Multimodal Imaging in Stem Cell Therapy for Retinal Disease
Abstract
1. Introduction
2. Imaging Techniques for Retinal Stem Cell Therapy
2.1. Preclinical Imaging Techniques
2.1.1. Cell Labeling
2.1.2. Optical Imaging
2.1.3. Magnetic Resonance Imaging (MRI)
2.1.4. Emerging Imaging Techniques with Potential for Retinal Stem Cell Therapy
2.2. Clinical Imaging Techniques
2.2.1. Optical Coherence Tomography (OCT)
2.2.2. Other Related Techniques
3. Multimodal Imaging
4. Challenges and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| RP | Retinitis pigmentosa |
| STGD1 | Stargardt macular degeneration |
| ESCs | Embryonic stem cells |
| iPSCs | Induced pluripotent stem cells |
| MSC | Mesenchymal stem cell |
| OCT | Optical coherence tomography |
| ICG | Indocyanine green |
| CFSE | 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester |
| DiI-AcLDL | 1,1′-dilinoleyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate-linked acetylated low-density lipoprotein |
| FI | Fluorescence imaging |
| FDA | Food and Drug Administration |
| NIR | Near-infrared |
| PAI | Photoacoustic imaging |
| GFP | Green fluorescent protein |
| FP | Fluorescent protein |
| EPC | Endothelial progenitor cell |
| hESC | Human embryonic stem cell |
| NP | Nanoparticle |
| MRI | Magnetic resonance imaging |
| CT | Computed tomography |
| GNP | Gold nanoparticle |
| SLO | Scanning laser ophthalmoscopy |
| FAOSLO | Fluorescence adaptive optics scanning laser ophthalmoscopy |
| fcSLO | Fluorescence confocal scanning laser ophthalmoscopy |
| PAM | Photoacoustic microscopy |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| SD-OCT | Spectral-domain OCT |
| SS-OCT | Swept-source OCT |
| PAI | Photoacoustic imaging |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| FP | Fundus photography |
| FAF | Fundus autofluorescence |
| ICGA | Indocyanine green angiography |
| FA | Fluorescein angiography |
| RPE | Retinal pigment epithelium |
| PPC | Photoreceptor precursor cell |
| FM | Fluorescence microscopy |
| CGNP | Chain-like GNP |
| GNC | Chain-like gold nanoparticle cluster |
| VEP | Visual evoked potential |
| ERG | Electroretinogram |
| D-FFOCT | Dynamic full-field optical coherence tomography |
References
- Voisin, A.; Pénaguin, A.; Gaillard, A.; Leveziel, N. Stem cell therapy in retinal diseases. Neural Regen. Res. 2023, 18, 1478–1485. [Google Scholar] [CrossRef]
- Wu, K.Y.; Dhaliwal, J.K.; Sasitharan, A.; Kalevar, A. Cell Therapy for Retinal Degenerative Diseases: Progress and Prospects. Pharmaceutics 2024, 16, 1299. [Google Scholar] [CrossRef]
- Radu, M.; Brănișteanu, D.C.; Pirvulescu, R.A.; Dumitrescu, O.M.; Ionescu, M.A.; Zemba, M. Exploring Stem-Cell-Based Therapies for Retinal Regeneration. Life 2024, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Coffey, P.J.; Girman, S.V.; Wang, S.; Hetherington, L.; Keegan, D.J.; Adamson, P.; Greenwood, J.; Lund, R.D.; Lund, R.D. Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat. Neurosci. 2002, 5, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Chung, S.J.; Yun, K.; Kim, B.; So, S.; Kang, S.; Kang, E.; Lee, J.Y. Long-term effects of human induced pluripotent stem cell-derived retinal cell transplantation in Pde6b knockout rats. Exp. Mol. Med. 2021, 53, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Ben M’Barek, K.; Habeler, W.; Plancheron, A.; Jarraya, M.; Regent, F.; Terray, A.; Yang, Y.; Chatrousse, L.; Domingues, S.; Masson, Y.; et al. Human ESC–derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci. Transl. Med. 2017, 9, eaai7471. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Song, W.K.; Park, K.M.; Kim, H.J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Mehat, M.S.; Sundaram, V.; Ripamonti, C.; Robson, A.G.; Smith, A.J.; Borooah, S.; Robinson, M.; Rosenthal, A.N.; Innes, W.; Weleber, R.G.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018, 125, 1765–1775. [Google Scholar] [CrossRef]
- Li, S.-Y.; Liu, Y.; Wang, L.; Wang, F.; Zhao, T.-T.; Li, Q.-Y.; Xu, H.-W.; Meng, X.-H.; Hao, J.; Zhou, Q.; et al. A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration: 5-years’ follow-up. Cell Prolif. 2021, 54, e13100. [Google Scholar] [CrossRef]
- Monville, C.; Bertin, S.; Devisme, C.; Brazhnikova, E.; Jaillard, C.; Walter, H.; Plancheron, A.; Jarraya, M.; Bejanariu, A.; Abbas, S. Phase I/II open-label study of implantation into one eye of hESC-derived RPE in patients with retinitis pigmentosa due to monogenic mutation: First safety results. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3829. [Google Scholar]
- Humayun, M.S.; Clegg, D.O.; Dayan, M.S.; Kashani, A.H.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; et al. Long-term Follow-up of a Phase 1/2a Clinical Trial of a Stem Cell-Derived Bioengineered Retinal Pigment Epithelium Implant for Geographic Atrophy. Ophthalmology 2024, 131, 682–691. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Mandai, M.; Gocho, K.; Hirami, Y.; Yamamoto, M.; Fujihara, M.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol. Retin. 2019, 3, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal Autologous Bone Marrow CD34+ Cell Therapy for Ischemic and Degenerative Retinal Disorders: Preliminary Phase 1 Clinical Trial Findings. Investig. Ophthalmol. Vis. Sci. 2015, 56, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Messias, A.; Voltarelli, J.C.; Scott, I.U.; Jorge, R. Intravitreal Injection of Autologous Bone Marrow–Derived Mononuclear Cells for Hereditary Retinal Dystrophy: A Phase I Trial. Retina 2011, 31, 1207–1214. [Google Scholar] [CrossRef]
