Envisioning Quantum Electrodynamic Frameworks Based on Bio-Photonic Cavities
Abstract
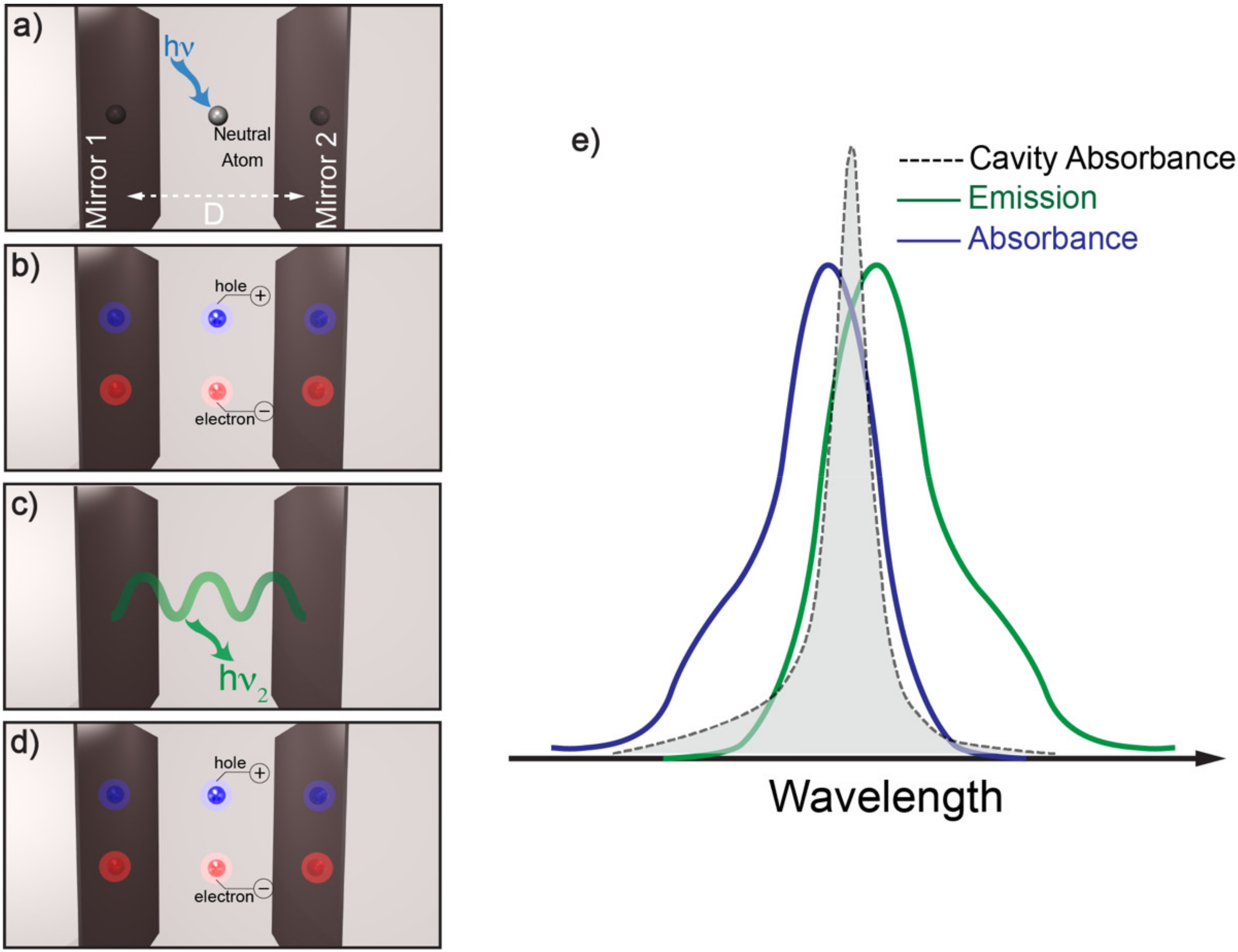
:1. Introduction
2. Resonant Meta-Surfaces
3. Biopolymers for Soft-Molding Processes
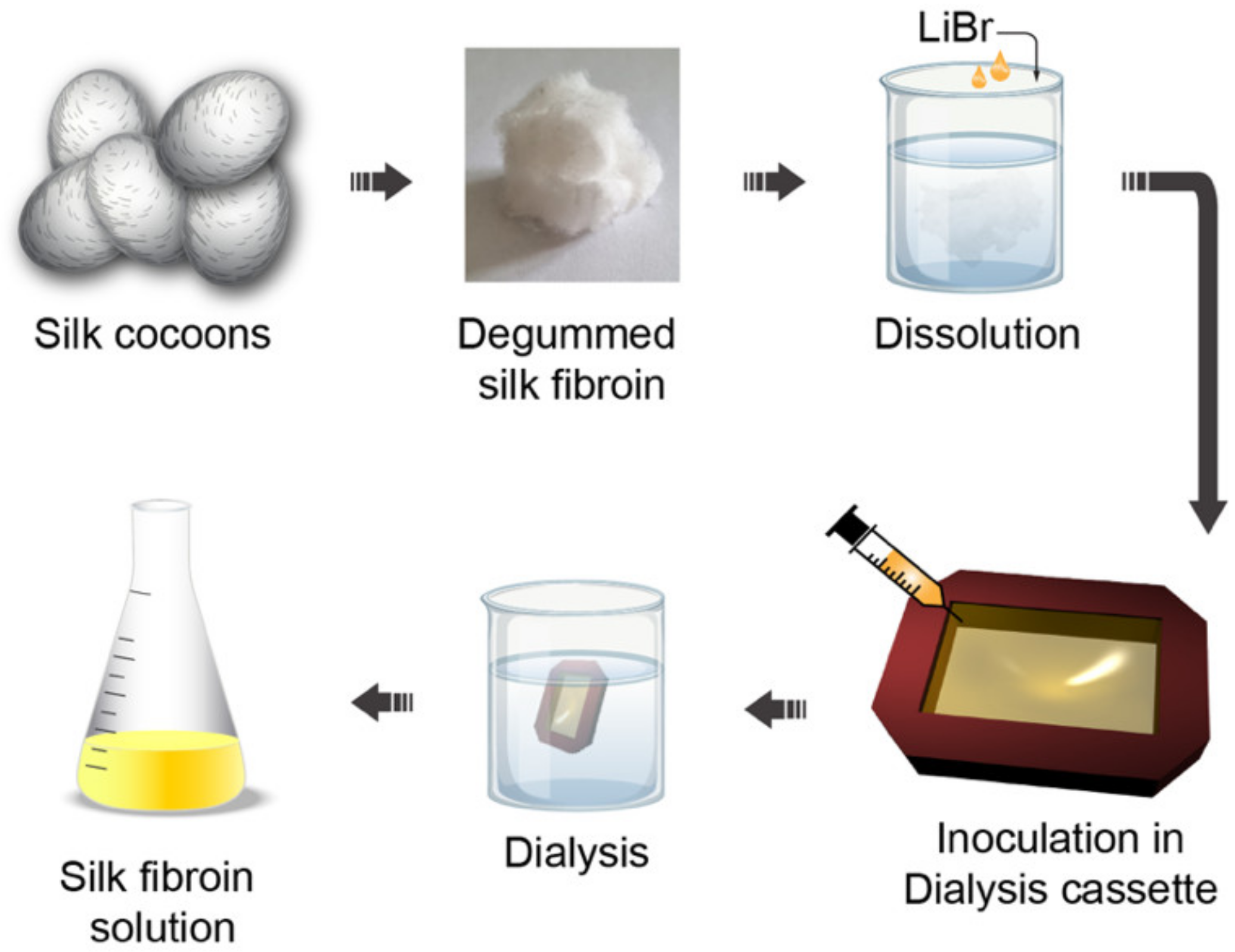
3.1. Silk
3.2. Cellulose
3.3. Hydrogels
4. Bio-Luminescent Gain Materials
4.1. Intrinsic Fluorophores: Aromatic Amino Acids and Enzyme Cofactors
4.2. Aggregation-Induced Emission (AIE) Materials
4.3. Fluorescent Proteins—Part A: Phycobiliproteins and Phytofluor
4.4. Fluorescent Proteins—Part B: Green Fluorescent Proteins and Similar
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fassioli, F.; Dinshaw, R.; Arpin, P.C.; Scholes, G.D. Photosynthetic Light Harvesting: Excitons and Coherence. J. R. Soc. Interface 2014, 11, 20130901. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; Wiley, J., Ed.; Wiley: Hoboken, NJ, USA, 1996; ISBN 978-0471415268. [Google Scholar]
- Snoke, D.W. Solid State Physics; Cambridge University Press: Cambridge, UK, 2020; ISBN 9781108123815. [Google Scholar]
- Einstein, A. Über Einen Die Erzeugung Und Verwandlung Des Lichtes Betreffenden Heuristischen Gesichtspunkt. Ann. Der Phys. 1905, 322, 132–148. [Google Scholar] [CrossRef]
- Scully, M.O.; Zubairy, M.S. Quantum Optics; Cambridge University Press: Cambridge, UK, 1997; p. 630. ISBN 0521434580. [Google Scholar]
- Kasap, S.O. Optoelectronics and Photonics: Principles and Practices; Prentice Hall: Hoboken, NJ, USA, 2001; p. 340. ISBN 9780201610871. [Google Scholar]
- Born, M.; Wolf, E. Principles of Optics Electromagnetic Theory of Propagation, Interference and Diffraction of Light; Pergamon Press: Oxford, UK, 1980; p. 952. ISBN 0521642221. [Google Scholar]
- Jacob, Z.; Smolyaninov, I.I.; Narimanov, E.E. Broadband Purcell Effect: Radiative Decay Engineering with Metamaterials. Appl. Phys. Lett. 2012, 100, 181105. [Google Scholar] [CrossRef] [Green Version]
- Jahani, S.; Zhao, H.; Jacob, Z. Switching Purcell Effect with Nonlinear Epsilon-near-Zero Media. Appl. Phys. Lett. 2018, 113, 021103. [Google Scholar] [CrossRef] [Green Version]
- Akselrod, G.M.; Argyropoulos, C.; Hoang, T.B.; Ciracì, C.; Fang, C.; Huang, J.; Smith, D.R.; Mikkelsen, M.H. Probing the Mechanisms of Large Purcell Enhancement in Plasmonic Nanoantennas. Nat. Photonics 2014, 8, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.J.; Sokhoyan, R.; Cheng, W.H.; Shirmanesh, G.K.; Davoyan, A.R.; Pala, R.A.; Thyagarajan, K.; Atwater, H.A. Dynamically Controlled Purcell Enhancement of Visible Spontaneous Emission in a Gated Plasmonic Heterostructure. Nat. Commun. 2017, 8, 1631. [Google Scholar] [CrossRef] [Green Version]
- Caligiuri, V.; Palei, M.; Imran, M.; Manna, L.; Krahne, R. Planar Double-Epsilon-Near-Zero Cavities for Spontaneous Emission and Purcell Effect Enhancement. ACS Photonics 2018, 5, 2287–2294. [Google Scholar] [CrossRef]
- Jeantet, A.; Chassagneux, Y.; Raynaud, C.; Roussignol, P.; Lauret, J.S.; Besga, B.; Estève, J.; Reichel, J.; Voisin, C. Widely Tunable Single-Photon Source from a Carbon Nanotube in the Purcell Regime. Phys. Rev. Lett. 2016, 116, 247402–247407. [Google Scholar] [CrossRef] [Green Version]
- Purcell, E.M. Spontaneous Emission Probabilities at Radio Frequencies. Proc. Am. Phys. Soc. 1946, 69, 681. [Google Scholar]
- Kristensen, P.T.; Van Vlack, C.; Hughes, S. Generalized Effective Mode Volume for Leaky Optical Cavities. Opt. Lett. 2012, 37, 1649. [Google Scholar] [CrossRef] [PubMed]
- Muljarov, E.A.; Langbein, W. Exact Mode Volume and Purcell Factor of Open Optical Systems. Phys. Rev. B 2016, 94, 235438. [Google Scholar] [CrossRef] [Green Version]
- Coccioli, R.; Boroditsky, M.; Kim, K.W.; Rahmat-Samii, Y.; Yablonovitch, E. Smallest Possible Electromagnetic Mode Volume in a Dielectric Cavity. IEE Proc. Optoelectron. 1998, 145, 391–396. [Google Scholar] [CrossRef]
- Li, X.; Smalley, J.S.T.; Li, Z.; Gu, Q. Effective Modal Volume in Nanoscale Photonic and Plasmonic Near-Infrared Resonant Cavities. Appl. Sci. 2018, 8, 1464. [Google Scholar] [CrossRef]
- Gao, J.; McMillan, J.F.; Wu, M.-C.; Zheng, J.; Assefa, S.; Wong, C.W. Demonstration of an Air-Slot Mode-Gap Confined Photonic Crystal Slab Nanocavity with Ultrasmall Mode Volumes. Appl. Phys. Lett. 2010, 96, 051123. [Google Scholar] [CrossRef] [Green Version]
- Bahari, B.; Tellez-Limon, R.; Kante, B. Directive and Enhanced Spontaneous Emission Using Shifted Cubes Nanoantenna. J. Appl. Phys. 2016, 120, 093106. [Google Scholar] [CrossRef] [Green Version]
- Nezhad, M.P.; Simic, A.; Bondarenko, O.; Slutsky, B.; Mizrahi, A.; Feng, L.; Lomakin, V.; Fainman, Y. Room-Temperature Subwavelength Metallo-Dielectric Lasers. Nature Photonics 2010, 4, 395–399. [Google Scholar] [CrossRef]
- Gérard, J.M.; Gayral, B. Strong Purcell Effect for InAs Quantum Boxes in Three-Dimensional Solid-State Microcavities. J. Lightwave Technol. 1999, 17, 2089–2095. [Google Scholar] [CrossRef]
- Zhang, L.; Gogna, R.; Burg, W.; Tutuc, E.; Deng, H. Photonic-Crystal Exciton-Polaritons in Monolayer Semiconductors. Nat. Commun. 2018, 9, 713. [Google Scholar] [CrossRef] [Green Version]
- Plumhof, J.D.; Stöferle, T.; Mai, L.; Scherf, U.; Mahrt, R.F. Room-Temperature Bose-Einstein Condensation of Cavity Exciton-Polaritons in a Polymer. Nat. Mater. 2014, 13, 247–252. [Google Scholar] [CrossRef]
- Skolnick, M.S.; Fisher, T.A.; Whittaker, D.M. Strong Coupling Phenomena in Quantum Microcavity Structures. Semicond. Sci. Technol. 1998, 13, 645–669. [Google Scholar] [CrossRef]
- Lidzey, D.G.; Bradley, D.D.C.; Skolnick, M.S.; Virgili, T.; Walker, S.; Whittaker, D.M. Strong Exciton-Photon Coupling in an Organic Semiconductor Microcavity. Nature 1998, 395, 53–55. [Google Scholar] [CrossRef]
- Hennessy, K.; Badolato, A.; Winger, M.; Gerace, D.; Atatüre, M.; Gulde, S.; Fält, S.; Hu, E.L.; Imamoǧlu, A. Quantum Nature of a Strongly Coupled Single Quantum Dot-Cavity System. Nature 2007, 445, 896–899. [Google Scholar] [CrossRef] [Green Version]
- Houdré, R.; Stanley, R.P.; Oesterle, U.; Ilegems, M.; Weisbuch, C. Room-Temperature Cavity Polaritons in a Semiconductor Microcavity. Phys. Rev. B 1994, 49, 16761–16764. [Google Scholar] [CrossRef] [PubMed]
- Carusotto, I.; Ciuti, C. Quantum Fluids of Light. Rev. Mod. Phys. 2013, 85, 299–366. [Google Scholar] [CrossRef] [Green Version]
- Yoshle, T.; Scherer, A.; Hendrickson, J.; Khitrova, G.; Gibbs, H.M.; Rupper, G.; Ell, C.; Shchekin, O.B.; Deppe, D.G. Vacuum Rabi Splitting with a Single Quantum Dot in a Photonic Crystal Nanocavity. Nature 2004, 432, 200–203. [Google Scholar] [CrossRef]
