Remedies to Avoid Failure Mechanisms of Lithium-Metal Anode in Li-Ion Batteries
Abstract
:1. Introduction
2. Liquid Electrolytes
2.1. Solvents
2.2. Salt–Solvent Interactions
2.3. Solvated Ionic Liquid Electrolytes
2.4. SEI-Forming Additives
2.5. Li Plating Additives
2.6. Nanostructured Electrolytes
3. Artificial SEI
3.1. Electrochemical Pretreatment
3.2. Chemical and Physical Pre
3.2.1. Gas Processing
3.2.2. Liquid Processing and Physical Pretreatment
3.2.3. Inorganic Layers
3.2.4. Organic Layers
3.2.5. Polymer Coating
3.3. Anchoring Li on 3D Current Collectors
3.3.1. Lithiophilic Matrix
3.3.2. Carbon Skeleton
3.3.3. Graphene Skeleton
3.3.4. Carbon Fiber-Based Skeleton
3.3.5. Carbon Nanotube Skeleton
3.3.6. Hierarchical Carbon Skeletons
3.4. Conductive Frameworks
3.4.1. Cu Skeleton
3.4.2. Ni Skeleton
3.4.3. Li–Si Alloy Skeletons
3.4.4. Other Li–Metal Alloying
3.4.5. Insulating Skeletons
3.4.6. Gradient Skeletons
3.4.7. Mxene-Based Skeletons
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two dimensional |
| 3D | Three dimensional |
| AB | Acetylene black |
| ALD | Atomic layer deposition |
| ASO | Aluminum silicate |
| BNS | Bare nickel scaffold |
| BTFE | Bis(2,2,2-trifluoroethyl) ether |
| CA | Caffeine acid |
| CC | Carbon cloth |
| CE | Coulombic efficiency |
| CF | Carbon fiber |
| CNTS | Carbon nanotubes |
| CuN | Cu nanowire |
| DEC | Diethyl carbonate |
| DFT | Density functional theory |
| DMC | Dimethyl carbonate |
| DME | 1,2-dimethoxyethane |
| DOL | 1,3-dioxolane |
| DSIL | Diluted solvate ionic liquid |
| EC | Ethylene carbonate |
| ERG | Edge-rich graphene |
| EVA | Ethylene-vinyl acetate |
| FEC | Fluoroethylene carbonate |
| G4 | Tetraethylene glycol dimethyl ether |
| GCD | Galvanostatic charge discharge |
| GCF | Graphene carbon fibers |
| GF | Glass fiber |
| HCE | Highly concentrated electrolytes |
| HDI | Hexamethylene diisocyanate |
| HFP | Hexafluoropropylene |
| LCE | Lithium carbonate equivalent metric ton |
| LFP | LiFePO4 |
| LIB | Lithium-ion battery |
| LiDFBP | Lithium difluoro (bisoxalato) phosphate |
| LiFSI | Lithium bis(fluorosulfonyl)imide |
| LIPs | Long-chain polysulfides |
| LLZO | Li7La3Zr2O12 |
| LLZTO | Li6.75La3Zr1.75Ta0.25O12 |
| LMB | Lithium-metal battery |
| LMPC | Lithium-metal polymer cell |
| LUMO | Lowest unoccupied molecular orbital |
| MLD | Molecular layer deposition |
| MOFs | Metal–organic frameworks |
| MS | Melamine sponge |
| NCA | Nickel-cobalt-aluminium cathode |
| NCS | Carbon nanosphere |
| NF | Ni foam |
| NGCF | Graphitic carbon foam |
| NHCNSs | N-doped hollow carbon nanospheres |
| NMC | Nickel-manganese-cobalt cathode |
| NMP | Methyl-pyrrolidone |
| NWs | Manowires |
| q-PET | Quaternized polyethylene terephthalate |
| PAN | Polyaniline |
| PDMS | Poly(dimethylsiloxane) |
| PEO | Polyethylene oxide |
| PEGA | Poly(ethylene glycol) methyl ether methacrylate |
| PFE | Pentafluoropropyl acrylate |
| PhDMCS | Dimethylphenylchlorosilane |
| PI | Polyimide |
| PMMA | Poly(methyl methacrylate) |
| PP | Polypropylene |
| PPA | Polyphosphoric acid |
| PPE | Temperature-responsive electrolyte |
| PVDF | Poly(vinylidene fluoride) |
| rGO | Reduced graphene oxide |
| SBR | Styrene butadiene rubber |
| SEI | Solid–electrolyte interphase |
| SIL | Solvated ionic liquid electrolytes |
| SL | Sulfolane |
| TEOS | Tetraethoxylane |
| TFSI | Tri[bis(trifluoromethane)sulfonimide] |
| TIPS | Triisopropylsilyl |
| TMCS | Chlorotrimethylsilane |
| TMS | Trimethylsilyl |
| VC | Vinylene carbonate |
| WGC | Wrinkled graphene cage |
References
- Marcelo Azevedo, N.C.; Hagenbruch, T.; Hoffman, K.; Lala, A.; Ramsbottom, O. Lithium and Cobalt: A Tale of Two Commodities. Available online: https://www.mckinsey.com/industries/metals-and-mining/our-insights/lithium-and-cobalt-a-tale-of-two-commodities (accessed on 15 June 2018).
- Swiss Resource Capital AG. Available online: https://libertyonelithium.com/pdf/Swiss%20Resource%20Capital%202018%20Lithium%20Report.pdf (accessed on 15 June 2018).
- Zheng, X.; Li, M.; El-Hady, D.A.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Hovington, P.; Lagacé, M.; Guerfi, A.; Bouchard, P.; Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. New lithium metal polymer solid state battery for an ultrahigh energy: Nano C-LiFePO4 versus nano Li1.2V3O8. Nano Lett. 2015, 15, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavornia, M.; Wu, J.; Liu, X. Review on polymer-based composite electrolytes for lithium batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Guo, C.; Naveed, A.; Lei, J.; Yang, J.; Nuli, Y.; Wang, J. Recent progress and perspective on lithium metal anode protection. Energy Storage Mater. 2018, 14, 199–221. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Borodin, O.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Zheng, J.; Yang, C.; Liou, S.C. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 2018, 13, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Hwang, J.G.; Yoon, C.S.; Jung, H.G.; Sun, Y.K. Stabilization of lithium-metal batteries based on the In-Situ formation of a stable solid electrolyte interphase layer. ACS Appl. Mater. Interfaces 2018, 10, 17985–17993. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ji, X.; Han, F.; Yue, J.; Chen, J.; Chen, L.; Deng, T.; Jiang, J.; Wang, C. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery. Sci. Adv. 2018, 4, 9245. [Google Scholar] [CrossRef] [Green Version]
- Markevich, E.; Salitra, G.; Chesneau, F.; Schmidt, M.; Aurbach, D. Very stable lithium Metal stripping-plating at a high rate and high areal capacity in fluoroethylene carbonate-based organic electrolyte solution. ACS Energy Lett. 2017, 2, 1321–1326. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Park, M.S.; Ma, S.B.; Lee, D.J.; Im, D.; Doo, S.G.; Yamamoto, O. A highly reversible lithium metal anode. Sci. Rep. 2014, 4, 3815–3822. [Google Scholar] [CrossRef]
- Lu, Y.; Tu, Z.; Archer, L.A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 2014, 13, 961–969. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Cheng, X.-B.; Tian, Y.; Chen, X.; Zhang, X.-Q.; Li, W.-J.; Huang, J.-Q.; Zhang, Q. Dual-layered film protected lithium metal anode to enable dendrite-free lithium deposition. Adv. Mater. 2018, 30, 1707629. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Xie, K.; Diao, Y.; Hong, X. Properties of surface film on lithium anode with LiNO3 as lithium salt in electrolyte solution for lithium–sulfur batteries. Electrochim. Acta 2012, 83, 78–86. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Yan, K.; Zheng, G.; Liang, Z.; Chiang, Y.M.; Cui, Y. The Synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 2015, 6, 7436. [Google Scholar] [CrossRef]
- Lang, S.-Y.; Shen, Z.-Z.; Hu, X.-C.; Shi, Y.; Guo, Y.-G.; Jia, F.-F.; Wang, F.-Y.; Wen, R.; Wan, L.-J. Tunable structure and dynamics of solid electrolyte interphase at lithium metal anode. Nano Energy 2020, 75, 104967. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, X.; Cheng, X.B.; Li, B.Q.; Shen, X.; Yan, C.; Huang, J.-Q.; Zhang, Q. Highly stable lithium metal batteries enabled by regulating the solvation of lithium ions in nonaqueous electrolytes. Angew. Chem. Int. Ed. 2018, 57, 5301–5305. [Google Scholar] [CrossRef]
- Amanchukwu, C.V.; Kong, X.; Qin, J.; Cui, Y.; Bao, Z. Nonpolar alkanes modify lithium-ion solvation for improved lithium deposition and stripping. Adv. Energy Mater. 2019, 9, 1902116. [Google Scholar] [CrossRef]
- von Wald Cresce, A.; Borodin, O.; Xu, K. Correlating Li+ solvation sheath structure with interphasial chemistry on graphite. J. Phys. Chem. C 2012, 116, 26111–26117. [Google Scholar] [CrossRef]
- Dey, A.N. Lithium anode film and organic and inorganic electrolyte batteries. Thin Solid Film. 1977, 43, 131–171. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.R.; Shen, X.; Zhang, Q. The origin of the reduced reductive stability of ion-solvent complexes on alkali and alkaline earth metal anodes. Angew. Chem. Int. Ed. 2018, 57, 16643–16647. [Google Scholar] [CrossRef]
- Chen, X.; Shen, X.; Li, B.; Peng, H.J.; Cheng, X.B.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q.; Zhang, Q. Ion-solvent complexes promote gas evolution from electrolytes on a sodium metal anode. Angew. Chem. Int. Ed. 2018, 57, 734–737. [Google Scholar] [CrossRef]
- Xing, L.; Borodin, O.; Smith, G.D.; Li, W. Density functional theory study of the role of anions on the oxidative decomposition reaction of propylene carbonate. J. Phys. Chem. A 2011, 115, 13896–13905. [Google Scholar] [CrossRef]
- Borodin, O. Molecular modeling of electrolytes. In Electrolytes for Lithium and Lithium-Ion Batteries; Jow, T.R., Xu, K., Borodin, O., Ue, M., Eds.; Springer: New York, NY, USA, 2014; Volume 58, pp. 371–401. [Google Scholar]
- Odziemkowski, M.; Irish, D. An electrochemical study of the reactivity at the lithium electrolyte/bare lithium metal interface I. Purified electrolytes. J. Electrochem. Soc. 1992, 139, 3063–3074. [Google Scholar] [CrossRef]
- Odziemkowski, M.; Irish, D. An electrochemical study of the reactivity at the lithium electrolyte/bare lithium metal interface II. Unpurified solvents. J. Electrochem. Soc. 1993, 140, 1546–1555. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Marin, T.W.; Zhu, T.; Abraham, D.P. Why bis (fluorosulfonyl) imide is a “magic anion” for electrochemistry. J. Phys. Chem. C 2014, 118, 19661–19671. [Google Scholar] [CrossRef]
- Kim, H.; Wu, F.; Lee, J.T.; Nitta, N.; Lin, H.T.; Oschatz, M.; Cho, W.I.; Kaskel, S.; Borodin, O.; Yushin, G. In situ formation of protective coatings on sulfur cathodes in lithium batteries with LiFSI-based organic electrolytes. Adv. Energy Mater. 2015, 5, 1401792–1401799. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Long, G.; Liu, S.; Li, G.; Gao, X. A LiFSI-LiTFSI binary-salt electrolyte to achieve high capacity and cycle stability for a Li–S battery. Chem. Commun. 2014, 50, 14647–14650. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuang, H.L.; Fan, L.; Gao, L.; Lu, Y. A “cation-anion regulation” synergistic anode host for dendrite-free lithium metal batteries. Sci. Adv. 2018, 4, 4410. [Google Scholar] [CrossRef] [Green Version]
