Raman Spectroscopy Enables Non-Invasive Identification of Mycotoxins p. Fusarium of Winter Wheat Seeds
Abstract
:1. Introduction
2. Materials and Methods
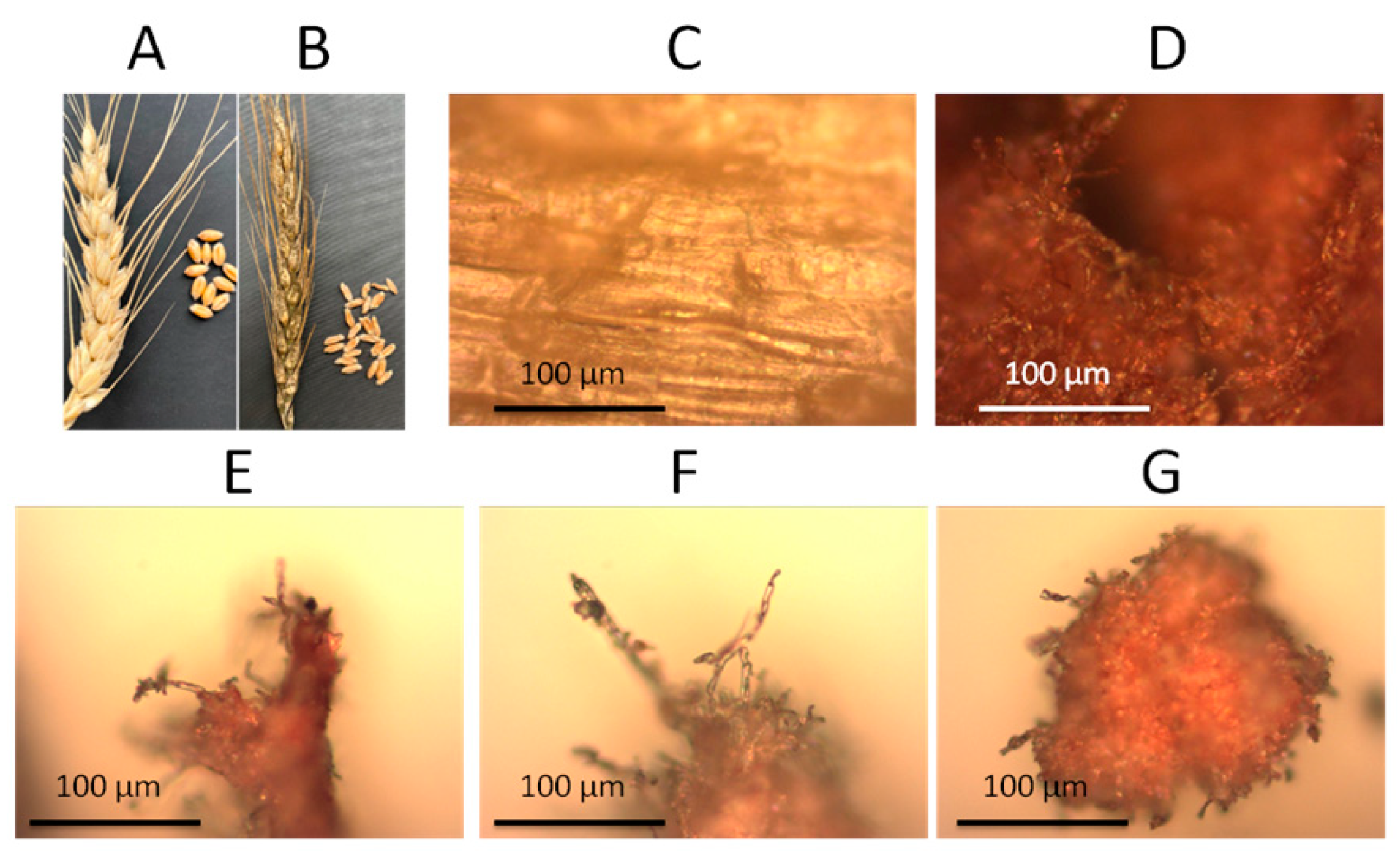
2.1. Materials
2.2. Methods
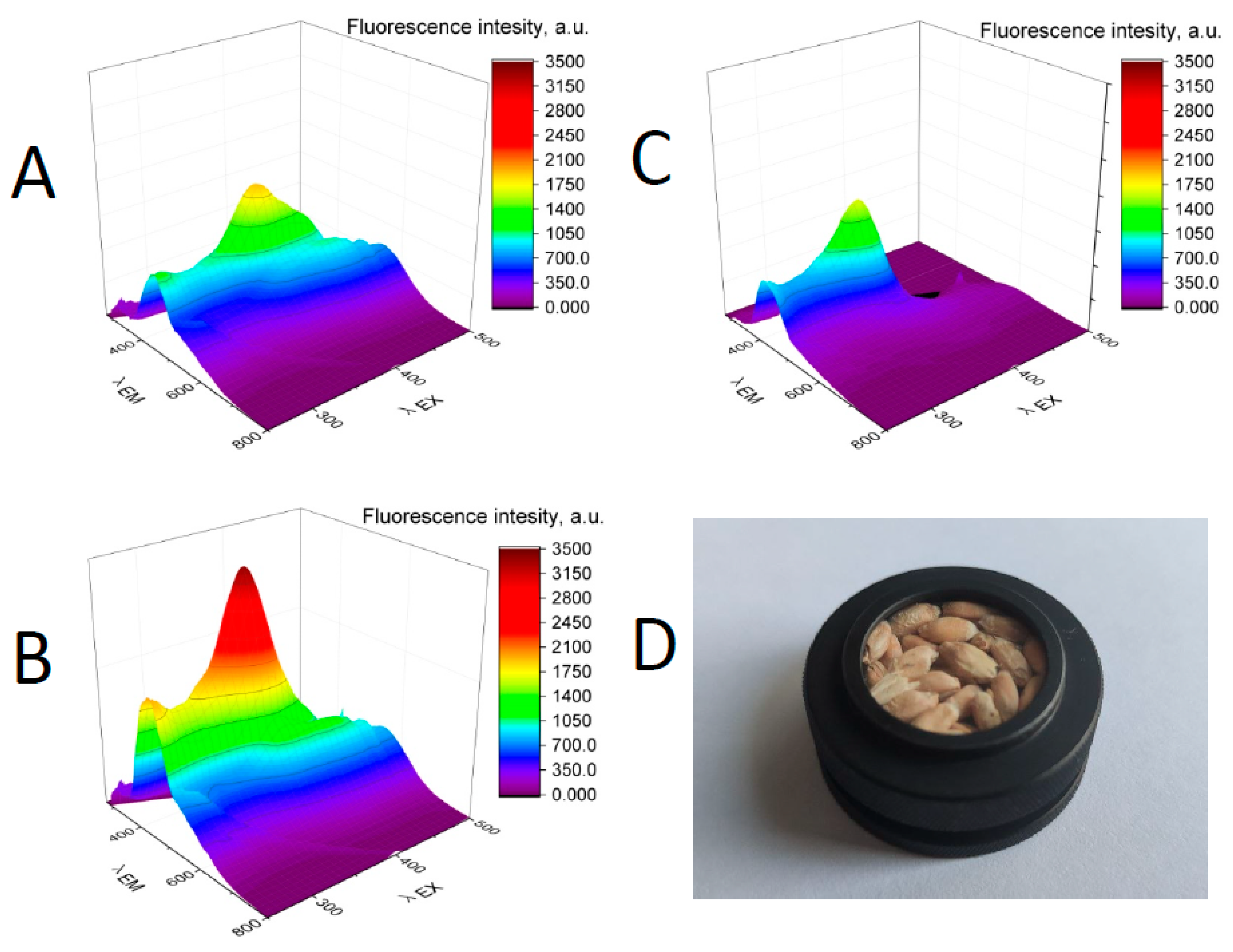
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farber, C.; Islam, A.S.M.F.; Septiningsih, E.M.; Thomson, M.J.; Kurouski, D. Non-Invasive Identification of Nutrient Components in Grain. Molecules 2021, 26, 3124. [Google Scholar] [CrossRef]
- Egging, V.; Nguyen, J.; Kurouski, D. Detection and Identification of Fungal Infections in Intact Wheat and Sorghum Grain Using a Hand-Held Raman Spectrometer. Anal. Chem. 2018, 90, 8616–8621. [Google Scholar] [CrossRef]
- Farber, C.; Kurouski, D. Detection and Identification of Plant Pathogens on Maize Kernels with a Hand-Held Raman Spectrometer. Anal. Chem. 2018, 90, 3009–3012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, L.; Farber, C.; Lei, J.X.; Zhu-Salzman, K.; Kurouski, D. Noninvasive and Nondestructive Detection of Cowpea Bruchid within Cowpea Seeds with a Hand-Held Raman Spectrometer. Anal. Chem. 2019, 91, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.; Pant, S.; Xing, Z.L.; Mandadi, K.; Kurouski, D. Rapid and noninvasive diagnostics of Huanglongbing and nutrient deficits on citrus trees with a handheld Raman spectrometer. Anal. Bioanal. Chem. 2019, 411, 3125–3133. [Google Scholar] [CrossRef]
- D’Angelo, D.L.; Bradley, C.A.; Ames, K.A.; Willyerd, K.T.; Madden, L.V.; Paul, P.A. Efficacy of Fungicide Applications during and After Anthesis against Fusarium Head Blight and Deoxynivalenol in Soft Red Winter Wheat. Plant Dis. 2014, 98, 1387–1397. [Google Scholar] [CrossRef] [Green Version]
