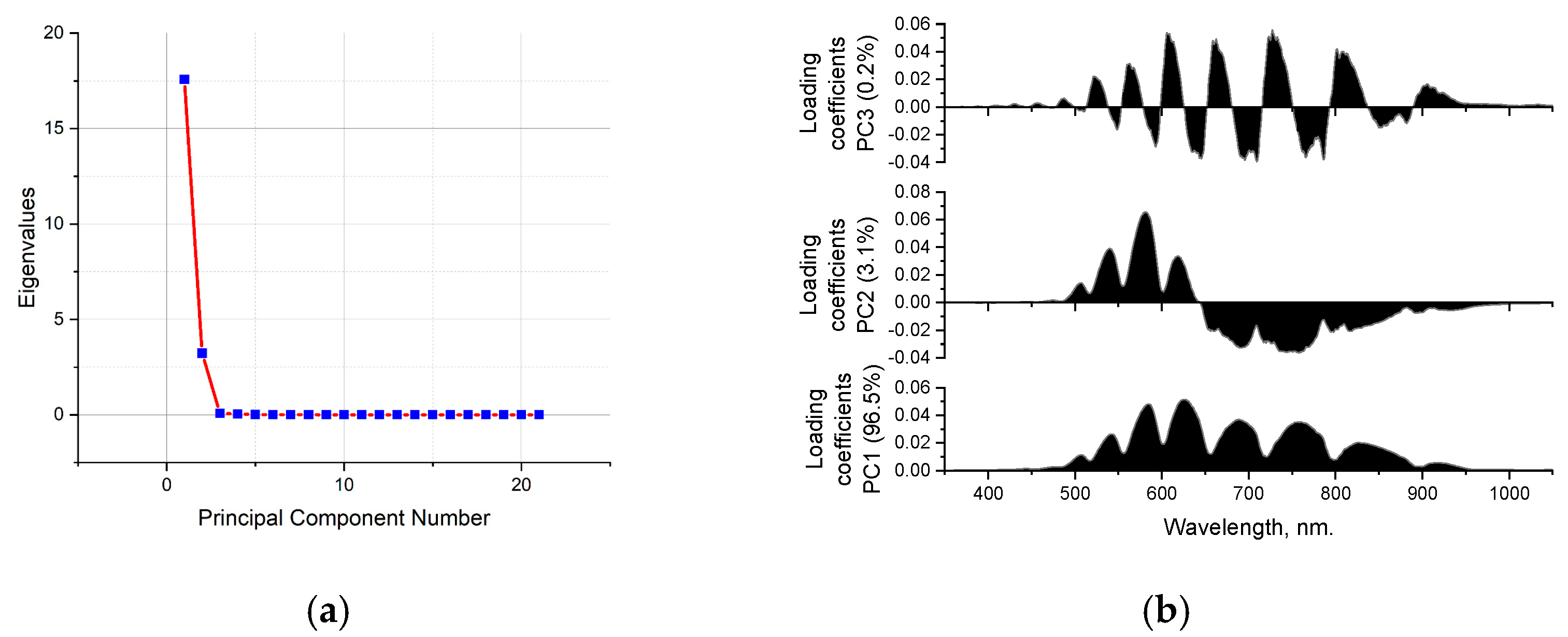
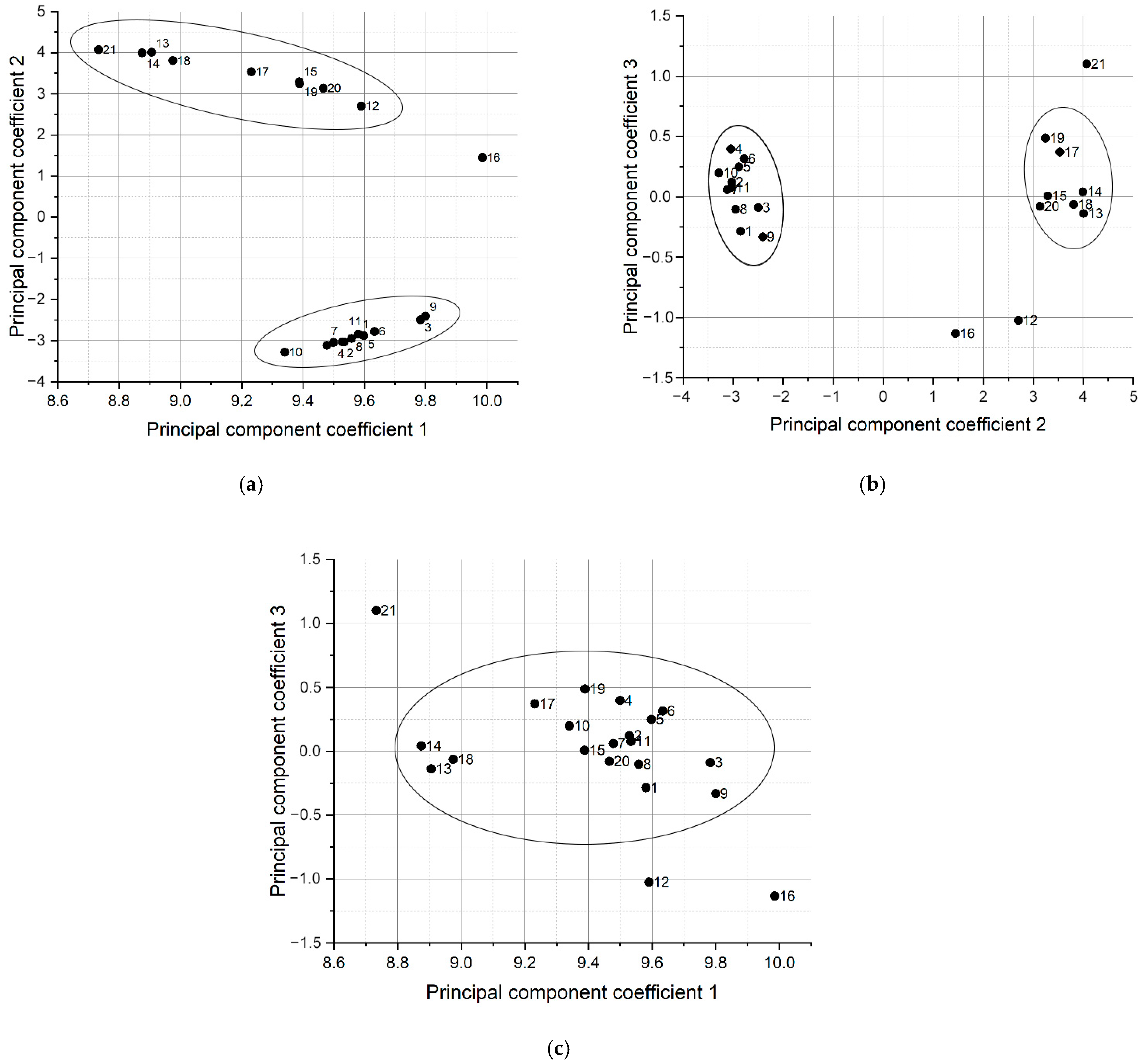
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaminsky, L.A.; German, C.; Imboden, M.; Ozemek, C.; Peterman, J.E.; Brubaker, P.H. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog. Cardiovasc. Dis. 2022, 70, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.F.; de Matos Soeiro, A.; Fernandes, J.L.; Pesaro, A.E.; Serrano, C.V., Jr. Coronary artery disease in women: A review on prevention, pathophysiology, diagnosis, and treatment. Vasc. Health Risk Manag. 2006, 2, 465–475. [Google Scholar] [CrossRef]
- Crandall, J.P.; Knowler, W.C.; Kahn, S.E.; Marrero, D.; Florez, J.C.; Bray, G.A.; Haffner, S.M.; Hoskin, M.; Nathan, D.M.; Diabetes Prevention Program Research Group. The prevention of type 2 diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 382–393. [Google Scholar] [CrossRef]
- Kramer, H. Diet and Chronic Kidney Disease. Adv. Nutr. 2019, 10, S367–S379. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/ru (accessed on 20 February 2025).