- Park, S.S.; Bauer, G.; Fury, B.; Abedi, M.; Perotti, N.; Colead-Bergum, D.; Nolta, J.A. Phase I Study of Intravitreal Injection of Autologous CD34+ Stem Cells from Bone Marrow in Eyes with Vision Loss from Retinitis Pigmentosa. Ophthalmol. Sci. 2025, 5, 100589. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Messias, A.; Messias, K.; Arcieri, R.S.; Ruiz, M.A.; Souza, N.F.; Martins, L.C.; Jorge, R. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell-clinical trial). Stem Cell Res. Ther. 2015, 6, 29. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef]
- Wiącek, M.P.; Gosławski, W.; Grabowicz, A.; Sobuś, A.; Kawa, M.P.; Baumert, B.; Paczkowska, E.; Milczarek, S.; Osękowska, B.; Safranow, K.; et al. Long-Term Effects of Adjuvant Intravitreal Treatment with Autologous Bone Marrow-Derived Lineage-Negative Cells in Retinitis Pigmentosa. Stem Cells Int. 2021, 2021, 6631921. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; del Cerro, M.; Jalali, S.; Rao, V.S.; Gullapalli, V.K.; Little, C.; Loreto, D.A.D.; Sharma, S.; Sreedharan, A.; del Cerro, C.; et al. The Transplantation of Human Fetal Neuroretinal Cells in Advanced Retinitis Pigmentosa Patients: Results of a Long-Term Safety Study. Exp. Neurol. 1999, 157, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.J.; Li, S.Y.; Qu, L.H.; Meng, X.H.; Wang, Y.; Xu, H.W.; Liang, Z.Q.; Yin, Z.Q. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res. Ther. 2017, 8, 209. [Google Scholar] [CrossRef]
- Kuppermann, B.D.; Boyer, D.S.; Mills, B.; Yang, J.; Klassen, H.J. Safety and Activity of a Single, Intravitreal Injection of Human Retinal Progenitor Cells (jCell) for Treatment of Retinitis Pigmentosa (RP). Investig. Ophthalmol. Vis. Sci. 2018, 59, 2987. [Google Scholar]
- Yang, J.; Yan, M.; Wang, Z.; Zhang, C.; Guan, M.; Sun, Z. Optical and MRI Multimodal Tracing of Stem Cells In Vivo. Mol. Imaging 2023, 2023, 4223485. [Google Scholar] [CrossRef]
- Park, S.; Nguyen, V.P.; Wang, X.; Paulus, Y.M. Gold Nanoparticles for Retinal Molecular Optical Imaging. Int. J. Mol. Sci. 2024, 25, 9315. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, Y.J.; Kang, J.H. Stem Cell Monitoring with a Direct or Indirect Labeling Method. Nucl. Med. Mol. Imaging 2016, 50, 275–283. [Google Scholar] [CrossRef]
- Kircher, M.F.; Gambhir, S.S.; Grimm, J. Noninvasive cell-tracking methods. Nat. Rev. Clin. Oncol. 2011, 8, 677–688. [Google Scholar] [CrossRef]
- Ma, D.J.; Lim, M.-S.; Park, U.C.; Park, J.-B.; Ji, S.Y.; Yu, H.G. Magnetic iron oxide nanoparticle labeling of photoreceptor precursors for magnetic resonance imaging. Tissue Eng. Part C Methods 2019, 25, 532–542. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Zhe, J.; Hu, J.; Ahmed, U.; Paulus, Y.M. Molecular and cellular imaging of the eye. Biomed. Opt. Express 2023, 15, 360–386. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.-R.; Bi, M.-C.; Yang, W.; Wang, D.; Liu, H.-L.; Liang, L.-L.; Li, X.-H.; Hao, Q.; Cui, Z.-H. Comparison of three fluorescence labeling and tracking methods of endothelial progenitor cells in laser-injured retina. Int. J. Ophthalmol. 2018, 11, 580. [Google Scholar]
- Shi, H.; Yang, W.; Cui, Z.H.; Lu, C.W.; Li, X.H.; Liang, L.L.; Song, E. Tracking of CFSE-labeled endothelial progenitor cells in laser-injured mouse retina. Chin. Med. J. 2011, 124, 751–757. [Google Scholar]
- Laver, C.R.J.; Metcalfe, A.L.; Szczygiel, L.; Yanai, A.; Sarunic, M.V.; Gregory-Evans, K. Bimodal in vivo imaging provides early assessment of stem-cell-based photoreceptor engraftment. Eye 2015, 29, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Aboualizadeh, E.; Phillips, M.J.; McGregor, J.E.; DiLoreto, D.A., Jr.; Strazzeri, J.M.; Dhakal, K.R.; Bateman, B.; Jager, L.D.; Nilles, K.L.; Stuedemann, S.A.; et al. Imaging Transplanted Photoreceptors in Living Nonhuman Primates with Single-Cell Resolution. Stem Cell Rep. 2020, 15, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Li, Y.; Henry, J.; Qian, T.; Zhang, W.; Wang, X.; Paulus, Y.M. In Vivo Subretinal ARPE-19 Cell Tracking Using Indocyanine Green Contrast-Enhanced Multimodality Photoacoustic Microscopy, Optical Coherence Tomography, and Fluorescence Imaging for Regenerative Medicine. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Desmettre, T.; Devoisselle, J.; Mordon, S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef]
- Filippi, M.; Garello, F.; Pasquino, C.; Arena, F.; Giustetto, P.; Antico, F.; Terreno, E. Indocyanine green labeling for optical and photoacoustic imaging of mesenchymal stem cells after in vivo transplantation. J. Biophotonics 2019, 12, e201800035. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Karoukis, A.J.; Qian, W.; Chen, L.; Perera, N.D.; Yang, D.; Zhang, Q.; Zhe, J.; Henry, J.; Liu, B.; et al. Multimodal Imaging-Guided Stem Cell Ocular Treatment. ACS Nano 2024, 18, 14893–14906. [Google Scholar] [CrossRef]
