Dynamic Measurement of Flowing Microparticles in Microfluidics Using Pulsed Modulated Digital Holographic Microscopy
Abstract
1. Introduction
2. Materials and Methods
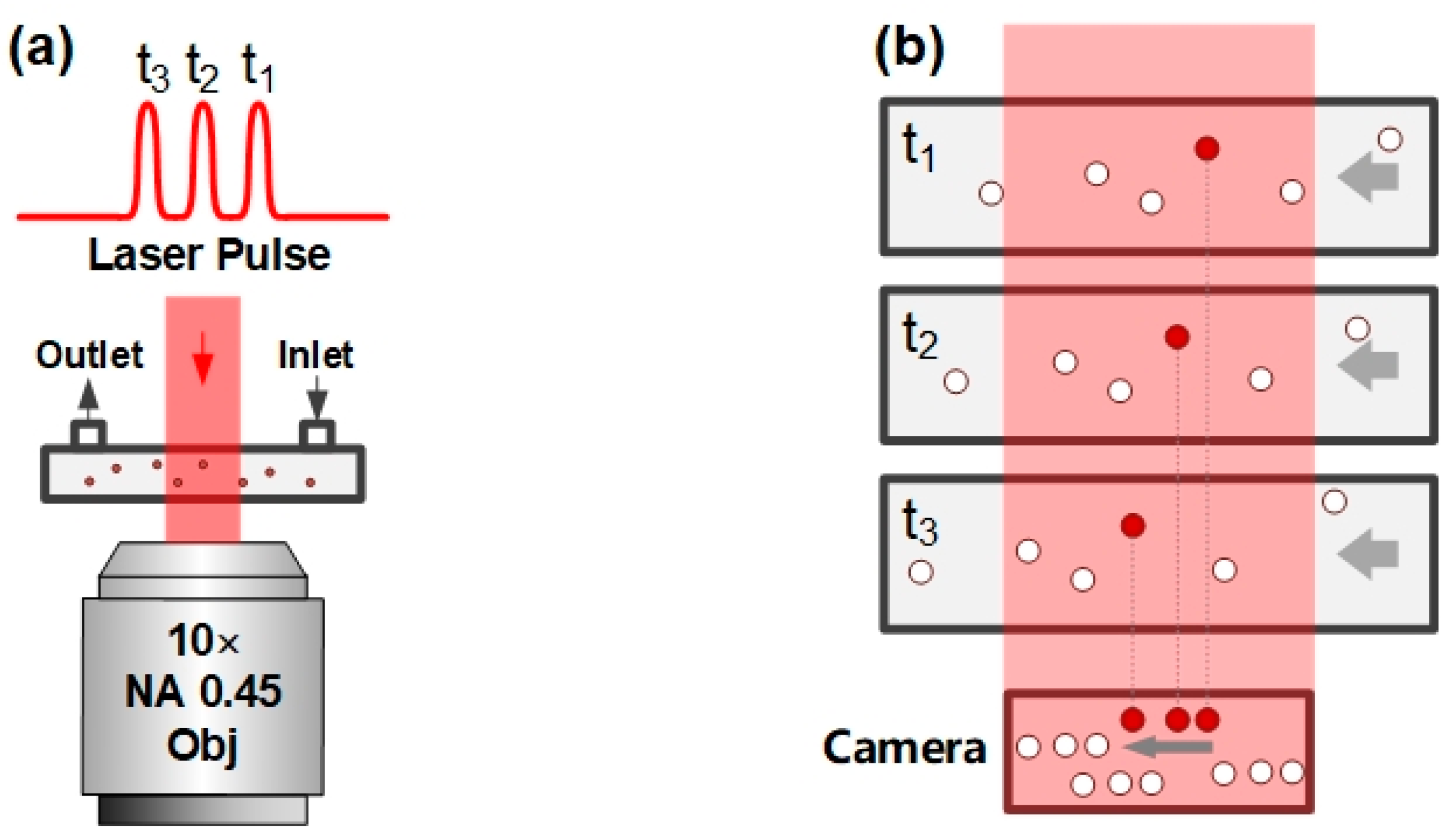
2.1. Principle of PM-DHM
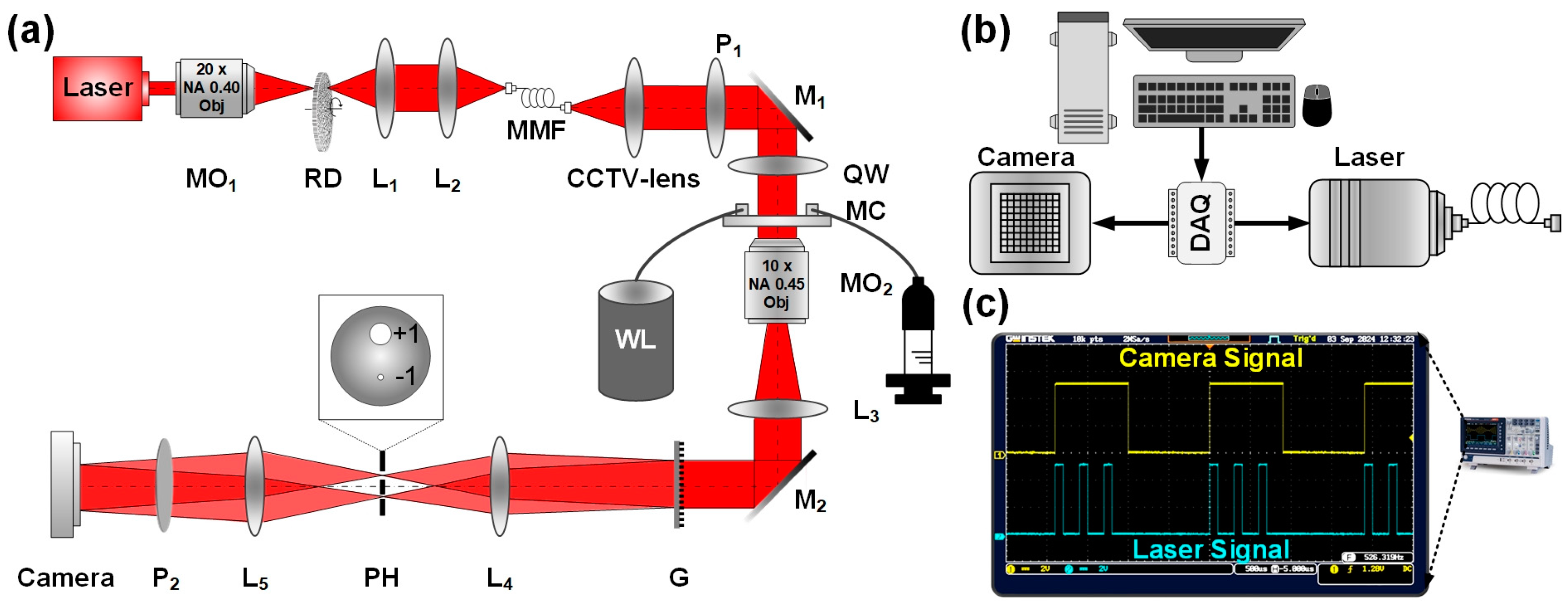
2.2. Optical Path and System Control
3. Results and Discussion
3.1. Measurement of Flowing PMMA Microspheres
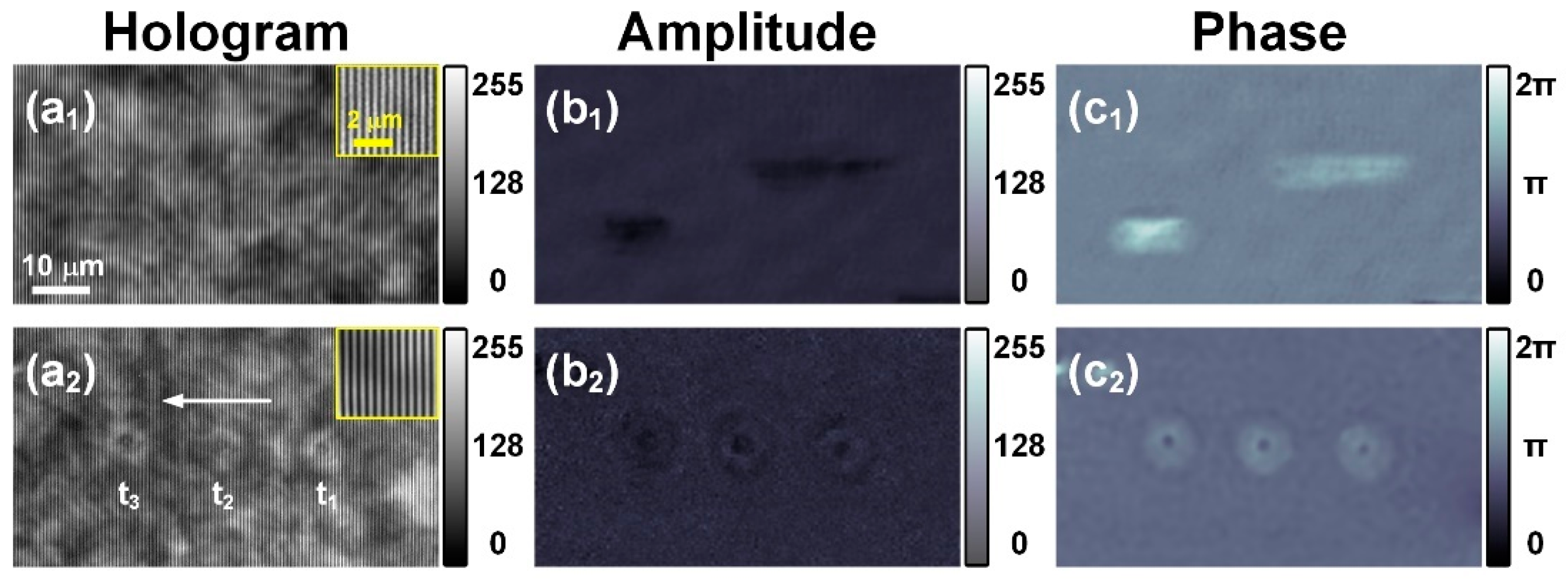
3.2. Measurement of Red Blood Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Barroso, Á.; Picazo-Bueno, J.Á.; Ketelhut, S.; Schnekenburger, J.; Kemper, B. Application of digital holographic microscopy for assessment of hydrodynamic focusing in a microfluidic chip. In Optica Digital Holography and Three-Dimensional Imaging 2024 (DH); Technical Digest Series; Optica Publishing Group: Washington, DC, USA, 2024; paper Tu2A.2. [Google Scholar]
- Zhou, H.; Yang, Z.; Yao, Z.; Cen, K. Application of digital holographic microscopy and microfluidic chips to the measurement of particle size distribution of fly ash after a wet electrostatic precipitator. Flow Meas. Instrum. 2018, 60, 24–29. [Google Scholar] [CrossRef]
- Cui, Y.T.; Wang, Z.; Sun, C.; Jiang, Z.Q. The measurement of flow rate of micro-fluid on-chip by digital holography. In Holography, Diffractive Optics, and Applications VII, Eighth International Conference on Graphic and Image Processing, Tokyo, Japan, 29–31 October 2016; SPIE: Bellingham, WA, USA, 2016; Volume 1002222. [Google Scholar]
- Evans, H.B.; Gorumlu, S.; Aksak, B.; Castillo, L.; Sheng, J. Holographic microscopy and microfluidics platform for measuring wall stress and 3D flow over surfaces textured by micro-pillars. Sci. Rep. 2016, 6, 28753. [Google Scholar] [CrossRef]
- Yu, X.; Hong, J.; Liu, C.G.; Kim, M.K. Review of digital holographic microscopy for three-dimensional profiling and tracking. Opt. Eng. 2014, 53, 112306. [Google Scholar] [CrossRef]
