MEMS Scanning Mirrors for Optical Coherence Tomography
Abstract
:1. Introduction
2. Requirements for MEMS Microscanners
3. Requirements for OCT Probes
4. Electrostatic MEMS Scanning Mirrors for OCT
4.1. Principles of Electrostatic Microactuators
4.2. Examples of Electrostatic OCT Probes
5. Electromagnetic MEMS Scanning Mirrors for OCT
5.1. Principles of Electromagnetic Microactuators
5.2. Examples of Electromagnetic OCT Probes
6. Electrothermal MEMS Scanning Mirrors for OCT
6.1. Principles of Electrothermal Microactuators
6.2. Examples of Electrothermal OCT Probes
7. Discussion on the Microscanner Design and Conclusions
Funding
Conflicts of Interest
References
- Petersen, K.E. Silicon as a mechanical materiel. IEEE 1982, 70, 420–457. [Google Scholar] [CrossRef]
- Motamedi, M.E. MOEMS: Micro-Opto-Electro-Mechanical Systems; SPIE Press Bellingham: Washington, DC, USA, 2005. [Google Scholar]
- Wu, M.C.; Solgaard, O.; Ford, J.E. Optical MEMS for Lightwave Communication. J. Lightwave Technol. 2006, 24, 4433–4454. [Google Scholar] [CrossRef] [Green Version]
- Neumann, H.; Kiesslich, R.; Wallace, M.B.; Neurath, M.F. Confocal laser endomicroscopy: Technical advances and clinical applications. Gastroenterology 2010, 139, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Gmitro, A.F.; Aziz, D. Confocal microscopy through a fiber-optic imaging bundle. Opt. Lett. 1993, 18, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Bargiel, S.; Baranski, M.; Wiemer, M.; Frömel, J.; Wang, W.S.; Gorecki, C. Technological Platform for Vertical Multi-Wafer Integration of Microscanners and Micro-Optical Components. Micromachines 2019, 10, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Koehler, M.J.; Vogel, T.; Elsner, P.; König, K.; Bückle, R.; Kaatz, M. In vivo measurement of the human epidermal thickness in different localizations by multiphoton laser tomography. Skin Res. Technol. 1010, 16, 259–264. [Google Scholar]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.D.; Kraus, M.F.; Potsaid, B.; Liu, J.J.; Choi, W.; Jayaraman, V.; Cable, A.E.; Hornegger, J.; Duker, J.S.; Fujimoto, J.G. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror. Biomed. Opt. Express 2014, 5, 293–311. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.; Tey, H.L. High-definition optical coherence tomography—An aid to clinical practice and research in dermatology. J. Dtsch. Dermatol. Ges. 2015, 13, 886–890. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.; Glaser, A.K.; Leigh, S.Y.; Chen, Y.; Wei, L.; Pillai, P.C.S.; Rosenberg, M.C.; Abeytunge, S.; Peterson, G.; Glazowski, C.; et al. Miniature in vivo MEMS-based line-scanned dual-axis confocal microscope for point-of-care pathology. Biomed. Opt. Express 2016, 2, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, P.R.; Hahb, D.; Fujino, M.; Piyawattanametha, W.; Wu, M.C. Scanning micromirrors: An overview. Proc. SPIE 2004, 5604, 195–207. [Google Scholar]
- Puliafito, C.A.; Hee, M.R.; Lin, C.P.; Reichel, E.; Schuman, J.S.; Duker, J.S.; A Izatt, J.; A Swanson, E.; Fujimoto, J.G. Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995, 102, 217–229. [Google Scholar] [CrossRef]
- Schuman, J.S.; Hee, M.R.; Arya, A.V.; Pedut-Kloizman, T.; Puliafito, C.A.; Fujimoto, J.G.; Swanson, E.A. Optical coherence tomography: A new tool for glaucoma diagnosis. Curr. Opin. Ophthalmol. 1995, 6, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Hitzenberger, C.K.; Drexler, W.; Leitgeb, R.A.; Findl, O.; Ferche, A.F. Key Developments for Partial Coherence Biometry and Optical Coherence Tomography in the Human Eye Made in Vienna. Investig. Ophthalmol. Vis. Sci. 2016, 57, 460–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivak, M.V.; Kobayashi, K.; Izatt, J.A.; Rollins, A.M.; Ung-Runyawee, R.; Chak, A.; Wong, R.C.; Isenberg, G.A.; Willis, J. High-resolution endoscopic imaging of the GI tract using optical coherence tomography. Gastrointest. Endosc. 2000, 51, 474–479. [Google Scholar] [CrossRef]
- Welzel, J. Optical coherence tomography in dermatology: A review. Ski. Res. Technol. 2001, 7, 1–9. [Google Scholar] [CrossRef]
- Pierce, M.C.; Sheridan, R.L.; Park, B.H.; Cense, B.; De Boer, J.F. Collagen denaturation can be quantified in burned human skin using polarization-sensitive optical coherence tomography. Burns 2004, 30, 511–517. [Google Scholar] [CrossRef]
- Gora, M.J.; Suter, M.J.; Tearney, G.J.; Li, X. Endoscopic optical coherence tomography: Technologies and clinical applications. Biomed. Opt. Express 2017, 8, 2405–2444. [Google Scholar] [CrossRef] [Green Version]
- Nine Point Medical. Available online: https://www.ninepointmedical.com/ (accessed on 30 December 2020).
- Cardiovascular. Available online: https://www.cardiovascular.abbott/us/en/home.html (accessed on 30 December 2020).