- Coppolaro, M.; Moccia, M.; Castaldi, G.; Alu, A.; Galdi, V. Surface-Wave Propagation on Non-Hermitian Metasurfaces with Extreme Anisotropy. IEEE Trans. Microw. Theory Tech. 2021, 69, 2060–2071. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhu, A.Y.; Capasso, F. Flat Optics with Dispersion-Engineered Metasurfaces. Nat. Rev. Mater. 2020, 5, 604–620. [Google Scholar] [CrossRef]
- Di Meo, V.; Moccia, M.; Sanità, G.; Crescitelli, A.; Lamberti, A.; Galdi, V.; Rendina, I.; Esposito, E. Advanced DNA Detection via Multispectral Plasmonic Metasurfaces. Front. Bioeng. Biotechnol. 2021, 9, 666121. [Google Scholar] [CrossRef]
- Yu, N.; Genevet, P.; Kats, M.A.; Aieta, F.; Tetienne, J.-P.; Capasso, F.; Gaburro, Z. Light Propagation with Phase Discontinuities: Generalized Laws of Reflection and Refraction. Science 2011, 334, 333–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaltout, A.M.; Shalaev, V.M.; Brongersma, M.L. Spatiotemporal Light Control with Active Metasurfaces. Science 2019, 364, 298–302. [Google Scholar] [CrossRef]
- Krasnok, A.; Tymchenko, M.; Alù, A. Nonlinear Metasurfaces: A Paradigm Shift in Nonlinear Optics. Mater. Today 2018, 21, 8–21. [Google Scholar] [CrossRef]
- Wu, P.C.; Tsai, W.-Y.; Chen, W.T.; Huang, Y.-W.; Chen, T.-Y.; Chen, J.-W.; Liao, C.Y.; Chu, C.H.; Sun, G.; Tsai, D.P. Versatile Polarization Generation with an Aluminum Plasmonic Metasurface. Nano Lett. 2016, 17, 445–452. [Google Scholar] [CrossRef]
- Caligiuri, V.; De Sio, L.; Petti, L.; Capasso, R.; Rippa, M.; Maglione, M.G.; Tabiryan, N.; Umeton, C. Electro-/All-Optical Light Extraction in Gold Photonic Quasi-Crystals Layered with Photosensitive Liquid Crystals. Adv. Opt. Mater. 2014, 2, 950–955. [Google Scholar] [CrossRef]
- Kamali, S.M.; Arbabi, E.; Arbabi, A.; Faraon, A. A Review of Dielectric Optical Metasurfaces for Wavefront Control. Nanophotonics 2018, 7, 1041–1068. [Google Scholar] [CrossRef]
- Ding, F.; Pors, A.; Bozhevolnyi, S.I. Gradient Metasurfaces: A Review of Fundamentals and Applications. Rep. Prog. Phys. 2017, 81, 026401. [Google Scholar] [CrossRef] [Green Version]
- Kruk, S.; Kivshar, Y. Functional Meta-Optics and Nanophotonics Governed by Mie Resonances. ACS Photonics 2017, 4, 2638–2649. [Google Scholar] [CrossRef] [Green Version]
- Mueller, J.P.B.; Rubin, N.A.; Devlin, R.C.; Groever, B.; Capasso, F.; Paulson, J.A. Metasurface Polarization Optics: Independent Phase Control of Arbitrary Orthogonal States of Polarization. Phys. Rev. Lett. 2017, 118, 113901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wu, P.C.; Su, V.-C.; Lai, Y.-C.; Chu, C.H.; Chen, J.-W.; Lu, S.-H.; Chen, J.; Xu, B.; Kuan, C.-H.; et al. Broadband Achromatic Optical Metasurface Devices. Nature Commun. 2017, 8, 187. [Google Scholar] [CrossRef]
- Lininger, A.; Zhu, A.Y.; Park, J.-S.; Palermo, G.; Chatterjee, S.; Boyd, J.; Capasso, F.; Strangi, G. Optical Properties of Metasurfaces Infiltrated with Liquid Crystals. Proc. Natl. Acad. Sci. USA 2020, 117, 20390–20396. [Google Scholar] [CrossRef] [PubMed]
- Tuz, V.R.; Khardikov, V.V.; Kivshar, Y.S. All-Dielectric Resonant Metasurfaces with a Strong Toroidal Response. ACS Photonics 2018, 5, 1871–1876. [Google Scholar] [CrossRef]
- Santiago-Cruz, T.; Fedotova, A.; Sultanov, V.; Weissflog, M.A.; Arslan, D.; Younesi, M.; Pertsch, T.; Staude, I.; Setzpfandt, F.; Chekhova, M. Photon Pairs from Resonant Metasurfaces. Nano Lett. 2021, 21, 4429. [Google Scholar] [CrossRef]
- Zou, C.; Sautter, J.; Setzpfandt, F.; Staude, I. Resonant Dielectric Metasurfaces: Active Tuning and Nonlinear Effects. J. Phys. D Appl. Phys. 2019, 52, 373002. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Miroshnichenko, A.E.; Brongersma, M.L.; Kivshar, Y.S.; Luk’yanchuk, B. Optically Resonant Dielectric Nanostructures. Science 2016, 354, aag2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staude, I.; Pertsch, T.; Kivshar, Y.S. All-Dielectric Resonant Meta-Optics Lightens Up. ACS Photonics 2019, 6, 802–814. [Google Scholar] [CrossRef]
- Pertsch, T.; Kivshar, Y. Nonlinear Optics with Resonant Metasurfaces. MRS Bull. 2020, 45, 210–220. [Google Scholar] [CrossRef]
- Overvig, A.; Alù, A. Wavefront-Selective Fano Resonant Metasurfaces. Adv. Photonics 2021, 3, 026002. [Google Scholar] [CrossRef]
- Zubyuk, V.; Carletti, L.; Shcherbakov, M.; Kruk, S. Resonant Dielectric Metasurfaces in Strong Optical Fields. APL Mater. 2021, 9, 60701. [Google Scholar] [CrossRef]
- Sadrieva, Z.F.; Sinev, I.S.; Koshelev, K.L.; Samusev, A.; Iorsh, I.V.; Takayama, O.; Malureanu, R.; Bogdanov, A.A.; Lavrinenko, A.V. Transition from Optical Bound States in the Continuum to Leaky Resonances: Role of Substrate and Roughness. ACS Photonics 2017, 4, 723–727. [Google Scholar] [CrossRef]
- Koshelev, K.; Favraud, G.; Bogdanov, A.; Kivshar, Y.; Fratalocchi, A. Nonradiating Photonics with Resonant Dielectric Nanostructures. Nanophotonics 2019, 8, 725–745. [Google Scholar] [CrossRef]
- Doeleman, H.M.; Monticone, F.; Den Hollander, W.; Alù, A.; Koenderink, A.F. Experimental Observation of a Polarization Vortex at an Optical Bound State in the Continuum. Nat. Photonics 2018, 12, 397–401. [Google Scholar] [CrossRef]
- Carletti, L.; Koshelev, K.; De Angelis, C.; Kivshar, Y. Giant Nonlinear Response at the Nanoscale Driven by Bound States in the Continuum. Phys. Rev. Lett. 2018, 121, 033903. [Google Scholar] [CrossRef] [Green Version]
- Yesilkoy, F.; Arvelo, E.R.; Jahani, Y.; Liu, M.; Tittl, A.; Cevher, V.; Kivshar, Y.; Altug, H. Ultrasensitive Hyperspectral Imaging and Biodetection Enabled by Dielectric Metasurfaces. Nat. Photonics 2019, 13, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.T.; Fu, Y.H.; Emani, N.K.; Pan, Z.; Bakker, R.M.; Paniagua-Domínguez, R.; Kuznetsov, A.I. Directional Lasing in Resonant Semiconductor Nanoantenna Arrays. Nat. Nanotechnol. 2018, 13, 1042–1047. [Google Scholar] [CrossRef]
- Rybin, M.V.; Koshelev, K.L.; Sadrieva, Z.F.; Samusev, K.B.; Bogdanov, A.A.; Limonov, M.F.; Kivshar, Y.S. High- Q Supercavity Modes in Subwavelength Dielectric Resonators. Phys. Rev. Lett. 2017, 119, 243901. [Google Scholar] [CrossRef] [Green Version]
