Photobiomodulation, Cells of Connective Tissue and Repair Processes: A Look at In Vivo and In Vitro Studies on Bone, Cartilage and Tendon Cells
Abstract
:1. Introduction
2. Methodology
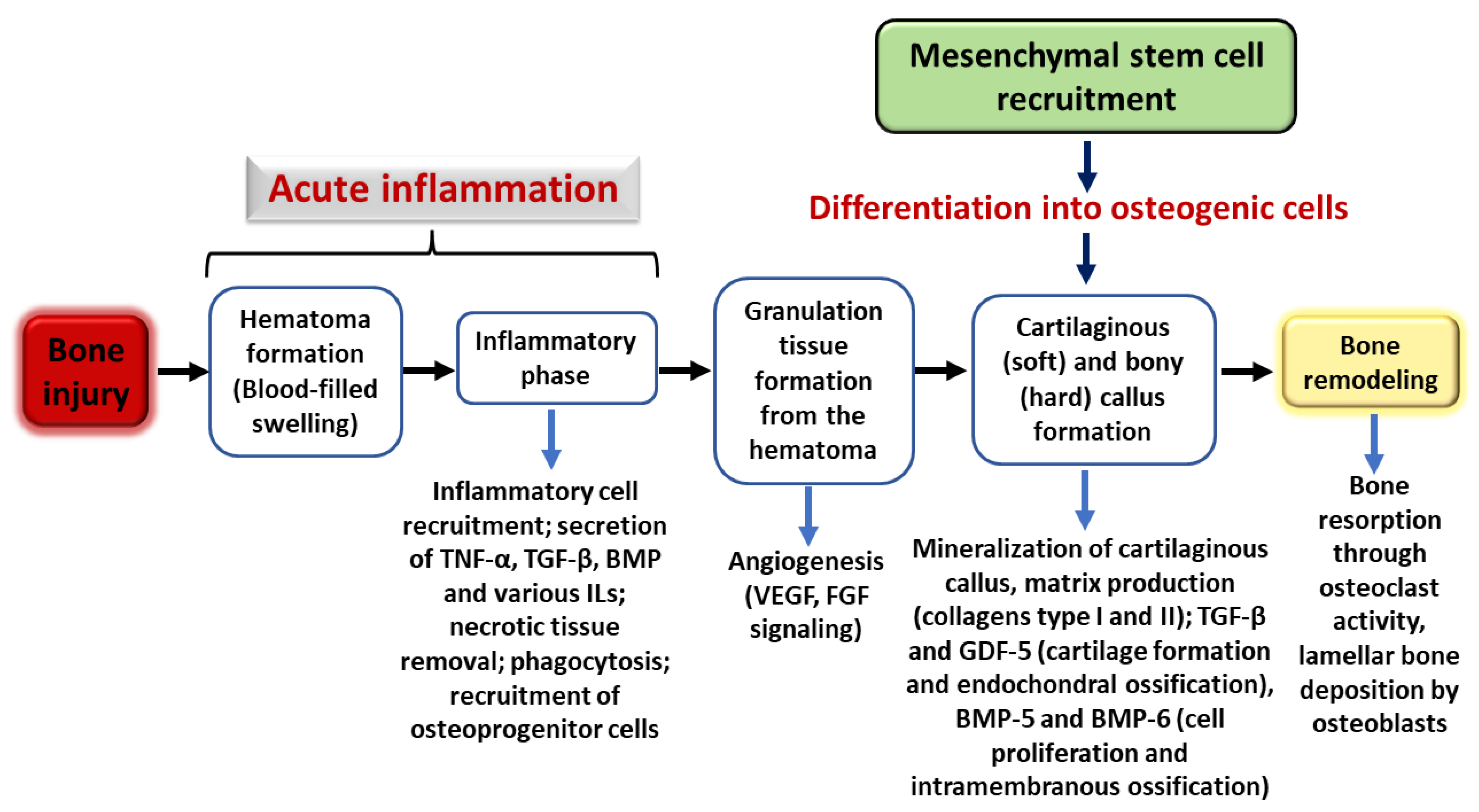
3. Bone Cells
4. Cartilage Cells
5. Tendon Cells
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderson, R.R.; Parrish, J.A. The optics of human skin. J. Investig. Dermatol. 1981, 77, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Son, T.; Lee, K.J.; Jung, B. Enhancement of light propagation depth in skin: Cross-validation of mathematical modeling methods. Lasers Med. Sci. 2009, 24, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Miyamura, Y.; Bowen, M.P.; Correa, G.; Ono, Y.; Goodarzi, H. Light, including ultraviolet. J. Autoimmun. 2010, 34, J247–J257. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 1–37. [Google Scholar] [CrossRef]
- Tosini, G.; Ferguson, I.; Tsubota, K. Effects of blue light on the circadian system and eye physiology. Mol. Vis. 2016, 22, 61–72. [Google Scholar]
- Young, A.R. Chromophores in human skin. Phys. Med. Biol. 1997, 42, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Cios, A.; Cieplak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of different wavelengths of laser irradiation on the skin cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.; Huang, Y.; Carroll, J.; Hamblin, M. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehfeld, A.; Nylander, M.; Karnov, K. Compendium of Histology. A Theoretical and Practical Guide; Springer Nature: Cham, Switzerland, 2017. [Google Scholar]
- Li, Y.; Wu, T.; Liu, S. Identification and distinction of tenocytes and tendon-derived stem cells. Front Cell Dev. Biol. 2021, 9, 629515. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature. 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M. An overview of bone cells and their regulating factors of differentiation. Malaysian. J. Med. Sci. 2008, 15, 4–12. [Google Scholar]
- Marsell, R.; Einhorn, T. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Mountziaris, P.; Tzouanas, S.; Mikos, A. Dose effect of tumor necrosis factor-α on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials 2010, 31, 1666. [Google Scholar] [CrossRef] [PubMed]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Bölükbaşı Ateş, G.; Ak Can, A.; Gülsoy, M. Investigation of photobiomodulation potentiality by 635 and 809 nm lasers on human osteoblasts. Lasers Med. Sci. 2017, 32, 591–599. [Google Scholar] [CrossRef]
- Bölükbaşı Ateş, G.; Ak, A.; Garipcan, B.; Gülsoy, M. Methylene blue mediated photobiomodulation on human osteoblast cells. Lasers Med. Sci. 2017, 32, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; do Vale Placa, R.; Sant’Ana, A.C.P.; Greghi, S.L.A.; Zangrando, M.S.R.; de Rezende, M.L.R.; Oliveira, R.C.; Damante, C.A. Laser and LED photobiomodulation effects in osteogenic or regular medium on rat calvaria osteoblasts obtained by newly forming bone technique. Lasers Med. Sci. 2021, 36, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Srivastava, K.; Gupta, A.; Saluja, T.S.; Kumar, S.; Mehrotra, D.; Singh, S.K. A quantitative method to determine osteogenic differentiation aptness of scaffold. J. Oral Biol. Craniofacial Res. 2020, 10, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Dalapria, V.; Marcos, R.L.; Bussadori, S.K.; Anselmo, G.; Benetti, C.; da Silva Santana, A.C.A.; Marinho, N.S.R.; Pinto, R.S.; de Sales, R.S.; de França, L.S.; et al. LED photobiomodulation therapy combined with biomaterial as a scaffold promotes better bone quality in the dental alveolus in an experimental extraction model. Lasers Med. Sci. 2022, 37, 1583–1592. [Google Scholar] [CrossRef]
- Bull, H.; Murray, P.G.; Thomas, D.; Fraser, A.M.; Nelson, P.N. Acid phosphatases. J. Clin. Pathol. Mol. Pathol. 2002, 55, 65–72. [Google Scholar] [CrossRef]
- Gonçalves, C.F.; Desiderá, A.d.C.; do Nascimento, G.C.; Issa, J.P.M.; Leite-Panissi, C.R.A. Experimental tooth movement and photobiomodulation on bone remodeling in rats. Lasers Med. Sci. 2016, 31, 1883–1890. [Google Scholar] [CrossRef]
- Gomes, M.F.; da Graças Vilela Goulart, M.; Giannasi, L.C.; Hiraoka, C.M.; de Fátima Santana Melo, G.; de Sousa, A.G.V.; Nóbrega, C.J.P.; Zangaro, R.A.; Salgado, M.A.C. Effects of the GaAlAs diode laser (780 nm) on the periodontal tissues during orthodontic tooth movement in diabetes rats: Histomorphological and immunohistochemical analysis. Lasers Med. Sci. 2017, 32, 1479–1487. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Xi, S.; Li, X.; Ding, L.; Li, M. The effects of photobiomodulation therapy on mouse pre-osteoblast cell line MC3T3-E1 proliferation and apoptosis via miR-503/Wnt3a pathway. Lasers Med. Sci. 2019, 34, 607–614. [Google Scholar] [CrossRef]
- Jullien, N.; Maudinet, A.; Leloutre, B.; Ringe, J.; Häupl, T.; Marie, P.J. Downregulation of ErbB3 by Wnt3a contributes to wnt-induced osteoblast differentiation in mesenchymal cells. J. Cell Biochem. 2012, 113, 2047–2056. [Google Scholar] [CrossRef]
- Karner, C.M.; Esen, E.; Chen, J.; Hsu, F.F.; Turk, J.; Long, F. Wnt protein signaling reduces nuclear Acetyl-CoA Levels to suppress gene expression during osteoblast differentiation. J. Biol. Chem. 2016, 291, 13028–13039. [Google Scholar] [CrossRef]
- Liu, L.; Liu, M.; Li, R.; Liu, H.; Du, L.; Chen, H.; Zhang, Y.; Zhang, S.; Liu, D. MicroRNA-503-5p inhibits stretch-induced osteogenic differentiation and bone formation. Cell Biol. Int. 2017, 41, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Liu, X.; Wang, J.; Chen, X.; Zhang, H.; Kim, S.H.; Cui, J.; Li, R.; Zhang, W.; Kong, Y.; et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther. Adv. Musculoskelet. Dis. 2013, 5, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Otsuka, T.; Kawabata, T.; Sakai, G.O.; Kim, W.O.O. Wnt3a downregulates thyroid hormone—Induced osteocalcin expression in osteoblasts. Exp. Ther. Med. 2019, 18, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Kang, Q.; Luu, H.H.; Park, J.K.; Luo, Q.; Song, W.