- Yun, W.S.; Cho, H.; Jeon, S.I.; Lim, D.K.; Kim, K. Fluorescence-Based Mono- and Multimodal Imaging for In Vivo Tracking of Mesenchymal Stem Cells. Biomolecules 2023, 13, 1787. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Li, C.; Huang, D.; Wang, Q.; Wang, Q. Recent Advances in Tracking the Transplanted Stem Cells Using Near-Infrared Fluorescent Nanoprobes: Turning from the First to the Second Near-Infrared Window. Adv. Healthc. Mater. 2018, 7, 1800497. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hwang, Y.; Kim, J.H.; Kim, Y.S.; Jung, B.K.; Kim, P.; Lee, H. In Vivo Fluorescence Retinal Imaging Following AAV2-Mediated Gene Delivery in the Rat Retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3390–3396. [Google Scholar] [CrossRef][Green Version]
- Müller, M.G.; Georgakoudi, I.; Zhang, Q.; Wu, J.; Feld, M.S. Intrinsic fluorescence spectroscopy in turbid media: Disentangling effects of scattering and absorption. Appl. Opt. 2001, 40, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V. Fluorescence molecular imaging. Annu. Rev. Biomed. Eng. 2006, 8, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.R.; Lamba, D.A.; Klesert, T.R.; Torre, A.L.; Hoshino, A.; Taylor, R.J.; Jayabalu, A.; Engel, A.L.; Khuu, T.H.; Wang, R.K.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Cells into the Subretinal Space of a Non-Human Primate. Transl. Vis. Sci. Technol. 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- McGill, T.J.; Stoddard, J.; Renner, L.M.; Messaoudi, I.; Bharti, K.; Mitalipov, S.; Lauer, A.; Wilson, D.J.; Neuringer, M. Allogeneic iPSC-Derived RPE Cell Graft Failure Following Transplantation Into the Subretinal Space in Nonhuman Primates. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1374–1383. [Google Scholar] [CrossRef]
- Tu, H.-Y.; Watanabe, T.; Shirai, H.; Yamasaki, S.; Kinoshita, M.; Matsushita, K.; Hashiguchi, T.; Onoe, H.; Matsuyama, T.; Kuwahara, A.; et al. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine 2019, 39, 562–574. [Google Scholar] [CrossRef]
- Chung, S.H.; Mollhoff, I.N.; Mishra, A.; Sin, T.N.; Ngo, T.; Ciulla, T.; Sieving, P.; Thomasy, S.M.; Yiu, G. Host Immune Responses after Suprachoroidal Delivery of AAV8 in Nonhuman Primate Eyes. Hum. Gene Ther. 2021, 32, 682–693. [Google Scholar] [CrossRef]
- Vandenberghe, L.H.; Bell, P.; Maguire, A.M.; Cearley, C.N.; Xiao, R.; Calcedo, R.; Wang, L.; Castle, M.J.; Maguire, A.C.; Grant, R.; et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 2011, 3, 88ra54. [Google Scholar] [CrossRef]
- Jurgielewicz, P.; Harmsen, S.; Wei, E.; Bachmann, M.H.; Ting, R.; Aras, O. New imaging probes to track cell fate: Reporter genes in stem cell research. Cell. Mol. Life Sci. 2017, 74, 4455–4469. [Google Scholar] [CrossRef]
- Shatos, M.A.; Mizumoto, K.; Mizumoto, H.; Kurimoto, Y.; Klassen, H.; Young, M.J. Multipotent Stem Cells from the Brain and Retina of Green Mice. e-biomed J. Regen. Med. 2001, 2, 13–15. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Fan, J.; Hung, W.; Li, W.; Yeh, J. Biocompatibility study of gold nanoparticles to human cells. In Proceedings of the 13th International Conference on Biomedical Engineering: ICBME 2008, Singapore, 3–6 December 2008; pp. 870–873. [Google Scholar]
- Betzer, O.; Meir, R.; Dreifuss, T.; Shamalov, K.; Motiei, M.; Shwartz, A.; Baranes, K.; Cohen, C.J.; Shraga-Heled, N.; Ofir, R.; et al. In-vitro Optimization of Nanoparticle-Cell Labeling Protocols for In-vivo Cell Tracking Applications. Sci. Rep. 2015, 5, 15400. [Google Scholar] [CrossRef] [PubMed]
- Adijanto, J.; Naash, M.I. Nanoparticle-based technologies for retinal gene therapy. Eur. J. Pharm. Biopharm. 2015, 95, 353–367. [Google Scholar] [CrossRef]
- Afrooz, A.N.; Sivalapalan, S.T.; Murphy, C.J.; Hussain, S.M.; Schlager, J.J.; Saleh, N.B. Spheres vs. rods: The shape of gold nanoparticles influences aggregation and deposition behavior. Chemosphere 2013, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Chemla, Y.; Betzer, O.; Markus, A.; Farah, N.; Motiei, M.; Popovtzer, R.; Mandel, Y. Gold nanoparticles for multimodal high-resolution imaging of transplanted cells for retinal replacement therapy. Nanomedicine 2019, 14, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Shu, Y.; Tang, Y.; Chen, H. Multifaceted application of nanoparticle-based labeling strategies for stem cell therapy. Nano Today 2020, 34, 100897. [Google Scholar] [CrossRef]
- Meir, R.; Popovtzer, R. Cell tracking using gold nanoparticles and computed tomography imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1480. [Google Scholar] [CrossRef]
- Dunpall, R.; Revaprasadu, N. Gold Fabricated Core–Shell Nanoparticles as Innovative Cancer Therapeutic Strategies to Improve Drug Delivery. 2017. Available online: https://books.rsc.org/books/edited-volume/1496/chapter-abstract/956312/Gold-fabricated-core-shell-nanoparticles-as?redirectedFrom=fulltext (accessed on 1 December 2024).