- Suo, L.; Hu, Y.-S.; Li, H.; Armand, M.; Chen, L. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481. [Google Scholar] [CrossRef]
- Yamada, Y.; Yaegashi, M.; Abe, T.; Yamada, A. A superconcentrated ether electrolyte for fast-charging Li-ion batteries. Chem. Commun. 2013, 49, 11194–11196. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Furukawa, K.; Sodeyama, K.; Kikuchi, K.; Yaegashi, M.; Tateyama, Y.; Yamada, A. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J. Am. Chem. Soc. 2014, 136, 5039–5046. [Google Scholar] [CrossRef]
- Ueno, K.; Yoshida, K.; Tsuchiya, M.; Tachikawa, N.; Dokko, K.; Watanabe, M. Glyme–lithium salt equimolar molten mixtures: Concentrated solutions or solvate ionic liquids. J. Phys. Chem. B 2012, 116, 11323–11331. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, S.; Lee, H.; Mei, D.; Engelhard, M.H.; Burton, S.D.; Zhao, W.; Zheng, J.; Li, Q.; Ding, M.S.; et al. Localized high-concentration sulfone electrolytes for high-efficiency lithium-metal batteries. Chem 2018, 4, 1877–1892. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Chen, S.; Zhao, W.; Song, J.; Engelhard, M.H.; Zhang, J.-G. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes. ACS Energy Lett. 2018, 3, 315–321. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, P.; Mei, D.; Engelhard, M.H.; Cartmell, S.S.; Polzin, B.J.; Wang, C.; Zhang, J.-G.; Xu, W. Highly stable operation of lithium metal batteries enabled by the formation of a transient high-concentration electrolyte layer. Adv. Energy Mater. 2016, 6, 1502151. [Google Scholar] [CrossRef]
- Camacho-Forero, L.E.; Smith, T.W.; Balbuena, P.B. Effects of high and low salt concentration in electrolytes at lithium–metal anode surfaces. J. Phys. Chem. C 2017, 121, 182–194. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, X.; Hou, L.P.; Li, B.-Q.; Cheng, X.-B.; Huang, J.-Q.; Zhang, Q. Regulating anions in the solvation sheath of lithium ions for stable lithium metal batteries. ACS Energy Lett. 2019, 4, 411–416. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Chiang, C.H.; Tateyama, Y.; Yamada, A. Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 2016, 7, 12032. [Google Scholar] [CrossRef]
- Ding, M.S.; von Cresce, A.; Xu, K. Conductivity, viscosity, and their correlation of a super-concentrated aqueous electrolyte. J. Phys. Chem. C 2017, 121, 2149–2153. [Google Scholar] [CrossRef]
- Wang, H.; Matsui, M.; Kuwata, H.; Sonoki, H.; Matsuda, Y.; Shang, X.; Takeda, Y.; Yamamoto, O.; Imanishi, N. A reversible dendrite-free high-areal-capacity lithium metal electrode. Nat. Commun. 2017, 8, 15106. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Shimizu, Y.; Hashinokuchi, M.; Inaba, M. Dilution of highly concentrated LiBF4/propylene carbonate electrolyte solution with fluoroalkyl ethers for 5-V LiNi0.5Mn1.5O4 positive electrodes. J. Electrochem. Soc. 2017, 164, A6412–A6416. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Mei, D.; Han, K.S.; Engelhard, M.H.; Zhao, W.; Xu, W.; Liu, J.; Zhang, J.G. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 2018, 30, 1706102. [Google Scholar] [CrossRef]
- Qiu, F.; Li, X.; Deng, H.; Wang, D.; Mu, X.; He, P.; Zhou, H. A concentrated ternary-salts electrolyte for high reversible Li metal battery with slight excess Li. Adv. Energy Mater. 2019, 9, 1803372. [Google Scholar] [CrossRef]
- Yu, L.; Chen, S.; Lee, H.; Zhang, L.; Engelhard, M.H.; Li, Q.; Jiao, S.; Liu, J.; Xu, W.; Zhang, J.-G. A localized high-concentration electrolyte with optimized solvents and lithium difluoro(oxalate)borate additive for stable lithium metal batteries. ACS Energy Lett. 2018, 3, 2059–2067. [Google Scholar] [CrossRef]
- Zheng, J.; Ji, G.; Fan, X.; Chen, J.; Li, Q.; Wang, H.; Yang, Y.; Demella, K.C.; Raghavan, S.R.; Wang, C. High-Fluorinated Electrolytes for Li–S Batteries. Adv. Energy Mater. 2019, 9, 1803774. [Google Scholar] [CrossRef]
- Huang, F.; Ma, G.; Wen, Z.; Jin, J.; Xu, S.; Zhang, J. Enhancing metallic lithium battery performance by tuning the electrolyte solution structure. J. Mater. Chem. A 2018, 6, 1612–1620. [Google Scholar] [CrossRef]
- Ma, G.; Wang, L.; He, X.; Zhang, J.; Chen, H.; Xu, W.; Ding, Y. Pseudoconcentrated electrolyte with high ionic conductivity and stability enables high-voltage lithium-ion battery chemistry. ACS Appl. Mater. Interfaces 2018, 1, 5446–5452. [Google Scholar] [CrossRef]
- Piao, N.; Ji, X.; Xu, H.; Fan, X.; Chen, L.; Liu, S.; Garaga, M.N.; Greenbaum, S.G.; Wang, L.; Wang, C.; et al. Countersolvent electrolytes for lithium-metal batteries. Adv. Energy Mater. 2020, 10, 1903568. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Cao, X.; Engelhard, M.H.; Liu, W.; Burton, S.D.; Lee, H.; Niu, C.; Matthews, B.E.; Zhu, Z.; et al. Enabling high-voltage lithium-metal batteries under practical conditions. Joule 2019, 3, 1662–1676. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, T.K.; Kim, S.; Lee, J.; Ahn, Y.; Kim, K.; Ma, H.; Park, G.; Lee, S.-M.; Kwak, S.K.; et al. Fluorine-incorporated interface enhances cycling stability of lithium metal batteries with Ni-rich NCM cathodes. Nano Energy 2020, 67, 104309. [Google Scholar] [CrossRef]
- Miao, R.; Yang, J.; Feng, X.; Jia, H.; Wang, J.; Nuli, Y. Novel dual-salts electrolyte solution for dendrite-free lithium-metal based rechargeable batteries with high cycle reversibility. J. Power Sources 2014, 271, 291–297. [Google Scholar] [CrossRef]
- Alvarado, J.; Schroeder, M.A.; Pollard, T.P.; Wang, X.; Lee, J.Z.; Zhang, M.; Wynn, T.; Ding, M.; Borodin, O.; Meng, Y.S.; et al. Bisalt ether electrolytes: A pathway towards lithium metal batteries with Ni-rich cathodes. Energy Environ. Sci. 2019, 12, 780–794. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Inoue, K.; Nakahara, K.; Yuge, R.; Noguchi, T.; Utsugi, K. Suppression of aluminum corrosion by using high concentration LiTFSI electrolyte. J. Power Sources 2013, 231, 234–238. [Google Scholar] [CrossRef]
- Yang, H.; Kwon, K.; Devine, T.M.; Evans, J.W. Aluminum corrosion in lithium batteries an investigation using the electrochemical quartz crystal microbalance. J. Electrochem. Soc. 2000, 147, 4399–4407. [Google Scholar] [CrossRef]
- Morita, M.; Shibata, T.; Yoshimoto, N.; Ishikawa, M. Anodic behavior of aluminum current collector in LiTFSI solutions with different solvent compositions. J. Power Sources 2003, 119–121, 784–788. [Google Scholar] [CrossRef]
- Chen, X.; Xu, W.; Engelhard, M.H.; Zheng, J.; Zhang, Y.; Ding, F.; Qian, J.; Zhang, J.-G. Mixed salts of LiTFSI and LiBOB for stable LiFePO4-based batteries at elevated temperatures. J. Mater. Chem. A 2014, 2, 2346–2352. [Google Scholar] [CrossRef]
- Xiang, H.; Shi, P.; Bhattacharya, P.; Chen, X.; Mei, D.; Bowden, M.E.; Zheng, J.; Zhang, J.-G.; Xu, W. Enhanced charging capability of lithium metal batteries based on lithium bis(trifluoromethanesulfonyl)imide-lithium bis(Oxalato) borate dual-salt electrolytes. J. Power Sources 2016, 318, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Engelhard, M.H.; Mei, D.; Jiao, S.; Polzin, B.J.; Zhang, J.G.; Xu, W. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2017, 2, 17012. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, S.; Li, Y.; Xin, S.; Manthiram, A.; Goodenough, J.B. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J. Am. Chem. Soc. 2016, 138, 9385–9388. [Google Scholar] [CrossRef]
- Terada, S.; Ikeda, K.; Ueno, K.; Dokko, K.; Watanabe, M. Liquid structures and transport properties of lithium bis(fluorosulfonyl)amide/glyme solvate ionic liquids for lithium batteries. Aust. J. Chem. 2019, 72, 70–80. [Google Scholar] [CrossRef]
- Hubble, D.; Qin, J.; Lin, F.; Murphy, I.A.; Jang, S.-H.; Yang, J.; Jen, A.K.Y. Designing solvate ionogel electrolytes with very high room-temperature conductivity and lithium transference number. J. Mater. Chem. 2018, 6, 24100–24106. [Google Scholar] [CrossRef]
- Wang, H.; Sunahiro, S.; Matsui, M.; Zhang, P.; Takeda, Y.; Yamamoto, O.; Imanishi, N. A Solvate ionic liquid as the anolyte for aqueous rechargeable Li-O2 batteries. ChemElectroChem 2015, 2, 1144–1151. [Google Scholar] [CrossRef]
- Ueno, K.; Murai, J.; Ikeda, K.; Tsuzuki, S.; Tsuchiya, M.; Tatara, R.; Mandai, T.; Umebayashi, Y.; Dokko, K.; Watanabe, M. Li+ solvation and ionic transport in lithium solvate ionic liquids diluted by molecular solvents. J. Phys. Chem. C 2015, 120, 15792–15802. [Google Scholar] [CrossRef]
- Wu, X.; Pan, K.; Jia, M.; Ren, Y.; He, H.; Zhang, L.; Zhang, S. Electrolyte for lithium protection: From liquid to solid. Green Energy Environ. 2019, 4, 360–374. [Google Scholar] [CrossRef]
- Heine, J.; Hilbig, P.; Qi, X.; Niehoff, P.; Winter, M.; Bieker, P. Fluoroethylene carbonate as electrolyte additive in tetraethylene glycol dimethyl ether based electrolytes for application in lithium Ion and lithium metal batteries. J. Electrochem. Soc. 2015, 162, A1094–A1101. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cheng, X.B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Fluoroethylene carbonate as an important component for the formation of an effective solid electrolyte interphase on anodes and cathodes for advanced Li-ion batteries. ACS Energy Lett. 2017, 2, 1337. [Google Scholar] [CrossRef] [Green Version]
- Jung, R.; Metzger, M.; Haering, D.; Solchenbach, S.; Marino, C.; Tsiouvaras, N.; Stinner, C.; Gasteiger, H.A. Consumption of fluoroethylene carbonate (FEC) on Si-C composite electrodes for Li-ion batteries. J. Electrochem. Soc. 2016, 163, A1705–A1716. [Google Scholar] [CrossRef]
- Shi, Q.; Zhong, Y.; Wu, M.; Wang, H.; Wang, H. High-capacity rechargeable batteries based on deeply cyclable lithium metal anodes. Proc. Natl Acad. Sci. USA 2018, 115, 5676–5680. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Yao, Y.-X.; Chen, X.; Cheng, X.-B.; Zhang, X.-Q.; Huang, J.-Q.; Zhang, Q. Lithium nitrate solvation chemistry in carbonate electrolyte sustains high-voltage lithium metal batteries. Angew. Chem. Int. Ed. 2018, 57, 14055–14059. [Google Scholar] [CrossRef]