- Santec. Swept-Source OCT System. Available online: https://www.santec.com/en/products/oct/swept-source-oct/ (accessed on 30 December 2020).
- Thor Labs. OCTH-1300-Handheld Scanner. Available online: https://www.thorlabs.com/thorproduct.cfm?partnumber=OCTH-1300 (accessed on 30 December 2020).
- MirrorcleTechnologies. Biomedical Imaging. Available online: https://www.mirrorcletech.com/wp/applications/#biomed (accessed on 30 December 2020).
- Wang, D.; Liang, P.; Samuelson, S.; Jia, H.; Ma, J.; Xie, H. Correction of image distortions in endoscopic optical coherence tomography based on two-axis scanning MEMS mirrors. Biomed. Opt. Express 2013, 4, 2066–2077. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.Y.; Keeler, E.G. Progress of MEMS Scanning Micromirrors for Optical Bio-Imaging. Micromachines 2015, 6, 1675–1689. [Google Scholar] [CrossRef] [Green Version]
- McCormick, D.; Jung, W.; Milanovic, V.; Chen, Z.; Tien, N. 3-D MEMS based real-time minimally invasive endoscopic optical coherence tomography. In Proceedings of the IEEE/LEOS International Conference on Optical MEMS and Their Applications Conference, Oulu, Finland, 25–26 August 2005; pp. 25–26. [Google Scholar]
- Urey, H.; Wine, D.W.; Osborn, T.D. Optical performance requirements for MEMS-scanner based microdisplays. Proc. SPIE 2000, 4178, 176–185. [Google Scholar]
- Hwang, K.; Seo, Y.-H.; Jeong, K.-H. Microscanners for optical endomicroscopic applications. Micro Nano Syst. Lett. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Urey, H.; Kan, C.; O Davis, W. Vibration mode frequency formulae for micromechanical scanners. J. Micromech. Microeng. 2005, 15, 1713–1721. [Google Scholar] [CrossRef]
- Holmstrom, S.T.S.; Baran, U.; Urey, H. MEMS Laser Scanners: A Review. J. Microelectromech. Syst. 2014, 23, 259–275. [Google Scholar] [CrossRef]
- Boppart, S.A.; Bouma, B.E.; Pitris, C.; Tearney, G.J.; Fujimoto, J.G.; Brezinski, M.E. Forward-imaging instruments *for optical coherence tomography. Opt. Lett. 1997, 22, 1618–1620. [Google Scholar] [CrossRef]