- Center for Disease Control and Prevention Federal Select Agent Program. Select Agent and Toxin List. 2017. Available online: www.selectagents.gov/sat/list.htm (accessed on 10 December 2021).
- Commission Regulation EC. 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 10 December 2021).
- Gagkaeva, T.Y.; Gavrilova, O.P.; Levitin, M.M.; Novozhilov, K.V. Fusarium of grain crops. Plant Prot. Quar. 2011, 5. [Google Scholar]
- Sarimov, R.M.; Lednev, V.N.; Sibirev, A.V.; Gudkov, S.V. The Use of Fluorescence Spectra for the Detection of Scab and Rot in Fruit and Vegetable Crops. Front. Phys. 2021, 8, 640887. [Google Scholar] [CrossRef]
- Levakova, O.V.; Barkovskaya, T.A.; Bannikova, M.I. New variety of winter soft wheat Felicia. Bull. Russ. Agric. Sci. 2020, 3. [Google Scholar] [CrossRef]
- Penkov, N.V.; Goltyaev, M.V.; Astashev, M.E.; Serov, D.A.; Moskovskiy, M.N.; Khort, D.O.; Gudkov, S.V. The Application of Terahertz Time-Domain Spectroscopy to Identification of Potato Late Blight and Fusariosis. Pathogens 2021, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E.; Bruskov, V.I.; Gudkov, S.V. The role of peroxiredoxin 6 in neutralization of X-ray mediated oxidative stress: Effects on gene expression, preservation of radiosensitive tissues and postradiation survival of animals. Free Radic. Res. 2017, 51, 148–166. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Spectral Analysis and Deconvolution of the Amide I Band of Proteins Presenting with High-Frequency Noise and Baseline Shifts. Appl. Spectrosc. 2020, 74, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Krimmer, M.; Farber, C.; Kurouski, D. Rapid and Noninvasive Typing and Assessment of Nutrient Content of Maize Kernels Using a Handheld Raman Spectrometer. ACS Omega 2019, 4, 16330–16335. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.R.; Alves, R.S.; Nascimbem, L.; Stephani, R.; Poppi, R.J.; de Oliveira, L.F.C. Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal. Bioanal. Chem. 2010, 397, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.G.M.; Farwell, D.W.; Webster, D. FT Raman microscopy of untreated natural plant fibres. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 1997, 53, 2383–2392. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, A.; Wong, Y.H.; Korsholm, S.S.; Bahring, N.H.B.; Bobone, S.; Tayyab, S.; van de Weert, M.; Stella, L. On the purported “backbone fluorescence” in protein three-dimensional fluorescence spectra. RSC Adv. 2016, 6, 112870–112876. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.L.; Hill, S.C.; Pinnick, R.G.; House, J.M.; Flagan, R.C.; Chang, R.K. Dual-excitation-wavelength fluorescence spectra and elastic scattering for differentiation of single airborne pollen and fungal particles. Atmos. Environ. 2011, 45, 1555–1563. [Google Scholar] [CrossRef]
- Raimondi, V.; Palombi, L.; Cecchi, G.; Lognoli, D.; Trambusti, M.; Gomoiu, I. Remote detection of laser-induced autofluorescence on pure cultures of fungal and bacterial strains and their analysis with multivariate techniques. Opt. Commun. 2007, 273, 219–225. [Google Scholar] [CrossRef]
- Raimondi, V.; Agati, G.; Cecchi, G.; Gomoiu, I.; Lognoli, D.; Palombi, L. In vivo real-time recording of UV-induced changes in the autofluorescence of a melanin-containing fungus using a microspectrofluorimeter and a low-cost webcam. Opt. Express 2009, 17, 22735–22746. [Google Scholar] [CrossRef] [PubMed]