- Li, S.; Jiang, M.; Xie, Y.; Xu, H.; Jia, J.; Li, J. Developing high performance lithium metal anode in liquid electrolytes: Challenge and progress. Adv. Mater. 2018, 30, 1706375. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Ji, X.; Chen, J.; Chen, L.; Fan, X.; Mu, D.; Wang, C. Lithium nitrate regulated sulfone electrolytes for lithium metal batteries. Angew. Chem. 2020, 132, 22378–22385. [Google Scholar] [CrossRef]
- Rong, G.; Zhang, X.; Zhao, W.; Qiu, Y.; Liu, M.; Ye, F.; Xu, Y.; Chen, J.; Hou, Y.; Li, W. Liquid-phase electrochemical scanning electron microscopy for in situ investigation of lithium dendrite growth and dissolution. Adv. Mater. 2017, 29, 1606187. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.-B.; Zhao, C.-Z.; Huang, J.-Q.; Yang, S.-T.; Zhang, Q. Lithium metal protection through in-situ formed solid electrolyte interphase in lithium-sulfur batteries: The role of polysulfides on lithium Anode. J. Power Sources 2016, 327, 212–220. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Yan, C.; Chen, X.; Guan, C.; Huang, J.-Q.; Peng, H.-J.; Zhang, R.; Yang, S.-T.; Zhang, Q. Implantable solid electrolyte interphase in lithium-metal batteries. Chem 2017, 2, 258–270. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Zheng, L.; Zhang, Z.; Li, M.; Chen, Z.; Cui, R.; Shen, Y.; Li, G.; Feng, R.; Zhang, S.; et al. Constructing multifunctional solid electrolyte interface via in-situ polymerization for dendrite-free and low N/P ratio lithium metal batteries. Nat. Commun. 2021, 12, 186. [Google Scholar] [CrossRef]
- Choudhury, S.; Tu, Z.; Stalin, S.; Vu, D.; Fawole, K.; Gunceler, D.; Sundararaman, R.; Archer, L.A. Electroless formation of hybrid lithium anodes for fast interfacial ion transport. Angew. Chem. Int. Ed. 2017, 56, 13070–13077. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Morita, M.; Matsuda, Y. In Situ scanning vibrating electrode technique for lithium metal anodes. J. Power Sources 1997, 68, 501–505. [Google Scholar] [CrossRef]
- Matsuda, Y. Behavior of lithium/electrolyte interface in organic solutions. J. Power Sources 1993, 43, 1–7. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ishlkawa, M.; Yoshitake, S.; Monla, M. Characterization of the lithium-organic electrolyte interface containing inorganic and organic additives by in situ techniques. J. Power Sources 1995, 54, 301–305. [Google Scholar] [CrossRef]
- Ye, H.; Yin, Y.-X.; Zhang, S.-F.; Shi, Y.; Liu, L.; Zeng, X.-X.; Wen, R.; Guo, Y.-G.; Wan, L.-J. Synergism of Al-containing solid electrolyte interphase layer and Al-based colloidal particles for stable lithium anode. Nano Energy 2017, 36, 411–417. [Google Scholar] [CrossRef]
- Lu, Y.; Tu, Z.; Shu, J.; Archer, L.A. Stable lithium electrodeposition in salt-reinforced electrolytes. J. Power Sources 2015, 279, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Biswal, P.; Kludze, A.; Rodrigues, J.; Deng, Y.; Moon, T.; Stalin, S.; Zhao, Q.; Yin, J.; Kourkoutis, L.F.; Archer, L.A. The early-stage growth and reversibility of Li electrodeposition in Br-rich electrolytes. Proc. Natl Acad. Sci. USA 2021, 118, e2012071118. [Google Scholar] [CrossRef]
- Stark, J.K.; Ding, Y.; Hohl, P.A. Dendrite-free electrodeposition and reoxidation of lithium-sodium alloy for metal-anode battery. J. Electrochem. Soc. 2011, 158, A1100–A1105. [Google Scholar] [CrossRef] [Green Version]
- Vega, J.A.; Zhou, J.; Kohl, P.A. Electrochemical comparison and deposition of lithium and potassium from phosphonium- and ammonium-TFSI ionic liquids. J. Electrochem. Soc. 2009, 156, A253–A259. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J.; et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Chen, X.; Cao, R.; Qian, J.; Xiang, H.; Zheng, J.; Zhang, J.-G.; Xu, W. Enhanced performance of Li|LiFePO4 cells using CsPF6 as an electrolyte additive. J. Power Sources 2015, 293, 1062–1067. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Xu, W.; Chen, X.; Zhang, J.; Shao, Y.; Engelhard, M.H.; Zhang, Y.; Blake, T.A.; Graff, G.L.; Liu, X. Effects of cesium cations in lithium deposition via self-healing electrostatic shield mechanism. J. Phys. Chem. C 2014, 118, 4043–4049. [Google Scholar] [CrossRef]
- Lu, Y.; Tikekar, M.; Mohanty, R.; Hendrickson, K.; Ma, L.; Archer, L.A. Stable cycling of lithium metal batteries using high transference number electrolytes. Adv. Energy Mater. 2015, 5, 1402073. [Google Scholar] [CrossRef]
- Tu, Z.; Nath, P.; Lu, Y.; Tikekar, M.D.; Archer, L.A. Nanostructured electrolytes for stable lithium electrodeposition in secondary batteries. Acc. Chem. Res. 2015, 48, 2947–2956. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Das, S.K.; Moganty, S.S.; Archer, L.A. Ionic liquid-nanoparticle hybrid electrolytes and their application in secondary lithium-metal batteries. Adv. Mater. 2012, 24, 4430–4435. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Mangal, R.; Agrawal, A.; Archer, L.A. A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles. Nat. Commun. 2015, 6, 10101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, J.L.; Yang, D.A.; Archer, L.A. High lithium transference number electrolytes via creation of 3-dimensional, charged, nanoporous networks from dense functionalized nanoparticle composites. Chem. Mater. 2013, 25, 834–839. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, R.; He, Y.-B.; Li, F.; Liu, M.; Li, B.; Yang, Q.-H.; Cai, Q.; Kang, F. SiO2 hollow nanosphere-based composite solid electrolyte for lithium metal batteries to suppress lithium dendrite growth and enhance cycle life. Adv. Energy Mater. 2016, 6, 1502214. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Zhuo, D.; Zheng, G.; Lu, Z.; Liu, K.; Cui, Y. Core–shell nanoparticle coating as an interfacial layer for dendrite-free lithium metal anodes. ACS Cent. Sci. 2017, 3, 135–140. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, Z.; Gray, J.L.; He, X.; Wang, D.; Chen, T.; Huang, Q.; Li, Y.C.; Wang, H.; Kim, S.H.; et al. Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 2019, 18, 384–389. [Google Scholar] [CrossRef]
- Shi, L.; Xu, A.; Zhao, T. First-principles investigations of the working mechanism of 2D h-BN as an interfacial layer for the anode of lithium metal batteries. ACS Appl. Mater. Interfaces 2017, 9, 1987–1994. [Google Scholar] [CrossRef]
- Shen, L.; Shi, P.; Hao, X.; Zhao, Q.; Ma, J.; He, Y.-B.; Kang, F. Progress on lithium dendrite suppression strategies from the interior to exterior by hierarchical structure designs. Small 2020, 16, 2000699. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.-J.; Jin, H.S.; No, H.; Kwack, H.; Chu, H.; Ye, F.; Lee, H.; Kim, H.-T. Tuning two interfaces with fluoroethylene carbonate electrolytes for high-performance Li/LCO batteries. ACS Omega 2019, 4, 3220–3227. [Google Scholar] [CrossRef]
- Liu, Q.-C.; Xu, J.-J.; Yuan, S.; Chang, Z.-W.; Xu, D.; Yin, Y.-B.; Li, L.; Zhong, H.-X.; Jiang, Y.-S.; Yan, J.-M. Artificial protection film on lithium metal anode toward long-cycle-life lithium–oxygen batteries. Adv. Mater. 2015, 27, 5241–5247. [Google Scholar] [CrossRef]
- Ma, L.; Kim, M.S.; Archer, L.A. Stable artificial solid electrolyte interphases for lithium batteries. Chem. Mater. 2017, 29, 4181–4189. [Google Scholar] [CrossRef]
- Tu, Z.; Choudhury, S.; Zachman, M.J.; Wei, S.; Zhang, K.; Kourkoutis, L.F.; Archer, L.A. Fast ion transport at solid-solid interfaces in hybrid battery anodes. Nat. Energy 2018, 3, 310–316. [Google Scholar] [CrossRef]
- Luo, Z.; Qiu, X.; Liu, C.; Li, S.; Wang, C.; Zou, G.; Hou, H.; Ji, X. Interfacial challenges towards stable Li metal anode. Nano Energy 2021, 79, 105507. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, H.Y.; Zhang, X.G.; Wang, W.W.; He, J.W.; Tang, S.; Yan, J.W.; Wu, D.Y.; Zheng, M.S.; Dong, Q.F. Lithiophilic faceted Cu (100) surfaces: High utilization of host surface and cavities for lithium metal anodes. Angew. Chem. Int. Ed. 2019, 58, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wen, Z.; Wu, M.; Shen, C.; Wang, Q.; Jin, J.; Wu, X. A lithium anode protection guided highly-stable lithium–sulfur battery. Chem. Commun. 2014, 50, 14209–14212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Wang, W.; Tang, H.; Bai, W.Q.; Ge, X.; Wang, X.L.; Gu, C.D.; Tu, J.P. An ex-situ nitridation route to synthesize Li3N-modified Li anodes for lithium secondary batteries. J. Power Sources 2015, 277, 304–311. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. Strategies based on nitride materials chemistry to stabilize Li metal anode. Adv. Sci. 2017, 4, 1600517. [Google Scholar] [CrossRef]
- Zhao, Y.; Amirmaleki, M.; Sun, Q.; Zhao, C.; Codirenzi, A.; Goncharova, L.V.; Wang, C.; Adair, K.; Li, X.; Yang, X.; et al. Natural SEI-inspired dual-protective layers via atomic/molecular layer deposition for long-life metallic lithium anode. Matter 2019, 1, 1215–1231. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Wu, F.; Liu, Y.; Wang, X.; Zhang, K.; Zheng, L.; Wang, Z.; Bai, Y.; Wu, C. Rational tuning of a Li4SiO4-based hybrid interface with unique stepwise prelithiation for dendrite-proof and high-rate lithium anodes. ACS Appl. Mater. Interfaces 2020, 12, 39362–39371. [Google Scholar] [CrossRef]
- Li, N.-W.; Yin, Y.-X.; Yang, C.-P.; Guo, Y.-G. An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Q.; Jia, W.; Chen, S.; Gao, P.; Li, J. Li metal coated with amorphous Li3PO4 via magnetron sputtering for stable and long-cycle life lithium metal batteries. J. Power Sources 2017, 342, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Zhuang, H.; Gao, L.; Lu, Y.; Archer, L.A. Regulating Li deposition at artificial solid electrolyte interphases. J. Mater. Chem. A 2017, 5, 3483–3492. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, X.; Xu, M.; Chen, Z.; Liu, M.; Cheng, X.; Li, W. A self-healing interface on lithium metal with lithium difluoro (bisoxalato) phosphate for enhanced lithium electrochemistry. J. Mater. Chem. A 2019, 7, 26002–26010. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, S.; Zhou, J.; Jin, Y.; Liu, Y.; Qin, Y.; Shen, C.; Han, W.; Wang, D. Volumetric variation confinement: Surface protective structure for high cyclic stability of lithium metal electrodes. J. Mater. Chem. A 2016, 4, 2427–2432. [Google Scholar] [CrossRef]