- Aggarwal, R.; Ranganathan, P.; Pramesh, C.S. Research studies on screening tests. Perspect. Clin. Res. 2022, 13, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Suaganya, T.; Robinson, S. Design and Analysis of 2D Photonic crystal based biosensor to detect different blood components. Photonic Sens. 2019, 9, 69–77. [Google Scholar] [CrossRef]
- Enejder, A.M.K.; Swartling, J.; Aruna, P.; Engels, S.A. Influence of cell shape and aggregate formation on the optical properties of flowing whole blood. Appl. Opt. 2003, 42, 1384–1394. [Google Scholar] [CrossRef]
- Liu, S.; Gao, W.; Li, H.; Dong, Y.; Zhang, H. Liquid-filled simplified hollow-core photonic crystal fiber. Opt. Laser Technol. 2014, 64, 140–144. [Google Scholar] [CrossRef]
- Hoffmann, J.J.M.L.; Urrechaga, E. Recent advances in laboratory hematology reflected by a decade of CCLM publications. Clin. Chem. Lab. Med. 2022, 61, 829–840. [Google Scholar] [CrossRef]
- Skibina, Y.S.; Tuchin, V.V.; Beloglazov, V.I.; Shteinmaeer, G.; Betge, I.L.; Wedell, R.; Langhoff, N. Photonic crystal fibres in biomedical investigations. Quantum Electron. 2011, 41, 284. [Google Scholar] [CrossRef]
- Sinibaldi, A.; Occhicone, A.; Munzert, P.; Danz, N.; Sonntag, F.; Michelotti, F. Label-Free monitoring of human IgG/Anti-IgG recognition using Bloch surface waves on 1D photonic crystals. Biosensors 2018, 8, 71. [Google Scholar] [CrossRef]
- Toma, A.D.; Brunetti, G.; Colapietro, P.; Ciminelli, C. High-resolved near-field sensing by means of dielectric grating with a box-bike resonance shape. IEEE Sens. J. 2024, 24, 6045–6053. [Google Scholar] [CrossRef]
- Lotfiani, A.; Jahromi, H.D.; Hamedi, S. Monolithic Silicon-Based Photovoltaic-Nanoplasmonic Biosensor with Enhanced Limit of Detection and Minimum Detectable Power. Light. Technol. 2022, 40, 1231–1237. [Google Scholar] [CrossRef]
- Khan, M.R.H.; Chowdhury, A.A.; Islam, M.R.; Hosen, M.S.; Mim, M.H.; Nishat, M.M. Wave-Shaped Microstructure Cancer Detection Sensor in Terahertz Band: Design and Analysis. Appl. Sci. 2023, 13, 5784. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, B.K.; Singh, R.; Chen, N.K.; Yang, Q.S.; Zhang, X.; Wang, W.; Zhang, B. LSPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed. Opt. Express 2019, 10, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Zanishevskaya, A.A.; Malinin, A.V.; Skibina, Y.S.; Tuchin, V.V.; Chainikov, M.V.; Beloglazov, V.I.; Silokhin, I.Y.; Ermakova, A.M. Determination of glucose concentration in biological liquids using photonic crystal waveguides. Opt. Spectrosc. 2013, 115, 228–232. [Google Scholar] [CrossRef]
- Ermatov, T.; Noskov, R.E.; Machnev, A.A.; Gnusov, I.; Atkin, V.; Lazareva, E.N.; German, S.V.; Kosolobov, S.S.; Zatsepin, T.S.; Sergeeva, O.V.; et al. Multispectral sensing of biological liquids with hollow-core microstructured optical fibres. Light Sci. Appl. 2020, 9, 173. [Google Scholar] [CrossRef]
- Haes, A.J.; Chang, L.; Klein, W.L.; Van Duyne, R.P. Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J. Am. Chem. Soc. 2005, 127, 2264–2271. [Google Scholar] [CrossRef]
- Kudryavtseva, O.M.; Skibina, Y.S.; Kozhevnikov, V.A.; Bugorkova, S.A.; Chainikov, M.V.; Gryaznov, A.Y.; Glukhov, D.E.; Konnova, S.S.; Shuvalov, A.A.; Shchukovskaya, T.N. Direct detection of antibodies to Yersinia pestis using glass microstructural waveguides as an express method for assessing seroconversion in individuals vaccinated against plague. Epidemiol. Vaccinal Prev. 2022, 21, 89–96. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Gharib, G.; Bütün, İ.; Muganlı, Z.; Kozalak, G.; Namlı, İ.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Bzheumikhov, K.A.; Gurtueva, I.A.; Margushev, Z.C.; Savoysky, Y.V. Features of the spectral properties of photonic crystal fibers with a hollow core filled with liquid. News Kabard.-Balkar. Sci. Cent. Russ. Acad. Sci. 2012, 3, 26–29. [Google Scholar]
- Pissadakis, S. Lab-in-a-fiber sensors: A review. Microelectronics 2019, 217, 111105. [Google Scholar] [CrossRef]
- Cheng, T.; Sakai, Y.; Suzuki, T.; Ohishi, Y. Fabrication and characterization of an all-solid tellurite–phosphate photonic bandgap fiber. Opt. Lett. 2015, 40, 2088–2090. [Google Scholar] [CrossRef] [PubMed]