- Konig, K.; Berns, M.W.; Tromberg, B.J. Time-resolved and steady-state fluorescence measurements of beta-nicotinamide adenine dinucleotide-alcohol dehydrogenase complex during UVA exposure. J. Photochem. Photobiol. B-Biol. 1997, 37, 91–95. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufosse, L.; Singh, S.K.; Singh, P.N. Fungal Pigments and Their Prospects in Different Industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khundzhua, D.A.; Patsaeva, S.V.; Terekhova, V.A.; Yuzhakov, V.I. Spectral Characterization of Fungal Metabolites in Aqueous Medium with Humus Substances. J. Spectrosc. 2013, 2013, 538608. [Google Scholar] [CrossRef] [Green Version]
- Fredlund, E.; Gidlund, A.; Pettersson, H.; Olsen, M.; Borjesson, T. Real-time PCR detection of Fusarium species in Swedish oats and correlation to T-2 and HT-2 toxin content. World Mycotoxin J. 2010, 3, 77–88. [Google Scholar] [CrossRef]
- Lang, M.; Stober, F.; Lichtenthaler, H.K. Fluorescence emission-spectra of plant-leaves and plant constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef]
- Harris, P.J.; Hartley, R.D. Detection of bound ferulic acid in cell-walls of gramineae by ultraviolet fluorescence microscopy. Nature 1976, 259, 508–510. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Schweiger, J. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J. Plant Physiol. 1998, 152, 272–282. [Google Scholar] [CrossRef]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]

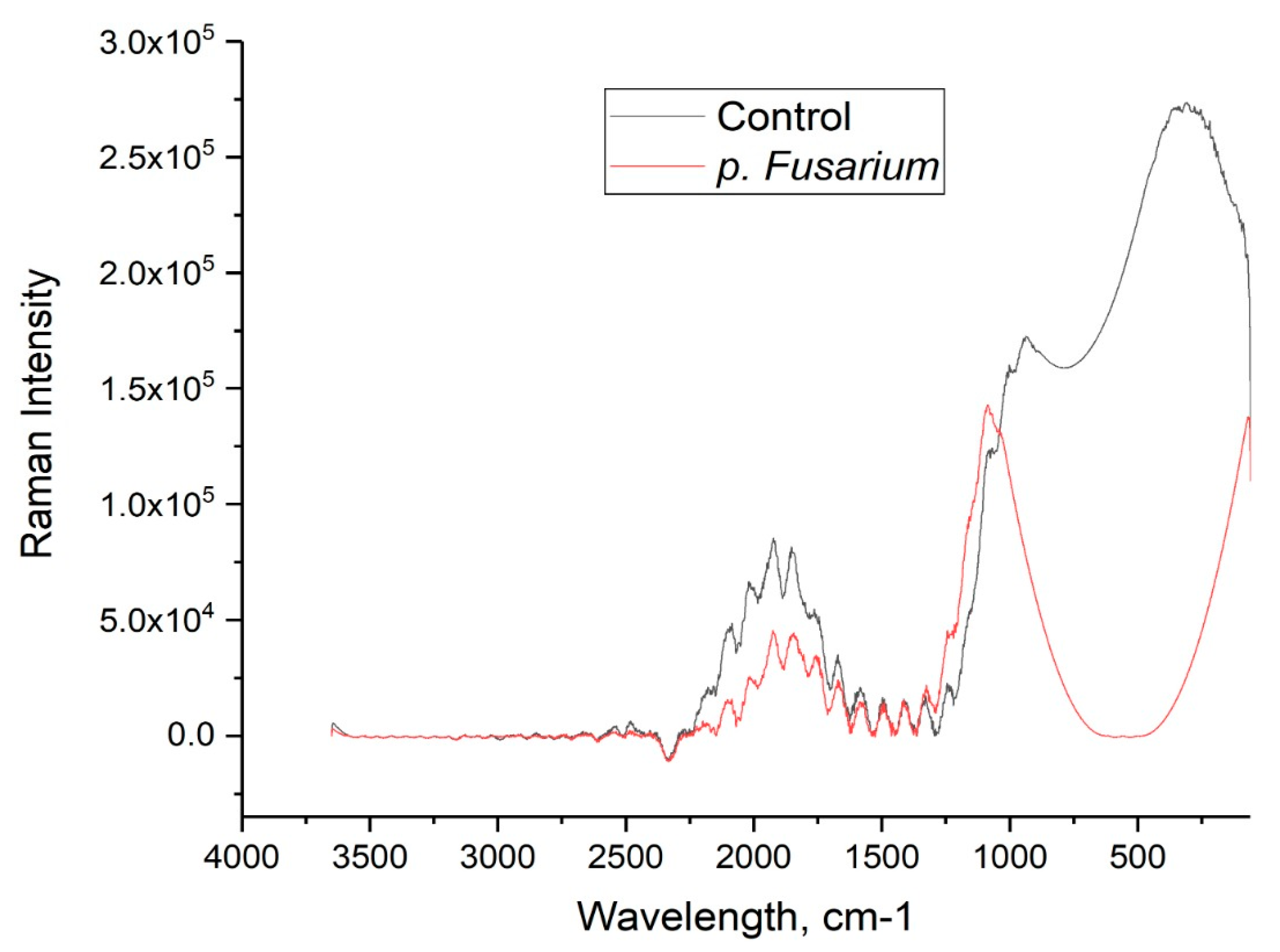
| Band (cm−1) | Vibrational Mode | Assignment |
|---|---|---|
| 479.04–481.42 | Deformations CCO and CCC; Associated with deformations of the skeleton of the glycosidic ring δ (C–C–C) + τ (C–O) notching C–C–C and bending C–O out of plane (CCO and CCC deformations; Related to glycosidic ring skeletal deformations δ (C–C–C) + τ (C–O) scissoring of C–C–C and out-of-plane bending of C–O) | Quantitative content of amylase [15,16]. Raman band at 480 cm−1, related to the ring vibration of starches [15,16]. |