- Jing, H.-K.; Kong, L.L.; Liu, S.; Li, G.-R.; Gao, X.-P. Protected lithium anode with porous Al2O3 layer for lithium-sulfur battery. J. Mater. Chem. A 2015, 3, 12213–12219. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wang, Q.; Li, W.; Wu, B.; Jia, W.; Wang, Y.; Li, J.; Li, H. Long lifespan lithium metal anodes enabled by Al2O3 sputter coating. Energy Storage Mater. 2018, 10, 16–23. [Google Scholar] [CrossRef]
- Chen, L.; Connell, J.G.; Nie, A.; Huang, Z.; Zavadil, K.R.; Klavetter, K.C.; Yuan, Y.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Libera, J.A. Lithium metal protected by atomic layer deposition metal oxide for high performance anodes. J. Mater. Chem. A 2017, 5, 12297–12309. [Google Scholar] [CrossRef]
- Kozen, A.C.; Lin, C.F.; Pearse, A.J.; Schroeder, M.A.; Han, X.; Hu, L.; Lee, S.B.; Rubloff, G.W.; Noked, M. Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 2015, 9, 5884–5892. [Google Scholar] [CrossRef]
- Kazyak, E.; Wood, K.N.; Dasgupta, N.P. Improved cycle life and stability of lithium metal anodes through ultrathin atomic layer deposition surface treatments. Chem. Mater. 2015, 27, 6457–6462. [Google Scholar] [CrossRef]
- Alaboina, P.K.; Rodrigues, S.; Rottmayer, M.; Cho, S.J. In situ dendrite suppression study of nanolayer encapsulated Li metal enabled by zirconia atomic layer deposition. ACS Appl. Mater. Interfaces 2018, 10, 32801–32808. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Yuen, P.Y.; Liu, K.; Xie, J.; Dauskart, R.H.; Cui, Y. An artificial solid electrolyte interphase with high Li-ion conductivity, mechanical strength, and flexibility for stable lithium metal anodes. Adv. Mater. 2017, 29, 1605531. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.B.; Yao, Y.X.; Shen, X.; Li, B.-Q.; Li, W.-J.; Zhang, R.; Huang, J.-Q.; Li, H.; Zhang, Q. An armored mixed conductor interphase on a dendrite-free lithium-metal anode. Adv. Mater. 2018, 30, 1804461–1804469. [Google Scholar] [CrossRef]
- Liu, S.; Ji, X.; Yue, J.; Hou, S.; Wang, P.; Cui, C.; Chen, J.; Shao, B.; Li, J.; Han, F.; et al. High interfacial-energy interphase promoting safe lithium metal batteries. J. Am. Chem. Soc. 2020, 142, 2438–2447. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building better batteries in the solid state: A review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.; Zhang, W.; Zhu, G.; Huang, Y.; Feng, Q.; Lu, Y. Design principles of the anode-electrolyte interface for all solid-state lithium metal batteries. Small Methods 2020, 4, 1900592. [Google Scholar] [CrossRef]
- Zhao, C.-Z.; Chen, P.-Y.; Zhang, R.; Chen, X.; Li, B.-Q.; Zhang, X.-Q.; Cheng, X.-B.; Zhang, Q. An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv. 2018, 4, 3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Xiao, Y.; Zhang, R.; Cheng, X.-B.; Zhao, C.-Z.; Zhang, X.-Q.; Yan, C.; Zhang, Q.; Huang, J.-Q. Dual-phase single-ion pathway interfaces for robust lithium metal in working batteries. Adv. Mater. 2019, 31, 1808392. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Ci, C.; Salpekar, D.; Ai, Q.; Chen, Q.; Guo, H.; Chen, L.; Zhang, X.; Cheng, J.; Kato, K.; et al. Stable lithium metal anode enabled by an artificial multi-phase composite protective film. J. Power Sources 2020, 448, 227547. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, A.Y.; Liu, G.; Woo, J.Y.; Kim, H.; Lee, J.K. Li4SiO4-based artificial passivation thin film for improving interfacial stability of Li metal anodes. ACS Appl. Mater. Interfaces 2018, 10, 8692–8701. [Google Scholar] [CrossRef] [PubMed]
- Neuhold, S.; Schroeder, D.J.; Vaughey, J.T. Effect of surface preparation and R-group size on the stabilization of lithium metal anodes with silanes. J. Power Sources 2012, 206, 295–300. [Google Scholar] [CrossRef]
- Marchioni, F.; Star, K.; Menke, E.; Buffeteau, T.; Servant, L.; Dunn, B.; Wudl, F. Protection of lithium metal surfaces using chlorosilanes. Langmuir 2007, 23, 11597–11602. [Google Scholar] [CrossRef]
- Li, B.; Xu, J.; Xiao, X. Reinforced composite film on lithium metal electrodes through aryl chlorosilane treatment. Langmuir 2019, 35, 16459–16465. [Google Scholar] [CrossRef]
- Luo, Y.; Li, T.; Zhang, H.; Yu, Y.; Hussain, A.; Yan, J.; Zhang, H.; Li, X. New insights into the formation of silicon–oxygen layer on lithium metal anode via in situ reaction with tetraethoxysilane. J. Energy Chem. 2021, 56, 14–22. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Three-dimensional stable lithium metal anode with nanoscale lithium islands embedded in ionically conductive solid matrix. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Shi, P.; Li, T.; Zhang, R.; Shen, X.-B.; Xu, R.; Huang, J.-Q.; Chen, X.-R.; Liu, H.; Zhang, Q. Lithiophilic LiC6 layers on carbon hosts enabling stable Li metal anode in working batteries. Adv. Mater. 2019, 31, 1807131. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.J.; Wang, H.C.; Gong, Z.L.; Wang, D.W.; Zheng, B.Z.; Zhu, J.P.; Lu, Y.X.; Hu, Y.S.; Guo, X.X.; Li, H.; et al. Drawing a soft interface: An effective interfacial modification strategy for garnet-type solid-state Li batteries. ACS Energy Lett. 2018, 3, 1212–1218. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Bai, W.-Q.; Wang, X.-L.; Xia, X.-H.; Gu, C.-D.; Tu, J.-P. In situ confocal microscopic observation on inhibiting the dendrite formation of a-CNx/Li electrode. J. Mater. Chem. A 2016, 4, 15597–15604. [Google Scholar] [CrossRef]
- Zheng, G.; Lee, S.W.; Liang, Z.; Lee, H.-W.; Yan, K.; Yao, H.; Wang, H.; Li, W.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Levi, E.; Gershinsky, G.; Aurbach, D.; Isnard, O.; Ceder, G. New insight on the unusually high ionic mobility in Chevrel phases. Chem. Mater. 2009, 21, 1390–1399. [Google Scholar] [CrossRef]
- Lu, K.; Gao, S.; Dick, R.J.; Sattar, Z.; Cheng, Y. A fast and stable Li metal anode incorporating an Mo6S8 artificial interphase with super Li-ion conductivity. J. Mater. Chem. A 2019, 7, 6038–6044. [Google Scholar] [CrossRef]
- Kang, D.; Hart, N.; Koh, J.; Ma, L.; Liang, W.; Xu, J.; Sardar, S.; Lemmon, J.P. Rearrange SEI with artificial organic layer for stable lithium metal anode. Energy Storage Mater. 2020, 24, 618–625. [Google Scholar] [CrossRef]
- Sun, Y.; Amirmaleki, X.; Zhao, Y.; Zhao, C.; Liang, J.; Wang, C.; Adair, K.R.; Li, J.; Cui, T.; Wang, G.; et al. Tailoring the mechanical and electrochemical properties of an artificial interphase for high-performance metallic lithium anode. Adv. Energy. Mater. 2020, 10, 2001139. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Yan, C.; Zhang, X.-Q.; Liu, H.; Zhang, Q. Electronic and ionic channels in working interfaces of lithium metal anodes. ACS Energy Lett. 2018, 3, 1564–1570. [Google Scholar] [CrossRef]
- Aurbach, D.; Weissman, I.; Zaban, A.; Chusid, O. Correlation between surface chemistry, morphology, cycling efficiency and interfacial properties of Li electrodes in solutions containing different Li salts. Electrochim. Acta 1994, 39, 51–71. [Google Scholar] [CrossRef]
- Aurbach, D.; Ein-Ely, Y.; Zaban, A. The surface chemistry of lithium electrodes in alkyl carbonate solutions. J. Electrochem. Soc. 1994, 141, L1–L3. [Google Scholar] [CrossRef]
- Aurbach, D.; Zaban, A.; Schechter, A.; Ein-Eli, Y.; Zinigrad, E.; Markovsky, B. The study of electrolyte solutions based on ethylene and diethyl carbonates for rechargeable Li batteries: I. Li metal anodes. J. Electrochem. Soc. 1995, 142, 2873–2882. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Wu, Z.; Li, J.; Zou, J.; Jiang, C.; Yang, J.; Wang, L.; Niu, X. The effects of lithium salt and solvent on lithium metal anode performance. Solid State Ionics 2018, 324, 144–149. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Wu, X.; Henderson, W.A. Lithium Metal Anodes and Rechargeable Lithium Metal Batteries; Springer: Cham, Switzerland, 2017; pp. 21–31. [Google Scholar]
- Thompson, R.S.; Schroeder, D.J.; Lopez, C.M.; Neuhold, S.; Vaughey, J.T. Stabilization of lithium metal anodes using silane-based coatings. Electrochem. Commun. 2011, 13, 1369–1372. [Google Scholar] [CrossRef]
- Zhu, B.; Jin, Y.; Hu, X.; Zheng, Q.; Zhang, S.; Wang, Q.; Zhu, J. Poly(dimethylsiloxane) thin film as a stable interfacial layer for high-performance lithium-metal battery anodes. Adv. Mater. 2017, 29, 1603755. [Google Scholar] [CrossRef]
- Liu, K.; Pei, A.; Lee, H.R.; Kong, B.; Liu, N.; Lin, D.; Liu, Y.; Liu, C.; Hsu, P.-C.; Bao, Z. Lithium metal anodes with an adaptive “solid-liquid” interfacial protective layer. J. Am. Chem. Soc. 2017, 139, 4815–4820. [Google Scholar] [CrossRef] [PubMed]
- Assegie, A.A.; Cheng, J.H.; Kuo, L.M.; Su, W.N.; Hwang, B.J. Polyethylene oxide film coating enhances lithium cycling efficiency of an anode-free lithium-metal battery. Nanoscale 2018, 10, 6125–6138. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, X.Q.; Cheng, X.-B.; Peng, H.-J.; Zhao, C.-Z.; Yan, C.; Huang, J.-Q. Artificial soft–rigid protective layer for dendrite-free lithium metal anode. Adv. Funct. Mater. 2018, 28, 1705838. [Google Scholar] [CrossRef]
- Guo, S.; Wang, L.; Jin, Y.; Piao, N.; Chen, Z.; Tian, G.; Li, J.; Zhao, C.; He, X. A polymeric composite protective layer for stable Li metal anodes. Nano Converg. 2020, 7, 21. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, D.; Zhou, X.; Wang, A.; Wang, D.; Zhu, J.; Shen, C.; Liu, Y.; Guo, B.; Wang, D. Amide-based interface layer with high toughness in-situ building on the Li metal anode. ACS Appl. Mater. Interfaces 2020, 12, 25826–25831. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, S.; Lv, Z.; Xu, G.; Huang, L.; Wang, Q.; Cui, Z.; Cui, G. A temperature- responsive electrolyte endowing superior safety characteristic of lithium metal batteries. Adv. Energy Mater. 2020, 10, 1903441. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, Y.; Yan, C.; Xu, R.; Ding, J.F.; Liang, J.; Peng, H.J.; Yuan, H.; Huang, J.Q. A bifunctional ethylene-vinyl acetate copolymer protective layer for dendrites-free lithium metal anodes. J. Energy Chem. 2020, 48, 203–207. [Google Scholar] [CrossRef]