- Koshelev, K.; Lepeshov, S.; Liu, M.; Bogdanov, A.; Kivshar, Y. Asymmetric Metasurfaces with High- Q Resonances Governed by Bound States in the Continuum. Phys. Rev. Lett. 2018, 121, 193903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodigala, A.; Lepetit, T.; Gu, Q.; Bahari, B.; Fainman, Y.; Kanté, B. Lasing Action from Photonic Bound States in Continuum. Nature 2017, 541, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lawrence, M.; Dionne, J.A. High Quality Factor Dielectric Metasurfaces for Ultraviolet Circular Dichroism Spectroscopy. ACS Photonics 2020, 7, 36–42. [Google Scholar] [CrossRef]
- Han, S.; Cong, L.; Srivastava, Y.K.; Qiang, B.; Rybin, M.V.; Kumar, A.; Jain, R.; Lim, W.X.; Achanta, V.G.; Prabhu, S.S.; et al. All-Dielectric Active Terahertz Photonics Driven by Bound States in the Continuum. Adv. Mater. 2019, 31, 1901921. [Google Scholar] [CrossRef]
- He, Y.; Guo, G.; Feng, T.; Xu, Y.; Miroshnichenko, A.E. Toroidal Dipole Bound States in the Continuum. Phys. Rev. B 2018, 98, 161112. [Google Scholar] [CrossRef]
- Gomis-Bresco, J.; Artigas, D.; Torner, L. Anisotropy-Induced Photonic Bound States in the Continuum. Nature Photonics 2017, 11, 232–236. [Google Scholar] [CrossRef]
- Koshelev, K.; Bogdanov, A.; Kivshar, Y. Meta-Optics and Bound States in the Continuum. Sci. Bull. 2019, 64, 836–842. [Google Scholar] [CrossRef] [Green Version]
- Azzam, S.I.; Shalaev, V.M.; Boltasseva, A.; Kildishev, A.V. Formation of Bound States in the Continuum in Hybrid Plasmonic-Photonic Systems. Phys. Rev. Lett. 2018, 121, 253901. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Yin, X.; Ni, L.; Soljačić, M.; Zhen, B.; Peng, C. Topologically Enabled Ultrahigh- Q Guided Resonances Robust to out-of-Plane Scattering. Nature 2019, 574, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Von Neuman, J.; Wigner, E.; von Neuman, J.; Wigner, E. Uber Merkwürdige Diskrete Eigenwerte. Uber Das Verhalten von Eigenwerten Bei Adiabatischen Prozessen. PhyZ 1929, 30, 467–470. [Google Scholar]
- Friedrich, H.; Wintgen, D. Interfering Resonances and Bound States in the Continuum. Phys. Rev. A 1985, 32, 3231. [Google Scholar] [CrossRef]
- Hsu, C.W.; Zhen, B.; Stone, A.D.; Joannopoulos, J.D.; Soljačić, M. Bound States in the Continuum. Nature Rev. Mater. 2016, 1, 16048. [Google Scholar] [CrossRef] [Green Version]
- Tittl, A.; Leitis, A.; Liu, M.; Yesilkoy, F.; Choi, D.-Y.; Neshev, D.N.; Kivshar, Y.S.; Altug, H. Imaging-Based Molecular Barcoding with Pixelated Dielectric Metasurfaces. Science 2018, 360, 1105–1109. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft Lithography for Micro- and Nanoscale Patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahadian, S.; Civitarese, R.; Bannerman, D.; Mohammadi, M.H.; Lu, R.; Wang, E.; Davenport-Huyer, L.; Lai, B.; Zhang, B.; Zhao, Y.; et al. Organ-On-A-Chip Platforms: A Convergence of Advanced Materials, Cells, and Microscale Technologies. Adv. Healthc. Mater. 2018, 7, 1700506. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D Printing of Conducting Polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [Green Version]
- Cataldi, U.; Caputo, R.; Kurylyak, Y.; Klein, G.; Chekini, M.; Umeton, C.; Bürgi, T. Growing Gold Nanoparticles on a Flexible Substrate to Enable Simple Mechanical Control of Their Plasmonic Coupling. J. Mater. Chem. C 2014, 2, 7927–7933. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with Designer Functionalities—Properties, Modifications Strategies, and Applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Z.; Sun, J.; Yan, Y.; Bu, M.; Huo, Y.; Li, Y.-F.; Hu, N. Recent Advances in Natural Functional Biopolymers and Their Applications of Electronic Skins and Flexible Strain Sensors. Polymers 2021, 13, 813. [Google Scholar] [CrossRef]
- Campbell, S.B.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond Polydimethylsiloxane: Alternative Materials for Fabrication of Organ-on-a-Chip Devices and Microphysiological Systems. ACS Biomater. Sci. Eng. 2020, 7, 2880–2899. [Google Scholar] [CrossRef]
- Shan, D.; Gerhard, E.; Zhang, C.; Tierney, J.W.; Xie, D.; Liu, Z.; Yang, J. Polymeric Biomaterials for Biophotonic Applications. Bioact. Mater. 2018, 3, 434–445. [Google Scholar] [CrossRef]
- Omenetto, F.G.; Kaplan, D.L. A New Route for Silk. Nat. Photonics 2008, 2, 641–643. [Google Scholar] [CrossRef]
- Guidetti, G.; Wang, Y.; Omenetto, F.G. Active Optics with Silk. Nanophotonics 2020, 10, 137–148. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx Mori Silk Fibroin. Nature Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Cyr, K.M.; Matsumoto, A.; Langer, R.; Borenstein, J.T.; Kaplan, D.L. Silk Fibroin Microfluidic Devices. Adv. Mater. 2007, 19, 2847–2850. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pradhan, S.; Agostinacchio, F.; Pal, R.K.; Greco, G.; Mazzolai, B.; Pugno, N.M.; Motta, A.; Yadavalli, V.K. Easy, Scalable, Robust, Micropatterned Silk Fibroin Cell Substrates. Adv. Mater. Interfaces 2019, 6, 1801822. [Google Scholar] [CrossRef]
- Pal, R.K.; Kurland, N.E.; Wang, C.; Kundu, S.C.; Yadavalli, V.K. Biopatterning of Silk Proteins for Soft Micro-Optics. ACS Appl. Mater. Interfaces 2015, 7, 8809–8816. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Aurelio, D.; Li, W.; Tseng, P.; Zheng, Z.; Li, M.; Kaplan, D.L.; Liscidini, M.; Omenetto, F.G. Modulation of Multiscale 3D Lattices through Conformational Control: Painting Silk Inverse Opals with Water and Light. Adv. Mater. 2017, 29, 1702769. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Li, M.; Garbarini, L.P.; Omenetto, F.G. Inkjet Printing of Patterned, Multispectral, and Biocompatible Photonic Crystals. Adv. Mater. 2019, 31, 1901036. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Marelli, B.; Brenckle, M.A.; Mitropoulos, A.N.; Gil, E.S.; Tsioris, K.; Tao, H.; Kaplan, D.L.; Omenetto, F.G. All-Water-Based Electron-Beam Lithography Using Silk as a Resist. Nat. Nanotechnol. 2014, 9, 306–310. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000.
- Bayer, I.S.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Pignatelli, F.; Ruffilli, R.; Cingolani, R.; Athanassiou, A. Direct Transformation of Edible Vegetable Waste into Bioplastics. Macromolecules 2014, 47, 5135–5143. [Google Scholar] [CrossRef]
- Caligiuri, V.; Tedeschi, G.; Palei, M.; Miscuglio, M.; Martin-Garcia, B.; Guzman-Puyol, S.; Hedayati, M.K.; Kristensen, A.; Athanassiou, A.; Cingolani, R.; et al. Biodegradable and Insoluble Cellulose Photonic Crystals and Metasurfaces. ACS Nano 2020, 14, 9502–9511. [Google Scholar] [CrossRef] [PubMed]
- Espinha, A.; Dore, C.; Matricardi, C.; Alonso, M.I.; Goñi, A.R.; Mihi, A. Hydroxypropyl Cellulose Photonic Architectures by Soft Nanoimprinting Lithography. Nat. Photonics 2018, 12, 343–348. [Google Scholar] [CrossRef]
