Evaluation of Three Iterative Algorithms for Phase Modulation Regarding Their Application in Concentrating Light Inside Biological Tissues for Laser Induced Photothermal Therapy
Abstract
:1. Introduction
2. Phase Control Algorithms
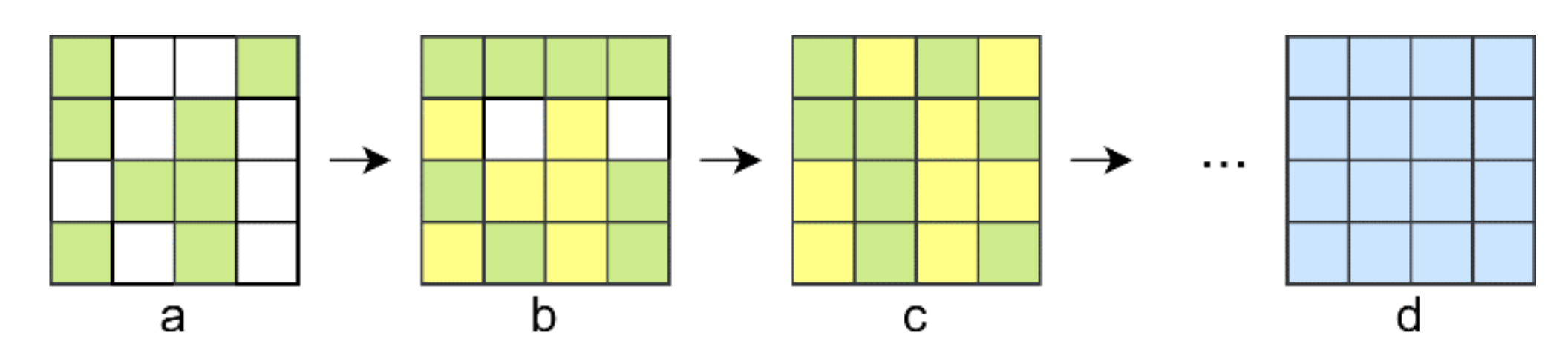
2.1. Four-Element Division Algorithm (FEDA)
2.2. Partitioning Algorithm (PA)
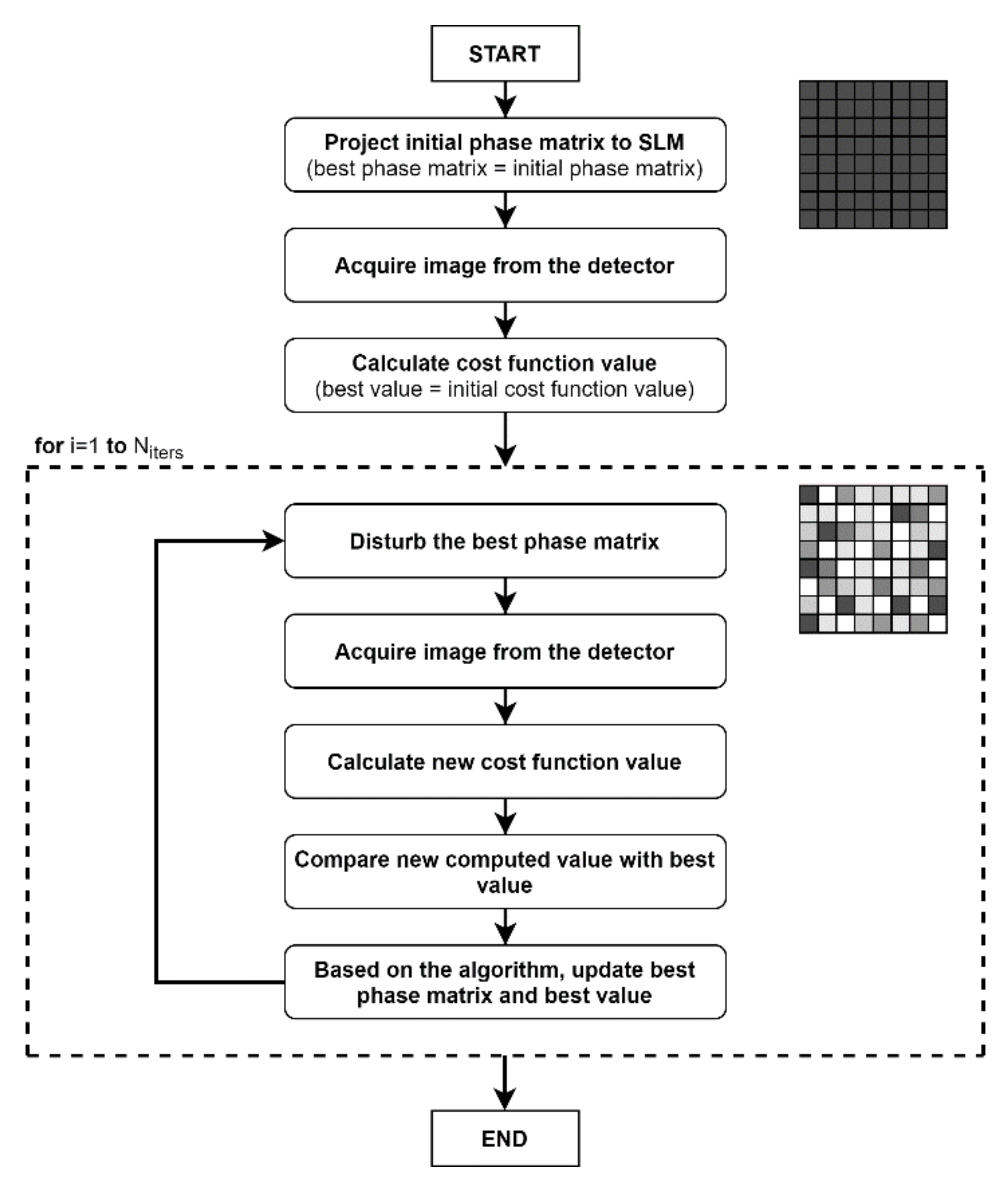
2.3. Simulated Annealing Algorithm (SAA)
2.4. Cost Functions
2.4.1. Weighted Average
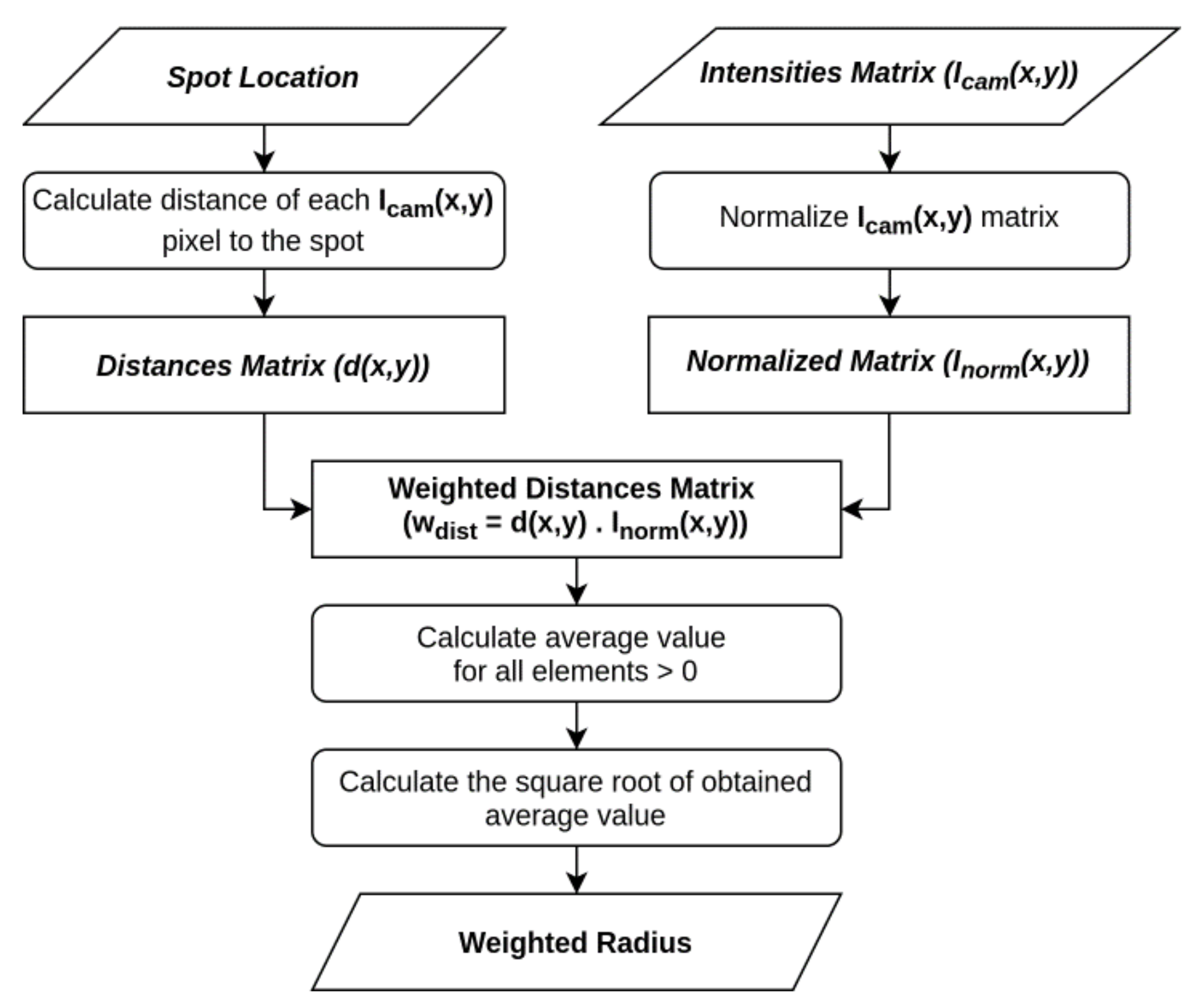
2.4.2. Weighted Radius
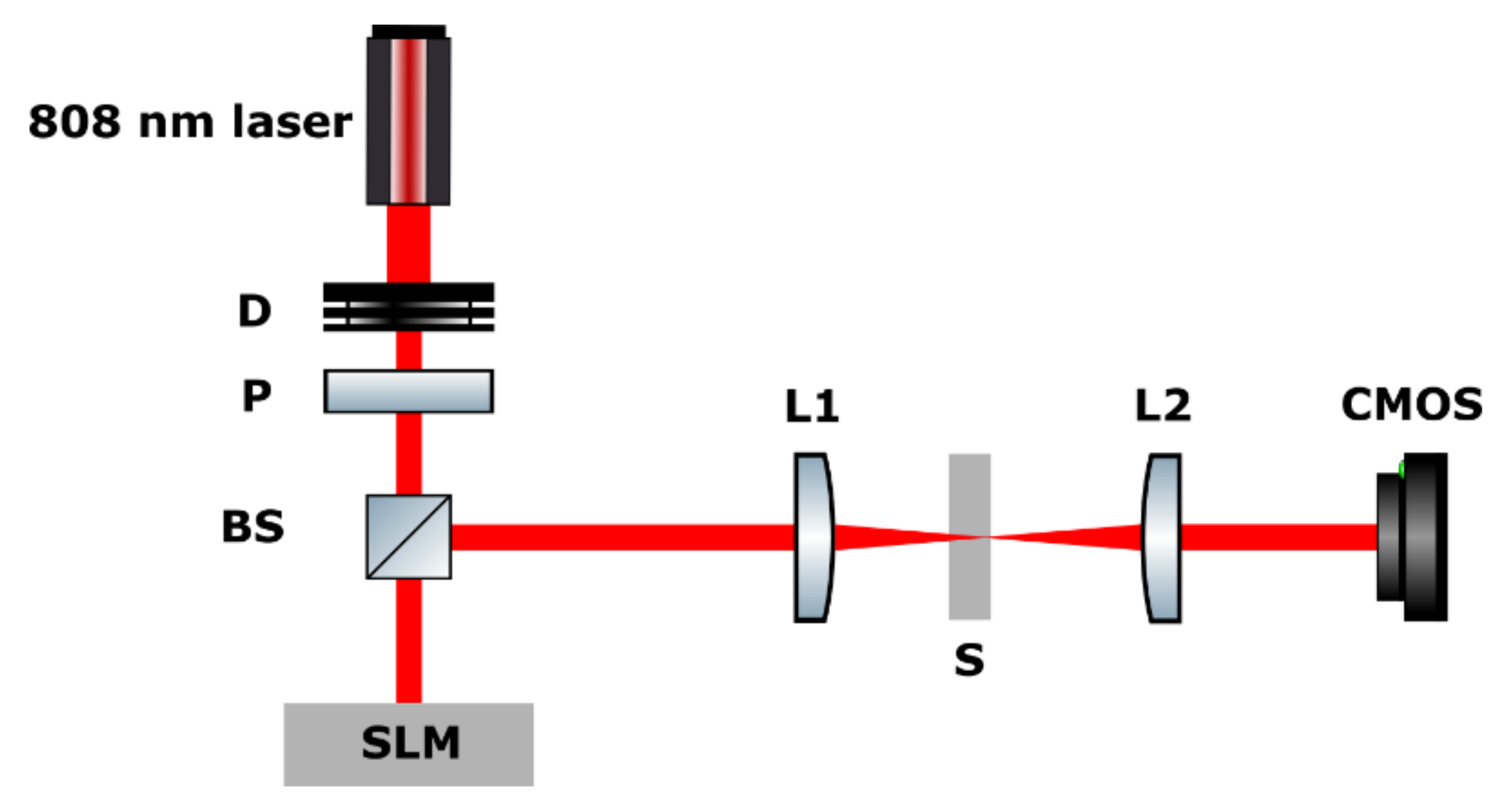
3. Experimental Procedures
- 1-
- A mixture of 1% agar-agar powder in distilled water was made;
- 2-
- The resulting mixture was heated until its boiling point;
- 3-
- The boiling mixture was left to cool at room temperature;
- 4-
- When the temperature reached a value below 50 °C, the right amount of milk was added to have a 5% milk concentrated mixture;
- 5-
- The resulting mixture was left to cool, for a minimum of 4 h, until use.
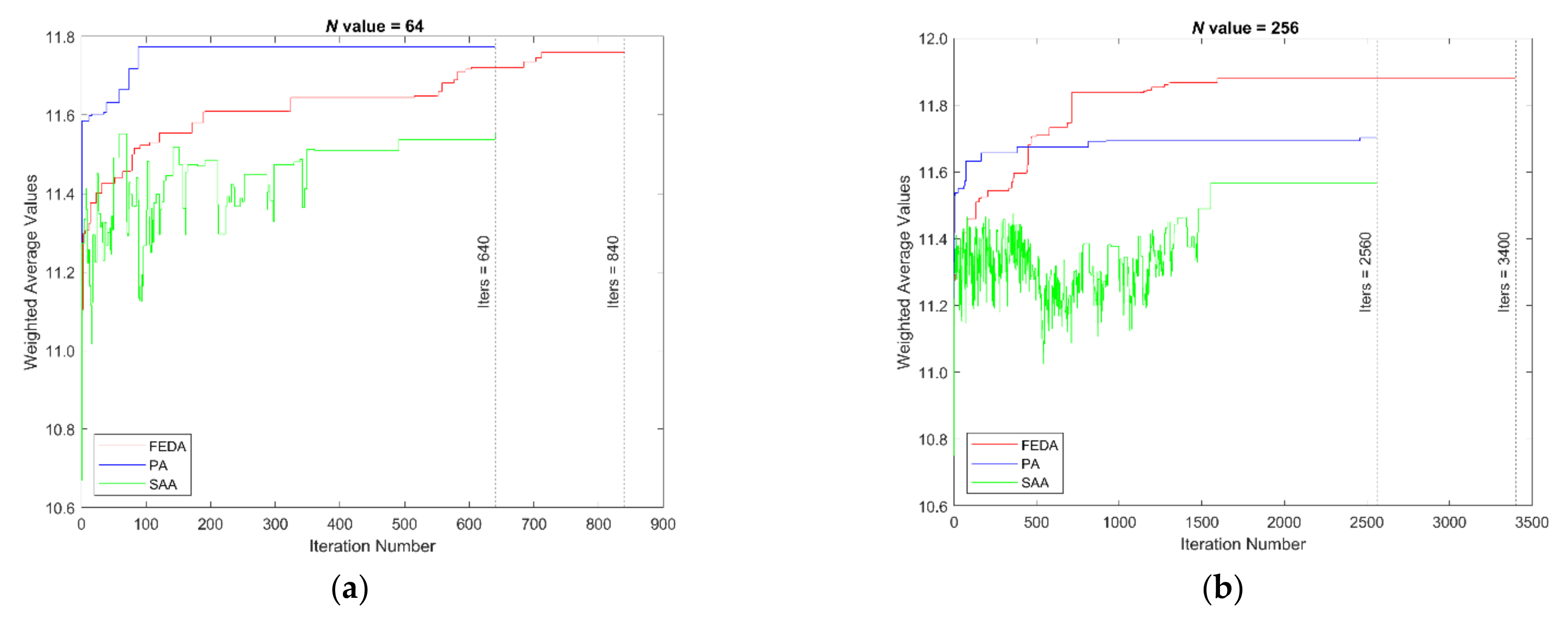
4. Results and Discussion
4.1. Weighted Average Results
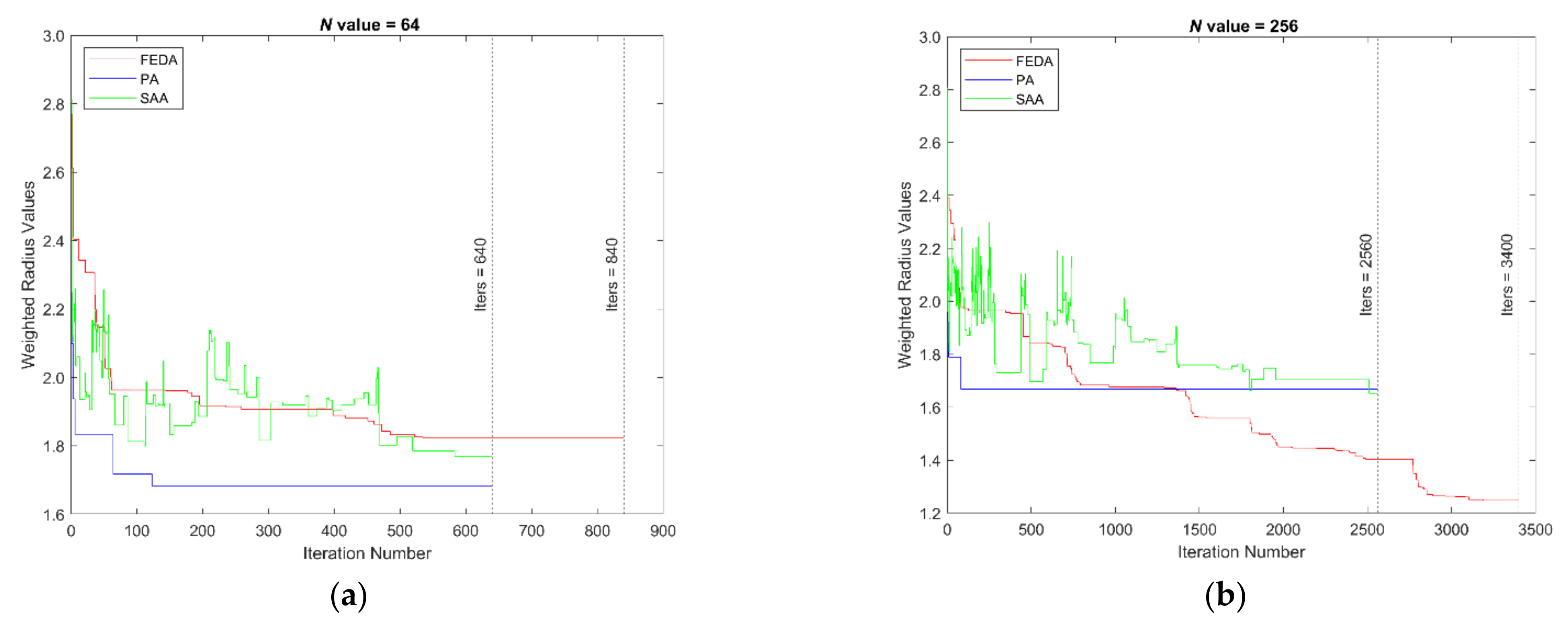
4.2. Weighted Radius Results