- Luo, J.; Lee, R.-C.; Jin, J.-T.; Weng, Y.-T.; Fang, C.-C.; Wu, N.-L. A dual-functional polymer coating on a lithium anode for suppressing dendrite growth and polysulfide shuttling in Li–S batteries. Chem. Commun. 2017, 53, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Kang, I.S.; Sun, Y.-K.; Song, J.-H.; Chung, S.-M.; Kim, D.-W. Cycling characteristics of lithium metal batteries assembled with a surface modified lithium electrode. J. Power Sources 2013, 244, 363–368. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Li, Y.C.; Huang, Q.; Mallouk, T.E.; Wang, D. Interfacial chemistry regulation via a skin-grafting strategy enables high-performance lithium-metal batteries. J. Am. Chem. Soc. 2017, 139, 15288–15291. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wang, C.; Pei, A.; Lopez, J.; Shi, F.; Chen, Z.; Sendek, A.D.; Lee, H.-W.; Lu, Z.; Schneider, H. High-performance lithium metal negative electrode with a soft and flowable polymer coating. ACS Energy Lett. 2016, 1, 1247–1255. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Chen, Z.; McDowell, M.T.; Cui, Y.; Bao, Z. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 2013, 5, 1042–1048. [Google Scholar] [CrossRef]
- Jin, C.; Sheng, O.; Chen, M.; Ju, Z.; Lu, G.; Liu, T.; Nai, J.; Liu, Y.; Wang, Y.; Tao, X. Armed lithium metal anodes with functional skeletons. Mater. Today Nano 2021, 13, 100103. [Google Scholar] [CrossRef]
- Li, N.; Wei, W.; Xie, K.; Tan, J.; Zhang, L.; Luo, X.; Yuan, K.; Song, Q.; Li, H.; Shen, C.; et al. Suppressing dendritic lithium formation using porous media in lithium metal-based batteries. Nano Lett. 2018, 18, 2067–2073. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review-SEI: Past, present and future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Zhan, J.; Wang, X.; Tu, J. A CNT cocoon on sodium manganate nanotubes forming a core/branch cathode coupled with a helical carbon nanofiber anode for enhanced sodium ion batteries. J. Mater. Chem. A 2016, 4, 11207–11213. [Google Scholar] [CrossRef]
- Liang, Z.; Zheng, G.; Liu, C.; Liu, N.; Li, W.; Yan, K.; Yao, H.; Hsu, P.C.; Chu, S.; Cui, Y. Polymer nanofiber-guided uniform lithium deposition for battery electrodes. Nano Lett. 2015, 15, 2910–2916. [Google Scholar] [CrossRef]
- Lang, J.; Song, J.; Qi, L.; Luo, Y.; Luo, X.; Wu, H. Uniform lithium deposition induced by polyacrylonitrile submicron fiber array for stable lithium metal anode. ACS Appl. Mater. Interfaces 2017, 9, 10360–10365. [Google Scholar] [CrossRef]
- Cheng, X.B.; Hou, T.Z.; Zhang, R.; Peng, H.Y.; Zhao, C.Z.; Huang, J.Q.; Zhang, Q. Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv. Mater. 2016, 28, 2888–2895. [Google Scholar] [CrossRef]
- Lee, H.; Song, J.; Kim, Y.J.; Park, J.K.; Kim, H.T. Structural modulation of lithium metal-electrolyte interface with three-dimensional metallic interlayer for high-performance lithium metal batteries. Sci. Rep. 2016, 6, 30830. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lin, D.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7, 10992. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, W.; Wang, C.; Li, Y.; Chen, C.; Song, J.; Dai, J.; Hitz, E.M.; Xu, S.; Yang, C.; et al. High-capacity, low-tortuosity, and channel-guided lithium metal anode. Proc. Natl. Acad. Sci. USA 2017, 114, 3584–3589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Chen, X.R.; Chen, X.; Cheng, X.B.; Zhang, X.Q.; Yan, C.; Zhang, Q. Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes. Angew. Chem. Int. Ed. 2017, 56, 7764–7768. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zhou, L.; Xiao, Z.; Zhou, S.; Li, L.; Zhi, L. A collaborative strategy for stable lithium metal anodes by using three-dimensional nitrogen-doped graphene foams. Nanoscale 2018, 10, 4675–4679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cheng, X.-B.; Zhao, C.-Z.; Peng, H.-J.; Shi, J.-L.; Huang, J.-Q.; Wang, J.; Wei, F.; Zhang, Q. Conductive nanostructured scaffolds render low local current density to inhibit lithium dendrite growth. Adv. Mater. 2016, 28, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Zhamu, A.; Chen, G.; Liu, C.; Neff, D.; Fang, Q.; Yu, Z.; Xiong, W.; Wang, Y.; Wang, X.; Jang, B.Z. Reviving rechargeable lithium metal batteries: Enabling next-generation high-energy and high-power cells. Energy Environ. Sci. 2012, 5, 5701–5707. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Peng, H.-J.; Huang, J.-Q.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Dual-phase lithium metal Anode containing a polysulfide-induced solid electrolyte interphase and nanostructured graphene framework for lithium–sulfur batteries. ACS Nano 2015, 9, 6373–6382. [Google Scholar] [CrossRef]
- Mukherjee, R.; Thomas, A.V.; Datta, D.; Singh, E.; Li, J.; Eksik, O.; Shenoy, V.B.; Koratkar, N. Defect-induced plating of lithium metal within porous graphene networks. Nat. Commun. 2014, 5, 3710. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-K.; Woo, S.-G.; Kim, J.-H.; Yu, J.-S.; Lee, S.-R.; Kim, Y.-J. Few-layer graphene island seeding for dendrite-free Li metal electrodes. ACS Appl. Mater. Interfaces 2016, 8, 26895–26901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Xia, X.-H.; Wang, D.-H.; Wang, X.-L.; Gu, C.-D.; Tu, J.-P. Integrated reduced graphene oxide multilayer/Li composite anode for rechargeable lithium metal batteries. RSC Adv. 2016, 6, 11657–11664. [Google Scholar] [CrossRef]
- Jin, S.; Xin, S.; Wang, L.; Du, Z.; Cao, L.; Chen, J.; Kong, X.; Gong, M.; Lu, J.; Zhu, Y. Covalently connected carbon nanostructures for current collectors in both the cathode and anode of Li–S batteries. Adv. Mater. 2016, 28, 9094–9102. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Y.; Liu, C.; Fan, S. The effect of the carbon nanotube buffer layer on the performance of a Li metal battery. Nanoscale 2016, 8, 11161–11167. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, B.; Hitz, E.; Luo, W.; Yao, Y.; Li, Y.; Dai, J.; Chen, C.; Wang, Y.; Yang, C. A carbon-based 3D current collector with surface protection for Li metal anode. Nano Res. 2017, 10, 1356–1365. [Google Scholar] [CrossRef]
- Matsuda, S.; Kubo, Y.; Uosak, K.; Nakanishi, S. Lithium-metal deposition/dissolution within internal space of CNT 3D matrix results in prolonged cycle of lithium-metal negative electrode. Carbon 2017, 119, 119–123. [Google Scholar] [CrossRef]
- Raji, A.-R.O.; Villegas Salvatierra, R.; Kim, N.D.; Fan, X.; Li, Y.; Silva, G.A.L.; Sha, J.; Tour, J.M. Lithium batteries with nearly maximum metal storage. ACS Nano 2017, 11, 6362–6369. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Fang, X.; Shen, C.; Liu, Y.; Zhou, C. A Carbon nanofiber network for stable lithium metal anodes with high Coulombic efficiency and long cycle life. Nano Res. 2016, 9, 3428–3436. [Google Scholar] [CrossRef]
- Ji, X.; Liu, D.-Y.; Prendiville, D.G.; Zhang, Y.; Liu, X.; Stucky, G.D. Spatially heterogeneous carbon-fiber papers as surface dendrite-free current collectors for lithium deposition. Nano Today 2012, 7, 10–20. [Google Scholar] [CrossRef]
- Zuo, T.-T.; Wu, X.-W.; Yang, C.-P.; Yin, Y.-X.; Ye, H.; Li, N.-W.; Guo, Y.-G. Graphitized carbon fibers as multifunctional 3D current collectors for high areal capacity Li anodes. Adv. Mater. 2017, 29, 1700389. [Google Scholar] [CrossRef]
- Ye, H.; Xin, S.; Yin, Y.-X.; Li, J.-Y.; Guo, Y.-G.; Wan, L.-J. Stable Li plating/stripping electrochemistry realized by a Hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons. J. Am. Chem. Soc. 2017, 139, 5916–5922. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Y.-X.; Li, J.-Y.; Li, N.-W.; Zeng, X.-X.; Ye, H.; Guo, Y.-G.; Wan, L.J. Free-standing hollow carbon fibers as high-capacity containers for stable lithium metal anodes. Joule 2017, 1, 563–575. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhen, Y.; Li, T.; Bettels, F.; He, T.; Peng, M.; Liang, Y.; Ding, F.; Zhang, L. High-capacity, dendrite-free, and ultrahigh-rate lithium-metal anodes based on monodisperse N-doped hollow carbon nanospheres. Small 2020, 16, 2004770. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Lee, H.R.; Yan, K.; Li, Y.; Shi, F.; Huang, W.; Pei, A.; Chen, G.; Subbaraman, R. Engineering stable interfaces for three-dimensional lithium metal anodes. Sci. Adv. 2018, 4, 5168. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-K.; Woo, S.-G.; Kim, J.-H.; Lee, S.-R.; Kim, Y.-J. Conductive porous carbon film as a lithium metal storage medium. Electrochim. Acta 2015, 176, 172–178. [Google Scholar] [CrossRef]
- Yue, X.-Y.; Bao, J.; Qiu, Q.-Q.; Luo, R.-J.; Wang, Q.-C.; Wu, X.-Y.; Zhou, Y.-N. Copper decorated ultralight 3D carbon skeleton derived from soybean for dendrite-free Li metal anode. Chem. Eng. J. 2020, 391, 123516. [Google Scholar] [CrossRef]
- Lang, J.L.; Jin, Y.; Luo, X.Y.; Liu, Z.L.; Song, J.N.; Long, Y.Z.; Qi, L.H.; Fang, M.H.; Li, Z.C.; Wu, H. Surface graphited carbon scaffold enables simple and scalable fabrication of 3D composite lithium metal anode. J. Mater. Chem. A 2017, 5, 19168. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Deng, Y.; Huang, Z.; Zhang, C.; He, Y.-B.; Lv, W.; Yang, Q.-H. A lightweight carbon nanofiber-based 3D structured matrix with high nitrogen-doping level for lithium metal anodes. Sci. China Mater. 2018, 62, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Zhai, P.; Wang, T.; Yang, W.; Cui, S.; Zhang, P.; Nie, A.; Zhang, Q.; Gong, Y. Uniform lithium deposition Assisted by single-atom doping toward high-performance lithium metal anodes. Adv. Energy Mater. 2019, 9, 1804019. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.-R.; Hou, T.-Z.; Li, B.-Q.; Cheng, X.-B.; Zhang, R.; Zhang, Q. Lithiophilicity chemistry of heteroatom-doped carbon to guide uniform lithium nucleation in lithium metal anodes. Sci. Adv. 2019, 5, 7728. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Li, Z.; Xie, W.; Li, J.; Rao, D.; Shao, M.; Zhang, B.; Wei, M. Oxygen-rich carbon nanotube networks for enhanced lithium metal anode. Energy Storage Mater. 2018, 15, 308–314. [Google Scholar] [CrossRef]