- Chen, F.; Si, P.; de la Zerda, A.; Jokerst, J.V.; Myung, D. Gold nanoparticles to enhance ophthalmic imaging. Biomater. Sci. 2021, 9, 367–390. [Google Scholar] [CrossRef]
- Agrawal, A.; Huang, S.; Wei Haw Lin, A.; Lee, M.H.; Barton, J.K.; Drezek, R.A.; Pfefer, T.J. Quantitative evaluation of optical coherence tomography signal enhancement with gold nanoshells. J. Biomed. Opt. 2006, 11, 041121. [Google Scholar] [CrossRef]
- Zagaynova, E.V.; Shirmanova, M.V.; Kirillin, M.Y.; Khlebtsov, B.N.; Orlova, A.G.; Balalaeva, I.V.; Sirotkina, M.A.; Bugrova, M.L.; Agrba, P.D.; Kamensky, V.A. Contrasting properties of gold nanoparticles for optical coherence tomography: Phantom, in vivo studies and Monte Carlo simulation. Phys. Med. Biol. 2008, 53, 4995–5009. [Google Scholar] [CrossRef]
- Liba, O.; SoRelle, E.D.; Sen, D.; de la Zerda, A. Contrast-enhanced optical coherence tomography with picomolar sensitivity for functional in vivo imaging. Sci. Rep. 2016, 6, 23337. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Li, Y.; Zhang, W.; Wang, X.; Paulus, Y.M. High-resolution multimodal photoacoustic microscopy and optical coherence tomography image-guided laser induced branch retinal vein occlusion in living rabbits. Sci. Rep. 2019, 9, 10560. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Lim, H.T.; Maekawa, T.; Vadakke Matham, M.; Sakthi Kumar, D. Gold nanocages entering into the realm of high-contrast photoacoustic ocular imaging. Nanoscale 2018, 10, 13959–13968. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Qian, W.; Zhe, J.; Henry, J.; Wang, M.; Liu, B.; Zhang, W.; Wang, X.; Paulus, Y.M. Renally Clearable Ultraminiature Chain-Like Gold Nanoparticle Clusters for Multimodal Molecular Imaging of Choroidal Neovascularization. Adv. Mater. 2023, 35, e2302069. [Google Scholar] [CrossRef]
- Nguyen, V.-P.; Li, Y.; Henry, J.; Zhang, W.; Wang, X.; Paulus, Y.M. Gold Nanorod Enhanced Photoacoustic Microscopy and Optical Coherence Tomography of Choroidal Neovascularization. ACS Appl. Mater. Interfaces 2021, 13, 40214–40228. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Fan, W.; Zhu, T.; Qian, W.; Li, Y.; Liu, B.; Zhang, W.; Henry, J.; Yuan, S.; Wang, X.; et al. Long-Term, Noninvasive In Vivo Tracking of Progenitor Cells Using Multimodality Photoacoustic, Optical Coherence Tomography, and Fluorescence Imaging. ACS Nano 2021, 15, 13289–13306. [Google Scholar] [CrossRef]
- Cho, W.K.; Kang, S.; Choi, H.; Rho, C.R. Topically administered gold nanoparticles inhibit experimental corneal neovascularization in mice. Cornea 2015, 34, 456–459. [Google Scholar] [CrossRef]
- Roh, Y.J.; Rho, C.R.; Cho, W.K.; Kang, S. The Antiangiogenic Effects of Gold Nanoparticles on Experimental Choroidal Neovascularization in Mice. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6561–6567. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in ocular applications and their potential toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef]
- Elkhenany, H.; Abd Elkodous, M.; Ghoneim, N.I.; Ahmed, T.A.; Ahmed, S.M.; Mohamed, I.K.; El-Badri, N. Comparison of different uncoated and starch-coated superparamagnetic iron oxide nanoparticles: Implications for stem cell tracking. Int. J. Biol. Macromol. 2020, 143, 763–774. [Google Scholar] [CrossRef]