4.3. Final Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, J.M.P.; Vilhena, H.; Rebordão, J.M. Finite element model for the simulation of laser activated micro- and nano-scale drug delivery systems. In Proceedings of the Third International Conference on Applications of Optics and Photonics, Faro, Portugal, 8–12 May 2017; Volume 10453, p. 104531Y. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Mirza, F.N.; Khatri, K.A. The use of lasers in the treatment of skin cancer: A review. J. Cosmet. Laser Ther. 2017, 19, 451–458. [Google Scholar] [CrossRef]
- Jain, S.M.; Pandey, K.; Lahoti, A.; Rao, P.K. Evaluation of skin and subcutaneous tissue thickness at insulin injection sites in Indian, insulin naïve, type-2 diabetic adult population. Indian J. Endocrinol. Metab. 2013, 17, 864–870. [Google Scholar] [CrossRef]
- Schena, E.; Saccomandi, P.; Fong, Y. Laser ablation for cancer: Past, present and future. J. Funct. Biomater. 2017, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Coelho, J.M.P.; Vieira, P.M.C.; Viana, A.S.; Gaspar, M.M.; Reis, C. Preliminary assays towards melanoma cells using phototherapy with gold-based nanomaterials. Nanomaterials 2020, 10, 1536. [Google Scholar] [CrossRef]