- Liang, F.; Lin, L.; Feng, Z.; Chu, C.; Pan, J.; Yang, J.; Qian, Y. Spatial separation of lithiophilic surface and superior conductivity for advanced Li metal anode: The case of acetylene black and N-doped carbon spheres. J. Mater. Chem A 2019, 7, 8765–8770. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Y.; Li, J.; Wang, S.; Guo, Y.; Wan, L. Uniform lithium nucleation/growth induced by lightweight nitrogen-doped graphitic carbon foams for high-performance lithium metal anodes. Adv. Mater. 2018, 30, 1706216. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Sheng, O.; Lu, Y.; Luo, J.; Yuan, H.; Zhang, W.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C.; et al. Metal oxide nanoparticles induced step-edge nucleation of stable Li metal anode working under an ultrahigh current density of 15 mA cm−2. Nano Energy 2018, 45, 203–209. [Google Scholar] [CrossRef]
- Sun, Q.; Zhai, W.; Hou, G.; Feng, J.; Zhang, L.; Si, P.; Guo, S.; Ci, L. In situ synthesis of a lithiophilic Ag-nanoparticles-decorated 3D porous carbon framework toward dendrite-free lithium metal anodes. ACS Sustain. Chem. Eng. 2018, 6, 15219–15227. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Pastel, G.; Kuang, Y.; Xie, H.; Li, Y.; Liu, B.; Luo, W.; Chen, C.; Hu, L. 3D wettable framework for dendrite-free alkali metal anodes. Adv. Energy Mater. 2018, 8, 1800635. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Hafez, A.M.; Jiao, Y.; Shi, J.; Ma, Y.; Cao, D.; Liu, Y.; Zhu, H. Stable metal anode enabled by porous lithium foam with superior ion accessibility. Adv. Mater. 2018, 30, 1802156. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, M.; Geng, H.; Zhang, Y.; Fang, Y.; Li, C.; Zhao, J. Three-dimensional graphene/Ag aerogel for durable and stable Li metal anodes in carbonate-based electrolytes. Chem. Eur. J. 2019, 25, 5036–5042. [Google Scholar] [CrossRef]
- Xue, P.; Liu, S.; Shi, X.; Sun, C.; Lai, C.; Zhou, Y.; Sui, D.; Chen, Y.; Liang, J. A Hierarchical silver-nanowire–graphene host enabling ultrahigh rates and superior long-term cycling of lithium-metal composite anodes. Adv. Mater. 2018, 30, 1804165. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tao, T.; Mateti, S.; Lu, S.; Chen, Y. Nanoflake arrays of lithiophilic metal oxides for the ultra-stable anodes of lithium-metal batteries. Adv. Funct. Mater. 2018, 28, 1803023. [Google Scholar] [CrossRef]
- Chen, X.R.; Li, B.Q.; Zhao, B.Q.; Zhang, R.; Zhang, Q. Synergetic coupling of lithiophilic sites and conductive scaffolds for dendrite-free lithium metal anodes. Small Methods 2019, 4, 1900177. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Li, Y.; Liu, Y.; Lin, D.; Zhu, C.; Chen, G.; Yang, A.; Yan, K.; Chen, H.; et al. Wrinkled graphene cages as hosts for high-capacity Li metal anodes shown by cryogenic electron microscopy. Nano Lett. 2019, 19, 1326–1335. [Google Scholar] [CrossRef]
- Pu, J.; Li, J.C.; Shen, Z.H.; Zhong, C.L.; Liu, J.Y.; Ma, H.X.; Zhu, J.; Zhang, H.G.; Braun, P.V. Interlayer lithium plating in Au nanoparticles pillared reduced graphene oxide for lithium metal anodes. Adv. Funct. Mater. 2018, 28, 1804133. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Z.; Xia, Z.; Jiang, T.; Wang, G.; Sun, J.; Sun, P.; Yan, C.; Zhang, L. (PECVD-derived graphene nanowall/lithium composite anodes towards highly stable lithium metal batteries. Energy Storage Mater. 2018, 22, 29–39. [Google Scholar] [CrossRef]
- Ren, F.; Lu, Z.; Zhang, H.; Huai, L.; Chen, X.; Wu, S.; Peng, Z.; Wang, D.; Ye, J. Pseudocapacitance induced uniform plating/stripping of Li metal anode in vertical graphene nanowalls. Adv. Funct. Mater. 2018, 28, 1805638. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, S.; Lu, K.; Li, Y.; Wang, R.; Cheng, Y.; Qin, W.; Wu, X. Stable high-capacity cycling of Li metal via directed and confined Li growth with robust composite sponge. J. Power Sources 2019, 428, 1–7. [Google Scholar] [CrossRef]
- Xie, K.; Wei, W.; Yuan, K.; Lu, W.; Guo, M.; Li, Z.; Song, Q.; Liu, X.; Wang, J.-G.; Shen, C. Toward dendrite-free lithium deposition via structural and interfacial synergistic effects of 3D graphene@Ni scaffold. ACS Appl. Mater. Interfaces 2016, 8, 26091–26097. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tang, S.; Kong, D.; Liu, S.; Chiou, K.; Zhi, L.; Huang, J.; Xia, Y.Y.; Luo, J. Bending-tolerant anodes for lithium-metal batteries. Adv. Mater. 2018, 30, 1703891. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Jin, Y.; Liu, K.; Tao, X.; Zhang, Q.; Zhang, X.; Cui, Y. Transforming from planar to three-dimensional lithium with flowable interphase for solid lithium metal batteries. Sci. Adv. 2017, 3, eaao0713. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Huang, W.; Song, X.; Wang, W.; Hou, Z.; Zhao, X.; Deng, K.; Ju, H.; Sun, Y.; Zhao, Y.; et al. Thermally reduced graphene paper with fast Li ion diffusion for stable Li metal anode. Electrochim. Acta 2019, 294, 413–422. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Li, S.; Guo, W.; Zhao, Y.; Dong, Q.; Lin, X.; Song, Z.; Tan, X.; Wang, C.; et al. Ultrahigh-capacity and long-life lithium–metal batteries enabled by engineering carbon nanofiber–stabilized graphene aerogel film host. Small 2018, 14, 1803310. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, X.; Yang, Y.-W.; Huang, J.; Liu, X.; Luo, J. Horizontal centripetal plating in the patterned voids of Li/graphene composites for stable lithium-metal anodes. Inside Chem. 2018, 4, 2192–2200. [Google Scholar] [CrossRef] [Green Version]
- Go, W.; Kim, M.H.; Park, J.; Lim, C.H.; Joo, S.H.; Kim, Y.; Lee, H.W. Nanocrevasse-rich carbon fibers for stable lithium and sodium metal anodes. Nano Lett. 2019, 19, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Yan, H.B.; Liu, K.D.; Xie, K.Y.; Li, W.; Gai, W.H.; Chen, G.H.; Li, H.J.; Shen, C.; Fu, Q.G.; et al. Vertically grown edge-rich graphene nanosheets for spatial control of Li nucleation. Adv. Energy Mater. 2018, 8, 1800564. [Google Scholar] [CrossRef]
- Xiong, W.-S.; Xia, Y.; Jiang, Y.; Qi, Y.; Sun, W.; He, D.; Liu, Y.; Zhao, X.-Z. Highly conductive and robust three-dimensional host with Excellent alkali metal infiltration boosts ultrastable lithium and sodium metal anodes. ACS Appl. Mater. Interfaces 2018, 10, 21254–21261. [Google Scholar] [CrossRef]
- Guo, C.; Yang, H.; Naveed, A.; Nuli, Y.; Yang, J.; Cao, Y.; Yang, H.; Yang, J. AlF3-Modified carbon nanofibers as a multifunctional 3D interlayer for stable lithium metal anodes. Chem. Commun. 2018, 54, 8347–8350. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Boyer, M.; Hwang, G.S.; Lee, J.-W. Lithium-coated carbon cloth for anode of Lithium rechargeable batteries with enhanced cycling stability. Electrochim. Acta 2019, 303, 78–84. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, Q.; Cheng, X.-B.; Liu, F.; Yang, Y.; Lu, Z.; Zhao, J.; Wu, J.; Liu, H.; Yang, S.; et al. Three-dimensional superlithiophilic interphase for dendrite-free lithium metal anodes. ACS Appl. Mater. Interfaces 2020, 12, 5767–5774. [Google Scholar] [CrossRef]
- Liu, S.; Xia, X.; Yao, Z.; Wu, J.; Zhang, L.; Deng, S.; Zhou, C.; Shen, S.; Wang, X.; Tu, J. Straw–brick-like carbon fiber cloth/lithium composite electrode as an advanced lithium metal anode. Small Methods 2018, 2, 1800035. [Google Scholar] [CrossRef]
- Tian, R.; Wan, S.; Guan, L.; Duan, H.; Guo, Y.; Li, H.; Liu, H. Oriented growth of Li metal for stable Li/carbon composite negative electrode. Electrochim. Acta 2018, 292, 227–233. [Google Scholar] [CrossRef]
- Yang, C.; Yao, Y.; He, S.; Xie, H.; Hitz, E.; Hu, L. Ultrafine silver nanoparticles for seeded lithium deposition toward stable lithium metal anode. Adv. Mater. 2017, 29, 1702714. [Google Scholar] [CrossRef]
- Xiang, J.; Yuan, L.; Shen, Y.; Cheng, Z.; Yuan, K.; Guo, Z.; Zhang, Y.; Chen, X.; Huang, Y. Improved rechargeability of lithium metal anode via controlling lithium-ion flux. Adv. Energy Mater. 2018, 8, 1802352. [Google Scholar] [CrossRef]
- Xiang, J.; Zhao, Y.; Yuan, L.; Chen, C.; Shen, Y.; Hu, F.; Hao, Z.; Liu, J.; Xu, B.; Huang, Y. A strategy of selective and dendrite-free lithium deposition for lithium batteries. Nano Energy 2017, 42, 262–268. [Google Scholar] [CrossRef]
- Niu, C.; Pan, H.; Xu, W.; Xiao, J.; Zhang, J.-G.; Luo, L.; Wang, C.; Mei, D.; Meng, J.; Wang, X. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 2019, 14, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xia, X.; Zhong, Y.; Deng, S.; Yao, Z.; Zhang, L.; Cheng, X.B.; Wang, X.; Zhang, Q.; Tu, J. 3D TiC/C core/shell nanowire skeleton for dendrite-free and long-life lithium metal anode. Adv. Energy Mater. 2018, 8, 1702322. [Google Scholar] [CrossRef]
- Sun, Z.; Jin, S.; Jin, H.; Du, Z.; Zhu, Y.; Cao, A.; Ji, H.; Wan, L.J. Robust expandable carbon nanotube scaffold for ultrahigh-capacity lithium-metal anodes. Adv. Mater. 2018, 30, 1800884. [Google Scholar] [CrossRef]
- Shuai, Y.; Lin, H.; Chen, K.; Chen, S.; He, X.; Ge, K.; Li, N.; Gan, F. The effects of carbon-modified electrode on stability of lithium metal deposition with high areal capacity and high Coulombic efficiency. Mater. Lett. 2017, 209, 71–74. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Najafabadi, H.S.; Chen, S.; Ren, Z. A highly flexible semi-tubular carbon film for stable lithium metal anodes in high-performance batteries. Nano Energy 2017, 38, 504–509. [Google Scholar] [CrossRef]
- Zuo, T.T.; Yin, Y.X.; Wang, S.H.; Wang, P.F.; Yang, X.; Liu, J.; Yang, C.P.; Guo, Y.G. Trapping lithium into hollow silica microspheres with a carbon nanotube core for dendrite-free lithium metal anodes. Nano Lett. 2018, 18, 297–301. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, S.; Yang, H.; Zhang, F.; Liu, J. Electron regulation enabled selective lithium deposition for stable anodes of lithium-metal batteries. J. Mater. Chem. 2019, 7, 2184–2191. [Google Scholar] [CrossRef]
- Liu, F.; Xu, R.; Hu, Z.; Ye, S.; Zeng, S.; Yao, Y.; Li, S.; Yu, Y. Regulating lithium nucleation via CNTs modifying carbon cloth film for stable Li metal anode. Small 2019, 15, 1803734. [Google Scholar] [CrossRef]
- Ye, L.; Liao, M.; Sun, H.; Yang, Y.; Tang, C.; Zhao, Y.; Wang, L.; Xu, Y.; Zhang, L.; Wang, B.; et al. Stabilizing lithium into cross-stacked nanotube sheets with an ultra-high specific capacity for lithium oxygen batteries. Angew. Chem. Int. Ed. 2019, 58, 2437–2442. [Google Scholar] [CrossRef]