- Oberländer, J.; Ayerbe, R.; Cabellos, J.; da Costa Marques, R.; Li, B.; Günday-Türeli, N.; Türeli, A.E.; Ofir, R.; Shalom, E.I.; Mailänder, V. Higher Loading of Gold Nanoparticles in PAD Mesenchymal-like Stromal Cells Leads to a Decreased Exocytosis. Cells 2022, 11, 2323. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Yin, L.; Liu, Z. The Roles of Nanoparticles in Stem Cell-Based Therapy for Cardiovascular Disease. Front. Bioeng. Biotechnol. 2020, 8, 947. [Google Scholar] [CrossRef]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 59–85. [Google Scholar]
- Morgan, J.; Huckfeldt, R.; Wong, R.O.L. Imaging techniques in retinal research. Exp. Eye Res. 2005, 80, 297–306. [Google Scholar] [CrossRef]
- Contag, C.H.; Contag, P.R.; Mullins, J.I.; Spilman, S.D.; Stevenson, D.K.; Benaron, D.A. Photonic detection of bacterial pathogens in living hosts. Mol. Microbiol. 1995, 18, 593–603. [Google Scholar] [CrossRef]
- Mao, W.; Bui, H.-T.D.; Cho, W.; Yoo, H.S. Spectroscopic techniques for monitoring stem cell and organoid proliferation in 3D environments for therapeutic development. Adv. Drug Deliv. Rev. 2023, 201, 115074. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Zhang, P.; Dolf, C.; Smit-McBride, Z.; Cary, W.; Nolta, J.A.; Zawadzki, R.J.; Marsh-Armstrong, N.; Park, S.S. Effects of intravitreal injection of human CD34+ bone marrow stem cells in a murine model of diabetic retinopathy. Exp. Eye Res. 2020, 190, 107865. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Li, Y.; Wu, X.; Wang, M.; Tan, X.; Paulus, Y.M.; Fan, X.; Wang, X. In vivo tracking of individual stem cells labeled with nanowire lasers using multimodality imaging. Biomed. Opt. Express 2022, 13, 4706–4717. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Paulus, Y.M. Photoacoustic ophthalmoscopy: Principle, application, and future directions. J. Imaging 2018, 4, 149. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Nguyen, V.P.; Derouin, K.; Xia, X.; Paulus, Y.; Wang, X. Ultralow energy photoacoustic microscopy for ocular imaging in vivo. J. Biomed. Opt. 2020, 25, 066003. [Google Scholar] [CrossRef]
- Donnelly, E.M.; Kubelick, K.P.; Dumani, D.S.; Emelianov, S.Y. Photoacoustic image-guided delivery of plasmonic-nanoparticle-labeled mesenchymal stem cells to the spinal cord. Nano Lett. 2018, 18, 6625–6632. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Nguyen, V.P.; Khan, N.W.; Xia, X.; Wang, X.; Paulus, Y.M. Retinal safety evaluation of photoacoustic microscopy. Exp. Eye Res. 2021, 202, 108368. [Google Scholar] [CrossRef]
- Hosseinaee, Z.; Le, M.; Bell, K.; Reza, P.H. Towards non-contact photoacoustic imaging [review]. Photoacoustics 2020, 20, 100207. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, J.; Lee, D.; Baik, J.W.; Kim, C. Review on practical photoacoustic microscopy. Photoacoustics 2019, 15, 100141. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, W.; Mordovanakis, A.; Wang, X.; Paulus, Y.M. Noninvasive chorioretinal imaging in living rabbits using integrated photoacoustic microscopy and optical coherence tomography. Opt. Express 2017, 25, 15947–15955. [Google Scholar] [CrossRef]