| 864 | δ(C–C–H) + δ(C–O–C) glycosidic bond; anomeric region | Starch (range of carbohydrates 410–1259 cm−1) [1] |
| 938 | δ(C–O–C) + δ(C–O–H) + ν(C–O) α-1,4 glycosidic linkages | Starch (range of carbohydrates 410–1259 cm−1) [1] |
| 1029–1031 | In-plane CH3 rocking of polyene aromatic ring of phenylalanine | Cellulose, phenylpropanoids [17] Carotenoids [18] |
| 1126 | ν (C–O) + ν(C–C) + δ(C–O–H) | Starch (range of carbohydrates 410–1259 cm−1) [1] |
| 1152 | ν(C–O–C), ν(C–C) in glycosidic linkage, asymmetric ring breath | Carbohydrates [19] |
| 1463–1458 | δ(CH) + δ(CH2) + δ(C–O–H) CH, CH2, and COH deformations | Carbohydrates [16] |
| 1597–1504 | Carotenoids [3] |
| Object | Protein, % | Water, % | Fat, % | Cellulose, % | Ash, % | Starch, % |
|---|---|---|---|---|---|---|
| Sample 1 | 10.16 | 11.97 | 1.56 | 2.06 | 1.42 | 60.30 |
| Sample 2 | 11.29 | 12.35 | 1.43 | 2.2 | 1.39 | 59.88 |
| Sample 3 | 10.55 | 12.26 | 1.49 | 1.84 | 1.30 | 60.67 |
| Sample 4 | 11.19 | 11.95 | 1.59 | 2.01 | 1.59 | 59.19 |
| Sample 5 | 11.54 | 12.04 | 1.42 | 2.15 | 1.52 | 60.25 |
| Sample 6 | 10.30 | 12.18 | 1.41 | 2.05 | 1.43 | 61.19 |
| Sample 7 | 11.45 | 12.16 | 1.44 | 2.09 | 1.41 | 61.49 |
| Sample 8 | 10.72 | 11.94 | 1.44 | 1.88 | 1.49 | 60.68 |
| Mean | 10.84 | 12.11 | 1.47 | 2.03 | 1.45 | 60.38 |
| SD | 0.536 | 0.154 | 0.068 | 0.125 | 0.088 | 0.727 |
| Object | Protein, % | Water, % | Fat, % | Cellulose, % | Ash, % | Starch, % |
|---|---|---|---|---|---|---|
| Sample 1 | 11.54 | 11.25 | 1.61 | 2.39 | 1.64 | 58.34 |
| Sample 2 | 12.74 | 11.08 | 1.70 | 2.58 | 1.77 | 57.83 |
| Sample 3 | 12.48 | 11.12 | 1.77 | 2.15 | 1.66 | 60.59 |
| Sample 4 | 11.75 | 10.44 | 1.82 | 2.41 | 1.76 | 59.80 |
| Sample 5 | 11.19 | 10.89 | 1.80 | 2.2 | 1.74 | 57.98 |