- Yang, G.; Tan, J.; Jin, H.; Kim, Y.H.; Yang, X.; Son, D.H.; Ahn, S.; Zhou, H.; Yu, C. Creating effective nanoreactors on carbon nanotubes with mechanochemical treatments for high-areal-capacity sulfur cathodes and lithium anodes. Adv. Funct. Mater. 2018, 28, 1800595. [Google Scholar] [CrossRef]
- Jin, S.; Sun, Z.; Guo, Y.; Qi, Z.; Guo, C.; Kong, X.; Zhu, Y.; Ji, H. High areal capacity and lithium utilization in anodes made of covalently connected graphite microtubes. Adv. Mater. 2017, 29, 1700783. [Google Scholar] [CrossRef]
- Wang, T.; Villegas Salvatierra, R.; Jalilov, A.S.; Tian, J.; Tour, J.M. Ultrafast charging high capacity asphalt–lithium metal batteries. ACS Nano 2017, 11, 10761–10767. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wang, Y.; Kang, T.; Liu, C.; Shen, Y.; Lu, W.; Wu, X.; Chen, L. A Li-dual carbon composite as stable anode material for Li batteries. Energy Storage Mater. 2018, 15, 116–123. [Google Scholar] [CrossRef]
- Xu, Y.; Li, T.; Wang, L.; Kang, K. Interlayered dendrite-free lithium plating for high-performance lithium-metal batteries. Adv. Mater. 2019, 31, 1901662. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, P.; Liu, Y.; Ouyang, Y.; Li, D.; Zhai, T.; Li, H.; Cui, Y. An autotransferable g-C3N4 Li+-modulating layer toward stable lithium anodes. Adv. Mater. 2019, 31, 1900342. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Fan, J.M.; Zhang, J.H.; Yang, J.F.; Yuan, R.M.; Chang, J.K.; Zheng, M.S.; Dong, Q.F. A honeycomb-like Co@N-C composite for ultrahigh sulfur loading Li–S batteries. ACS Nano 2017, 11, 11417–11424. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, J.H.; Jeoung, S.G.; Moon, H.R. Transformation of metal–organic frameworks/coordination polymers into functional nanostructured materials: Experimental approaches based on mechanistic insights. Acc. Chem. Res. 2017, 50, 2684–2692. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Tan, R.; Wang, H.; Yang, L.; Hu, J.; Chen, H.; Pan, F. A metal–organic-framework-based electrolyte with nanowetted interfaces for high-energy-density solid-state lithium battery. Adv. Mater. 2018, 30, 1704436. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Sun, Y.; Yi, J.; He, Y.; Qiao, Y.; Zhou, H. High-power Li-metal anode enabled by metal-organic framework modified electrolyte. Joule 2018, 2, 2117–2132. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yin, Y.X.; Li, J.Y.; Guo, Y.G.; Wan, L.J. Ladderlike carbon nanoarrays on 3D conducting skeletons enable uniform lithium nucleation for stable lithium metal anodes. Chem. Commun. 2018, 54, 5330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, A.; Lv, R.; Luo, J. A corrosion-resistant current collector for lithium metal anodes. Energy Storage Mater. 2019, 18, 199–204. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, G.; Lv, W.; Deng, Y.; Zhang, Y.; Zhang, C.; Kang, F.; Yang, Q.-H. Seeding lithium seeds towards uniform lithium deposition for stable lithium metal anodes. Nano Energy 2019, 61, 47–53. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, W.; Zhou, G.; Huang, Z.; Zhang, Y.; Lyu, R.; Wu, H.; Yun, Q.; Kang, F.; Yang, Q. Vertically aligned lithiophilic CuO nanosheets on a Cu collector to stabilize lithium deposition for lithium metal batteries. Adv. Energy Mater. 2018, 8, 1703404. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, S.; Lu, Y. 3D Porous Cu current collector/Li-metal composite anode for stable lithium-metal batteries. Adv. Funct. Mater. 2017, 27, 1606422. [Google Scholar] [CrossRef]
- An, Y.; Fei, H.; Zeng, G.; Xu, X.; Ci, L.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Vacuum distillation derived 3D porous current collector for stable lithium–metal batteries. Nano Energy 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, K.; Lv, Z.; Xu, X.; Hou, G.; Cao, H.; Wu, L.; Zheng, G.; Deng, Y. Three-dimensional ordered macroporous Cu current collector for lithium metal anode: Uniform nucleation by seed crystal. J. Power Sources 2018, 403, 82–89. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, D.; Zhao, J.; Lu, Z.; Liu, Y.; Liu, C.; Lu, Y.; Wang, H.; Yan, K.; Tao, X.; et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl Acad. Sci. USA 2016, 113, 2862–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhu, X.; Guan, Y.; Zhang, J.; Ai, F.; Zhang, W.; Xiang, Y.; Vijayan, S.; Li, G.; Huang, Y.; et al. ZnO/carbon framework derived from metal-organic frameworks as a stable host for lithium metal anodes. Energy Storage Mater. 2018, 11, 191–196. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Luo, W.; Liu, B.; Li, Y.; Xu, S.; Hamann, T.; McOwen, D.; Dai, J.; Luo, W.; et al. Continuous plating/stripping behavior of solid-state lithium metal anode in a 3D ion-conductive framework. Proc. Natl Acad. Sci. USA 2018, 115, 3770–3775. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Tang, T.; Asif, M.; Huang, X.; Hou, Y. 3D Porous Cu current collectors derived by hydrogen bubble dynamic template for enhanced Li metal anode performance. Adv. Funct. Mater. 2019, 29, 1808468. [Google Scholar] [CrossRef]
- Umh, H.N.; Park, J.; Yeo, J.; Jung, S.; Nam, I.; Yi, J. Lithium metal anode on a copper dendritic superstructure. Electrochem. Commun. 2019, 99, 27–31. [Google Scholar] [CrossRef]
- Shen, F.; Zhang, F.; Zheng, Y.; Fan, Z.; Li, Z.; Sun, Z.; Xuan, Y.; Zhao, B.; Lin, Z.; Gui, X.; et al. Direct growth of 3D host on Cu foil for stable lithium metal anode. Energy Storage Mater. 2018, 13, 323–328. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Gao, H.; Niu, J.; Ma, W.; Qin, J.; Peng, Z.; Zhang, Z. A self-supported, three-dimensional porous copper film as a current collector for advanced lithium metal batteries. J. Mater. Chem. 2019, 7, 1092–1098. [Google Scholar] [CrossRef]
- Zhao, H.; Lei, D.; He, Y.-B.; Yuan, Y.; Yun, Q.; Ni, B.; Lv, W.; Li, B.; Yang, Q.-H.; Kang, F.; et al. Compact 3D copper with uniform porous structure derived by electrochemical dealloying as dendrite-free lithium metal anode current collector. Adv. Energy Mater. 2018, 8, 1800266. [Google Scholar] [CrossRef]
- Yun, Q.; He, Y.B.; Lv, W.; Zhao, Y.; Li, B.; Kang, F.; Yang, Q.H. Chemical dealloying derived 3D porous current collector for Li metal anodes. Adv. Mater. 2016, 28, 6932–6939. [Google Scholar] [CrossRef]
- Wang, S.H.; Yin, Y.X.; Zuo, T.T.; Dong, W.; Li, J.Y.; Shi, J.L.; Zhang, C.H.; Li, N.W.; Li, C.J.; Guo, Y.G. Stable Li metal anodes via regulating lithium plating/stripping in vertically aligned microchannels. Adv. Mater. 2017, 29, 1703729. [Google Scholar] [CrossRef]
- Yang, C.-P.; Yin, Y.-X.; Zhang, S.-F.; Li, N.-W.; Guo, Y.-G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef]
- Lu, L.L.; Ge, J.; Yang, J.N.; Chen, S.M.; Yao, H.B.; Zhou, F.; Yu, S.H. Free-standing copper nanowire network current collector for Improving lithium anode performance. Nano Lett. 2016, 16, 4431–4437. [Google Scholar] [CrossRef]
- Yan, K.; Sun, B.; Munroe, P.; Wang, G. Three-dimensional pie-like current collectors for dendrite-free lithium metal anodes. Energy Storage Mater. 2018, 11, 127–133. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, K.; Han, Y.; Sun, Z.; Zhang, H.; Xu, L.; Ma, Y.; Chen, Y. A nitrogen-doped-carbon/ZnO modified Cu foam current collector for high-performance Li metal batteries. J. Mater. Chem. A 2019, 7, 5712–5718. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, W.; Ming, H.; Cao, G.; Fan, L.Z.; Zhang, H. Chemical energy release driven lithiophilic layer on 1 m2 commercial brass mesh toward highly stable lithium metal batteries. Nano Lett. 2019, 19, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, Z.; Yan, H.; Wang, R.; Lu, K.; Cheng, Y.; Qin, W.; Wu, X. High rate and cycling stable Li metal anodes enabled with aluminum-zinc oxides modified copper foam. J. Energy Chem. 2020, 41, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhang, C.; Li, B.; Xiong, S.; Song, J. Low-volume-change, dendrite-free lithium metal anodes enabled by lithophilic 3D matrix with LiF-enriched surface. J. Mater. Chem. A 2019, 7, 6090–6098. [Google Scholar] [CrossRef]
- Kim, J.Y.; Liu, G.; Tran, M.X.; Ardhi, R.E.A.; Kim, H.; Lee, J.K. Synthesis and characterization of a hierarchically structured three-dimensional conducting scaffold for highly stable Li metal anodes. J. Mater. Chem. A 2019, 7, 12882–12892. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, C.; Lv, W.; Zhou, G.; Zhang, Y.; Deng, Y.; Wu, H.; Kang, F.; Yang, Q.-H. Realizing stable lithium deposition by in situ grown Cu2S nanowires inside commercial Cu foam for lithium metal anodes. J. Mater. Chem. A 2019, 7, 727–732. [Google Scholar] [CrossRef]
- Pei, F.; Fu, A.; Ye, W.; Peng, J.; Fang, X.; Wang, M.S.; Zheng, N. Robust lithium metal anodes realized by lithiophilic 3D porous current collectors for constructing high-energy lithium-sulfur batteries. ACS Nano 2019, 13, 8337–8346. [Google Scholar] [CrossRef]
- Zhu, W.; Deng, W.; Zhao, F.; Liang, S.; Zhou, X.; Liu, Z. Graphene network nested Cu foam for reducing size of lithium metal towards stable metallic lithium anode. Energy Storage Mater. 2019, 21, 107–114. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, J.; Chen, X.; Zhang, X.D.; Li, J.Y.; Huang, L.B.; Zhang, X.; Shi, J.L.; Yin, Y.X.; Zhang, Q.; et al. Uniform nucleation of lithium in 3D current collectors via bromide intermediates for stable cycling lithium metal batteries. J. Am. Chem. Soc. 2018, 140, 18051–18057. [Google Scholar] [CrossRef]
- Yu, L.; Canfield, N.L.; Chen, S.; Lee, H.; Ren, X.; Engelhard, M.H.; Li, Q.; Liu, J.; Xu, W.; Zhang, J.-G. Enhanced stability of lithium metal anode by using a 3D porous nickel substrate. ChemElectroChem 2018, 5, 761–769. [Google Scholar] [CrossRef]
- Park, G.-K.; Kang, H.; Lee, G.W. Fabrication and characterization of Li-coated nickel mesh for anode of lithium-metal batteries. J. Alloys Compd. 2019, 790, 847–852. [Google Scholar] [CrossRef]