- Gao, P.; Yuan, C. Resolution enhancement of digital holographic microscopy via synthetic aperture: A review. Light. Adv. Manuf. 2022, 3, 105–112. [Google Scholar] [CrossRef]
- Tahara, T.; Quan, X.Y.; Otani, R.; Takaki, Y.; Matoba, O. Digital holography and its multidimensional imaging applications: A review. Microscopy 2018, 67, 55–67. [Google Scholar] [CrossRef]
- Wang, H.Y.; Dong, Z.; Wang, X.; Lou, Y.L.; Xi, S.X. Phase compensation in digital holographic microscopy using a quantitative evaluation metric. Opt. Commun. 2019, 430, 262–267. [Google Scholar] [CrossRef]
- Min, J.W.; Yao, B.L.; Ketelhut, S.; Engwer, C.; Greve, B.; Kemper, B. Simple and fast spectral domain algorithm for quantitative phase imaging of living cells with digital holographic microscopy. Opt. Lett. 2017, 42, 227–230. [Google Scholar] [CrossRef]
- Osten, W.G.; Faridian, A.; Gao, P.; Körner, K.; Naik, D.; Pedrini, G.; Singh, A.K.; Takeda, M.; Wilke, M. Recent advances in digital holography [Invited]. Appl. Opt. 2014, 53, G44–G63. [Google Scholar] [CrossRef]
- Kim, M.K. Principles and techniques of digital holographic microscopy. SPIE Rev. 2010, 1, 018005. [Google Scholar] [CrossRef]
- Zhuo, K.Q.; Wang, Y.; Wang, Y.; Wen, K.; Liu, M.; Ma, Y.; Zheng, J.J.; Gao, P. Partially Coherent Illumination Based Point-Diffraction Digital Holographic Microscopy Study Dynamics of Live Cells. Front. Phys. 2021, 9, 796935. [Google Scholar] [CrossRef]
- Byeon, H.; Go, T.; Lee, S.J. Deep learning-based digital in-line holographic microscopy for high resolution with extended field of view. Opt. Laser Technol. 2019, 113, 77–86. [Google Scholar] [CrossRef]