-X.; Jiang, W.; Luo, X.; Li, X.; Yin, H.; et al. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol. Cell Biol. 2006, 26, 2955–2964. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt pathway in bone repair and regeneration—What do we know so far. Front. Cell Dev. Biol. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, J.; Xu, L.; Zhang, J.; Chan, K.; Pan, X.; Li, G. MiR-503 promotes bone formation in distraction osteogenesis through suppressing smurf1 expression. Sci. Rep. 2017, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Mergoni, G.; Vescovi, P.; Belletti, S.; Uggeri, J.; Nammour, S.; Gatti, R. Effects of 915 nm laser irradiation on human osteoblasts: A preliminary in vitro study. Lasers Med. Sci. 2018, 33, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Incerti-Parenti, S.; Cepollaro, S.; Checchi, L.; Fini, M. Photobiomodulation with low-level diode laser promotes osteoblast migration in an in vitro micro wound model. J. Biomed. Opt. 2015, 20, 078002. [Google Scholar] [CrossRef]
- Parenti, S.; Panseri, S.; Gracco, A.; Sandri, M.; Tampieri, A.; Bonetti, G. Effect of low-level laser irradiation on osteoblast-like cells cultured on porous hydroxyapatite scaffolds. Annali dell’Istituto Superiore di Sanità 2013, 49, 255–260. [Google Scholar] [CrossRef]
- Kaur, G.; Dufour, J.M. Cell lines. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Na, S.; TruongVo, T.; Jiang, F.; Joll, J.E.; Guo, Y.; Utreja, A.; Chen, J. Dose analysis of photobiomodulation therapy on osteoblast, osteoclast, and osteocyte. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Scalize, P.H.; de Sousa, L.G.; Gonçalves, L.M.N.; Pitol, D.L.; Palinkas, M.; Coppi, A.A.; Righetti, M.A.; Ricardo, V.; Bombonato-Prado, K.F.; Regalo, S.C.H.; et al. Low-level laser therapy enhances the number of osteocytes in calvaria bone defects of ovariectomized rats. Anim. Model. Exp. Med. 2019, 2, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, S.; Ito, K.; Hofmann, S. Cell sources for human in vitro bone models. Curr. Osteoporos. Rep. 2021, 19, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Pathria, M.N.; Chung, C.B.; Resnick, D.L. Acute and stress-related injuries of bone and cartilage: Pertinent anatomy, basic biomechanics, and imaging perspective. Radiology 2016, 280, 21–38. [Google Scholar] [CrossRef]
- Bos, P.K.; Van Osch, G.J.V.M.; Frenz, D.A.; Verhaar, J.A.N.; Verwoerd-Verhoef, H.L. Growth factor expression in cartilage wound healing: Temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthr. Cartil. 2001, 9, 382–389. [Google Scholar] [CrossRef]
- Trevisan, E.S.; Martignago, C.C.S.; Assis, L.; Tarocco, J.C.; Salman, S.; Dos Santos, L.; Liebano, R.; Tim, C.R. Effectiveness of led photobiomodulation therapy on treatment with knee osteoarthritis: A rat study. Am. J. Phys. Med. Rehabil. 2020, 99, 725–732. [Google Scholar] [CrossRef]