- Ryabchun, A.; Bobrovsky, A. Cholesteric Liquid Crystal Materials for Tunable Diffractive Optics. Adv. Opt. Mater. 2018, 6, 1800335. [Google Scholar] [CrossRef]
- Mitov, M. Cholesteric Liquid Crystals in Living Matter. Soft Matter 2017, 13, 4176–4209. [Google Scholar] [CrossRef] [PubMed]
- Finkelmann, H.; Kim, S.T.; Muaeoz, A.; Palffy-Muhoray, P.; Taheri, B. Tunable Mirrorless Lasing in Cholesteric Liquid Crystalline Elastomers. Adv. Mater. 2001, 13, 1069–1072. [Google Scholar] [CrossRef]
- Green, M.M.; Peterson, N.C.; Sato, T.; Teramoto, A.; Cook, R.; Lifson, S. A Helical Polymer with a Cooperative Response to Chiral Information. Science 1995, 268, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Chilaya, G.; Chanishvili, A.; Petriashvili, G.; Barberi, R.; Bartolino, R.; De Santo, M.P.; Matranga, M.A.; Collings, P. Light Control of Cholesteric Liquid Crystals Using Azoxy-Based Host Materials. Mol. Cryst. Liq. Cryst. 2006, 453, 123–140. [Google Scholar] [CrossRef]
- Chanishvili, A.; Chilaya, G.; Petriashvili, G.; Barberi, R.; Bartolino, R.; Cipparrone, G.; Mazzulla, A. Laser Emission from a Dye-Doped Cholesteric Liquid Crystal Pumped by Another Cholesteric Liquid Crystal Laser. Appl. Phys. Lett. 2004, 85, 3378–3380. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Vignolini, S. So Much More than Paper. Nat. Photonics 2019, 13, 365–367. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcot, L.R.; Gröschel, A.H.; Linder, M.B.; Rojas, O.J.; Ikkala, O. Self-Assembly of Native Cellulose Nanostructures. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH: Hoboken, NJ, USA, 2017; pp. 123–174. [Google Scholar]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH: Hoboken, NJ, USA, 2017; pp. 1–49. [Google Scholar]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Honorato-Rios, C.; Bruckner, J.; Schütz, C.; Wagner, S.; Tosheva, Z.; Bergström, L.; Lagerwall, J.P.F. Cholesteric liquid crystal formation in suspensions of cellulose nanocrystals. In Liquid Crystals with Nano and Microparticles; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2016; Volume 2, pp. 871–897. ISBN 9789814619264. [Google Scholar]
- Beck, S.; Méthot, M.; Bouchard, J. General Procedure for Determining Cellulose Nanocrystal Sulfate Half-Ester Content by Conductometric Titration. Cellulose 2015, 22, 101–116. [Google Scholar] [CrossRef]
- Abitbol, T.; Kloser, E.; Gray, D.G. Estimation of the Surface Sulfur Content of Cellulose Nanocrystals Prepared by Sulfuric Acid Hydrolysis. Cellulose 2013, 20, 785–794. [Google Scholar] [CrossRef]
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal Self-Ordering of Cellulose Microfibrils in Aqueous Suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Kadar, R.; Spirk, S.; Nypelo, T. Cellulose Nanocrystal Liquid Crystal Phases: Progress and Challenges in Characterization Using Rheology Coupled to Optics, Scattering, and Spectroscopy. ACS Nano 2021, 15, 7931–7945. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.; Bruckner, J.R.; Honorato-Rios, C.; Tosheva, Z.; Anyfantakis, M.; Lagerwall, J.P.F. From Equilibrium Liquid Crystal Formation and Kinetic Arrest to Photonic Bandgap Films Using Suspensions of Cellulose Nanocrystals. Crystals 2020, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.X.; Hamad, W.Y.; MacLachlan, M.J. Polymer and Mesoporous Silica Microspheres with Chiral Nematic Order from Cellulose Nanocrystals. Angew. Chem. Int. Ed. 2016, 55, 12460–12464. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Inui, O.; Horii, F.; Tsuji, M. Phase Separation Behavior in Aqueous Suspensions of Bacterial Cellulose Nanocrystals Prepared by Sulfuric Acid Treatment. Langmuir 2009, 25, 497–502. [Google Scholar] [CrossRef]
- Wang, P.X.; Hamad, W.Y.; MacLachlan, M.J. Structure and Transformation of Tactoids in Cellulose Nanocrystal Suspensions. Nat. Commun. 2016, 7, 11515. [Google Scholar] [CrossRef]
- Schütz, C.; Van Rie, J.; Eyley, S.; Gençer, A.; Van Gorp, H.; Rosenfeldt, S.; Kang, K.; Thielemans, W. Effect of Source on the Properties and Behavior of Cellulose Nanocrystal Suspensions. ACS Sustain. Chem. Eng. 2018, 6, 8317–8324. [Google Scholar] [CrossRef]
- Zhu, B.; Johansen, V.E.; Kamita, G.; Guidetti, G.; Bay, M.M.; Parton, T.G.; Frka-Petesic, B.; Vignolini, S. Hyperspectral Imaging of Photonic Cellulose Nanocrystal Films: Structure of Local Defects and Implications for Self-Assembly Pathways. ACS Nano 2020, 14, 15361–15373. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Ikada, Y.; Moritera, T.; Ogura, Y.; Honda, Y. Collagen-Immobilized Hydrogel as a Material for Lamellar Keratoplasty. J. Appl. Biomater. Off. J. Soc. Biomater. 1991, 2, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Marquis, J.C.; Nohria, A.; Emmanual, J.; Mikos, A.G.; Langer, R. Neocartilage Formation in Vitro and in Vivo Using Cells Cultured on Synthetic Biodegradable Polymers. J. Biomed. Mater. Res. 1993, 27, 11–23. [Google Scholar] [CrossRef]
- Sui, Z.; King, W.J.; Murphy, W.L. Protein-Based Hydrogels with Tunable Dynamic Responses. Adv. Funct. Mater. 2008, 18, 1824–1831. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical Applications of Layer-by-Layer Assembly: From Biomimetics to Tissue Engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef] [Green Version]
- McGuigan, A.P.; Sefton, M.V. Vascularized Organoid Engineered by Modular Assembly Enables Blood Perfusion. Proc. Natl. Acad. Sci. USA 2006, 103, 11461–11466. [Google Scholar] [CrossRef] [Green Version]
- Nerem, R.M. Cellular Engineering. Ann. Biomed. Eng. 1991, 19, 529–545. [Google Scholar] [CrossRef]
- Langer, R. New Methods of Drug Delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Gluzband, Y.A.; Grodzinsky, A.J.; Kimura, J.H.; Hunziker, E.B. Chondrocytes in Agarose Culture Synthesize a Mechanically Functional Extracellular Matrix. J. Orthop. Res. 1992, 10, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, A.S.; Hubbell, J.A. Poly(Ethylene Oxide)-Graft-Poly(L-Lysine) Copolymers to Enhance the Biocompatibility of Poly(L-Lysine)-Alginate Microcapsule Membranes. Biomaterials 1992, 13, 863–870. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.; Peppas, N.A. Advances in Biomaterials, Drug Delivery, and Bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Franzesi, G.T.; Ni, B.; Ling, Y.; Khademhosseini, A. A Controlled-Release Strategy for the Generation of Cross-Linked Hydrogel Microstructures. J. Am. Chem. Soc. 2006, 128, 15064–15065. [Google Scholar] [CrossRef] [PubMed]