- Goud, B.K.; Shinde, D.D.; Udupa, D.V.; Krishna, C.M.; Rao, K.D.; Sahoo, N.K. Low cost digital holographic microscope for 3-D cell imaging by integrating smartphone and DVD optical head. Opt. Lasers Eng. 2019, 114, 1–6. [Google Scholar] [CrossRef]
- Kubota, T. 48 Years with holography. Opt. Rev. 2014, 21, 883–892. [Google Scholar] [CrossRef]
- Potter, J.C.; Xiong, Z.; McLeod, E. Clinical and biomedical applications of lensless holographic microscopy. Laser Photonics Rev. 2024, 18, 2400197. [Google Scholar] [CrossRef]
- Ozcan, A.; McLeod, E. Lensless imaging and sensing. Annu. Rev. Biomed. Eng. 2016, 18, 77–102. [Google Scholar] [CrossRef]
- Liao, Z.R.; Zhang, Y.; Li, Y.R.; Miao, Y.F.; Gao, S.M.; Lin, F.K.; Deng, Y.L.; Geng, L.N. Microfluidic chip coupled with optical biosensors for simultaneous detection of multiple analytes: A review. Biosens. Bioelectron. 2019, 126, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Pousti, M.; Zarabadi, M.P.; Amirdehi, M.A.; Paquet-Mercier, F.; Greener, J. Microfluidic bioanalytical flow cells for biofilm studies: A review. Analyst 2019, 144, 68–86. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef]
- Chen, P.; Kuo, K. High-throughput microfluidic systems for disease detection. J. Chin. Inst. Eng. 2013, 37, 670–675. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Q.; Liu, C.; Qiao, X.; Shao, C.S.; Su, X.T. Label-free light-sheet microfluidic cytometry for the automatic identification of senescent cells. Biomed. Opt. Express 2018, 9, 1692–1703. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, T.Q.; Lei, Y.Z.; Zhang, L.L.; Ma, L.; Liu, M.; An, S.; Zheng, J.J.; Zhuo, K.Q.; Kong, L.; et al. Panoramic quantitative phase imaging of adherent live cells in a microfluidic environment. Biomed. Opt. Express 2023, 14, 5182–5198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.Y.; Bai, G.F.; Zhu, D.X.; Xu, Q.; Zhang, X.D.; Li, T.R.; Qian, Y.Y.; Bu, C.W. Pump-free SERS microfluidic chip based on an identification-competition strategy for ultrasensitive and efficient simultaneous detection of liver cancer-related microRNAs. Biomed. Opt. Express 2024, 15, 6469–6485. [Google Scholar] [CrossRef] [PubMed]