- Balbinot, G.; Schuch, C.P.; Nascimento, P.S.d.; Lanferdini, F.J.; Casanova, M.; Baroni, B.M.; Vaz, M.A. Photobiomodulation therapy partially restores cartilage integrity and reduces chronic pain behavior in a rat model of osteoarthritis: Involvement of spinal glial modulation. Cartilage 2021, 13 (Suppl. S2), 1309S–1321S. [Google Scholar] [CrossRef]
- Bozhokin, M.S.; Vcherashnii, D.B.; Yastrebov, S.G.; Beilinson, L.L.; Zherebtsova, J.V.; Khotin, M.G. Low-intensity photobiomodulation at 632.8 nm increases tgfβ3, col2a1, and sox9 gene expression in rat bone marrow mesenchymal stem cells in vitro. Lasers Med. Sci. 2022, 37, 435–441. [Google Scholar] [CrossRef]
- Dahlin, R.L.; Ni, M.; Meretoja, V.V.; Kasper, F.K.; Mikos, A.G. TGF-β3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials 2014, 35, 123–132. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, P.; Wu, Y.; Xiong, S.; Sun, H.; Xia, Q.; Shi, L.; Liu, H.; Ouyang, H.W. Programmed application of transforming growth factor β3 and Rac1 inhibitor NSC23766 committed hyaline cartilage differentiation of adipose-derived stem cells for osteochondral defect repair. Stem. Cells Transl. Med. 2014, 3, 1242–1251. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1−SMAD1 interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Ma, X.; Zhao, T. Mechanism of TGF-β3 promoting chondrogenesis in human fat stem cells. Biochem. Biophys. Res. Commun. 2020, 530, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.K. Exercise as an adjuvant to cartilage regeneration therapy. Int. J. Mol. Sci. 2020, 21, 9471. [Google Scholar] [CrossRef]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Muniz Renno, A.C. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef]
- Milares, L.P.; Assis, L.; Siqueira, A.; Claudino, V.; Domingos, H.; Almeida, T.; Tim, C.; Renno, A.C. Effectiveness of an aquatic exercise program and low-level laser therapy on articular cartilage in an experimental model of osteoarthritis in rats. Connect. Tissue Res. 2016, 57, 398–407. [Google Scholar] [CrossRef]
- Assis, L.; Tim, C.; Magri, A.; Fernandes, K.R.; Vassão, P.G.; Renno, A.C.M. Interleukin-10 and collagen type II immunoexpression are modulated by photobiomodulation associated to aerobic and aquatic exercises in an experimental model of osteoarthritis. Lasers Med. Sci. 2018, 33, 1875–1882. [Google Scholar] [CrossRef]
- Tim, C.R.; Martignago, C.C.S.; Assis, L.; Neves, L.M.; Andrade, A.L.; Silva, N.C.; Parizotto, N.; Pinto, K.Z.; Rennó, A.C. Effects of photobiomodulation therapy in chondrocyte response by in vitro experiments and experimental model of osteoarthritis in the knee of rats. Lasers Med. Sci. 2022, 37, 1677–1686. [Google Scholar] [CrossRef]
- Sakata, S.; Kunimatsu, R.; Tsuka, Y.; Nakatani, A.; Gunji, H.; Yanoshita, M.; Kado, I.; Ito, S.; Putranti, N.A.R.; Prasetya, R.C.; et al. High-frequency near-infrared diode laser irradiation suppresses IL-1β-induced inflammatory cytokine expression and NF-κB signaling pathways in human primary chondrocytes. Lasers Med. Sci. 2022, 37, 1193–1201. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB in defense and disease NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κb signaling pathways in osteoarthritic cartilage destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Luesma, M.J.; Cantarero, I.; Sánchez-Cano, A.I.; Rodellar, C.; Junquera, C. Ultrastructural evidence for telocytes in equine tendon. J. Anat. 2021, 238, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222. [Google Scholar] [CrossRef]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and ligament healing and current approaches to tendon and ligament regeneration. J. Orthop. Res. 2020, 38, 7–12. [Google Scholar] [CrossRef]
- Tsai, W.C.; Hsu, C.C.; Pang, J.H.S.; Lin, M.S.; Chen, Y.H.; Liang, F.C. Low-level laser irradiation stimulates tenocyte migration with up-regulation of dynamin II expression. PLoS ONE 2012, 7, e38235. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.M.; De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012, 13, 75–88. [Google Scholar] [CrossRef]
- Atalay, M.; Oksala, N.; Lappalainen, J.; Laaksonen, D.; Sen, C.; Roy, S. Heat shock proteins in diabetes and wound healing. Curr. Protein Pept. Sci. 2009, 10, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Murrell, G.A.C. Heat shock proteins in tendinopathy: Novel molecular regulators. Mediat. Inflamm. 2012, 2012, 436203. [Google Scholar] [CrossRef]
- Evangelista, A.N.; dos Santos, F.F.; de Oliveira Martins, L.P.; Gaiad, T.P.; Machado, A.S.D.; Rocha-Vieira, E.; Costa, K.B.; Santos, A.P.; Oliveira, M.X. Photobiomodulation therapy on expression of HSP70 protein and tissue repair in experimental acute Achilles tendinitis. Lasers Med. Sci. 2021, 36, 1201–1208. [Google Scholar] [CrossRef]
- de Freitas Dutra Júnior, E.; Hidd, S.M.C.M.; Amaral, M.M.; Filho, A.L.M.M.; Assis, L.; Ferreira, R.S.; Barraviera, B.; Martignago, C.C.S.; Figueredo-Silva, J.; de Oliveira, R.A.; et al. Treatment of partial injury of the calcaneus tendon with heterologous fibrin biopolymer and/or photobiomodulation in rats. Lasers Med. Sci. 2022, 37, 971–981. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Cassaro, C.V.; Shindo, J.V.T.C.; Coletta, B.B.D.; Pomini, K.T.; De Oliveira Rosso, M.P.; Campos, L.M.G.; Ferreira, R.S., Jr.; Barraviera, B.; Buchaim, R.L. Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties: A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190038. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.R.; Da Silva, F.S.; Bortolin, R.H.; Da Silva Marques, D.E.; Ramos, G.V.; Marqueti, R.C.; Da Silva, N.B.; Medeiros, K.C.D.P.; Corrêa, M.A.; Lima, J.P.M.S.; et al. Effect of photobiomodulation and exercise on early remodeling of the Achilles tendon in streptozotocin-induced diabetic rats. PLoS ONE 2019, 14, e0211643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzyoud, J.A.M.; Al Najjar, S.A.; Talat, S.; Abu-Irmaileh, B.; Bustanji, Y.; Al-Shudiefat, A.A.R.S. Effect of light-emitting diodes, platelet-rich plasma, and their combination on the activity of sheep tenocytes. Lasers Med. Sci. 2019, 34, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.-M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, T.; Wang, J.H.C. Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats. Oncotarget 2016, 7, 8498–8512. [Google Scholar] [CrossRef] [Green Version]