- Lopes, A.; Gomes, R.; Castiñeras, M.; Coelho, J.M.P.; Santos, J.P.; Vieira, P. Probing deep tissues with laser-induced thermotherapy using near-infrared light. Lasers Med. Sci. 2020, 35, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging: Progress continues in the development of smaller, more penetrable probes for biological imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Coelho, J.M.P.; Gabriel, A.; Vieira, P.; Oliveira Silva, C.; Reis, C. Wavefront shaping using a deformable mirror for focusing inside optical tissue phantoms. In Proceedings of the Second International Conference on Applications of Optics and Photonics, Aveiro, Portugal, 26–30 May 2014; Volume 9286, p. 92863Z. [Google Scholar] [CrossRef]
- Yu, H.; Park, J.; Lee, K.; Yoon, J.; Kim, K.; Lee, S.; Park, Y. Recent advances in wavefront shaping techniques for biomedical applications. Curr. Appl. Phys. 2015, 15, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Huang, L.; Zentgraf, T.; Wang, Y. Optical wavefront shaping based on functional metasurfaces. Nanophotonics 2020, 9, 987–1002. [Google Scholar] [CrossRef]
- Tay, J.W.; Lai, P.; Suzuki, Y.; Wang, L.V. Ultrasonically encoded wavefront shaping for focusing into random media. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Gomes, R.; Vieira, P.; Coelho, J.M.P. Optical simulation of laser beam phase-shaping focusing optimization in biological tissues. In Proceedings of the 8th Ibero American Optics Meeting/11th Latin American Meeting on Optics, Lasers, and Applications, Porto, Portugal, 22–26 July 2013; Volume 8785, p. 8785DE. [Google Scholar] [CrossRef]