- Sun, J.; Xie, H. MEMS-Based Endoscopic Optical Coherence Tomography. Int. J. Opt. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zaeh, M.; Moesl, J.; Musiol, J.; Oefele, F. Material Processing with Remote Technology—Revolution or Evolution? Phys. Procedia 2010, 5, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Pelsue, K. Precision, Post-Objective, Two-Axis, Galvanometer Scanning. Proc. SPIE 1983, 390, 70–79. [Google Scholar] [CrossRef]
- A Flusberg, B.; Cocker, E.D.; Piyawattanametha, W.; Jung, J.C.; Cheung, E.L.M.; Schnitzer, M.J. Fiber-optic fluorescence imaging. Nat. Methods 2005, 2, 941–950. [Google Scholar] [CrossRef]
- Tanguy, Q. Design and Fabrication of a MEMS Scanner for OCT Imaging Endo-Microscopic Probe. Ph.D. Thesis, Université Bourgogne Franche-Comté, Besançon, France, February 2018. [Google Scholar]
- Petersen, K.E. Silicon torsional scanning mirror. IBM J. Res. Dev. 1980, 24, 631–637. [Google Scholar] [CrossRef]
- Tang, W.C.; Nguyen, T.C.H.; Juan, W.H.; Howe, R.T. Electrostatic-comb drive of lateral polysilicon resonator. Sens. Actuators A Phys. 1990, 21, 328–331. [Google Scholar] [CrossRef]
- Selvakumar, A.; Najafi, K.; Juan, W.; Pang, S. Vertical comb array microactuators. In Proceedings of the IEEE Micro Electro Mechanical Systems, Amsterdam, The Netherlands, 29 January–2 February 1995; pp. 43–48. [Google Scholar]
- Degani, O.; Socher, E.; Lipson, A.; Lejtner, T.; Setter, D.; Kaldor, S.; Nemirovsky, Y. Pull-in study of an electrostatic torsion microactuator. J. Microelectromech. Syst. 1998, 7, 373–379. [Google Scholar] [CrossRef]
- Jung, W.; McCormick, D.T.; Zhang, J.; Wang, L.; Tien, N.C.; Chen, Z. Three-dimensional endoscopic optical coherence tomography by use of a two-axis microelectromechanical scanning mirror. Appl. Phys. Lett. 2006, 88, 163901. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Teo, J.H.S.; Xu, Y.; Premachandran, C.S.; Chen, N.; Kotlanka, R.; Olivo, M.; Sheppard, C.J.R. A two axes scanning SOI MEMS micromirror for endoscopic bioimaging. J. Micromech. Microeng. 2007, 18, 025001. [Google Scholar] [CrossRef]