- Bissardon, C.; Mermet, X.; Quintard, C.; Sanjuan, F.; Fouillet, Y.; Bottausci, F.; Carriere, M.; Rivera, F.; Blandin, P. Selective plane illumination microscope dedicated to volumetric imaging in microfluidic chambers. Biomed. Opt. Express 2022, 13, 5261–5274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.S.; Luo, J.H.; Hua, Z.L.; Feng, X.; Cao, X.W. SERS microfluidic chip integrated with double amplified signal off-on strategy for detection of microRNA in NSCLC. Biomed. Opt. Express 2024, 15, 594–607. [Google Scholar] [CrossRef]
- Gangaram-Panday, N.H.; Nijenhuis, L.H.; Fine, I.; Reiss, I.K.M.; Weteringen, W.V. A novel non-invasive method of measuring microcirculatory perfusion and blood velocity in infants: A pilot study. Sci. Rep. 2022, 12, 7459. [Google Scholar] [CrossRef]
- Ye, F.; Yin, S.C.; Li, M.R.; Li, Y.J.; Zhong, J.G. In-vivo full-field measurement of microcirculatory blood flow velocity based on intelligent object identification. J. Biomed. Opt. 2020, 25, 1–11. [Google Scholar] [CrossRef]
- Secomb, T.W. Blood Flow in the Microcirculation. Annu. Rev. Fluid Mech. 2017, 49, 443–461. [Google Scholar] [CrossRef]
- Omori, T.; Imai, Y.; Kikuchi, K.J.; Ishikawa, T.; Yamaguchi, T. Hemodynamics in the Microcirculation and in Microfluidics. Ann. Biomed. Eng. 2015, 43, 238–257. [Google Scholar] [CrossRef]
- Stavrakis, S.; Holzner, G.; Choo, J.; deMello, A. High-throughput microfluidic imaging flow cytometry. Curr. Opin. Biotechnol. 2019, 55, 36–43. [Google Scholar] [CrossRef]
- Tripathi, V.R.; Tibdewal, M.N.; Mishra, R. A survey on Motion Artifact Correction in Magnetic Resonance Imaging for Improved Diagnostics. SN Comput. Sci. 2024, 5, 281. [Google Scholar] [CrossRef]