| Cells of Interest | Study Design | Laser Parameters | Main Outcome/s | Reference | |||
|---|---|---|---|---|---|---|---|
| Wavelength | Fluency | Power Output or Power Density | Irradiation Time | ||||
| Bone | |||||||
| Osteoblasts | in vitro | 635 nm (diode laser) 809 nm (diode laser) | 0 J/cm2 0.5 J/cm2 1 J/cm2 2 J/cm2 | 50 mW | 10, 20, 40 s | No change in cell viability or cell proliferation between irradiated and control groups. PBM had no effect on ALP activity. | Bölükbaşı Ateş (2017) [21] |
| Osteoblasts | in vitro | 635 nm (diode laser) | 0 J/cm2 0.5 J/cm2 1 J/cm2 2 J/cm2 | 50 mW/cm2 | 10, 20, 40 s | Decreased cell viability at 72 h when in irradiated osteoblasts previously incubated in MB. Increased ALP activity in groups with MB and PBM on day 7. Decreased mineralization reported in all treated groups. | Bölükbaşı Ateş (2017b) [22] |
| Osteoblasts | in vitro | 660 nm (AlGaInP) 808 nm (GaAlAs) 637 ± 15 nm (LED) | 5 J/cm2 8.3 J/cm2 | 40 mW | 3 s 5 s | Increased cell viability and wound closure occurred in groups exposed to the 660 nm laser and LED. All groups exposed to 5 s irradiation showed increased viability, greater cell density, and faster closure of the wound gap. PBM increased ALP activity. | Cardoso et al. (2021) [23] |
| Osteoblast; osteocyte | in vivo (Wistar rats) | 850 nm (LED) | 2.14 J/cm2 | 100 mW | 60 s | Groups treated with LED displayed better bone remodeling and maturation, but not bone formation, with and without the biomaterial scaffold. Increased ALP and decreased AcP activity reported in the LED groups with biomaterial. | Dalapria et al. (2022) [25] |
| Osteoblast | in vivo (Wistar rats) | 780 nm (GaAlAs) | 10 J/cm2 | 40 mW | 10 s | Increased osteoblast numbers and enhanced bone formation in the area surrounding the central incisors in groups with a fitted orthodontic appliance (orthodontic force) and PBM exposure. | Gonçalves et al. (2016) [27] |
| Osteoblast; osteocyte | in vivo (Wistar rats) | 780 nm (GaAlAs) | 640 J/cm2 | 20 mW | 40 s | PBM enhanced bone remodeling of the alveolar bone. At days 7 and 14, the number of osteopontin-positive osteocytes was higher in the groups receiving laser treatment (in normoglycemic and diabetic rats). PBM increased the number of osteoprotegerin-positive osteoblasts in the groups receiving laser treatment (in normoglycemic and diabetic rats). | Gomes et al. (2017) [28] |
| Osteoblast | in vitro | 808 nm (GaAlAs) | 3.75 J/cm2 | 0.401 W, 0.042 W/cm2 | 90 s | PBM down-regulated miR-503 expression and up-regulated Wnt3a expression. miR-503 stimulated apoptosis and caspase-3 expression, but repressed cell proliferation and decreased the expression of Wnt3a, β-catenin, Runx2 and Bcl-2. | Li et al. (2019) [29] |