- Kumar, K.; Condit, J.C.; McElroy, A.; Kemp, N.J.; Hoshino, K.; Milner, T.E.; Zhang, X. Fast 3D in vivo swept-source optical coherence tomography using a two-axis MEMS scanning micromirror. J. Opt. A Pure Appl. Opt. 2008, 10, 044013. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Fu, L.; Wang, X.; Gong, Z.; Samuelson, S.; Duan, C.; Jia, H.; Ma, J.S.; Xie, H. Endoscopic swept source optical coherence tomography based on a two-axis microelectromechanical system mirror. J. Biomed. Opt. 2013, 18, 086005. [Google Scholar] [CrossRef]
- Aguirre, A.D.; Hertz, P.R.; Chen, Y.; Fujimoto, J.G.; Piyawattanametha, W.; Fan, L.; Wu, M.C. Two-axis MEMS scanning catheter for ultrahigh resolution three-dimensional and en face imaging. Opt. Express 2007, 15, 2445–2453. [Google Scholar] [CrossRef] [Green Version]
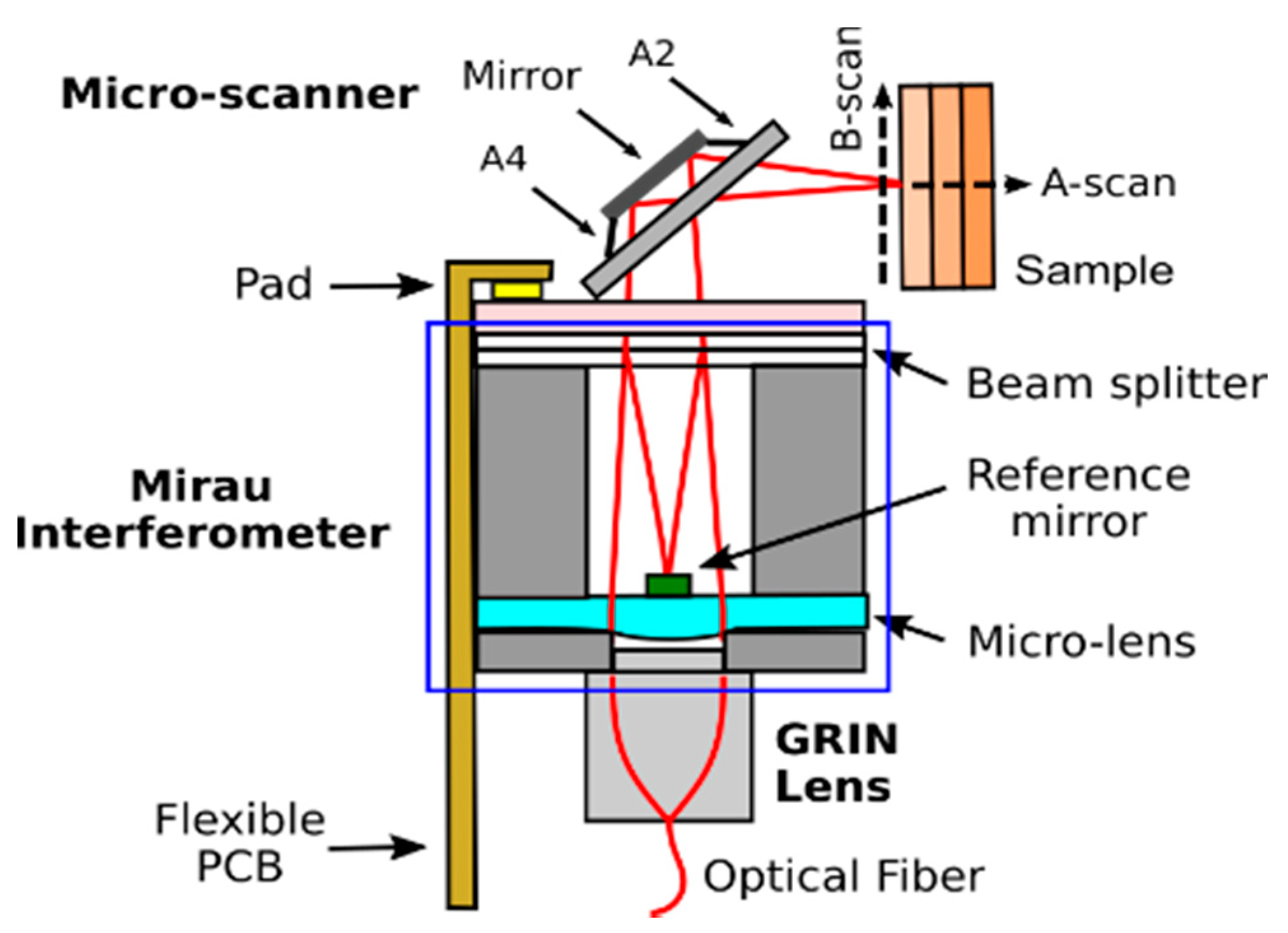
- Gorecki, C.; Lullin, J.; Perrin, S.; Bargiel, S.; Albero, J.; Gaiffe, O.; Rutkowski, J.; Cote, J.M.; Krauter, J.; Osten, W.; et al. Micromachined phase-shifted array-type Mirau interferometer for swept-source OCT imaging: Design, microfabrication and experimental validation. Biomed. Opt. Express 2019, 10, 1111–1125. [Google Scholar] [CrossRef]
- Lullin, J.; Bargiel, S.; Lemoal, P.; Perrin, S.; Albero, J.; Passilly, N.; Froehly, L.; Lardet-Vieudrin, F.; Gorecki, C. An electrostatic vertical microscanner for phase modulating array-type Mirau microinterferometry. J. Micromech. Microeng. 2015, 25, 115013. [Google Scholar] [CrossRef] [Green Version]
- Miyajima, H. Development of a MEMS electromagnetic optical scanner for a commercial laser scanning microscope. J. Micro/Nanolithograph. MEMS MOEMS 2004, 3, 348. [Google Scholar] [CrossRef]