- Chillcce, E.F.; Cordeiro, C.M.D.B.; Barbosa, L.C.; Cruz, C.B. Tellurite photonic crystal fiber made by a stack-and-draw technique. J. Non-Cryst. Solids 2006, 352, 3423–3428. [Google Scholar] [CrossRef]
- Ravi Kanth Kumar, V.V.; George, A.K.; Reeves, W.H.; Knight, J.C.; Russell, P.S.J.; Omenetto, F.G.; Taylor, A.J. Extruded soft glass photonic crystal fiber for ultrabroad supercontinuum generation. Opt. Express 2002, 10, 1520–1525. [Google Scholar] [CrossRef]
- Yajima, T.; Yamamoto, J.; Ishii, F.; Hirooka, T.; Yoshida, M.; Nakazawa, M. Low-loss photonic crystal fiber fabricated by a slurry casting method. Opt. Express 2013, 21, 30500–30506. [Google Scholar] [CrossRef]
- El Hamzaoui, H.; Bigot, L.; Bouwmans, G.; Razdobreev, I.; Bouazaoui, M.; Capoen, B. From molecular precursors in solution to microstructured optical fiber: A Sol-gel polymeric route. Opt. Mater. Express 2011, 1, 234–242. [Google Scholar] [CrossRef]
- Schuster, K.; Unger, S.; Aichele, C.; Lindner, F.; Grimm, S.; Litzkendorf, D.; Kobelke, J.; Bierlich, J.; Wondraczek, K.; Bartelt, H. Material and technology trends in fiber optics. Adv. Opt. Technol. 2014, 3, 447–468. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yang, Z.; Wilkinson, J.S.; Zhou, X. Optical biosensors based on refractometric sensing schemes: A review. Biosens. Bioelectron. 2019, 144, 111693. [Google Scholar] [CrossRef]
- Alam, M.K.; Vadivel, K.; Natesan, A.; Sghaireen, M.G.; Ganji, K.K.; Srivastava, K.C.; Nashwan, S.; Khader, Y. Design of highly sensitive biosensors using hollow-core microstructured fibers for plasma sensing in aids with human metabolism. Opt. Quantum Electron. 2023, 55, 188. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.B. Issues and recommendations for exploratory factor analysis and principal component analysis. Res. Soc. Adm. Pharm. 2021, 17, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Ritov, Y.; Gilula, Z. Analysis of contingency tables by correspondence models subject to order constraints. Am. Stat. Assoc. 1993, 88, 1380–1387. [Google Scholar] [CrossRef]
- Du, L.; Liu, K.; Yao, X.; Risacher, S.L.; Han, J.; Saykin, A.J.; Guo, L.; Shen, L. Multi—Task sparse canonical correlation analysis with application to multi-modal brain imaging genetics. Trans. Comput. Biol. Bioinform. 2021, 18, 227–239. [Google Scholar] [CrossRef]
- Leistritz, L.; Weiss, T.; Bär, K.J.; De Vico Fallani, F.; Babiloni, F.; Witte, H.; Lehmann, T. Network redundancy analysis of effective brain networks: A comparison of healthy controls and patients with major depression. PLoS ONE 2013, 8, e60956. [Google Scholar] [CrossRef]
- Ashenova, A.; Daniyarov, A.; Molkenov, A.; Sharip, A.; Zinovyev, A.; Kairov, U. Meta-analysis of esophageal cancer transcriptomes using independent component analysis. Front. Genet. 2021, 12, 683632. [Google Scholar] [CrossRef] [PubMed]
- David, C.C.; Jacobs, D.J. Principal component analysis: A method for determining the essential dynamics of proteins. Methods Mol. Biol. 2014, 1084, 193–226. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Q.; Hussain, M.; Wu, S.; Chen, J.; Tang, Z. Application of principal component analysis to classify textile fibers based on UV-vis diffuse reflectance spectroscopy. Zhurnal Prikl. Spektrosk. 2017, 84, 368–372. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Zhang, G.; Fan, S.; Ge, W.; Hu, W.; Huang, P.; Hou, D.; Zheng, S. Characterization and discrimination of human colorectal cancer cells using terahertz spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 256, 119713. [Google Scholar] [CrossRef]
- Basova, E.M.; Litvinenko, Y.N.; Polotnyanko, N.A. Identification of drug manufacturers using IR spectroscopy and the principal component method. J. Bull. Int. Univ. Nat. 2021, 2, 7–15. [Google Scholar]
- MU4.2.2039-05 “Technique of Collecting and Transporting Biomaterials Tomicrobiological Laboratories”. Available online: https://www.rospotrebnadzor.ru/bitrix/redirect.php?event1=file&event2=download&event3=mu-4.2.2039_05.doc&goto=/upload/iblock/df9/mu-4.2.2039_05.doc (accessed on 20 February 2025).