| Osteoblast | in vitro | 915 nm (GaAlAs) | 5 J/cm2 15 J/cm2 45 J/cm2 | 1.5 W 0.12 W/cm2 1.25 W/cm2 | 0.12 W/cm2 (41.7, 125 and 375 s) 1.25 W/cm2 (4, 12 and 36 s) | Osteoblast proliferation did not change in the groups receiving PBM (single treatment per day for 3 days) at 5, 15 and 45 J/cm2 and the control group. PBM stimulated bone nodule formation in groups treated with 5 J/cm2 and 0.12 W/cm2 as compared to control groups. | Mergoni et al. (2018) [38] |
| Osteoblasts, osteocytes and osteoclasts | in vitro | 940 nm (LED) | 0 J/cm2 1 J/cm2 5 J/cm2 7.5 J/cm2 | 1.67 mW/cm2 8.33 mW/cm2 | 10 min | PBM increased osteoblast proliferation after 48 h post-irradiation (1 J/cm2 promoted 100% increase, while 5 J/cm2 promoted a 25% increase). PBM did not affect osteocyte proliferation. Osteoclast differentiation and resorption activity stimulated at 1 J/cm2. Osteocyte and osteoclast viability decreased when irradiated with a dose of 5 J/cm2, while PBM at 7.5 J/cm2 decreased osteoblast viability. | Na et al. (2018) [42] |
| Osteocytes | in vivo (Wistar rats) | 780 nm (GaAlAs) | 0 J/cm2 20 J/cm2 30 J/cm2 | 70 mW | 20 J/cm2 (100 s) 30 J/cm2 (150 s) | PBM at higher fluencies promoted bone formation (increased trabecular surface area) and increased osteocyte number. | Scalize et al. (2019) [43] |
| Saos-2 human osteoblast-like cells | in vitro | 915 nm (GaAlAs) | 5 J/cm2 10 J/cm2 15 J/cm2 | 6 W ± 20% | 48, 96, 144 s | Wound closure occurred faster (after 72 h) in groups treated with 5 J/cm2 and 10 J/cm2 and after 96 h in the 15 J/cm2 as compared to the control. PBM did not influence cell viability for each experimental period. PBM increased COL1A1 gene expression and decreased TGF-β1 expression (5 and 15 J/cm2). | Tschon et al. (2015) [39] |
| Cartilage | |||||||
| Chondrocytes | in vivo (Wistar rats) | 808 nm (GaAIAs) | 50 J/cm2 | 50 mW | 28 s | Decreased caspase-3 expression in groups treated with irradiation coupled with exercise. Decreased IL-β and MMP-13 expression in groups receiving irradiation, exercise or both. | Assis et al. (2016) [56] |
| Chondrocytes | in vivo (Wistar rats) | 808 nm (GaAIAs) | 50 J/cm2 | 50 mW | 28 s | IL-10 and COL-2 expression increased in response to aerobic and aquatic exercise, with and without PBM intervention. Aerobic exercise with and without PBM stimulated TGF-β expression. | Assis et al. (2018) [58] |
| Chondrocytes | in vivo (Wistar rats) | 850 nm (GaAIAs) | 57.14 J/cm2 | 100 mW/1.43 W/cm2 | 40 s per site | PBM stimulated cartilage regeneration. | Balbinot et al. (2021) [48] |
| Chondrocytes | in vivo (Wistar rats) | 808 nm (GaAIAs) | 50 J/cm2 | 50 mW | 28 s | Aquatic exercise, with or without PBM, resulted in better tissue organization as well as improved chondrocyte organization along the articular surface. Aquatic exercise coupled with PBM decreased MMP-13 expression. | Milares et al. (2016) [57] |
| Chondrocytes | in vitro | 910 nm (GaAs) | 8 J/cm2 | 300 mW | 256 s | PBM decreased inflammatory cytokine expression (IL1β and IL-6) and NF-κB in IL1β-treated chondrocytes. | Sakata et al. (2022) [60] |