- Kanngiesser, J.; Roth, B. Wavefront shaping concepts for application in optical coherence tomography—A review. Sensors 2020, 20, 7044. [Google Scholar] [CrossRef]
- Fayyaz, Z.; Mohammadian, N.; Salimi, F.; Fatima, A.; Tabar, M.R.R.; Avanaki, M.R.N. Simulated annealing optimization in wavefront shaping controlled transmission. Appl. Opt. 2018, 57, 6233. [Google Scholar] [CrossRef]
- Tseng, S.H.; Yang, C. 2-D PSTD Simulation of optical phase conjugation for turbidity suppression. Opt. Express 2007, 15, 16005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayyaz, Z.; Salimi, F.; Mohammadian, N.; Fatima, A.; Rahimi Tabar, M.R.; Avanaki, M.R.N. Wavefront shaping using simulated annealing algorithm for focusing light through turbid media. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing, San Francisco, CA, USA, 28 January–1 February 2018; Volume 10494, p. 104946M. [Google Scholar] [CrossRef]
- Fayyaz, Z.; Mohammadian, N.; Avanaki, M.R.N. Comparative assessment of five algorithms to control an SLM for focusing coherent light through scattering media. In Proceedings of thePhotons Plus Ultrasound: Imaging and Sensing, San Francisco, CA, USA, 28 January–1 February 2018; Volume 10494, p. 104946I. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, X.; Zuo, H.; Pang, L. Focusing light through random scattering media by four-element division algorithm. Opt. Commun. 2018, 407, 301–310. [Google Scholar] [CrossRef]
- Vellekoop, I.M.; Mosk, A.P. Phase control algorithms for focusing light through turbid media. Opt. Commun. 2008, 281, 3071–3080. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Zuo, H.; Yang, Z.; Zhang, X.; Pang, L. Focusing light through random scattering media by simulated annealing algorithm. J. Appl. Phys. 2018, 124, 083104. [Google Scholar] [CrossRef]
- Vellekoop, I.M.; Aegerter, C.M. Focusing light through living tissue. In Proceedings of the Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XIV, San Francisco, CA, USA, 25–27 January 2010; Volume 7554, p. 755430. [Google Scholar] [CrossRef] [Green Version]
- Ntombela, L.; Adeleye, B.; Chetty, N. Low-cost fabrication of optical tissue phantoms for use in biomedical imaging. Heliyon 2020, 6, e03602. [Google Scholar] [CrossRef]