- State System of Sanitary-Epidemiological Regulation of the Russian Federation. Federal Sanitary Rules, Norms and Hygienic Standards, SanPiN 2.1.7.2790-10. Sanitary and Epide-Miological Requirements for the Management of Medical Waste. Available online: https://docs.cntd.ru/document/902251609 (accessed on 20 February 2025).
- Zheltikov, A.M. Colors of thin films, antiresonant phenomena in optical systems, and the limiting loss of modes in hollow optical waveguides. Phys.-Uspekhi 2008, 51, 591–600. [Google Scholar] [CrossRef]
- Dhanabalan, S.S.; Sriram, S.; Walia, S.; Raja Avaninathan, S.; Flores Carrasco, M.; Bhaskaran, M. Wearable Label–Free Optical Biodetectors: Progress and Perspectives. Adv. Photonics Res. 2020, 2, 2000076. [Google Scholar] [CrossRef]
- Esbensen, K. Analysis of Multidimensional Data; Selected Chapters; Publishing House IPCP RAS: Chernogolovka, Russia, 2005; pp. 160–170. [Google Scholar]
- Alekseev, S.G.; Ivanov, A.V.; Sviridov, S.V.; Petrova, G.P.; Petrusevic, M.; Boiko, A.V.; Ten, D.I. Multiparametrical Testing of Blood Proteins Solutions with Diagnostic Purpose. Proc. SPIE 2005, 5973, 597301. [Google Scholar]
- Chaikov, L.L. Dynamics of statistically confident particle sizes and concentrations in blood plasma obtained by the dynamic light scattering method. J. Biomed. Opt. 2015, 20, 57003. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, M.N.; Chaikov, L.L.; Kazaryan, M.A.; Bulychev, N.A. Application of light scattering methods in biomedicine and ecology. Int. Sci. J. Altern. Energy Ecol. 2019, 80–103. [Google Scholar] [CrossRef]
- Márquez-Islas, R.; Pérez-Pacheco, A.; Quispe-Siccha, R.; Salazar-Nieva, L.B.; García-Valenzuela, A. Visual assessment of blood plasma versus optical transmittance and refractive index measurements for quantifying lipemia. Diagnostics 2022, 12, 510. [Google Scholar] [CrossRef]
- Khozeymeh, F.; Melli, F.; Capodaglio, S.; Corradini, R.; Benabid, F.; Vincetti, L.; Cucinotta, A. Hollow-core fiber-based biosensor: A platform for lab-in-fiber optical biosensors for DNA detection. Sensors 2022, 22, 5144. [Google Scholar] [CrossRef]
- Zanishevskaya, A.A.; Malinin, A.V.; Skibina, Y.S.; Tuchin, V.V.; Chaynikov, M.V.; Beloglazov, V.I.; Silokhin, I.Y.; Ermakova, A.M. Determination of glucose concentration in biological fluids using photonic crystal waveguides. Opt. Spectrosc. 2013, 115, 266–271. [Google Scholar] [CrossRef]
- Skibina, J.S.; Malinin, A.V.; Zanishevskaya, A.A.; Tuchin, V.V. Photonic Crystal Waveguide Sensing. In Portable Biosensing of Food Toxicants and Environmental Pollutants, 1st ed.; Nikolelis, D.P., Varzakas, T., Erdem, A., Nikoleli, G.-P., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–32. [Google Scholar]