| Chondrocytes | in vitro in vivo (Wistar rats) | 808 nm | 28 J/cm2 (in vitro only) 50 J/cm2 | 50 mW | 16 s (in vitro only) 28 s | PBM at a higher energy dose stimulated chondrocyte proliferation (in vitro). Decreased IL-1β expression in PBM groups after 4 and 8 weeks. Greater IL-10, COL-2 and IL-4 expression in PBM group after 8 weeks of treatment. Increased gene expression in TGF-β, COL-2, aggrecan after 4 weeks of PBM treatment (in vivo). | Tim et al. (2022) [59] |
| Chondrocytes | in vivo (Wistar rats | 850 nm (GaAIAs) | Not given | 200 mW/0.4 mW/cm2 | 30 s | Groups treated with PBM showed enhanced COL-2 and TGFβ expression as compared to control. | Trevisan et al. (2020) [47] |
| Tendon | |||||||
| Tenocytes | in vitro | 630 nm (small probe) 625 nm (large probe) 850 nm (large probe) | 4 J/cm2 | 4150 mW (small probe) 1200 mW (large probe) | 18 min | PBM alone did not change cell viability; however, PBM increased viability of cells grown in a platelet-rich plasma culture medium. LED application increased the closure of the wound gap. | Alzyoud et al. (2019) [74] |
| Tenocytes | in vivo (Wistar rats) | 660 nm | 4 J/cm2 | 10 mW/ 250 mW/cm2 | 16 s | PBM and exercise increased COL-1 immunoreactivity and resulted provided better cellular alignment. MMP3 and MMP13 expression was reduced in the PBM groups. | de Oliveira et al. (2019) [73] |
| Tenocytes | in vivo (Wistar rats) | 630 ± 20nm | 9 J/cm2 | 300 mW/ 0.3 W/cm2 | 30 s | LED increased HSP70 expression and collagen production | Evangelista et al. (2021) [70] |
| Tenocytes | in vivo (Wistar rats) | 660 nm | 6 J/cm2 | 0.04 W/ 1 W/cm2 | 5.70 s | Heterologous fibrin polymer and PBM, either alone or coupled together, were successful at decreasing edema. After 7 days, the PBM group showed greater tendon injury, which reduced after 14 and 21 days. No differences in collagen quantification were found in treated and control groups over the 3-week period. | de Freitas Dutra Júnior et al. (2022) [71] |
| Tenocytes | in vitro | 660 nm | 1 J/cm2 1.5 J/cm2 2 J/cm2 | 50 mW | 5.2 min 7.8 min 10.4 min | PBM stimulated cell migration and wound closure. Dynamin-2 expression up-regulated in groups exposed to PBM. Dynasore treatment reduced cell migration in the 2 J/cm2 irradiated group | Tsai et al. (2012) [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh-Kader, A.; Houreld, N.N. Photobiomodulation, Cells of Connective Tissue and Repair Processes: A Look at In Vivo and In Vitro Studies on Bone, Cartilage and Tendon Cells. Photonics 2022, 9, 618. https://doi.org/10.3390/photonics9090618
Shaikh-Kader A, Houreld NN. Photobiomodulation, Cells of Connective Tissue and Repair Processes: A Look at In Vivo and In Vitro Studies on Bone, Cartilage and Tendon Cells. Photonics. 2022; 9(9):618. https://doi.org/10.3390/photonics9090618
Chicago/Turabian StyleShaikh-Kader, Asma, and Nicolette Nadene Houreld. 2022. "Photobiomodulation, Cells of Connective Tissue and Repair Processes: A Look at In Vivo and In Vitro Studies on Bone, Cartilage and Tendon Cells" Photonics 9, no. 9: 618. https://doi.org/10.3390/photonics9090618