- Kono, T.; Yamada, J. In Vivo measurement of optical properties of human skin for 450–800 nm and 950–1600 nm wavelengths. Int. J. Thermophys. 2019, 40, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Stocker, S.; Foschum, F.; Krauter, P.; Bergmann, F.; Hohmann, A.; Happ, C.S.; Kienle, A. Broadband optical properties of milk. Appl. Spectrosc. 2017, 71, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Quijano, N.; Fanjul-Vélez, F.; Salas-García, I.; Arce-Diego, J.L. Optical characterization of lipid-based tissue phantoms. In Proceedings of the 21st International Conference Radioelektronika, Brno, Czech Republic, 19–20 April 2011; pp. 107–110. [Google Scholar] [CrossRef]
- Aernouts, B.; Van Beers, R.; Watté, R.; Huybrechts, T.; Lammertyn, J.; Saeys, W. Visible and near-infrared bulk optical properties of raw milk. J. Dairy Sci. 2015, 98, 6727–6738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegelberger, G. ICNIRP guidelines on limits of exposure to laser radiation of wavelengths between 180 nm and 1000 µm. Health Phys. 2013, 105, 271–295. [Google Scholar]
- Ziegelberger, G.; van Rongen, E.; Croft, R.; Feychting, M.; Green, A.C.; Hirata, A.; d’Inzeo, G.; Marino, C.; Miller, S.; Oftedal, G.; et al. Principles for non-ionizing radiation protection. Health Phys. 2020, 118, 477–482. [Google Scholar]



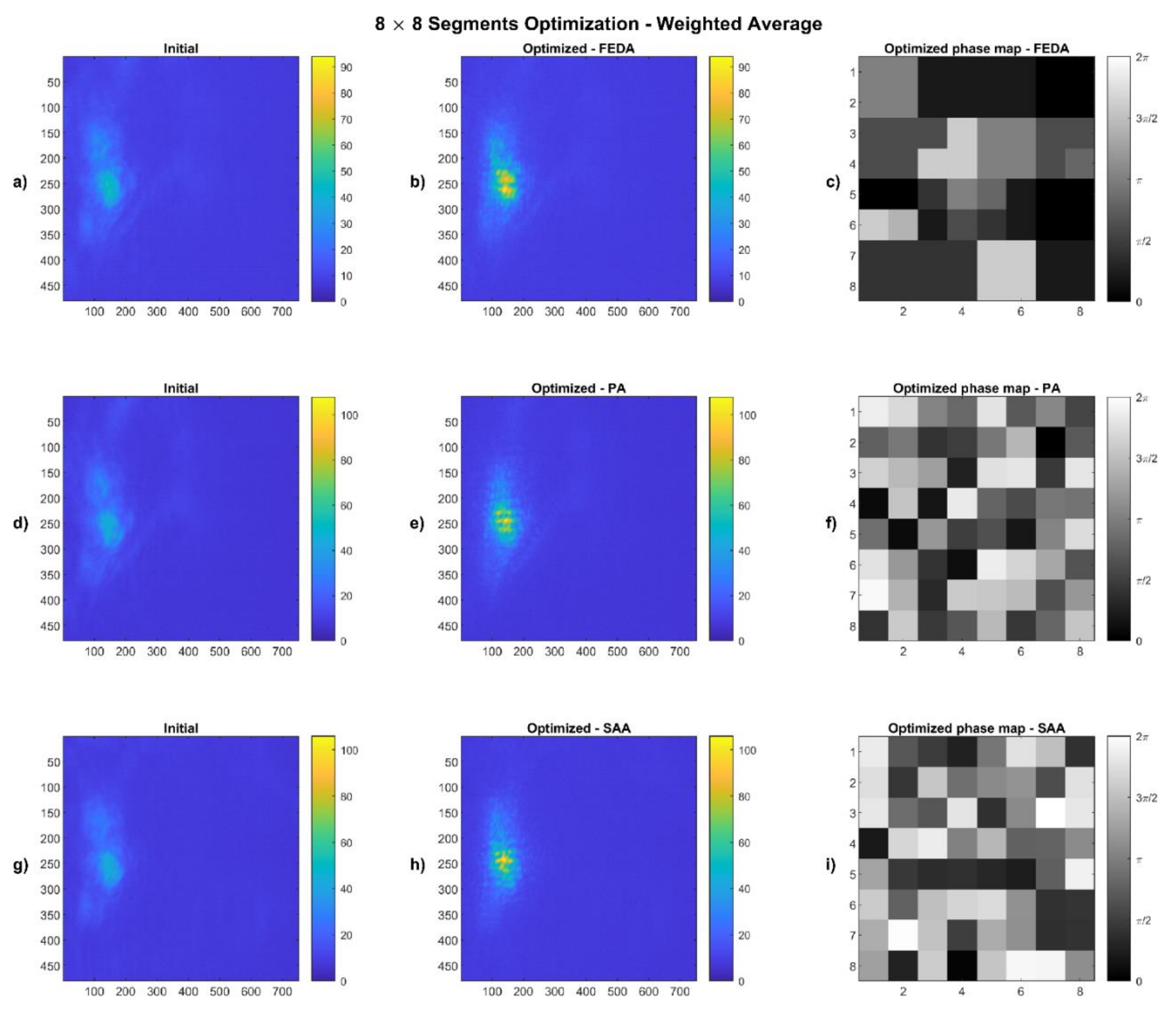
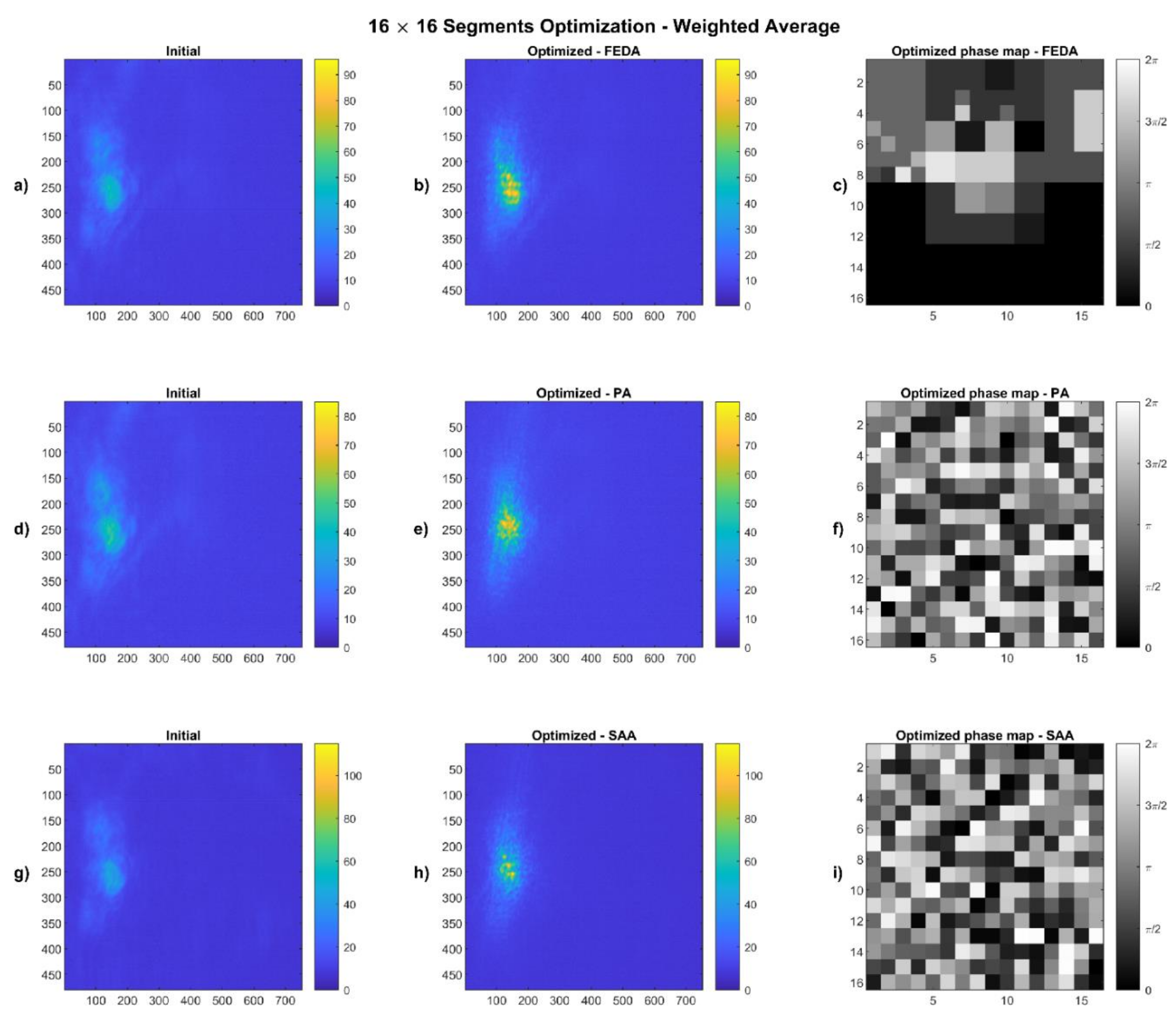