- Cabodi, M.; Choi, N.W.; Gleghorn, J.P.; Lee, C.S.D.; Bonassar, L.J.; Stroock, A.D. A Microfluidic Biomaterial. J. Am. Chem. Soc. 2005, 127, 13788–13789. [Google Scholar] [CrossRef]
- Balog, E.R.M.; Ghosh, K.; Park, Y.I.; Hartung, V.; Sista, P.; Rocha, R.C.; Wang, H.L.; Martinez, J.S. Stimuli-Responsive Genetically Engineered Polymer Hydrogel Demonstrates Emergent Optical Responses. ACS Biomater. Sci. Eng. 2016, 2, 1135–1142. [Google Scholar] [CrossRef]
- Wang, E.; Desai, M.S.; Lee, S.W. Light-Controlled Graphene-Elastin Composite Hydrogel Actuators. Nano Lett. 2013, 13, 2826–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; InTech: London, UK, 2011. [Google Scholar]
- Alonso, M.; Reboto, V.; Guiscardo, L.; San Martín, A.; Rodríguez-Cabello, J.C. Spiropyran Derivative of an Elastin-like Bioelastic Polymer: Photoresponsive Molecular Machine to Convert Sunlight into Mechanical Work. Macromolecules 2000, 33, 9480–9482. [Google Scholar] [CrossRef]
- Annabi, N.; Mithieux, S.M.; Weiss, A.S.; Dehghani, F. The Fabrication of Elastin-Based Hydrogels Using High Pressure CO2. Biomaterials 2009, 30, 1–7. [Google Scholar] [CrossRef]
- Sun, Z.; Qin, G.; Xia, X.; Cronin-Golomb, M.; Omenetto, F.G.; Kaplan, D.L. Photoresponsive Retinal-Modified Silk-Elastin Copolymer. J. Am. Chem. Soc. 2013, 135, 3675–3679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.L.; Dong, W.F.; Niu, L.G.; Jiang, T.; Liu, D.X.; Zhang, L.; Wang, Y.S.; Chen, Q.D.; Kim, D.P.; Sun, H.B. Protein-Based Soft Micro-Optics Fabricated by Femtosecond Laser Direct Writing. Light Sci. Appl. 2014, 3, e129. [Google Scholar] [CrossRef]
- Dong, L.; Agarwal, A.K.; Beebe, D.J.; Jiang, H. Adaptive Liquid Microlenses Activated by Stimuli-Responsive Hydrogels. Nature 2006, 442, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Melikov, R.; Press, D.A.; Kumar, B.G.; Dogru, I.B.; Sadeghi, S.; Chirea, M.; Yilgör, I.; Nizamoglu, S. Silk-Hydrogel Lenses for Light-Emitting Diodes. Sci. Rep. 2017, 7, 7258. [Google Scholar] [CrossRef] [Green Version]
- Mayer, M.; Yang, J.; Gitlin, I.; Gracias, D.H.; Whitesides, G.M. Micropatterned Agarose Gels for Stamping Arrays of Proteins and Gradients of Proteins. GM Proteom. 2004, 4, 2366. [Google Scholar] [CrossRef]
- Jain, A.; Yang, A.H.J.; Erickson, D. Gel-Based Optical Waveguides with Live Cell Encapsulation and Integrated Microfluidics. Opt. Lett. 2012, 37, 1472. [Google Scholar] [CrossRef]
- Fujiwara, E.; Cabral, T.D.; Sato, M.; Oku, H.; Cordeiro, C.M.B. Agarose-Based Structured Optical Fibre. Sci. Rep. 2020, 10, 7035. [Google Scholar] [CrossRef]
- Gao, R.; Jiang, Y.; Ding, W. Agarose Gel Filled Temperature-Insensitive Photonic Crystal Fibers Humidity Sensor Based on the Tunable Coupling Ratio. Sens. Actuators B Chem. 2014, 195, 313–319. [Google Scholar] [CrossRef]
- Gambino, S.; Mazzeo, M.; Genco, A.; Di Stefano, O.; Savasta, S.; Patanè, S.; Ballarini, D.; Mangione, F.; Lerario, G.; Sanvitto, D.; et al. Exploring Light-Matter Interaction Phenomena under Ultrastrong Coupling Regime. ACS Photonics 2014, 1, 1042–1048. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and Stabilizing Luminescence from Halide Perovskites with Potassium Passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [Green Version]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Press, D.; De Greve, K.; McMahon, P.L.; Ladd, T.D.; Friess, B.; Schneider, C.; Kamp, M.; Höfling, S.; Forchel, A.; Yamamoto, Y. Ultrafast Optical Spin Echo in a Single Quantum Dot. Nat. Photonics 2010, 4, 367–370. [Google Scholar] [CrossRef]
- Lund-Hansen, T.; Stobbe, S.; Julsgaard, B.; Thyrrestrup, H.; Sünner, T.; Kamp, M.; Forchel, A.; Lodahl, P. Experimental Realization of Highly Efficient Broadband Coupling of Single Quantum Dots to a Photonic Crystal Waveguide. Phys. Rev. Lett. 2008, 101, 113903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klenovský, P.; Schliwa, A.; Bimberg, D. Electronic States of (InGa)(AsSb)/GaAs/GaP Quantum Dots. Phys. Rev. B 2019, 100, 115424. [Google Scholar] [CrossRef] [Green Version]
- Grundmann, M.; Stier, O.; Bimberg, D. InAs/GaAs Pyramidal Quantum Dots: Strain Distribution, Optical Phonons, and Electronic Structure. Phys. Rev. B 1995, 52, 11969–11981. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Palei, M.; Caligiuri, V.; Kudera, S.; Krahne, R. Robust and Bright Photoluminescence from Colloidal Nanocrystal/Al2O3 Composite Films Fabricated by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2018, 10, 22356–22362. [Google Scholar] [CrossRef] [Green Version]
- Castelli, A.; Dhanabalan, B.; Polovitsyn, A.; Caligiuri, V.; Di Stasio, F.; Scarpellini, A.; Brescia, R.; Palei, M.; Martín-García, B.; Prato, M.; et al. Core/Shell CdSe/CdS Bone-Shaped Nanocrystals with a Thick and Anisotropic Shell as Optical Emitters. Adv. Opt. Mater. 2019, 8, 1901463. [Google Scholar] [CrossRef]
- Verlick, S.F. Fluorescence Spectra and Polarization of Glyceraldehyde-3-Phosphate and Lactic Dehydrogenase Coenzyme Complexes. J. Biol. Chem. 1958, 233, 1455–1467. [Google Scholar] [CrossRef]
- Schaefer, P.M.; Kalinina, S.; Rueck, A.; von Arnim, C.A.F.; von Einem, B. NADH Autofluorescence—A Marker on Its Way to Boost Bioenergetic Research. Cytom. Part A 2019, 95, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, S.; Tu, H.; Chaney, E.J.; Sun, Y.; Zhao, Y.; Bower, A.J.; Liu, Y.Z.; Marjanovic, M.; Sinha, S.; Pu, Y.; et al. Intravital Imaging by Simultaneous Label-Free Autofluorescence-Multiharmonic Microscopy. Nat. Commun. 2018, 9, 2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blacker, T.S.; Mann, Z.F.; Gale, J.E.; Ziegler, M.; Bain, A.J.; Szabadkai, G.; Duchen, M.R. Separating NADH and NADPH Fluorescence in Live Cells and Tissues Using FLIM. Nat. Commun. 2014, 5, 3936. [Google Scholar] [CrossRef] [Green Version]
- Rocheleau, J.V.; Head, W.S.; Piston, D.W. Quantitative NAD(P)H/Flavoprotein Autofluorescence Imaging Reveals Metabolic Mechanisms of Pancreatic Islet Pyruvate Response. J. Biol. Chem. 2004, 279, 31780–31787. [Google Scholar] [CrossRef] [Green Version]
- Ying, W. NAD+/NADH and NADP+/NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolenc, O.I.; Quinn, K.P. Evaluating Cell Metabolism through Autofluorescence Imaging of NAD(P)H and FAD. Antioxid. Redox Signal. 2019, 30, 875–889. [Google Scholar] [CrossRef]