- Huang, R.; Hong, K.; Yang, D.L.; Huang, G.H. Motion artifacts removal and evaluation techniques for functional near-infrared spectroscopy signals: A review. Front. Neurosci. 2022, 16, 878750. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, M.; Maclaren, J.; Herbst, M. Motion artifacts in MRI: A complex problem with many partial solutions. J. Magn. Reson. Imaging 2015, 42, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, D.; Pizarro, D.; Mazo, M. Single frame correction of motion artifacts in PMD-based time of flight cameras. Image Vis. Comput. 2014, 32, 1127–1143. [Google Scholar] [CrossRef]
- Xiong, Z.; Potter, C.J.; McLeod, E. High-speed lens-free holographic sensing of protein molecules using quantitative agglutination assays. ACS Sens. 2021, 6, 1208–1217. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wang, Y.; Zhuo, K.Q.; Liu, M.; Nienhaus, G.U.; Gao, P. Two-beam phase correlation spectroscopy: A label-free holographic method to quantify particle flow in biofluids. Photonics Res. 2023, 11, 757. [Google Scholar] [CrossRef]
- Le, A.V.; Fenech, M. Image-Based Experimental Measurement Techniques to Characterize Velocity Fields in Blood Microflows. Front. Physiol. 2022, 13, 886675. [Google Scholar] [CrossRef]
- Wereley, S.T.; Meinhart, C.D. Recent Advances in Micro-Particle Image Velocimetry. Annu. Rev. Fluid Mech. 2010, 42, 557–576. [Google Scholar] [CrossRef]
- Williams, S.J.; Park, C.; Wereley, S.T. Advances and applications on microfluidic velocimetry techniques. Microfluid. Nanofluidics 2010, 8, 709–726. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Available online: https://biology.stackexchange.com/questions/93703/what-is-the-mass-of-a-single-erythrocyte (accessed on 24 May 2020).