- Asada, N.; Takeuchi, M.; Vaganov, V.; Belov, N.; Hout, S.I.; Sluchak, I. Silicon micro-optical scanner. Sens. Actuators A Phys. 2000, 83, 284–290. [Google Scholar] [CrossRef]
- Judy, J.W.; Muller, R.S. Magnetically actuated, addressable microstructures. J. Microelectromech. Syst. 1997, 6, 249–256. [Google Scholar] [CrossRef]
- Miyajima, H.; Asaoka, N.; Arima, M.; Minamoto, Y.; Murakami, K.; Tokuda, K.; Matsumoto, K. A Durable, Shock Resistant Electromagnetic Optical Scanner with Polyimide-Based Hinges. J. Microelectromech. Syst. 2001, 10, 418–424. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.; Kim, J.Y.; Lim, G.; Kim, J.; Kim, C. A 2-axis Polydimethylsiloxane (PDMS) based electromagnetic MEMS scanning mirror for optical coherence tomography. Proc. SPIE 2016, 9698, 969812. [Google Scholar]
- Kim, K.H.; Park, B.H.; Maguluri, G.N.; Lee, T.W.; Rogomentich, F.J.; Bancu, M.G.; Bouma, B.E.; De Boer, J.F.; Bernstein, J.J. Two-axis magnetically-driven MEMS scanning catheter for endoscopic high-speed optical coherence tomography. Opt. Express 2007, 15, 18130–18140. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Abe, Y.; Iwamatsu, S.; Kobayashi, S.; Takahashi, Y.; Sato, T. Electromagnetically Driven Two-Axis Optical Beam Steering MEMS Mirror and Its Dependence of Actuation on Magnetic Field. Electron. Commun. Jpn. 2011, 94, 24–31. [Google Scholar] [CrossRef]
- Potekhina, A.; Wang, C. Review of Electrothermal Actuators and Applications. Actuators 2019, 8, 69. [Google Scholar] [CrossRef] [Green Version]
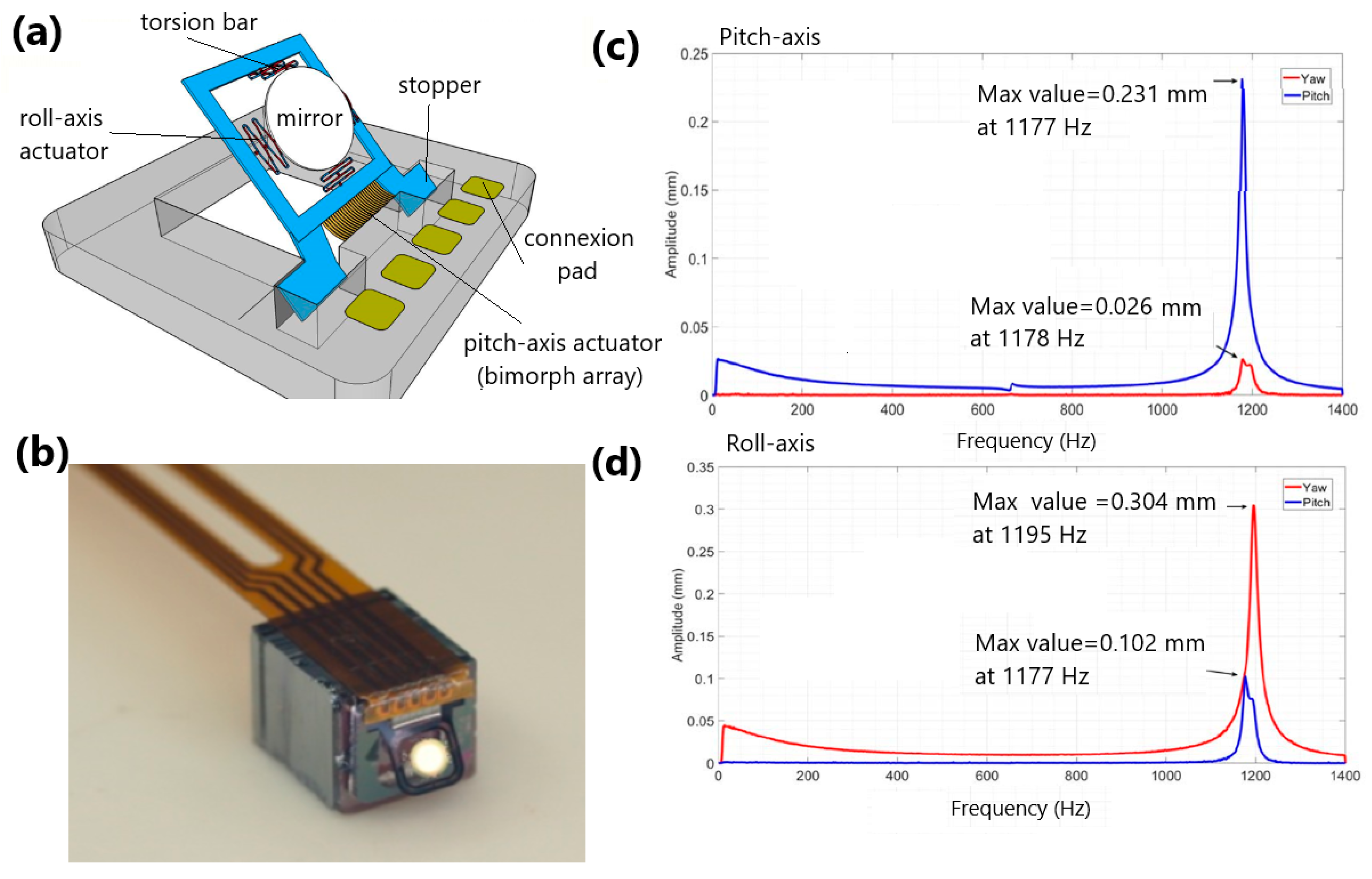
- Tanguy, Q.A.; Bargiel, S.; Xie, H.; Passilly, N.; Barthès, M.; Gaiffe, O.; Rutkowski, J.; Lutz, P.; Gorecki, C. Design and Fabrication of a 2-Axis Electrothermal MEMS Micro-Scanner for Optical Coherence Tomography. Micromachines 2017, 8, 146. [Google Scholar] [CrossRef]
- Sun, J.; Guo, S.; Wu, L.; Liu, L.; Choe, S.-W.; Sorg, B.S.; Xie, H. 3D In Vivo optical coherence tomography based on a low-voltage, large-scan-range 2D MEMS mirror. Opt. Express 2010, 18, 12065–12075. [Google Scholar] [CrossRef]