- Niendorf, T.; Beenakker, J.W.M.; Langner, S.; Erb-Eigner, K.; Bach Cuadra, M.; Beller, E.; Millward, J.M.; Niendorf, T.M.; Stachs, O. Ophthalmic Magnetic Resonance Imaging: Where Are We (Heading To)? Curr. Eye Res. 2021, 46, 1251–1270. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013, 3, 595. [Google Scholar] [CrossRef]
- Dysli, C.; Wolf, S.; Berezin, M.Y.; Sauer, L.; Hammer, M.; Zinkernagel, M.S. Fluorescence lifetime imaging ophthalmoscopy. Prog. Retin. Eye Res. 2017, 60, 120–143. [Google Scholar] [CrossRef]
- Xue, Y.; Browne, A.W.; Tang, W.C.; Delgado, J.; McLelland, B.T.; Nistor, G.; Chen, J.T.; Chew, K.; Lee, N.; Keirstead, H.S.; et al. Retinal Organoids Long-Term Functional Characterization Using Two-Photon Fluorescence Lifetime and Hyperspectral Microscopy. Front. Cell. Neurosci. 2021, 15, 796903. [Google Scholar] [CrossRef]
- Ayten, M.; Straub, T.; Kaplan, L.; Hauck, S.M.; Grosche, A.; Koch, S.F. CD44 signaling in Müller cells impacts photoreceptor function and survival in healthy and diseased retinas. J. Neuroinflamm. 2024, 21, 190. [Google Scholar] [CrossRef]
- Mochimaru, H.; Takahashi, E.; Tsukamoto, N.; Miyazaki, J.; Yaguchi, T.; Koto, T.; Kurihara, T.; Noda, K.; Ozawa, Y.; Ishimoto, T.; et al. Involvement of Hyaluronan and Its Receptor CD44 with Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4410–4415. [Google Scholar] [CrossRef]
- Karakoçak, B.B.; Laradji, A.; Primeau, T.; Berezin, M.Y.; Li, S.; Ravi, N. Hyaluronan-Conjugated Carbon Quantum Dots for Bioimaging Use. ACS Appl. Mater. Interfaces 2021, 13, 277–286. [Google Scholar] [CrossRef]
- Ishikura, M.; Muraoka, Y.; Kadomoto, S.; Nishigori, N.; Kogo, T.; Numa, S.; Nakano, E.; Hata, M.; Ishihara, K.; Ooto, S.; et al. Evaluation of Foveal Cone and Muller Cells in Epiretinal Membrane using Adaptive Optics OCT. Ophthalmol. Sci. 2024, 4, 100362. [Google Scholar] [CrossRef]
- Ishikura, M.; Muraoka, Y.; Hirami, Y.; Tu, H.-Y.; Mandai, M. Adaptive Optics Optical Coherence Tomography Analysis of Induced Pluripotent Stem Cell-Derived Retinal Organoid Transplantation in Retinitis Pigmentosa. Cureus 2024, 16, e64962. [Google Scholar] [CrossRef]
- .Liu, Y.; Xu, H.W.; Wang, L.; Li, S.Y.; Zhao, C.J.; Hao, J.; Li, Q.Y.; Zhao, T.T.; Wu, W.; Wang, Y.; et al. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov. 2018, 4, 50. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Tuekprakhon, A.; Sangkitporn, S.; Trinavarat, A.; Pawestri, A.R.; Vamvanij, V.; Ruangchainikom, M.; Luksanapruksa, P.; Pongpaksupasin, P.; Khorchai, A.; Dambua, A. Intravitreal autologous mesenchymal stem cell transplantation: A non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Res. Ther. 2021, 12, 52. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Nguyen, V.P.; Rosen, R.; Wang, X.; Xia, X.; Paulus, Y.M. Real-time OCT guidance and multimodal imaging monitoring of subretinal injection induced choroidal neovascularization in rabbit eyes. Exp. Eye Res. 2019, 186, 107714. [Google Scholar] [CrossRef]
- Kashani, A.H.; Uang, J.; Mert, M.; Rahhal, F.; Chan, C.; Avery, R.L.; Dugel, P.; Chen, S.; Lebkowski, J.; Clegg, D.O.; et al. Surgical Method for Implantation of a Biosynthetic Retinal Pigment Epithelium Monolayer for Geographic Atrophy: Experience from a Phase 1/2a Study. Ophthalmol. Retin. 2020, 4, 264–273. [Google Scholar] [CrossRef]