- Arroyo Ortega, J.; Cole, D.; Kukura, P. Interferometric scattering microscopy and its combination with single-molecule fluorescence imaging. Nat. Protoc. 2016, 11, 617–633. [Google Scholar] [CrossRef]
- Malm, J.; Giannaras, D.; Riehle, M.O.; Gadegaard, N.; Sjövall, P. Fixation and Drying Protocols for the Preparation of Cell Samples for Time-of-Flight Secondary Ion Mass Spectrometry Analysis. Anal. Chem. 2009, 81, 7197–7205. [Google Scholar] [CrossRef]
- Jafarfard, M.R.; Moon, S.; Tayebi, B.; Kim, D.Y. Dual-wavelength diffraction phase microscopy for simultaneous measurement of refractive index and thickness. Opt. Lett. 2014, 39, 2908–2911. [Google Scholar] [CrossRef]
- Schedin, S.; Pedrini, G.; Tiziani, H.J.; Mendoza Santoyo, F. Simultaneous three-dimensional dynamic deformation measurements with pulsed digital holography. Appl. Opt. 1999, 38, 7056. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhai, H.; Mu, G. Pulsed digital holography system recording ultrafast process of the femtosecond order. Opt. Lett. 2006, 31, 1636. [Google Scholar] [CrossRef] [PubMed]
- Icha, J.; Weber, M.; Waters, J.C.; Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays 2017, 39, 1700003. [Google Scholar] [CrossRef]
- Ashman, R.; Gu, M. Effect of ultrashort pulsed illumination on foci caused by a Fresnel zone plate. Appl. Opt. 2003, 42, 1852–1855. [Google Scholar] [CrossRef] [PubMed]
- Dover, J.S.; Bhatia, A.C.; Stewart, B.; Arndt, K.A. Topical 5-Aminolevulinic Acid Combined with Intense Pulsed Light in the Treatment of Photoaging. Arch. Dermatol. 2005, 141, 1247–1252. [Google Scholar] [CrossRef]
- Kang, S.; Duocastella, M.; Arnold, C.B. Variable optical elements for fast focus control. Nat. Photonics 2020, 14, 533–542. [Google Scholar] [CrossRef]