- Alam, S.R.; Wallrabe, H.; Svindrych, Z.; Chaudhary, A.K.; Christopher, K.G.; Chandra, D.; Periasamy, A. Investigation of Mitochondrial Metabolic Response to Doxorubicin in Prostate Cancer Cells: An NADH, FAD and Tryptophan FLIM Assay. Sci. Rep. 2017, 7, 10451. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Pouli, D.; Alonzo, C.A.; Varone, A.; Karaliota, S.; Quinn, K.P.; Mönger, K.; Karalis, K.P.; Georgakoudi, I. Mapping Metabolic Changes by Noninvasive, Multiparametric, High-Resolution Imaging Using Endogenous Contrast. Sci. Adv. 2018, 4, eaap9302. [Google Scholar] [CrossRef] [Green Version]
- Churchich, J.E. Fluorescence Properties of Pyridoxamine 5-Phosphate. BBA Biophys. Incl. Photosynth. 1965, 102, 280–288. [Google Scholar] [CrossRef]
- Hughes, C.F.; Ward, M.; Tracey, F.; Hoey, L.; Molloy, A.M.; Pentieva, K.; McNulty, H. B-Vitamin Intake and Biomarker Status in Relation to Cognitive Decline in Healthy Older Adults in a 4-Year Follow-up Study. Nutrients 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Pilicer, S.L.; Bakhshi, P.R.; Bentley, K.W.; Wolf, C. Biomimetic Chirality Sensing with Pyridoxal-5′-Phosphate. J. Am. Chem. Soc. 2017, 139, 1758–1761. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, Y.; Anand, T.; Babu, L.T.; Paira, P.; Crisponi, G.; Sk, A.K.; Kumar, R.; Sahoo, S.K. Three-in-One Type Fluorescent Sensor Based on a Pyrene Pyridoxal Cascade for the Selective Detection of Zn(II), Hydrogen Phosphate and Cysteine. Dalton Trans. 2018, 47, 742–749. [Google Scholar] [CrossRef]
- Halawa, M.I.; Gao, W.; Saqib, M.; Kitte, S.A.; Wu, F.; Xu, G. Sensitive Detection of Alkaline Phosphatase by Switching on Gold Nanoclusters Fluorescence Quenched by Pyridoxal Phosphate. Biosens. Bioelectron. 2017, 95, 8–14. [Google Scholar] [CrossRef]
- Passannante, A.J.; Avioli, L.V. Studies on the Ultraviolet Fluorescence of Vitamin D and Related Compounds in Acid-Alcohol Solutions. Anal. Biochem. 1966, 15, 287–295. [Google Scholar] [CrossRef]
- Zähringer, K. The Use of Vitamins as Tracer Dyes for Laser-Induced Fluorescence in Liquid Flow Applications. Exp. Fluids 2014, 55, 1712. [Google Scholar] [CrossRef]
- Rubin, M. Fluorometry and Phosphorimetry in Clinical Chemistry. Adv. Clin. Chem. 1970, 13, 161–269. [Google Scholar] [CrossRef]
- Xue, X.; Hu, L.; Hou, Z. Study on Fluorescence Spectra of B Vitamins; Atlantis Press: Amsterdam, The Netherlands, 2016; pp. 85–90. [Google Scholar]
- Zhang, Y.; Yang, J. Design Strategies for Fluorescent Biodegradable Polymeric Biomaterials. J. Mater. Chem. B 2013, 1, 132–148. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.C.B.; de Melo, J.S.S. Aggregation-Induced Emission: From Small Molecules to Polymers—Historical Background, Mechanisms and Photophysics. Top. Curr. Chem. 2021, 379, 15. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: A Trailblazing Journey to the Field of Biomedicine. ACS Appl. Bio Mater. 2018, 1, 1768–1786. [Google Scholar] [CrossRef]
- Xi, Y.; Gao, W.; Chen, J.; Zhang, S.; Yang, T.; Gao, B. Biocompatible Fluorescent Probe with the Aggregation-Induced Emission Characteristic for Live Cell Imaging. In Proceedings of the International Symposium on Materials Application and Engineering (SMAE 2016), Chiang Mai, Thailand, 20 August 2016. [Google Scholar] [CrossRef]
- Xu, D.; Liu, M.; Zou, H.; Tian, J.; Huang, H.; Wan, Q.; Dai, Y.; Wen, Y.; Zhang, X.; Wei, Y. A New Strategy for Fabrication of Water Dispersible and Biodegradable Fluorescent Organic Nanoparticles with AIE and ESIPT Characteristics and Their Utilization for Bioimaging. Talanta 2017, 174, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Wu, Y.T.; Kuo, M.Y.; Chang, Y.T.; Shin, C.C.; Wu, T.C.; Tai, C.C.; Cheng, T.H.; Liu, W.S. Synthesis, Structure, and Photophysical Properties of Highly Substituted 8,8a-Dihydrocyclopenta[a]Indenes. Angew. Chem. Int. Ed. 2008, 47, 9891–9894. [Google Scholar] [CrossRef]
- Shimizu, M.; Takeda, Y.; Higashi, M.; Hiyama, T. 1,4-Bis(Alkenyl)-2,5-Dipiperidinobenzenes: Minimal Fluorophores Exhibiting Highly Efficient Emission in the Solid State. Angew. Chem. Int. Ed. 2009, 48, 3653–3656. [Google Scholar] [CrossRef] [PubMed]
- An, B.K.; Kwon, S.K.; Jung, S.D.; Park, S.Y. Enhanced Emission and Its Switching in Fluorescent Organic Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef] [PubMed]
- Birkedal, H.; Pattison, P. Bis[4-(Salicylideneamino)Phenyl]-Methane. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, o139–o141. [Google Scholar] [CrossRef] [Green Version]
- Mutai, T.; Tomoda, H.; Ohkawa, T.; Yabe, Y.; Araki, K. Switching of Polymorph-Dependent ESIPT Luminescence of an Imidazo[1,2-a]Pyridine Derivative. Angew. Chem. Int. Ed. 2008, 47, 9522–9524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, F.; Fan, Y.; Song, W.; Mu, X.; Zhang, H.; Wang, Y. Hydrogen-Bonded Dimer Stacking Induced Emission of Aminobenzoic Acid Compounds. Chem. Commun. 2009, 3199–3201. [Google Scholar] [CrossRef]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Acetylenic Polymers: Syntheses, Structures, and Functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Shi, J.B.; Zhi, J.G.; Tong, B.; Dong, Y.P. Aggregation-Induced Emission Enhancement in Poly(Phenyleneethynylene) s Bearing Aniline Groups. Chin. J. Polym. Sci. 2012, 30, 443–450. [Google Scholar] [CrossRef]
- Pucci, A.; Rausa, R.; Ciardelli, F. Aggregation-Induced Luminescence of Polyisobutene Succinic Anhydrides and Imides. Macromol. Chem. Phys. 2008, 209, 900–906. [Google Scholar] [CrossRef]
- Ruff, Y.; Lehn, J.M. Glycodynamers: Fluorescent Dynamic Analogues of Polysaccharides. Angew. Chem. Int. Ed. 2008, 47, 3556–3559. [Google Scholar] [CrossRef] [PubMed]
- Ruff, Y.; Buhler, E.; Candau, S.J.; Kesselman, E.; Talmon, Y.; Lehn, J.M. Glycodynamers: Dynamic Polymers Bearing Oligosaccharides Residues - Generation, Structure, Physicochemical, Component Exchange, and Lectin Binding Properties. J. Am. Chem. Soc. 2010, 132, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission of Silole Molecules and Polymers: Fundamental and Applications. J. Inorg. Organomet. Polym. Mater. 2009, 19, 249–285. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Solntsevn, K.M.; Tolbert, L.M. Activation and Tuning of Green Fluorescent Protein Chromophore Emission by Alkyl Substituent-Mediated Crystal Packing. J. Am. Chem. Soc. 2009, 131, 662–670. [Google Scholar] [CrossRef]
- Debler, E.W.; Kaufmann, G.F.; Meijler, M.M.; Heine, A.; Mee, J.M.; Pljevaljčić, G.; Di Bilio, A.J.; Schultz, P.G.; Millar, D.P.; Janda, K.D.; et al. Deeply Inverted Electron-Hole Recombination in a Luminescent Antibody-Stilbene Complex. Science 2008, 319, 1232–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Debler, E.W.; Millar, D.P.; Deniz, A.A.; Wilson, I.A.; Schultz, P.G. The Effects of Antibodies on Stilbene Excited-State Energetics. Angew. Chem. Int. Ed. 2006, 45, 7763–7765. [Google Scholar] [CrossRef]