- Wu, L.; Xie, H. A large vertical displacement electrothermal bimorph microactuator with very small lateral shift. Sens. Actuators A Phys. 2008, A 145–146, 371–379. [Google Scholar] [CrossRef]
- Struk, P.; Bargiel, S.; Tanguy, Q.A.A.; Ramirez, F.E.G.; Passilly, N.; Lutz, P.; Gaiffe, O.; Xie, H.; Gorecki, C. Swept-source optical coherence tomography microsystem with an integrated Mirau interferometer and electrothermal micro-scanner. Opt. Lett. 2018, 43, 4847–4850. [Google Scholar] [CrossRef] [PubMed]
- Struk, P.; Bargiel, S.; Froehly, L.; Baranski, M.; Passilly, N.; Albero, J.; Gorecki, C. Swept source optical coherence tomography endomicroscope based on vertically integrated Mirau micro interferometer: Concept and technology. IEEE Sens. J. 2015, 15, 7061–7070. [Google Scholar] [CrossRef]
- Albero, J.; Perrin, S.; Bargiel, S.; Passilly, N.; Baranski, M.; Gauthier-Manuel, L.; Bernard, F.; Lullin, J.; Froehly, L.; Krauter, J.; et al. Dense arrays of millimeter-sized glass lenses fabricated at wafer-level. Opt. Express 2015, 23, 11702–11712. [Google Scholar] [CrossRef] [PubMed]
- Tanguy, Q.A.A.; Gaiffe, O.; Passilly, N.; Cote, J.-M.; Cabodevila, G.; Bargiel, S.; Lutz, P.; Xie, H.; Gorecki, C. Real-time Lissajous imaging with a low-voltage 2-axis MEMS scanner based on electrothermal actuation. Opt. Express 2020, 28, 8512–8527. [Google Scholar] [CrossRef] [PubMed]
- García-Ramírez, F.E.; Bargiel, S.; Gaiffe, O.; Tanguy, Q.A.; Struk, P.; Cote, J.M.; Passilly, N.; Lutz, P.; Gorecki, C.; Xie, H. Characterization of an integrated MOEMS scanning probe towards real-time Lissajous-based SS-OCT imaging for endoscopic applications. In Proceedings of the OSA Biophotonics Congress—Biomedical Optics, Fort Lauderdale, FL, USA, 20 April 2020. [Google Scholar]