| Sample 6 | 11.84 | 11.00 | 1.80 | 2.51 | 1.8 | 56.60 |
| Sample 7 | 12.79 | 11.19 | 1.68 | 2.64 | 1.72 | 57.80 |
| Sample 8 | 11.10 | 10.53 | 2.09 | 2.50 | 2.23 | 53.70 |
| Mean | 11.91 ** | 10.95 *** | 1.77 *** | 2.44 *** | 1.77 *** | 57.82 ** |
| SD | 0.668 | 0.302 | 0.144 | 0.174 | 0.186 | 2.084 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskovskiy, M.N.; Sibirev, A.V.; Gulyaev, A.A.; Gerasimenko, S.A.; Borzenko, S.I.; Godyaeva, M.M.; Noy, O.V.; Nagaev, E.I.; Matveeva, T.A.; Sarimov, R.M.; et al. Raman Spectroscopy Enables Non-Invasive Identification of Mycotoxins p. Fusarium of Winter Wheat Seeds. Photonics 2021, 8, 587. https://doi.org/10.3390/photonics8120587
Moskovskiy MN, Sibirev AV, Gulyaev AA, Gerasimenko SA, Borzenko SI, Godyaeva MM, Noy OV, Nagaev EI, Matveeva TA, Sarimov RM, et al. Raman Spectroscopy Enables Non-Invasive Identification of Mycotoxins p. Fusarium of Winter Wheat Seeds. Photonics. 2021; 8(12):587. https://doi.org/10.3390/photonics8120587
Chicago/Turabian StyleMoskovskiy, Maksim N., Aleksey V. Sibirev, Anatoly A. Gulyaev, Stanislav A. Gerasimenko, Sergey I. Borzenko, Maria M. Godyaeva, Oleg V. Noy, Egor I. Nagaev, Tatiana A. Matveeva, Ruslan M. Sarimov, and et al. 2021. "Raman Spectroscopy Enables Non-Invasive Identification of Mycotoxins p. Fusarium of Winter Wheat Seeds" Photonics 8, no. 12: 587. https://doi.org/10.3390/photonics8120587
APA StyleMoskovskiy, M. N., Sibirev, A. V., Gulyaev, A. A., Gerasimenko, S. A., Borzenko, S. I., Godyaeva, M. M., Noy, O. V., Nagaev, E. I., Matveeva, T. A., Sarimov, R. M., & Simakin, A. V. (2021). Raman Spectroscopy Enables Non-Invasive Identification of Mycotoxins p. Fusarium of Winter Wheat Seeds. Photonics, 8(12), 587. https://doi.org/10.3390/photonics8120587