- Ebner, M.; Koch, P.A.; Haucke, V. Phosphoinositides in the control of lysosome function and homeostasis. Biochem. Soc. Trans. 2019, 47, 1173–1185. [Google Scholar] [CrossRef]
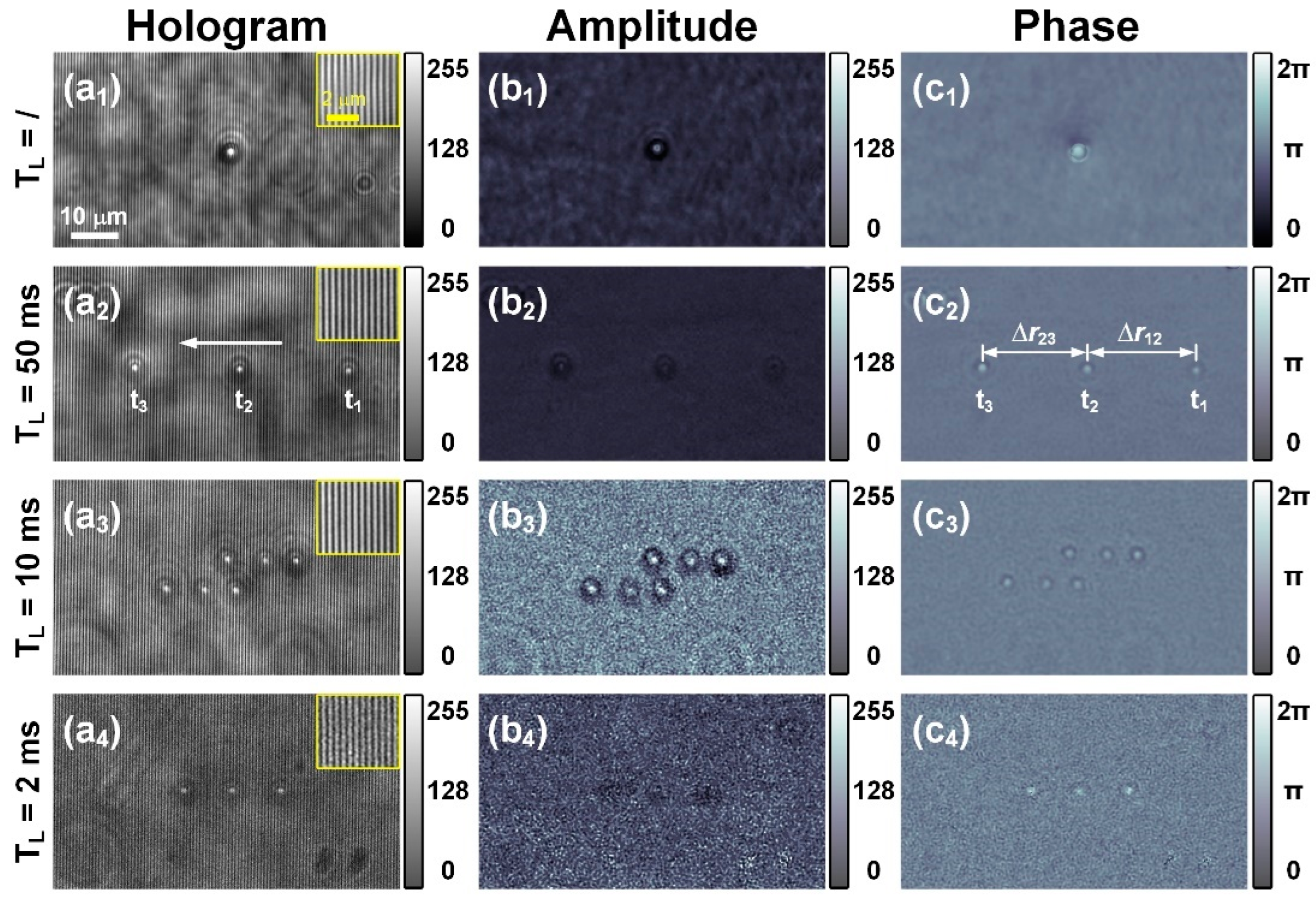

| Case | (a2) | (a3) | (a4) |
|---|---|---|---|
| TL (ms) | 50 | 10 | 2 |
| Ton (ms) | 0.5 | 0.1 | 0.02 |
| v12 (mm/s) | 0.46 | 0.65 | 5.29 |
| v23 (mm/s) | 0.45 | 0.73 | 5.12 |
| a (mm/s2) | −0.32 | 8.10 | −85 |
| F (fN) | −0.38 | 9.56 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Li, Y.; Wang, X.; Zhuo, K.; Ma, Y.; An, S.; Zheng, J.; Wen, K.; Yan, L.; Gao, P. Dynamic Measurement of Flowing Microparticles in Microfluidics Using Pulsed Modulated Digital Holographic Microscopy. Photonics 2025, 12, 411. https://doi.org/10.3390/photonics12050411
Lei Y, Li Y, Wang X, Zhuo K, Ma Y, An S, Zheng J, Wen K, Yan L, Gao P. Dynamic Measurement of Flowing Microparticles in Microfluidics Using Pulsed Modulated Digital Holographic Microscopy. Photonics. 2025; 12(5):411. https://doi.org/10.3390/photonics12050411
Chicago/Turabian StyleLei, Yunze, Yuge Li, Xiaofang Wang, Kequn Zhuo, Ying Ma, Sha An, Juanjuan Zheng, Kai Wen, Lihe Yan, and Peng Gao. 2025. "Dynamic Measurement of Flowing Microparticles in Microfluidics Using Pulsed Modulated Digital Holographic Microscopy" Photonics 12, no. 5: 411. https://doi.org/10.3390/photonics12050411
APA StyleLei, Y., Li, Y., Wang, X., Zhuo, K., Ma, Y., An, S., Zheng, J., Wen, K., Yan, L., & Gao, P. (2025). Dynamic Measurement of Flowing Microparticles in Microfluidics Using Pulsed Modulated Digital Holographic Microscopy. Photonics, 12(5), 411. https://doi.org/10.3390/photonics12050411