- Luo, S.; Wang, D.; Tang, J.; Zhou, L.; Duan, C.; Wang, N.; Liu, H.; Zhu, Y.; Li, G.; Zhao, H.; et al. Circumferential-scanning endoscopic optical coherence tomography probe based on a circular array of six 2-axis MEMS mirrors. Biomed. Opt. Express 2018, 9, 2104–2114. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Xie, H. A millimeter-tunable-range microlens for endoscopic biomedical imaging applications. IEEE J. Quantum Electron. 2010, 46, 1237–1244. [Google Scholar] [CrossRef]
- Liu, L.; Wang, E.; Zhang, X.; Liang, W.; Li, X.; Xie, H. MEMS-based 3D confocal scanning microendoscope using MEMS scanners for both lateral and axial scan. Sens. Actuators A 2014, 215, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yu, X.; Feng, P.X.-L.; Li, J.; Xie, H. A MEMS lens scanner based on serpentine electrothermal bimorph actuators for large axial tuning. Opt. Express 2020, 28, 23439. [Google Scholar] [CrossRef]
- Pengwang, E.; Rabenorosoa, M.; Rakotondrabe, N. Andreff, “Scanning Micromirror Platform Based on MEMS Technology for Medical Application”. Micromachines 2016, 7, 24. [Google Scholar] [CrossRef]
- Tsai, J.C.; Lu, L.C.; Hsu, W.C.; Sun, C.W.; Wu, M.C. Linearization of a two-axis MEMS scanner driven by vertical comb-drive actuators. J. Micromech. Microeng. 2008, 18, 015015. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.; Klose, T.; Drabe, C.; Schenk, H. Fabrication and characterization of a dynamically flat high resolution micro-scanner. J. Opt. A Pure Appl. Opt. 2008, 10, 044005. [Google Scholar] [CrossRef]
- Shaeffer, D.K. MEMS inertial sensors: A tutorial overview. IEEE Commun. Mag. 2013, 51, 100–109. [Google Scholar] [CrossRef]



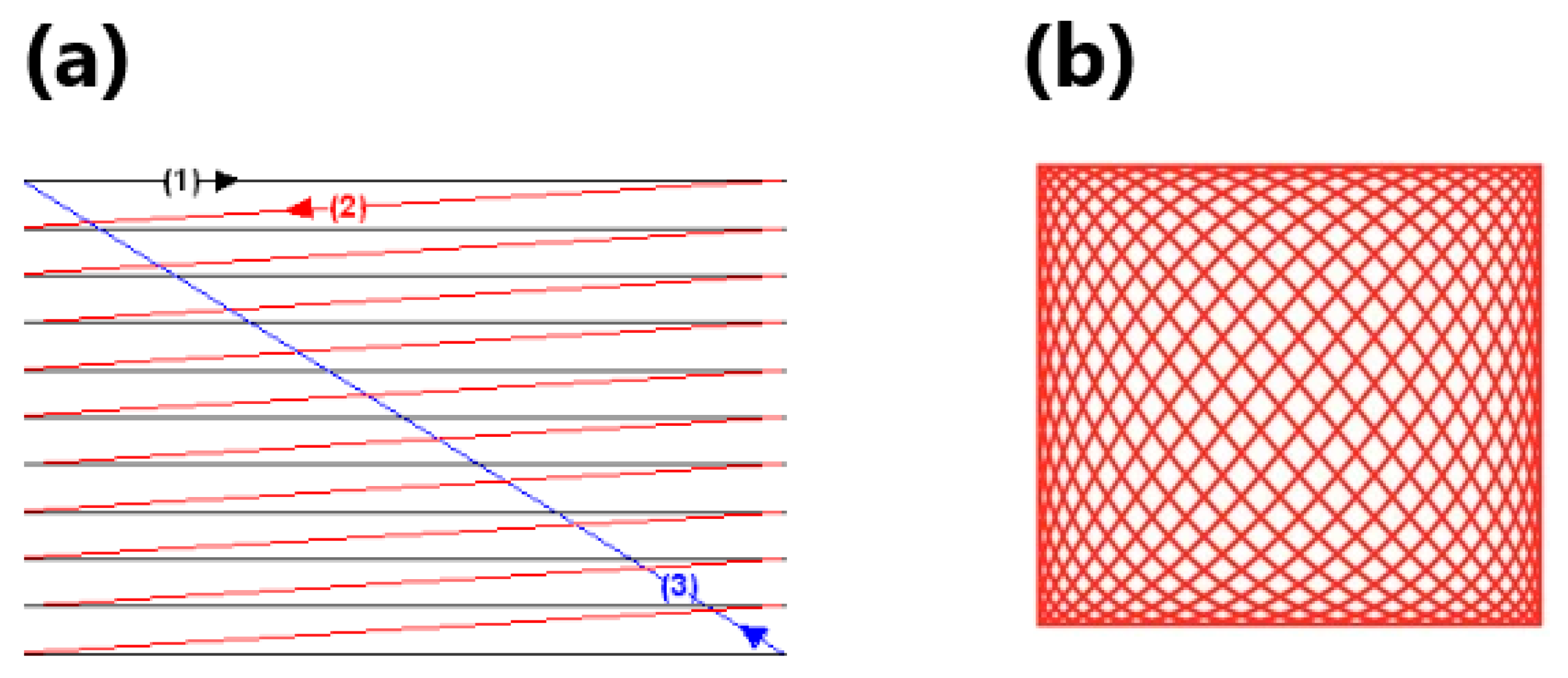









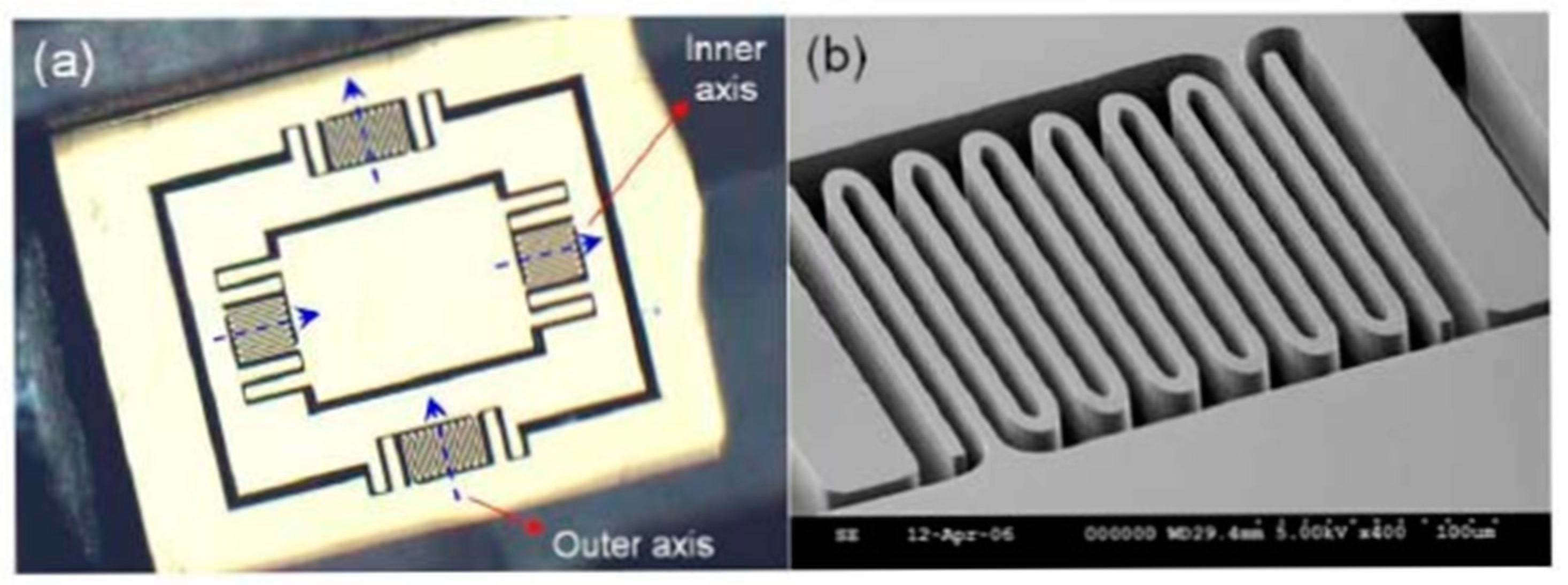


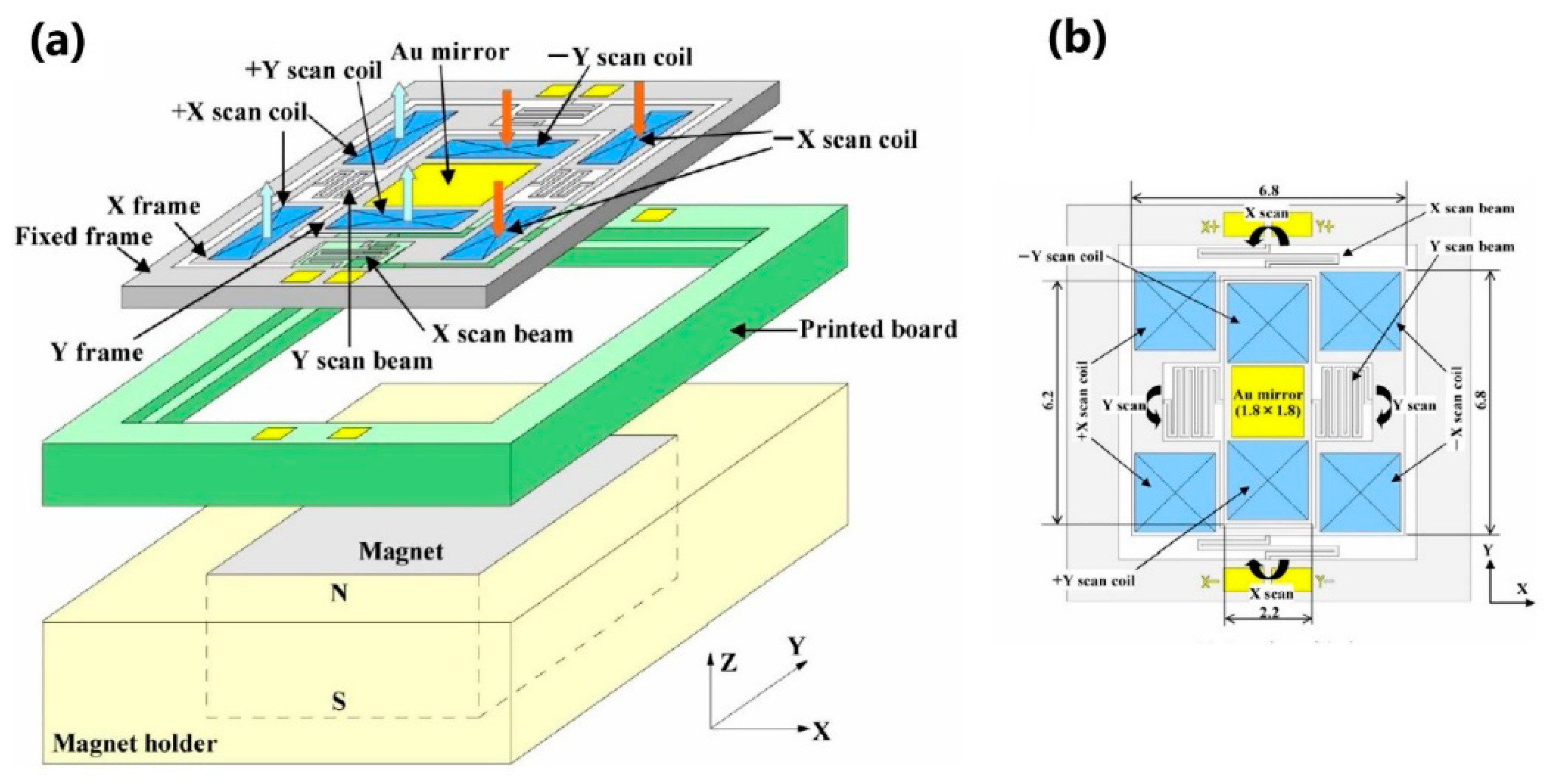

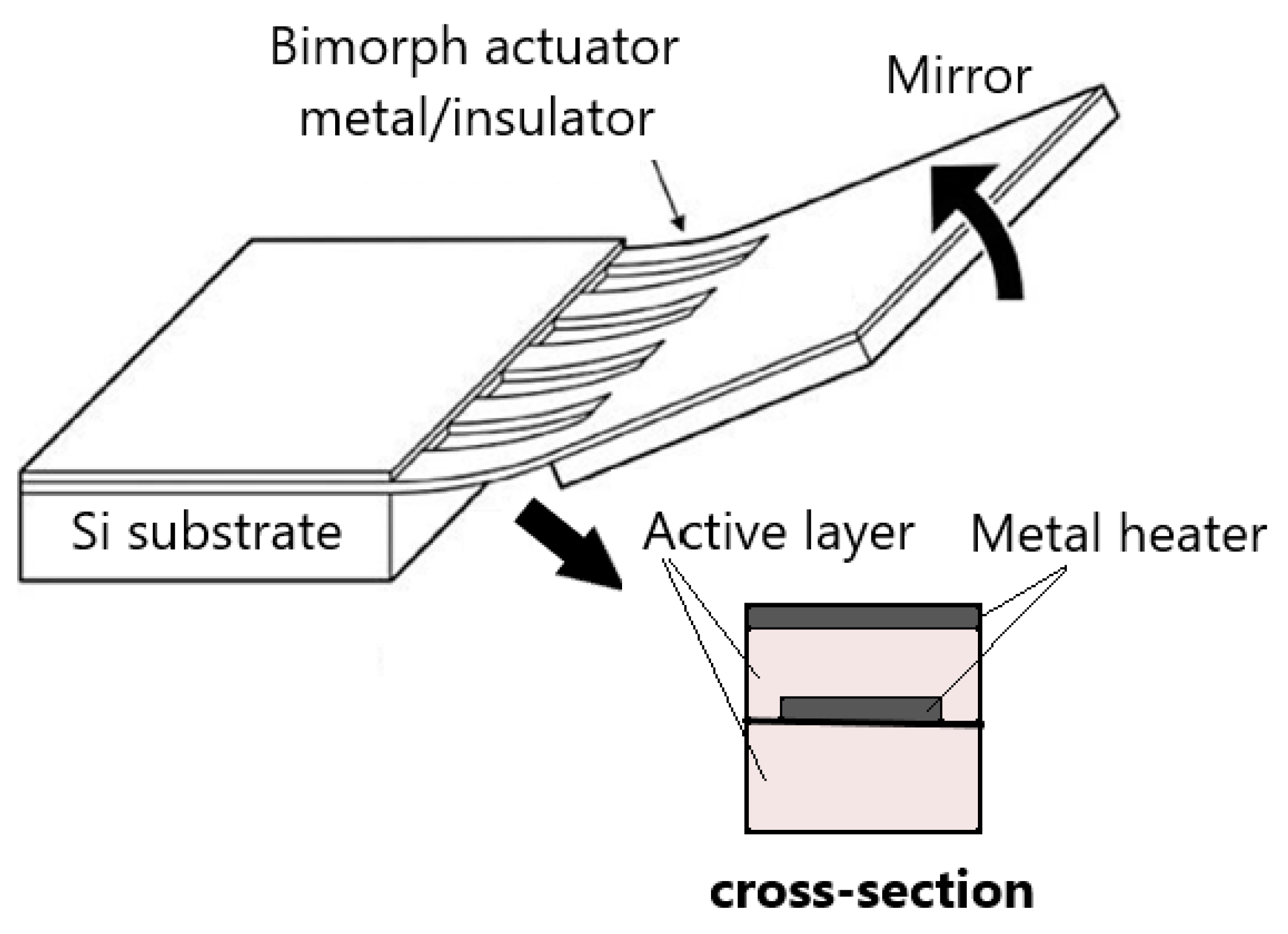




| Mirror Size (mm)/Data from [46] | Angular Deflection (°)/Data from [46] | Resonance Frequency (Hz)/Data from [46] | Advantages | Drawbacks | |
|---|---|---|---|---|---|
| Electrostatic all | Fast response Low power consumption Large scan angle | Pull-in effect High driving voltage (50–100 V) Low force | |||