- Nikolac, N. Lipemia: Causes, Interference Mechanisms, Detection and Management. Biochem. Medica 2014, 24, 57–67. [Google Scholar] [CrossRef]
- Febryani, N.; Amalia, I.N.; Anggraeni, I.D.; Nugraha, G. Study of hemoglobin levels on hemolysis sample. Indones. J. Med. Lab. Sci. Technol. 2019, 1, 74–79. [Google Scholar] [CrossRef]
- Huynh, T.; Lai, M.J.; Liu, Y.L. Spectral Analysis Methods Based on Background Subtraction and Curvature calculation used in the detection or quantification of hemolysis and icterus in Blood-derived clinical samples. Cureus 2017, 9, 1965. [Google Scholar] [CrossRef] [PubMed]
- Ayyanar, N.; Zanishevskaya, A.A.; Lepilin, P.A.; Shuvalov, A.A.; Skibina, J.S. Protein Detection Using Hollow Core Microstructured Optical Fiber. IEEE Sens. J. 2024, 20, 32172–32178. [Google Scholar] [CrossRef]
- Hamedi, S.; Jahromi, H.D.; Lotfiani, A. Artificial intelligence-aided nanoplasmonic iosensor modeling. Eng. Appl. Artif. Intell. 2023, 118, 105646. [Google Scholar] [CrossRef]
- Hamedi, S.; Jahromi, H.D. Performance analysis of all-optical logical gate using artificial neural network. Expert Syst. Appl. 2021, 178, 115029. [Google Scholar] [CrossRef]
- Jahromi, H.D.; Hamedi, S. Artificial intelligence approach for calculating electronic and optical properties of nanocomposites. Mater. Res. Bull. 2021, 141, 111371. [Google Scholar] [CrossRef]





| λmax, nm Calculated | λmin, nm Calculated | λmax, nm Experimental | λmin, nm Experimental | SD λmax | SD λmin |
|---|---|---|---|---|---|
| 865 | 837 | 861 | 834 | 2.08 | 1.53 |
| 811 | 786 | 809 | 784 | 1.04 | 1.4 |
| 763 | 741 | 762 | 739 | 0.76 | 1.22 |
| 721 | 701 | 721 | 699 | 1.73 | 1.1 |
| 683 | 665 | 683 | 665 | 0.75 | 1.15 |
| 648 | 633 | 648 | 631 | 0.64 | 1.13 |
| 618 | 603 | 618 | 601 | 0.6 | 1.08 |
| 589 | 576 | 590 | 575 | 1.26 | 0.7 |
| 564 | 552 | 564 | 552 | 1.0 | 0.75 |
| 540 | 529 | 541 | 531 | 0.5 | 1.15 |
| Serum No. | High-Density Lipoproteins, mmol/L | Triglycerides, mmol/L | Albumin, g/L | Magnesium, mmol/L | Iron, µmol/L |
|---|---|---|---|---|---|
| 1 | 1.01 ± 0.09 | 1.20 ± 0.07 | 44.01 ± 0.84 | 0.8 ± 0.04 | 20.9 ± 1.15 |
| 2 | 1.35 ± 0.1 | 0.96 ± 0.03 | 49.22 ± 0.76 | 0.79 ± 0.03 | 16.1 ± 1.01 |
| 3 | 1.78 ± 0.12 | 1.93 ± 0.08 | 43.36 ± 0.89 | 0.79 ± 0.05 | 15.2 ± 0.9 |
| 4 | 1.54 ± 0.08 | 1.32 ± 0.04 | 42.02 ± 0.9 | 0.79 ± 0.05 | 15.8 ± 1.19 |
| 5 | 2.03 ± 0.07 | 2.01 ± 0.1 | 51.30 ± 0.63 | 0.77 ± 0.03 | 17.4 ± 1.16 |
| 6 | 1.85 ± 0.03 | 1.37 ± 0.02 | 41.26 ± 0.59 | 0.79 ± 0.04 | 19.1 ± 1.50 |