- Glazer, A.N. Light Harvesting by Phycobilisomes. Annu. Rev. Biophys. Biophys. Chem. 1985, 14, 47–77. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer US: New York, NY, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Glazer, A.N. Phycobiliproteins—a Family of Valuable, Widely Used Fluorophores. J. Appl. Phycol. 1994, 6, 105–112. [Google Scholar] [CrossRef]
- Gantt, E. Structure and Function of Phycobilisomes: Light-Harvesting Pigment Complexes in Red and Blue-Green Algae. Int. Rev. Cytol. 1980, 56, 45–80. [Google Scholar]
- DeLange, R.J.; Glazer, A.G. Phycoerythrin Fluorescence-Based Assay for Peroxy Radicals: A Screen for Biologically Relevant Protective Agents. Anal. Biochem. 1989, 177, 300–306. [Google Scholar] [CrossRef]
- Grabowski, J.; Gantt, E. Photophysical Properties of Phycobiliproteins from Phycobilisomes: Fluorescence Lifetimes, Quantum Yields, and Polarization Spectra. Photochem. Photobiol. 1978, 28, 39–45. [Google Scholar] [CrossRef]
- Cohen-Bazire, G.; Beguin, S.; Rimon, S.; Glazer, A.N.; Brown, D.M. Physico-Chemical and Immunological Properties of Allophycocyanins. Arch. Microbiol. 1977, 111, 225–238. [Google Scholar] [CrossRef]
- Glazer, A.N.; Stryer, L. Phycofluor Probes. Trends Biochem. Sci. 1984, 9, 423–427. [Google Scholar] [CrossRef]
- Rüdiger, W.; Thümmler, F. The phytochrome chromophore. In Photomorphogenesis in Plants; Springer: Dordrecht, The Netherlands, 1994; pp. 51–69. [Google Scholar]
- Murphy, J.T.; Lagarias, J.C. The Phytofluors: A New Class of Fluorescent Protein Probes. Curr. Biol. 1997, 7, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.J.; Lagarias, J.C. Harnessing Phytochrome’s Glowing Potential. Proc. Natl. Acad. Sci. USA 2004, 101, 17334–17339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambetta, G.A.; Lagarias, J.C. Genetic Engineering of Phytochrome Biosynthesis in Bacteria. Proc. Natl. Acad. Sci. USA 2001, 98, 10566–10571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green Fluorescent Protein as a Marker for Gene Expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cody, C.W.; Prasher, D.C.; Westler, W.M.; Prendergast, F.G.; Ward, W.W. Chemical Structure of the Hexapeptide Chromophore of the Aequorea Green-Fluorescent Protein. Biochemistry 1993, 32, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.G.; Flynn, G.C. Chromophore Formation in Green Fluorescent Protein. Biochemistry 1997, 36, 6786–6791. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, Purification and Properties of Aequorin, a Bioluminescent. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Shimomura, O. A Short Story of Aequorin. Biol. Bull. 1995, 189, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Remington, S.J. Green Fluorescent Protein: A Perspective. Protein Sci. 2011, 20, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Barondeau, D.P.; Putnam, C.D.; Kassmann, C.J.; Tainer, J.A.; Getzoff, E.D. Mechanism and Energetics of Green Fluorescent Protein Chromophore Synthesis Revealed by Trapped Intermediate Structures. Proc. Natl. Acad. Sci. USA 2003, 100, 12111–12116. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Kumar, P.; Lee, K.F. Generation of Photonic Entanglement in Green Fluorescent Proteins. Nat. Commun. 2017, 8, 1934. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Zhou, Y.; Qiao, Z.; Eng Aik, C.; Tu, W.C.; Wu, X.; Chen, Y.C. Stimulated Chiral Light-Matter Interactions in Biological Microlasers. ACS Nano 2021, 15, 8975. [Google Scholar] [CrossRef]
- Karl, M.; Meek, A.; Murawski, C.; Tropf, L.; Keum, C.; Schubert, M.; Samuel, I.D.W.; Turnbull, G.A.; Gather, M.C. Distributed Feedback Lasers Based on Green Fluorescent Protein and Conformal High Refractive Index Oxide Layers. Laser Photonics Rev. 2020, 14, 2000101. [Google Scholar] [CrossRef]
- Dogru, I.B.; Min, K.; Umar, M.; Bahmani Jalali, H.; Begar, E.; Conkar, D.; Firat Karalar, E.N.; Kim, S.; Nizamoglu, S. Single Transverse Mode Protein Laser. Appl. Phys. Lett. 2017, 111, 231103. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.J.; Gather, M.C.; Song, J.-J.; Yun, S.H. Lasing from Fluorescent Protein Crystals. Opt. Express 2014, 22, 31411. [Google Scholar] [CrossRef]
- Gather, M.C.; Yun, S.H. Single-Cell Biological Lasers. Nat. Photonics 2011, 5, 406–410. [Google Scholar] [CrossRef]
- Gather, M.C.; Yun, S.H. Lasing from Escherichia Coli Bacteria Genetically Programmed to Express Green Fluorescent Protein. Opt. Lett. 2011, 36, 3299–3301. [Google Scholar] [CrossRef]
- Weber, M.D.; Niklaus, L.; Pröschel, M.; Coto, P.B.; Sonnewald, U.; Costa, R.D. Bioinspired Hybrid White Light-Emitting Diodes. Adv. Mater. 2015, 27, 5493–5498. [Google Scholar] [CrossRef] [PubMed]
- Bajar, B.T.; Wang, E.S.; Zhang, S.; Lin, M.Z.; Chu, J. A Guide to Fluorescent Protein FRET Pairs. Sensors 2016, 16, 1488. [Google Scholar] [CrossRef] [PubMed]
- Zajac, J.M.; Schubert, M.; Roland, T.; Keum, C.; Samuel, I.D.W.; Gather, M.C. Time-Resolved Studies of Energy Transfer in Thin Films of Green and Red Fluorescent Proteins. Adv. Funct. Mater. 2018, 28, 1706300. [Google Scholar] [CrossRef]
- Lelimousin, M.; Noirclerc-Savoye, M.; Lazareno-Saez, C.; Paetzold, B.; Le Vot, S.; Chazal, R.; Macheboeuf, P.; Field, M.J.; Bourgeois, D.; Royant, A. Intrinsic Dynamics in ECFP and Cerulean Control Fluorescence Quantum Yield. Biochemistry 2009, 48, 10038–10046. [Google Scholar] [CrossRef]
- Goedhart, J.; Van Weeren, L.; Hink, M.A.; Vischer, N.O.E.; Jalink, K.; Gadella, T.W.J. Bright Cyan Fluorescent Protein Variants Identified by Fluorescence Lifetime Screening. Nat. Methods 2010, 7, 137–139. [Google Scholar] [CrossRef]
- Rizzo, M.A.; Springer, G.H.; Granada, B.; Piston, D.W. An Improved Cyan Fluorescent Protein Variant Useful for FRET. Nat. Biotechnol. 2004, 22, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, J.; Von Stetten, D.; Noirclerc-Savoye, M.; Lelimousin, M.; Joosen, L.; Hink, M.A.; Van Weeren, L.; Gadella, T.W.J.; Royant, A. Structure-Guided Evolution of Cyan Fluorescent Proteins towards a Quantum Yield of 93%. Nat. Commun. 2012, 3, 751. [Google Scholar] [CrossRef] [PubMed]