| Linear comb | ~1 | <10 Ave scan: 8.5 Ave piston: 29 µm | 250–5000 (ave 1800) | ||
| Vertical comb | ~1 | >10 Ave scan: 14 Ave piston: 94 µm | 150–10,000 (ave 5000) | ||
| Characteristics | electrostatic attractive force between conductors | a pair of electrodes with air gap | requires mechanical resonance to enhance scan angle | Small temperature dependance | Silicon DRIE and metallization |
| Electromagnetic | 0.8–3.5 | Maximum: 20 Ave scan: 15 Ave piston: 5 µm | 100–1000 | Larger driving Lower driving voltage Large scan angle | High power consumption External magnets increasing the size Electromagnetic interferences |
| Characteristics | Lorentz force between current and magnetic field | coil and permanent magnet | size limited by magnet | Multiple layers of metal and insulator for coil | Small temperature dependance |
| Electrothermal | 0.5–1 | ~25, maximum 64 Ave scan: 27° Ave piston: 270 µm | 117–1000 | Large scan angle Low driving voltage High fill-factor | High power consumption Slow response |
| Characteristics | thermal expansion by Joule effect | materials with different thermal expansion coefficients | multiple layers of metal and insulator for heater | Requires relatively high power | Bimorph sensitive to temperature change |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorecki, C.; Bargiel, S. MEMS Scanning Mirrors for Optical Coherence Tomography. Photonics 2021, 8, 6. https://doi.org/10.3390/photonics8010006
Gorecki C, Bargiel S. MEMS Scanning Mirrors for Optical Coherence Tomography. Photonics. 2021; 8(1):6. https://doi.org/10.3390/photonics8010006
Chicago/Turabian StyleGorecki, Christophe, and Sylwester Bargiel. 2021. "MEMS Scanning Mirrors for Optical Coherence Tomography" Photonics 8, no. 1: 6. https://doi.org/10.3390/photonics8010006
APA StyleGorecki, C., & Bargiel, S. (2021). MEMS Scanning Mirrors for Optical Coherence Tomography. Photonics, 8(1), 6. https://doi.org/10.3390/photonics8010006