| 7 | 2.05 ± 0.09 | 1.52 ± 0.1 | 53.03 ± 0.69 | 0.77 ± 0.02 | 14.7 ± 1.02 |
| 8 | 1.12 ± 0.10 | 1.43 ± 0.07 | 41.02 ± 0.74 | 0.78 ± 0.01 | 15.2 ± 1.30 |
| 9 | 2.07 ± 0.08 | 0.96 ± 0.03 | 50.56 ± 0.89 | 0.81 ± 0.06 | 15.6 ± 0.56 |
| 10 | 1.07 ± 0.04 | 1.15 ± 0.04 | 46.23 ± 0.6 | 0.78 ± 0.02 | 20.7 ± 0.63 |
| 11 | 1.59 ± 0.05 | 1.54 ± 0.05 | 45.36 ± 0.55 | 0.79 ± 0.03 | 18.6 ± 0.89 |
| 12 | 1.11 ± 0.03 | 1.98 ± 0.03 | 43.95 ± 0.45 | 0.90 ± 0.03 | 8.91 ± 1.16 |
| 13 | 2.03 ± 0.12 | 0.71 ± 0.01 | 42.96 ± 0.98 | 0.84 ± 0.06 | 10.1 ± 1.03 |
| 14 | 1.28 ± 0.09 | 1.23 ± 0.02 | 40.46 ± 0.66 | 0.86 ± 0.08 | 11.7 ± 0.64 |
| 15 | 1.09 ± 0.02 | 2.17 ± 0.09 | 40.71 ± 0.076 | 1.00 ± 0.02 | 13.6 ± 0.75 |
| 16 | 1.18 ± 0.03 | 1.73 ± 0.03 | 42.69 ± 0.58 | 0.83 ± 0.09 | 5.74 ± 0.56 |
| 17 | 1.12 ± 0.02 | 0.64 ± 0.04 | 50.36 ± 0.35 | 0.98 ± 0.03 | 5.71 ± 0.43 |
| 18 | 1.52 ± 0.05 | 1.27 ± 0.02 | 48.21 ± 0.45 | 0.84 ± 0.04 | 15.21 ± 0.95 |
| 19 | 1.53 ± 0.02 | 0.71 ± 0.03 | 46.62 ± 0.63 | 0.85 ± 0.03 | 17.19 ± 1.23 |
| 20 | 2.01 ± 0.15 | 0.58 ± 0.05 | 42.43 ± 0.79 | 0.84 ± 0.02 | 12.21 ± 1.34 |
| 21 | 1.11 ± 0.03 | 0.99 ± 0.04 | 46.35 ± 0.58 | 0.83 ± 0.01 | 17.22 ± 1.57 |
| Norm values | 0.9–2.10 | 1–2.3 | 32–46 | 0.66–1.07 | 9.0–30.4 |
| Serum No. | Glucose mmol/L | Cholesterol mmol/L | Low-Density Lipoproteins mmol/L | Creatinine µmol/L | Alaninetransferase units/L | Aspartate Transferase units/L | Creatine Kinase units/L |
|---|---|---|---|---|---|---|---|
| 1 | 2.81 ± 0.19 | 4.74 ± 0.18 | 0.91 ± 0.09 | 57.30 ± 0.96 | 18.02 ± 0.73 | 20.31 ± 1.24 | 33.36 ± 1.98 |
| 2 | 3.63 ± 0.20 | 5.00 ± 0.20 | 1.37 ± 0.12 | 68.20 ± 0.88 | 10.31 ± 0.56 | 12.54 ± 0.91 | 53.72 ± 2.35 |
| 3 | 2.82 ± 0.17 | 4.16 ± 0.15 | 1.92 ± 0.13 | 112.32 ± 1.23 | 35.26 ± 1.21 | 30.43 ± 1.25 | 63.39 ± 2.14 |
| 4 | 3.43 ± 0.25 | 5.88 ± 0.19 | 1.78 ± 0.18 | 78.82 ± 0.92 | 32.49 ± 1.46 | 31.54 ± 2.13 | 67.52 ± 3.25 |
| 5 | 3.62 ± 0.18 | 4.57 ± 0.17 | 2.79 ± 0.21 | 89.02 ± 0.12 | 29.13 ± 1.25 | 25.82 ± 2.26 | 106.36 ± 3.71 |
| 6 | 3.16 ± 0.17 | 5.63 ± 0.16 | 2.63 ± 0.23 | 95.52 ± 0.45 | 15.42 ± 1.49 | 17.29 ± 1.12 | 152.24 ± 2.23 |
| 7 | 2.94 ± 0.19 | 4.94 ± 0.15 | 2.01 ± 0.19 | 117.43 ± 1.26 | 23.28 ± 1.73 | 23.53 ± 1.93 | 37.21 ± 0.24 |
| 8 | 2.99 ± 0.21 | 4.71 ± 0.18 | 1.99 ± 0.20 | 47.57 ± 1.58 | 37.32 ± 4.79 | 39.62 ± 1.52 | 63.82 ± 1.24 |
| 9 | 3.23 ± 0.22 | 4.82 ± 0.19 | 1.34 ± 0.12 | 55.73 ± 1.52 | 23.41 ± 1.22 | 21.74 ± 1.41 | 76.28 ± 1.29 |
| 10 | 2.61 ± 0.24 | 5.58 ± 0.20 | 1.78 ± 0.16 | 115.02 ± 2.31 | 39.62 ± 1.58 | 35.28 ± 1.23 | 54.34 ± 1.42 |