- Erard, M.; Fredj, A.; Pasquier, H.; Beltolngar, D.B.; Bousmah, Y.; Derrien, V.; Vincent, P.; Merola, F. Minimum Set of Mutations Needed to Optimize Cyan Fluorescent Proteins for Live Cell Imaging. Mol. Biosyst. 2013, 9, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Villoing, A.; Ridhoir, M.; Cinquin, B.; Erard, M.; Alvarez, L.; Vallverdu, G.; Pernot, P.; Grailhe, R.; Mérola, F.; Pasquier, H. Complex Fluorescence of the Cyan Fluorescent Protein: Comparisons with the H148D Variant and Consequences for Quantitative Cell Imaging. Biochemistry 2008, 47, 12483–12492. [Google Scholar] [CrossRef]
- Tramier, M.; Zahid, M.; Mevel, J.C.; Masse, M.J.; Coppey-Moisan, M. Sensitivity of CFP/YFP and GFP/MCherry Pairs to Donor Photobleaching on FRET Determination by Fluorescence Lifetime Imaging Microscopy in Living Cells. Microsc. Res. Tech. 2006, 69, 933–939. [Google Scholar] [CrossRef]
- Espagne, A.; Erard, M.; Madiona, K.; Derrien, V.; Jonasson, G.; Lévy, B.; Pasquier, H.; Melki, R.; Mèrola, F. Cyan Fluorescent Protein Carries a Constitutive Mutation That Prevents Its Dimerization. Biochemistry 2011, 50, 437–439. [Google Scholar] [CrossRef]
- Markwardt, M.L.; Kremers, G.J.; Kraft, C.A.; Ray, K.; Cranfill, P.J.C.; Wilson, K.A.; Day, R.N.; Wachter, R.M.; Davidson, M.W.; Rizzo, M.A. An Improved Cerulean Fluorescent Protein with Enhanced Brightness and Reduced Reversible Photoswitching. PLoS ONE 2011, 6, 17896. [Google Scholar] [CrossRef]
- Kremers, G.J.; Goedhart, J.; Van Munster, E.B.; Gadella, T.W.J. Cyan and Yellow Super Fluorescent Proteins with Improved Brightness, Protein Folding, and FRET Förster Radius. Biochemistry 2006, 45, 6570–6580. [Google Scholar] [CrossRef]
- Pletneva, N.V.; Pletnev, V.Z.; Souslova, E.; Chudakov, D.M.; Lukyanov, S.; Martynov, V.I.; Arhipova, S.; Artemyev, I.; Wlodawer, A.; Dauter, Z.; et al. Yellow Fluorescent Protein PhiYFPv (Phialidium): Structure and Structure-Based Mutagenesis. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T.; Ibata, K.; Park, E.S.; Kubota, M.; Mikoshiba, K.; Miyawaki, A. A Variant of Yellow Fluorescent Protein with Fast and Efficient Maturation for Cell-Biological Applications. Nat. Biotechnol. 2002, 20, 87–90. [Google Scholar] [CrossRef]
- Rekas, A.; Alattia, J.R.; Nagai, T.; Miyawaki, A.; Ikura, M. Crystal Structure of Venus, a Yellow Fluorescent Protein with Improved Maturation and Reduced Environmental Sensitivity. J. Biol. Chem. 2002, 277, 50573–50578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kredel, S.; Oswald, F.; Nienhaus, K.; Deuschle, K.; Röcker, C.; Wolff, M.; Heilker, R.; Nienhaus, G.U.; Wiedenmann, J. MRuby, a Bright Monomeric Red Fluorescent Protein for Labeling of Subcellular Structures. PLoS ONE 2009, 4, 4391. [Google Scholar] [CrossRef] [PubMed]
- Hense, A.; Nienhaus, K.; Nienhaus, G.U. Exploring Color Tuning Strategies in Red Fluorescent Proteins. Photochem. Photobiol. Sci. 2015, 14, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Dedecker, P.; De Schryver, F.C.; Hofkens, J. Fluorescent Proteins: Shine on, You Crazy Diamond. J. Am. Chem. Soc. 2013, 135, 2387–2402. [Google Scholar] [CrossRef] [PubMed]
- Kredel, S.; Nienhaus, K.; Oswald, F.; Wolff, M.; Ivanchenko, S.; Cymer, F.; Jeromin, A.; Michel, F.J.; Spindler, K.D.; Heilker, R.; et al. Optimized and Far-Red-Emitting Variants of Fluorescent Protein EqFP611. Chem. Biol. 2008, 15, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Hense, A.; Prunsche, B.; Gao, P.; Ishitsuka, Y.; Nienhaus, K.; Nienhaus, G.U. Monomeric Garnet, a Far-Red Fluorescent Protein for Live-Cell STED Imaging. Sci. Rep. 2015, 5, 18006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shcherbo, D.; Murphy, C.S.; Ermakova, G.V.; Solovieva, E.A.; Chepurnykh, T.V.; Shcheglov, A.S.; Verkhusha, V.V.; Pletnev, V.Z.; Hazelwood, K.L.; Roche, P.M.; et al. Far-Red Fluorescent Tags for Protein Imaging in Living Tissues. Biochem. J. 2009, 418, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Chung, P.-H.; Tregidgo, C.; Suhling, K. Determining a Fluorophore’s Transition Dipole Moment from Fluorescence Lifetime Measurements in Solvents of Varying Refractive Index. Methods Appl. Fluoresc. 2016, 4, 045001. [Google Scholar] [CrossRef] [Green Version]
- Uudsemaa, M.; Trummal, A.; de Reguardati, S.; Callis, P.R.; Rebane, A. TD-DFT Calculations of One- and Two-Photon Absorption in Coumarin C153 and Prodan: Attuning Theory to Experiment. Phys. Chem. Chem. Phys. 2017, 19, 28824–28833. [Google Scholar] [CrossRef]
- Pandey, N.; Gahlaut, R.; Arora, P.; Joshi, N.K.; Joshi, H.C.; Pant, S. Study of Dipole Moments of Some Coumarin Derivatives. J. Mol. Struct. 2014, 1061, 175–180. [Google Scholar] [CrossRef]
- Nifosì, R.; Mennucci, B.; Filippi, C. The Key to the Yellow-to-Cyan Tuning in the Green Fluorescent Protein Family Is Polarisation. Phys. Chem. Chem. Phys. 2019, 21, 18988–18998. [Google Scholar] [CrossRef] [PubMed]








| Polymer | Silk | Cellulose | Hydrogel | PDMS | |
|---|---|---|---|---|---|
| Property | |||||
| Mechanical property | Excellent | Good | Good | Good | |
| Optical clarity | Excellent | Excellent | Very Good | Moderate/Negative | |
| Biocompatibility | Excellent | Excellent | Excellent | Good | |
| Biodegradability | Excellent | Excellent | Excellent | Negative | |
| Low absorption | Excellent | Excellent | Moderate | Negative | |
| Tunable fluorescence | Moderate | Negative | |||
| Rapid prototyping | Moderate | Moderate | Moderate | Negative | |
| Molecule | tdm (D) | Reference |
|---|---|---|
| Rhodamine 123 | 8.1 | Chung et al. [249] |
| PM546 | 7.1 | Chung et al. [249] |
| Coumarin (C153) | 6.3 | Uudesmaa et al. [250] |
| 6MMC (Coumarin derivative) | 6.4–9.8 | Pandey et al. [251] |
| phiYFP | 10.2 | Nifosì et al. [252] |
| EYFP | 10.1 | Nifosì et al. [252] |
| Dronpa | 10.46 | Nifosì et al. [252] |
| GFP-S65T | 10.5 | Nifosì et al. [252] |
| mTFP0.7 | 9.915 | Nifosì et al. [252] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caligiuri, V.; Leone, F.; Annesi, F.; Pane, A.; Bartolino, R.; De Luca, A. Envisioning Quantum Electrodynamic Frameworks Based on Bio-Photonic Cavities. Photonics 2021, 8, 470. https://doi.org/10.3390/photonics8110470
Caligiuri V, Leone F, Annesi F, Pane A, Bartolino R, De Luca A. Envisioning Quantum Electrodynamic Frameworks Based on Bio-Photonic Cavities. Photonics. 2021; 8(11):470. https://doi.org/10.3390/photonics8110470
Chicago/Turabian StyleCaligiuri, Vincenzo, Francesca Leone, Ferdinanda Annesi, Alfredo Pane, Roberto Bartolino, and Antonio De Luca. 2021. "Envisioning Quantum Electrodynamic Frameworks Based on Bio-Photonic Cavities" Photonics 8, no. 11: 470. https://doi.org/10.3390/photonics8110470