- Terasaki, H.; Sonoda, S.; Tomita, M.; Sakamoto, T. Recent Advances and Clinical Application of Color Scanning Laser Ophthalmoscope. J. Clin. Med. 2021, 10, 718. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef]
- Slakter, J.S.; Yannuzzi, L.A.; Guyer, D.R.; Sorenson, J.A.; Orlock, D.A. Indocyanine-green angiography. Curr. Opin. Ophthalmol. 1995, 6, 25–32. [Google Scholar] [CrossRef]
- Tsang, S.H.; Sharma, T. Fluorescein angiography. In Atlas of Inherited Retinal Diseases; Springer: Berlin/Heidelberg, Germany, 2018; pp. 7–10. [Google Scholar]
- Ahmed, I.; Johnston Jr, R.J.; Singh, M.S. Pluripotent stem cell therapy for retinal diseases. Ann. Transl. Med. 2021, 9, 1279. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; Ingram, A.; Dang, W. One-year follow-up in a phase 1/2a clinical trial of an allogeneic RPE cell bioengineered implant for advanced dry age-related macular degeneration. Transl. Vis. Sci. Technol. 2021, 10, 13. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Oner, A.; Gonen, Z.B.; Sinim, N.; Cetin, M.; Ozkul, Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: A phase I clinical safety study. Stem Cell Res. Ther. 2016, 7, 178. [Google Scholar] [CrossRef]
- Nishida, M.; Tanaka, Y.; Tanaka, Y.; Amaya, S.; Tanaka, N.; Uyama, H.; Masuda, T.; Onishi, A.; Sho, J.; Yokota, S.; et al. Human iPS cell derived RPE strips for secure delivery of graft cells at a target place with minimal surgical invasion. Sci. Rep. 2021, 11, 21421. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Karoukis, A.J.; Hu, J.; Wei, Z.; Yang, D.; Fahim, A.T.; Wang, X.; Paulus, Y.M. Selective nanosecond laser removal of retinal pigment epithelium for cell therapy. Sci. Rep. 2024, 14, 19457. [Google Scholar] [CrossRef]
- Bischof, J.; Fletcher, G.; Verkade, P.; Kuntner, C.; Fernandez-Rodriguez, J.; Chaabane, L.; Rose, L.A.; Walter, A.; Vandenbosch, M.; van Zandvoort, M.A.M.J.; et al. Multimodal bioimaging across disciplines and scales: Challenges, opportunities and breaking down barriers. npj Imaging 2024, 2, 5. [Google Scholar] [CrossRef]
- Jahnavi, G.S.; Sivaswamy, J. Multimodal Registration of Retinal Images. In Proceedings of the Computer Vision, Pattern Recognition, Image Processing, and Graphics, Singapore, 16–19 December 2017; Springer: Singapore, 2018; pp. 314–324. [Google Scholar]
- Szewczuk, A.; Zaleska-Żmijewska, A.; Dziedziak, J.; Szaflik, J.P. Clinical Application of Adaptive Optics Imaging in Diagnosis, Management, and Monitoring of Ophthalmological Diseases: A Narrative Review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2023, 29, e941926. [Google Scholar] [CrossRef]
- Ren, C.; Hao, S.; Wang, F.; Matt, A.; Amaral, M.M.; Yang, D.; Wang, L.; Zhou, C. Dynamic contrast optical coherence tomography (DyC-OCT) for label-free live cell imaging. Commun. Biol. 2024, 7, 278. [Google Scholar] [CrossRef]
- Scholler, J.; Mazlin, V.; Thouvenin, O.; Groux, K.; Xiao, P.; Sahel, J.-A.; Fink, M.; Boccara, C.; Grieve, K. Probing dynamic processes in the eye at multiple spatial and temporal scales with multimodal full field OCT. Biomed. Opt. Express 2019, 10, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Scholler, J.; Groux, K.; Goureau, O.; Sahel, J.-A.; Fink, M.; Reichman, S.; Boccara, C.; Grieve, K. Dynamic full-field optical coherence tomography: 3D live-imaging of retinal organoids. Light Sci. Appl. 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, É. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2019, 39, 302–327. [Google Scholar] [CrossRef]