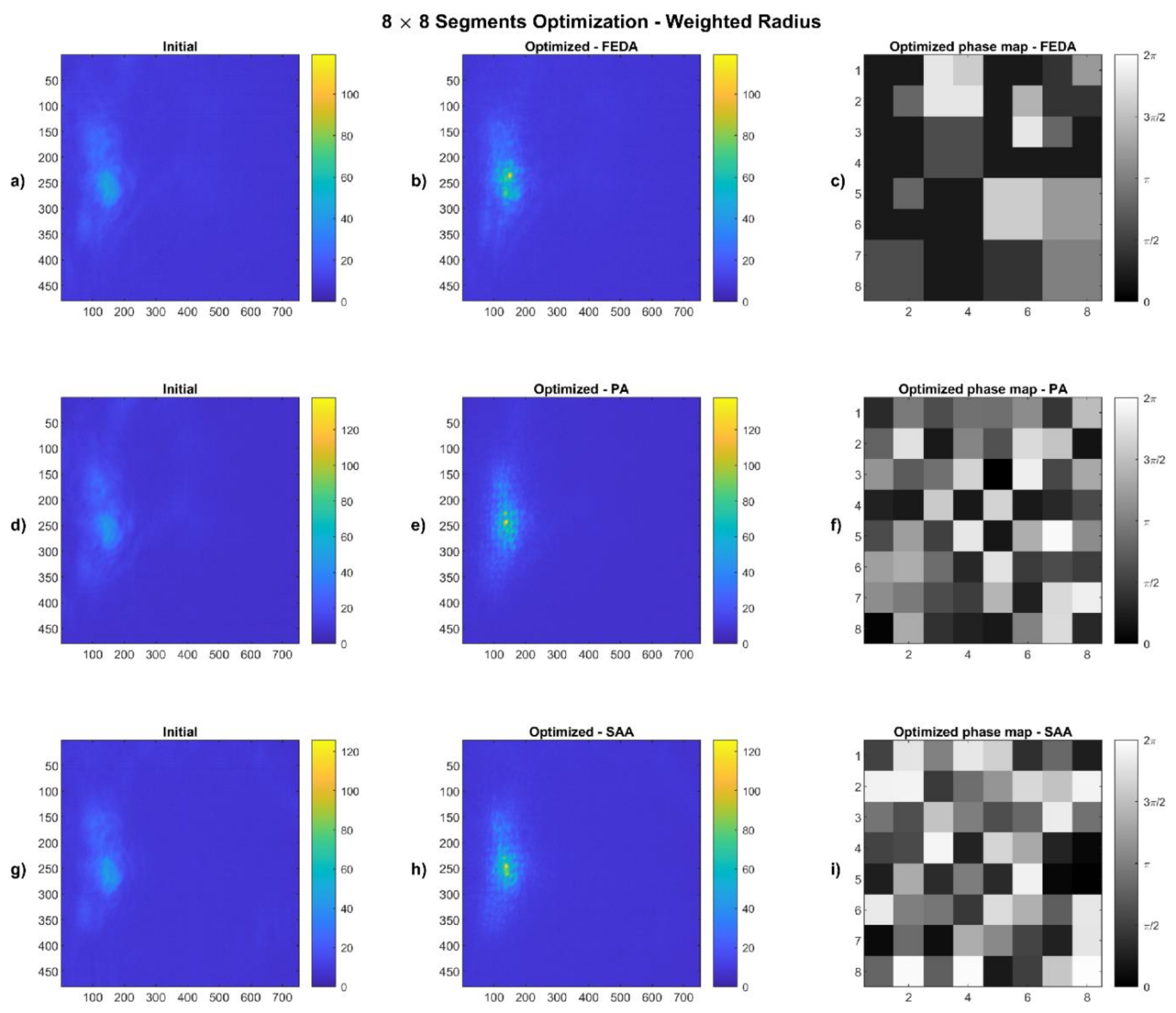

| Algorithm | Segmentation | Max. I Value (0–255) | Av. Intensity in Spot | Improvement | Weighted Radius (Outside the Spot) | Improvement | |
|---|---|---|---|---|---|---|---|
| FEDA | 8 × 8 | Initial | 52 | 30.75 | 45% | 3.43 | 17% |
| Optimized | 94 | 44.38 | 2.84 | ||||
| 16 × 16 | Initial | 52 | 30.83 | 51% | 3.48 | 26% | |
| Optimized | 96 | 46.57 | 2.59 | ||||
| PA | 8 × 8 | Initial | 52 | 30.92 | 49% | 3.26 | 17% |
| Optimized | 108 | 45.93 | 2.72 | ||||
| 16 × 16 | Initial | 53 | 31.04 | 34% | 3.31 | 19% | |
| Optimized | 85 | 41.47 | 2.70 | ||||
| SAA | 8 × 8 | Initial | 50 | 29.90 | 50% | 3.47 | 21% |
| Optimized | 106 | 44.52 | 2.73 | ||||
| 16 × 16 | Initial | 51 | 29.93 | 39% | 3.44 | 24% | |
| Optimized | 115 | 41.60 | 2.61 |
| Algorithm | Segmentation | Max. I Value (0–255) | Av. Intensity in Spot | Improvement | Weighted Radius (Outside the Spot) | Improvement | |
|---|---|---|---|---|---|---|---|
| FEDA | 8 × 8 | Initial | 51 | 30.64 | 30% | 3.53 | 18% |
| Optimized | 119 | 39.90 | 2.90 | ||||
| 16 × 16 | Initial | 51 | 30.64 | 46% | 3.53 | 25% | |
| Optimized | 253 | 44.73 | 2.65 | ||||
| PA | 8 × 8 | Initial | 52 | 30.75 | 41% | 3.27 | 15% |
| Optimized | 138 | 43.34 | 2.77 | ||||