| 11 | 2.82 ± 0.15 | 4.14 ± 0.16 | 3.25 ± 0.18 | 44.89 ± 1.25 | 30.28 ± 1.48 | 32.17 ± 1.52 | 78.15 ± 2.29 |
| 12 | 10.91 ± 0.43 | 4.12 ± 0.15 | 2.84 ± 0.16 | 122.2 ± 1.21 | 18.53 ± 1.34 | 18.38 ± 1.13 | 100.43 ± 2.41 |
| 13 | 4.93 ± 0.32 | 4.00 ± 0.34 | 1.56 ± 0.15 | 76.72 ± 0.88 | 46.15 ± 2.46 | 51.29 ± 2.41 | 95.34 ± 1.69 |
| 14 | 3.75 ± 0.19 | 4.78 ± 0.28 | 2.59 ± 0.17 | 112.13 ± 0.96 | 23.43 ± 1.48 | 35.45 ± 2.19 | 84.51 ± 1.98 |
| 15 | 6.83 ± 0.21 | 4.61 ± 0.25 | 2.86 ± 0.18 | 94.92 ± 0.87 | 39.93 ± 1.26 | 36.97 ± 1.49 | 300.01 ± 2.75 |
| 16 | 12.51 ± 0.35 | 3.82 ± 0.23 | 1.80 ± 0.9 | 142.21 ± 0.65 | 18.74 ± 1.42 | 15.68 ± 0.81 | 125.23 ± 2.15 |
| 17 | 5.53 ± 0.19 | 5.65 ± 0.23 | 3.45 ± 0.23 | 90.24 ± 1.56 | 19.19 ± 1.52 | 20.62 ± 0.96 | 77.26 ± 1.72 |
| 18 | 6.42 ± 0.23 | 6.04 ± 0.31 | 3.97 ± 0.25 | 113.41 ± 2.31 | 45.26 ± 2.46 | 75.35 ± 2.65 | 254.19 ± 2.47 |
| 19 | 4.55 ± 0.16 | 5.53 ± 0.22 | 3.65 ± 0.31 | 86.32 ± 1.13 | 17.52 ± 1.34 | 11.92 ± 0.49 | 89.45 ± 2.56 |
| 20 | 5.57 ± 0.24 | 4.94 ± 0.28 | 2.68 ± 0.32 | 97.49 ± 1.54 | 21.39 ± 1.57 | 16.42 ± 0.95 | 136.26 ± 3.12 |
| 21 | 5.82 ± 0.32 | 5.99 ± 0.24 | 3.94 ± 0.36 | 112.25 ± 2.21 | 20.19 ± 1.96 | 23.35 ± 2.15 | 160.17 ± 2.49 |
| Norm values | 3.9–6.1 | 3.3–5.0 | <3.5 | 44–124 | 5–40 | 5–40 | 26–174 |
| Cluster # | Sample # |
|---|---|
| 1 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 |
| 2 | 13, 14, 15, 17, 18, 19, 20 |
| Dropped samples | 12, 16, 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konnova, S.S.; Lepilin, P.A.; Zanishevskaya, A.A.; Gryaznov, A.Y.; Kosheleva, N.A.; Ilinskaya, V.P.; Skibina, J.S.; Tuchin, V.V. Microstructured Waveguide Sensors for Point-of-Care Health Screening. Photonics 2025, 12, 399. https://doi.org/10.3390/photonics12040399
Konnova SS, Lepilin PA, Zanishevskaya AA, Gryaznov AY, Kosheleva NA, Ilinskaya VP, Skibina JS, Tuchin VV. Microstructured Waveguide Sensors for Point-of-Care Health Screening. Photonics. 2025; 12(4):399. https://doi.org/10.3390/photonics12040399
Chicago/Turabian StyleKonnova, Svetlana S., Pavel A. Lepilin, Anastasia A. Zanishevskaya, Alexey Y. Gryaznov, Natalia A. Kosheleva, Victoria P. Ilinskaya, Julia S. Skibina, and Valery V. Tuchin. 2025. "Microstructured Waveguide Sensors for Point-of-Care Health Screening" Photonics 12, no. 4: 399. https://doi.org/10.3390/photonics12040399
APA StyleKonnova, S. S., Lepilin, P. A., Zanishevskaya, A. A., Gryaznov, A. Y., Kosheleva, N. A., Ilinskaya, V. P., Skibina, J. S., & Tuchin, V. V. (2025). Microstructured Waveguide Sensors for Point-of-Care Health Screening. Photonics, 12(4), 399. https://doi.org/10.3390/photonics12040399