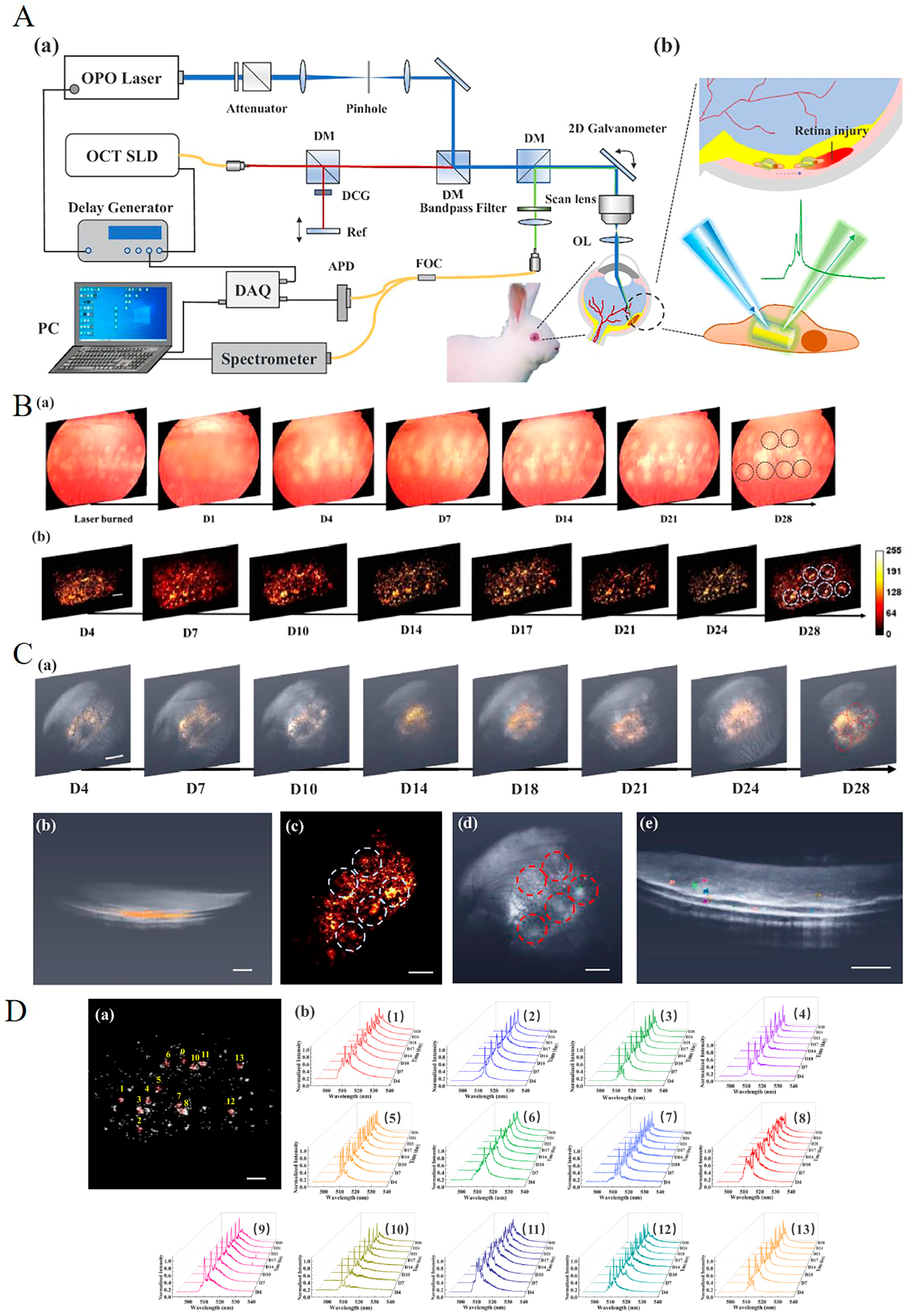
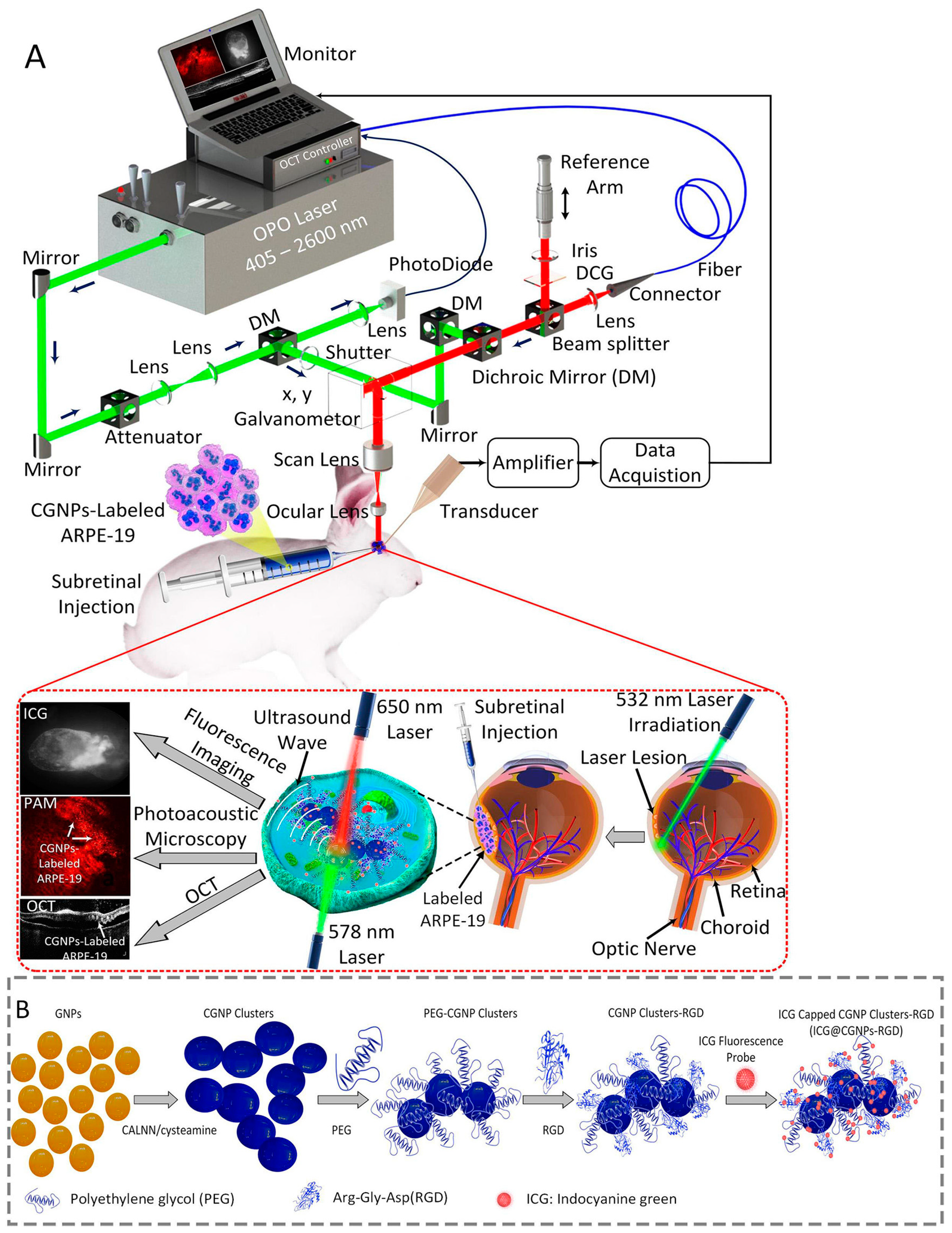
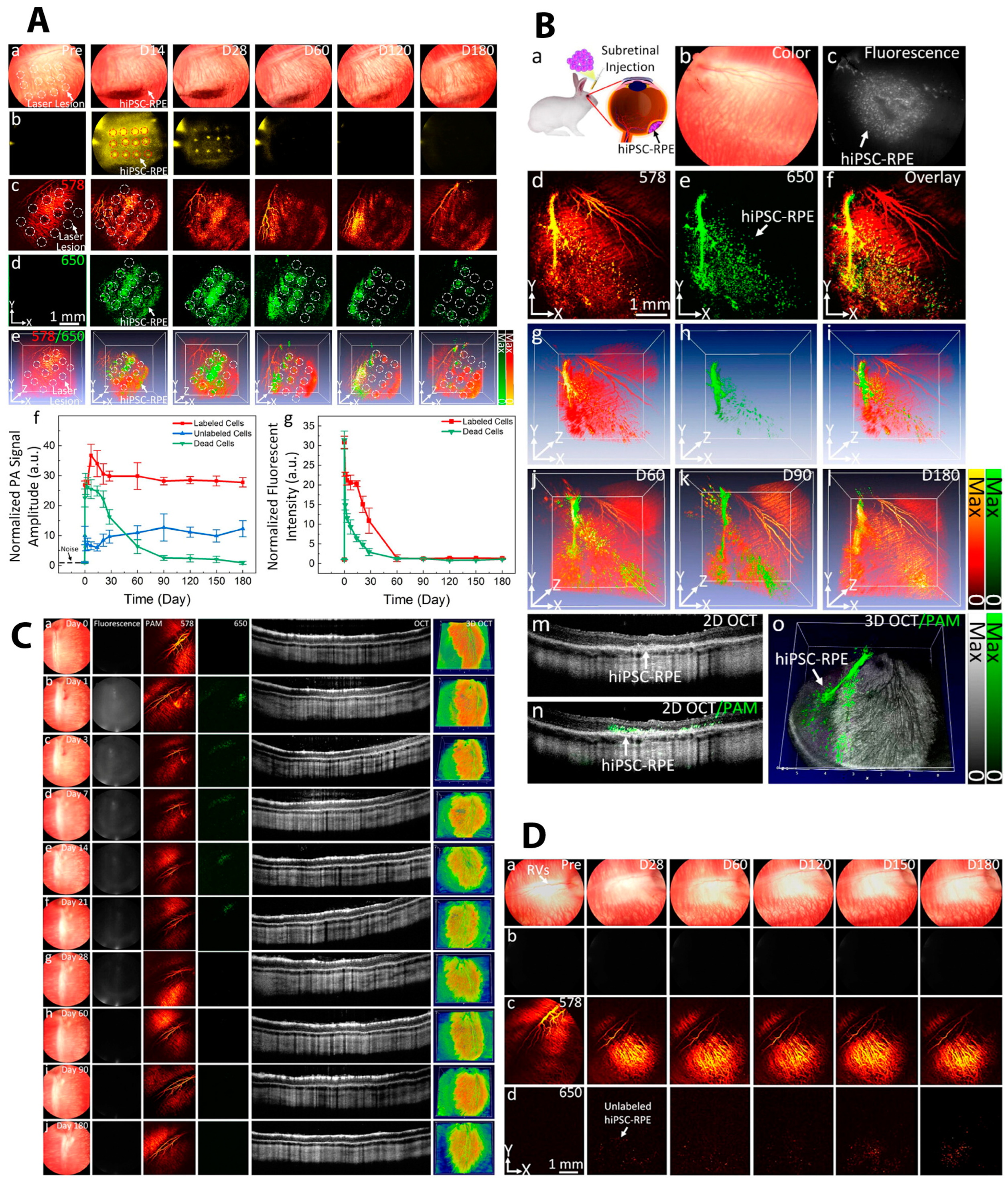
| Labeling Agent | Typical Labeling Agents | Advantages | Disadvantages | Imaging Techniques | |
|---|---|---|---|---|---|
| Direct | Fluorescent dye | ICG | Simple; applicable for multiple imaging modalities | Photobleaching; phototoxicity; not suitable for long-term monitoring; autofluorescence interference; false positives | FI |
| Nanoparticle | GNPs | Relatively simple; easy to synthesize and modify to achieve different optical properties; easy to combine with other labeling agents; can be used for a variety of imaging techniques; potential for long-term imaging; GNPs have antiangiogenic and anti-inflammatory properties | Potential toxicity and impact on cell function; exocytosis; false positives; potential long-term side effects | OCT | |
| Indirect | Reporter gene | SPIONs | More stable and long-lasting imaging signals | Complexity; high cost; delayed signal expression; immune response; toxicity; genome alteration and gene silencing | FI; PAI |
| Imaging Techniques | Advantages | Disadvantages | Applications in Retinal Stem Cell Therapy | |
|---|---|---|---|---|
| Preclinical | FI | High resolution; real-time imaging; multi-fluorescence detection ability | 2D imaging | Tracking stem cells |
| PAI | High resolution; high sensitivity; greater imaging depth; 3D imaging | Difficult to distinguish transplanted cells from the background; artifacts | Tracking stem cells | |
| MRI | 3D imaging; no depth limitation; capable of penetrating opaque tissues; long-term imaging | Limited sectional structure of the retina; small scanning range; high cost and complexity of setting up the system | Tracking stem cells | |
| Clinical | OCT | High resolution; real-time imaging; cross-sectional imaging; 3D imaging; non-invasive; widely used in clinical trials | Photobleaching; phototoxicity; autofluorescence interference | Monitoring morphology change; assisting cell delivery |
| Others (FP, SLO, FAF, ICGA, FA) | Widely used in clinical trials; safe for long-term monitoring | Relatively low resolution for retinal imaging; advantages come from cell-labeling agents (toxicity, degradation); complex and costly; contraindications of MRI | Assessing efficacy and safety of stem cell therapy | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Paulus, Y.M. Multimodal Imaging in Stem Cell Therapy for Retinal Disease. Photonics 2025, 12, 413. https://doi.org/10.3390/photonics12050413
Zheng M, Paulus YM. Multimodal Imaging in Stem Cell Therapy for Retinal Disease. Photonics. 2025; 12(5):413. https://doi.org/10.3390/photonics12050413
Chicago/Turabian StyleZheng, Mi, and Yannis M. Paulus. 2025. "Multimodal Imaging in Stem Cell Therapy for Retinal Disease" Photonics 12, no. 5: 413. https://doi.org/10.3390/photonics12050413
APA StyleZheng, M., & Paulus, Y. M. (2025). Multimodal Imaging in Stem Cell Therapy for Retinal Disease. Photonics, 12(5), 413. https://doi.org/10.3390/photonics12050413