| 16 × 16 | Initial | 52 | 30.61 | 33% | 3.41 | 18% | |
| Optimized | 142 | 40.61 | 2.78 | ||||
| SAA | 8 × 8 | Initial | 51 | 29.71 | 38% | 3.70 | 27% |
| Optimized | 126 | 41.01 | 2.70 | ||||
| 16 × 16 | Initial | 51 | 29.72 | 34% | 3.58 | 24% | |
| Optimized | 145 | 39.84 | 2.71 |
| Algorithm | + | − |
|---|---|---|
| FEDA |
|
|
| PA |
|
|
| SAA |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerreiro, J.; Vieira, P.; Coelho, J.M.P. Evaluation of Three Iterative Algorithms for Phase Modulation Regarding Their Application in Concentrating Light Inside Biological Tissues for Laser Induced Photothermal Therapy. Photonics 2021, 8, 355. https://doi.org/10.3390/photonics8090355
Guerreiro J, Vieira P, Coelho JMP. Evaluation of Three Iterative Algorithms for Phase Modulation Regarding Their Application in Concentrating Light Inside Biological Tissues for Laser Induced Photothermal Therapy. Photonics. 2021; 8(9):355. https://doi.org/10.3390/photonics8090355
Chicago/Turabian StyleGuerreiro, João, Pedro Vieira, and João M. P. Coelho. 2021. "Evaluation of Three Iterative Algorithms for Phase Modulation Regarding Their Application in Concentrating Light Inside Biological Tissues for Laser Induced Photothermal Therapy" Photonics 8, no. 9: 355. https://doi.org/10.3390/photonics8090355