- Zou, P.; Chiang, S.-W.; Li, J.; Wang, Y.; Wang, X.; Wu, D.; Nairan, A.; Kang, F.; Yang, C. Ni@Li2O co-axial nanowire based reticular anode: Tuning electric field distribution for homogeneous lithium deposition. Energy Storage Mater. 2019, 18, 155–164. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Ke, X.; Zhang, Z.; Wu, W.; Lin, G.; Zhou, Z.; Shi, Z. Coupling of triporosity and strong Au-Li interaction to enable dendrite-free lithium plating/stripping for long-life lithium metal anodes. J. Mater. Chem. A 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Kang, H.-K.; Woo, S.-G.; Kim, J.-H.; Lee, S.-R.; Lee, D.-G.; Yu, J.-S. Three-dimensional monolithic corrugated graphene/Ni foam for highly stable and efficient Li metal electrode. J. Power Sources 2019, 413, 467–475. [Google Scholar] [CrossRef]
- Song, R.; Wang, B.; Xie, Y.; Ruan, T.; Wang, F.; Yuan, Y.; Wang, D.; Dou, S. A 3D conductive scaffold with lithiophilic modification for stable lithium metal batteries. J. Mater. Chem. 2018, 6, 17967–17976. [Google Scholar] [CrossRef]
- Lu, Z.; Liang, Q.; Wang, B.; Tao, Y.; Zhao, Y.; Lv, W.; Liu, D.; Zhang, C.; Weng, Z.; Liang, J.; et al. Graphitic carbon nitride induced micro-electric field for dendrite-free lithium metal anodes. Adv. Energy Mater. 2019, 9, 1803186. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, G.; Yan, K.; Xie, J.; Li, Y.; Liao, L.; Jin, Y.; Liu, K.; Hsu, P.C.; Wang, J.; et al. Air-stable and freestanding lithium alloy/graphene foil as an alternative to lithium metal anodes. Nat. Nanotechnol. 2017, 12, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Weng, S.; Yang, G.; Li, Y.; Li, H.; Su, D.; Gu, L.; Wang, Z.; Wang, X.; Chen, L. Interplay between solid-electrolyte interphase and (in)active LixSi in silicon anode. Cell Rep. Phys. Sci. 2021, 2, 100668. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Peng, H.-J.; Huang, J.-Q.; Wei, F.; Zhang, Q. Dendrite-free nanostructured anode: Entrapment of lithium in a 3D fibrous matrix for ultra-stable lithium–sulfur batteries. Small 2014, 10, 4257–4263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Wang, A.; Huang, Y.; Yuan, K.; Yu, Z.; Qiu, J.; Yang, Y. Improved cycle stability and high security of Li-B alloy anode for lithium-sulfur battery. J. Mater. Chem. A 2014, 2, 11660–11665. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, S.; Tang, C.; Zhai, Q.; Zhang, X.; Wang, R. Li-B Alloy as an anode material for stable and long-life lithium metal batteries. Energies 2018, 11, 2512. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zeng, Q.; Lv, R.; Lv, W.; Yang, Q.-H.; Amal, R.; Wang, D.-W. A Li-ion sulfur full cell with ambient resistant Al-Li alloy anode. Energy Storage Mater. 2018, 15, 209–217. [Google Scholar] [CrossRef]
- Wang, H.; Lin, D.; Liu, Y.; Li, Y.; Cui, Y. Ultrahigh-current density anodes with interconnected Li metal reservoir through overlithiation of mesoporous AlF3 framework. Sci. Adv. 2017, 3, 1701301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Li, S.; Liu, L.; Zhang, W.; Gao, L.; Fu, Y.; Chen, F.; Li, J.; Zhuang, H.L.; Lu, Y. Enabling stable lithium metal anode via 3D inorganic skeleton with superlithiophilic interphase. Adv. Energy Mater. 2018, 8, 1802350. [Google Scholar] [CrossRef]
- Jagannathan, M.; Chandran, K.S.R. Electrochemical charge/discharge behavior and phase transitions during cell cycling of Li(Mg) alloy anodes for high capacity Li ion batteries. J. Electrochem. Soc. 2013, 160, A1922–A1926. [Google Scholar] [CrossRef]
- Kong, L.L.; Wang, L.; Ni, Z.C.; Liu, S.; Li, G.R.; Gao, X.P. Lithium–magnesium alloy as a stable anode for lithium–sulfur battery. Adv. Funct. Mater. 2019, 29, 1808756. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, W.; Zhong, C.; Deng, N.; Cheng, B. Multifunctional LaF3 doped pomegranate-like porous carbon nanofibers with high-speed transfer channel and strong polar interface for high stability lithium sulfur battery. Chem. Eng. J. 2021, 403, 126449. [Google Scholar] [CrossRef]
- Matsuda, S.; Kubo, Y.; Uosaki, K.; Nakanishi, S. Insulative microfiber 3D matrix as a host material minimizing volume change of the anode of Li metal batteries. ACS Energy Lett. 2017, 2, 924–929. [Google Scholar] [CrossRef]
- Fan, L.; Zhuang, H.L.; Zhang, W.; Fu, Y.; Liao, Z.; Lu, Y. Stable lithium electrodeposition at ultra-high current densities enabled by 3D PMF/Li composite anode. Adv. Energy Mater. 2018, 8, 1703360. [Google Scholar] [CrossRef]
- Xu, B.; Zhai, H.; Liao, X.; Qie, B.; Mandal, J.; Gong, T.; Tan, L.; Yang, X.; Sun, K.; Cheng, Q.; et al. Porous insulating matrix for lithium metal anode with long cycling stability and high power. Energy Storage Mater. 2019, 17, 31–37. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Yuan, L.; Li, Z.; Huang, Y. Stable lithium metal anode enabled by 3D soft host. ACS Appl. Mater. Interfaces 2020, 12, 28337–28344. [Google Scholar] [CrossRef]
- Zou, P.; Wang, Y.; Chiang, S.W.; Wang, X.; Kang, F.; Yang, C. Directing lateral growth of lithium dendrites in micro-compartmented anode arrays for safe lithium metal batteries. Nat. Commun. 2018, 9, 464. [Google Scholar] [CrossRef]
- Cao, D.; Xing, Y.; Tantratian, K.; Wang, X.; Ma, Y.; Mukhopadhyay, A.; Cheng, Z.; Zhang, Q.; Jiao, Y.; Chen, L.; et al. 3D printed high-performance lithium metal microbatteries enabled by nanocellulose. Adv. Mater. 2019, 31, 1807313. [Google Scholar] [CrossRef]
- Ju, Z.; Jin, C.; Yuan, H.; Yang, T.; Sheng, O.; Liu, T.; Liu, Y.; Wang, Y.; Ma, F.; Zhang, W.; et al. A fast-ion conducting interface enabled by aluminum silicate fibers for stable Li metal batteries. Chem. Eng. J. 2021, 408, 128016. [Google Scholar] [CrossRef]
- Li, J.; Zou, P.; Chiang, S.W.; Yao, W.; Wang, Y.; Liu, P.; Liang, C.; Kang, F.; Yang, C. A conductive-dielectric gradient framework for stable lithium metal anode. Energy Storage Mater. 2020, 24, 700–706. [Google Scholar] [CrossRef]
- Zhang, C.; Lyu, R.; Lv, W.; Li, H.; Jiang, W.; Li, J.; Gu, S.; Zhou, G.; Huang, Z.; Zhang, Y. A lightweight 3D Cu nanowire network with phosphidation gradient as current collector for high-density nucleation and stable deposition of lithium. Adv. Mater. 2019, 31, 1904991. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Q.; Chen, Q.; Xu, W.; Xie, Q.; Cai, Y.; Ma, Y.; Qiao, Z.; Luo, Q.; Lin, J.; et al. 3D lithiophilic–lithiophobic–lithiophilic dual-gradient porous skeleton for highly stable lithium metal anode. J. Mater. Chem. 2020, 8, 313–322. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, X.; Guan, Y.; Xiang, Y.; Li, M.; Zhang, W.; Zhu, X.; Ming, H.; Lu, L.; Qiu, J.; et al. Lithiophilic-lithiophobic gradient interfacial layer for a highly stable lithium metal anode. Nat. Commun. 2018, 9, 3729. [Google Scholar] [CrossRef] [Green Version]
- Nan, Y.; Li, S.; Shi, Y.; Yang, S.; Li, B. Gradient-distributed nucleation seeds on conductive host for a dendrite-free and high-rate lithium metal anode. Small 2019, 15, 1903520. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ke, X.; Chen, Y.; Huang, X.; Shi, Z.; Guo, Z. Lithiophobic-lithiophilic composite architecture through co-deposition technology toward high-performance lithium metal batteries. Nano Energy 2019, 63, 103854. [Google Scholar] [CrossRef]
- Pu, J.; Li, J.; Zhang, K.; Zhang, T.; Li, C.; Ma, H.; Zhu, J.; Braun, P.V.; Lu, J.; Zhang, H. Conductivity and lithiophilicity gradients guide lithium deposition to mitigate short circuits. Nat. Commun. 2019, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, D.; Liu, Y.; Yu, Y.X.; Li, S.M.; Yang, S.B. Flexible Ti3C2 MXene-lithium film with lamellar structure for ultrastable metallic lithium anodes. Nano Energy 2017, 39, 654–661. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, R.; Wang, A.; Guo, W.; Liu, X.; Luo, J. MXene aerogel scaffolds for high-rate lithium metal anodes. Angew. Chem. Int. Ed. 2018, 57, 15028–15033. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, B. Multi-storey corridor structured host for a large area capacity and high-rate metallic lithium anode. Electrochim. Acta 2021, 365, 137341. [Google Scholar] [CrossRef]
- Wei, C.; Fei, H.; Tian, Y.; An, Y.; Guo, H.; Feng, J.; Qian, Y. Isotropic Li nucleation and growth achieved by an amorphous liquid metal nucleation seed on MXene framework for dendrite-free Li metal anode. Energy Storage Mater. 2020, 26, 223–233. [Google Scholar] [CrossRef]
- Chen, Y.C.; Ouyang, C.Y.; Song, L.J.; Sun, Z.L. Electrical and lithium-ion dynamics in three main components of solid electrolyte interphase from density functional theory study. J. Phys. Chem. C 2011, 115, 7044–7049. [Google Scholar] [CrossRef]
- Iddir, H.; Curtiss, L.A. Li ion diffusion mechanisms in bulk monoclinic Li2CO3 crystals from density functional studies. J. Phys. Chem. C 2010, 114, 20903–20906. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H. A Climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Cheng, Y.-T.; Qi, Y. General method to predict voltage-dependent ionic conduction in a solid electrolyte coating on electrodes. Phys. Rev. B 2015, 91, 134116. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Qi, Y.; Li, H.; Hector, L.G. Defect Thermodynamics and diffusion mechanisms in Li2CO3 and implications for the solid electrolyte interphase in Li-ion batteries. J. Phys. Chem. C 2013, 117, 8579–8593. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B. Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J. Electrochem. Soc. 2016, 163, A138–A149. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Q.; Liu, X.; Tang, T.; Bock, D.C.; Bruck, A.M.; Tallman, K.R.; Housel, L.M.; Kiss, A.M.; Marschilok, A.C. Nonplanar electrode architectures for ultrahigh areal capacity batteries. ACS Energy Lett. 2018, 4, 271–275. [Google Scholar] [CrossRef]
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 2018, 3, 16–21. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Zheng, J.; Kim, M.S.; Tu, Z.; Choudhury, S.; Tang, T.; Archer, L.A. Regulating electrodeposition morphology of lithium: Towards commercially relevant secondary Li metal batteries. Chem. Soc. Rev. 2020, 49, 2701–2750. [Google Scholar] [CrossRef]




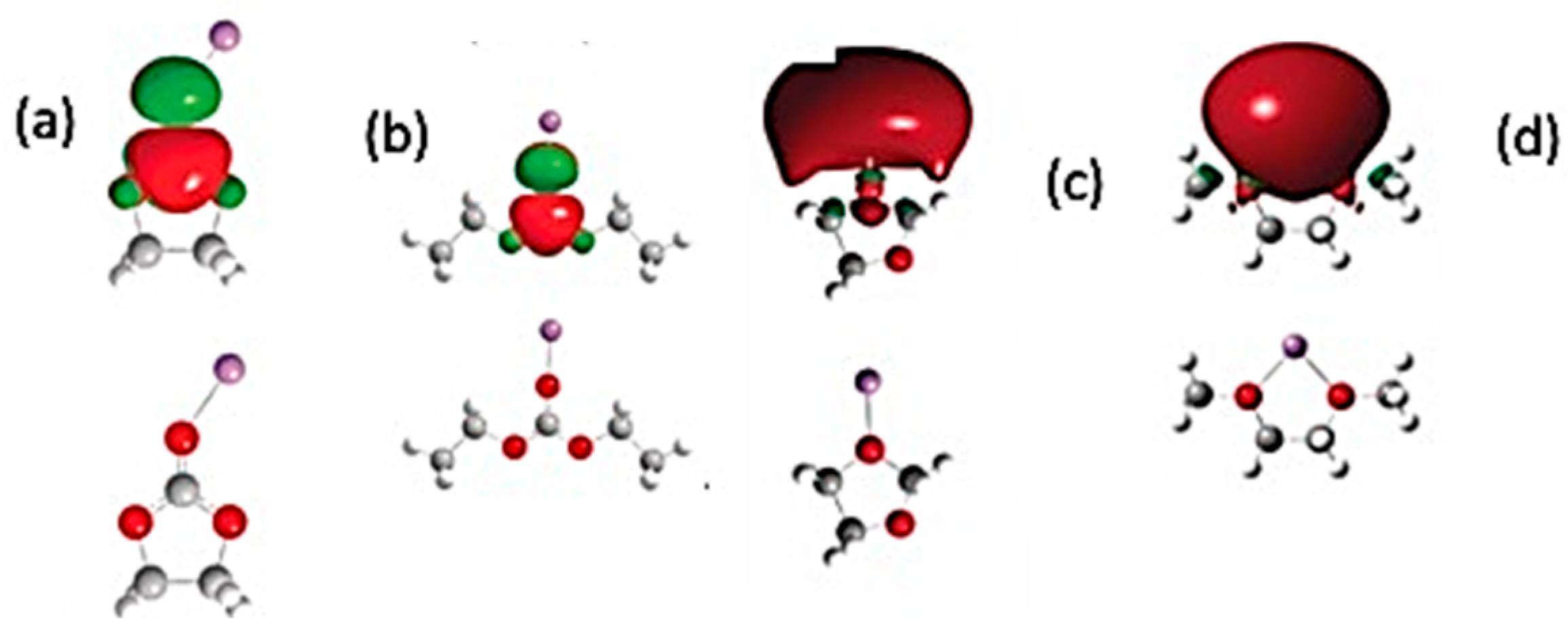

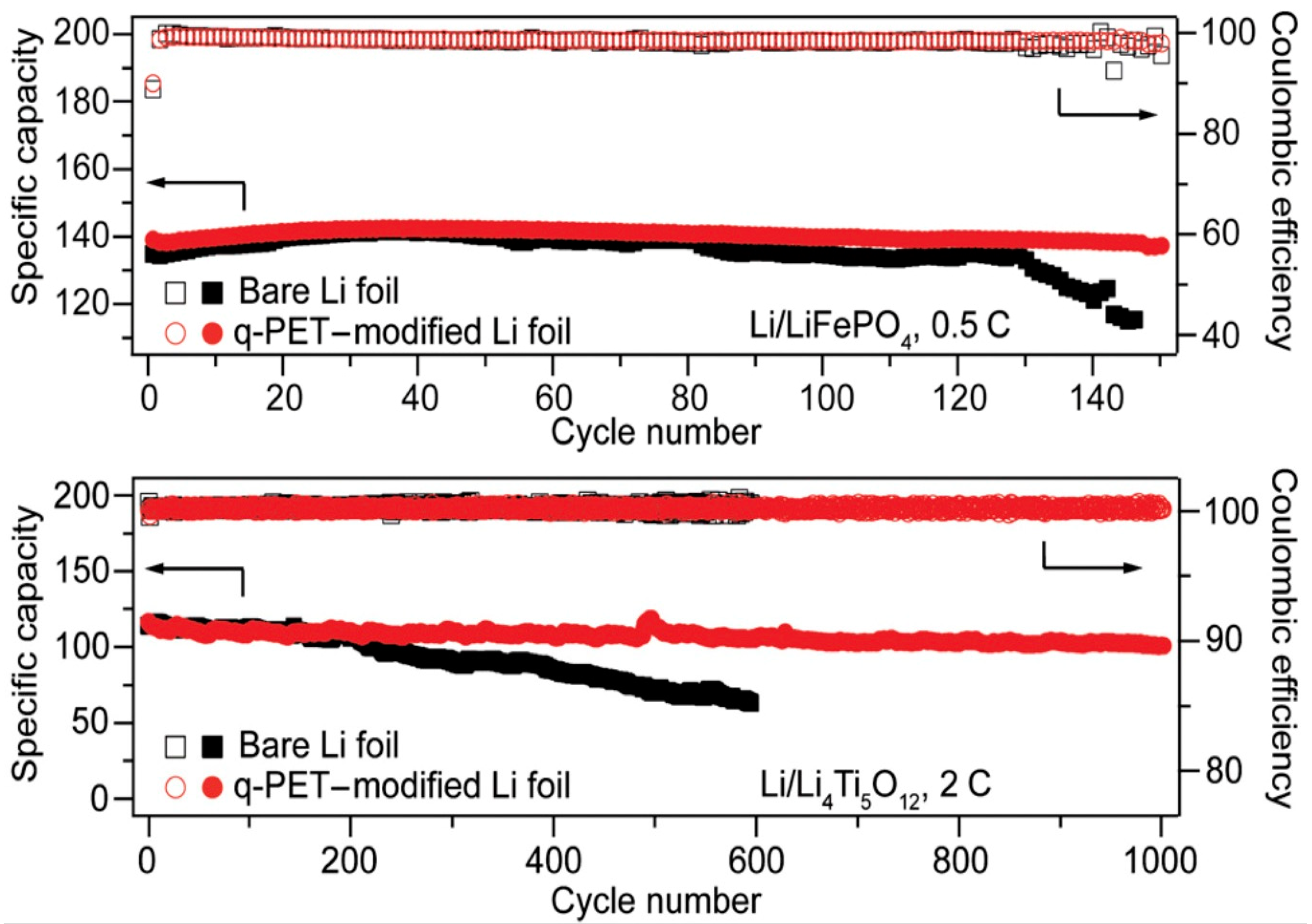

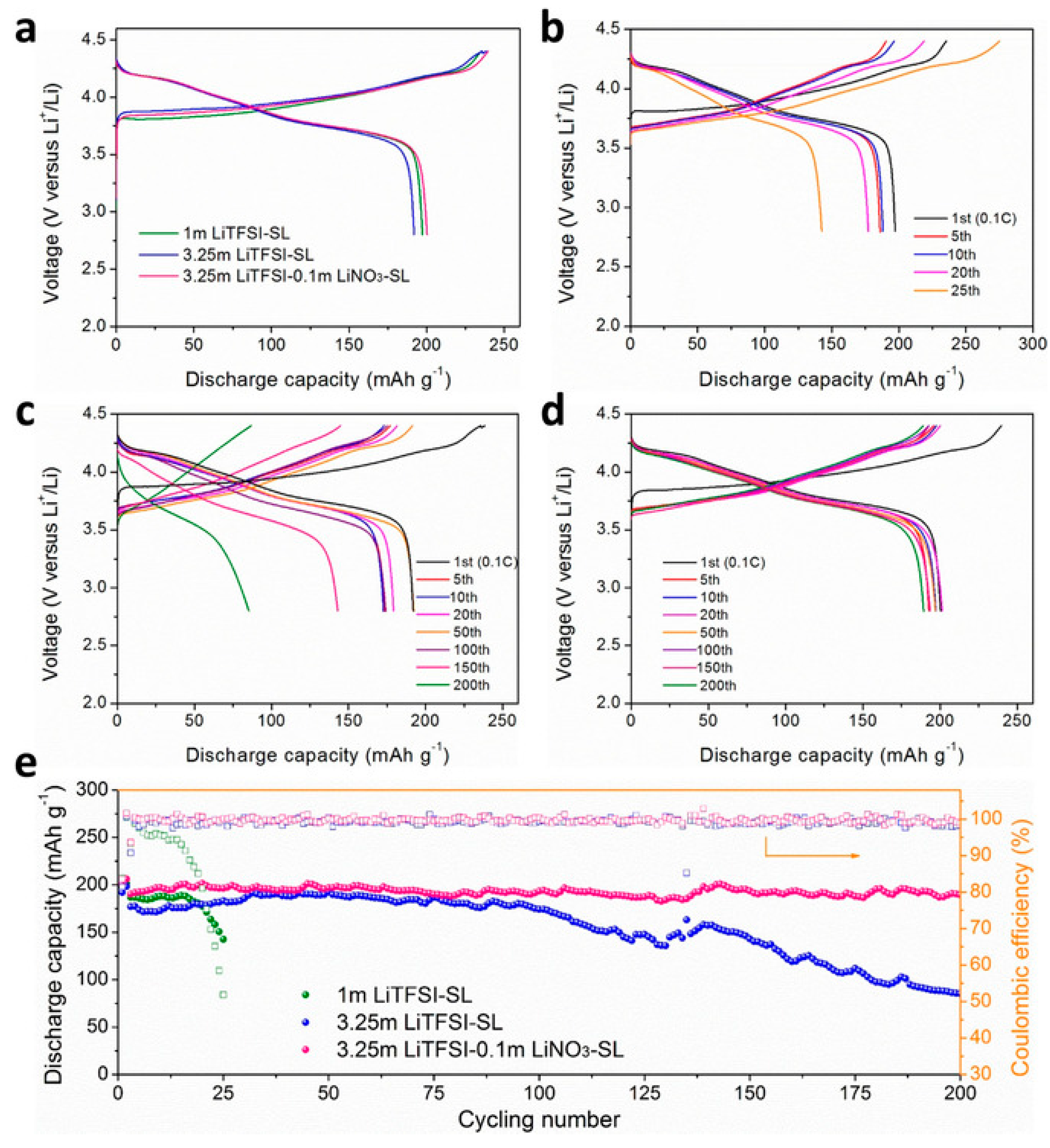

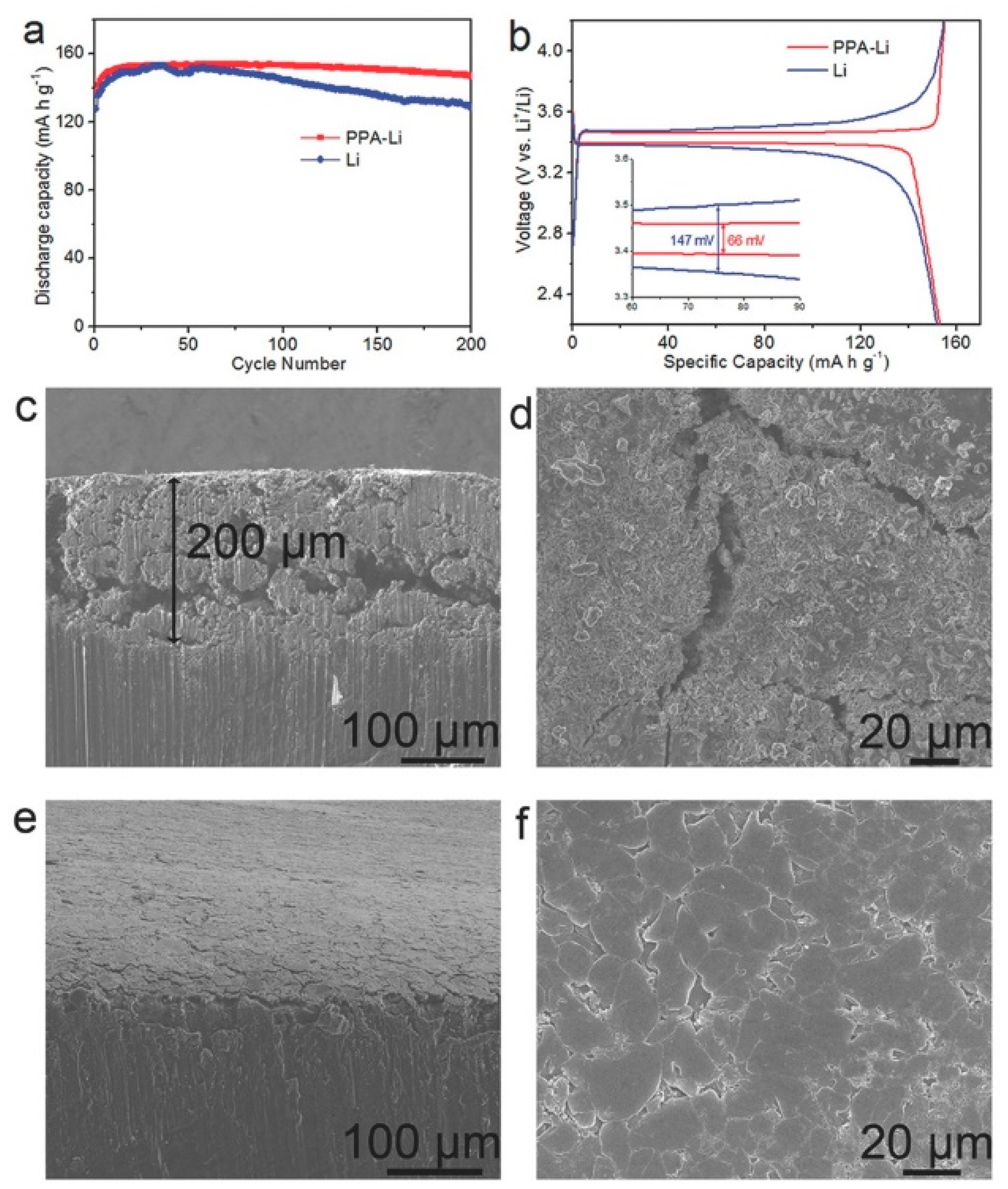
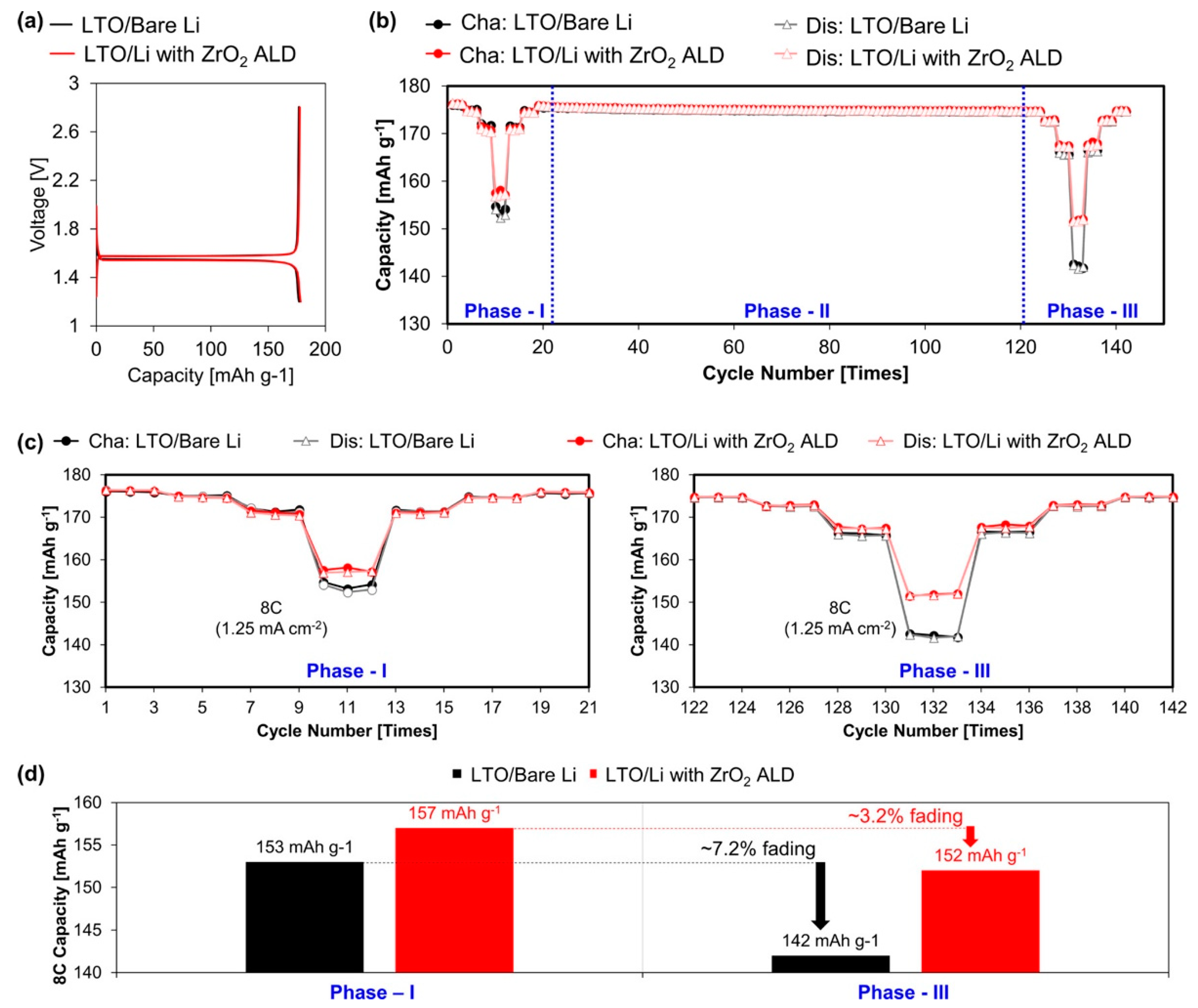
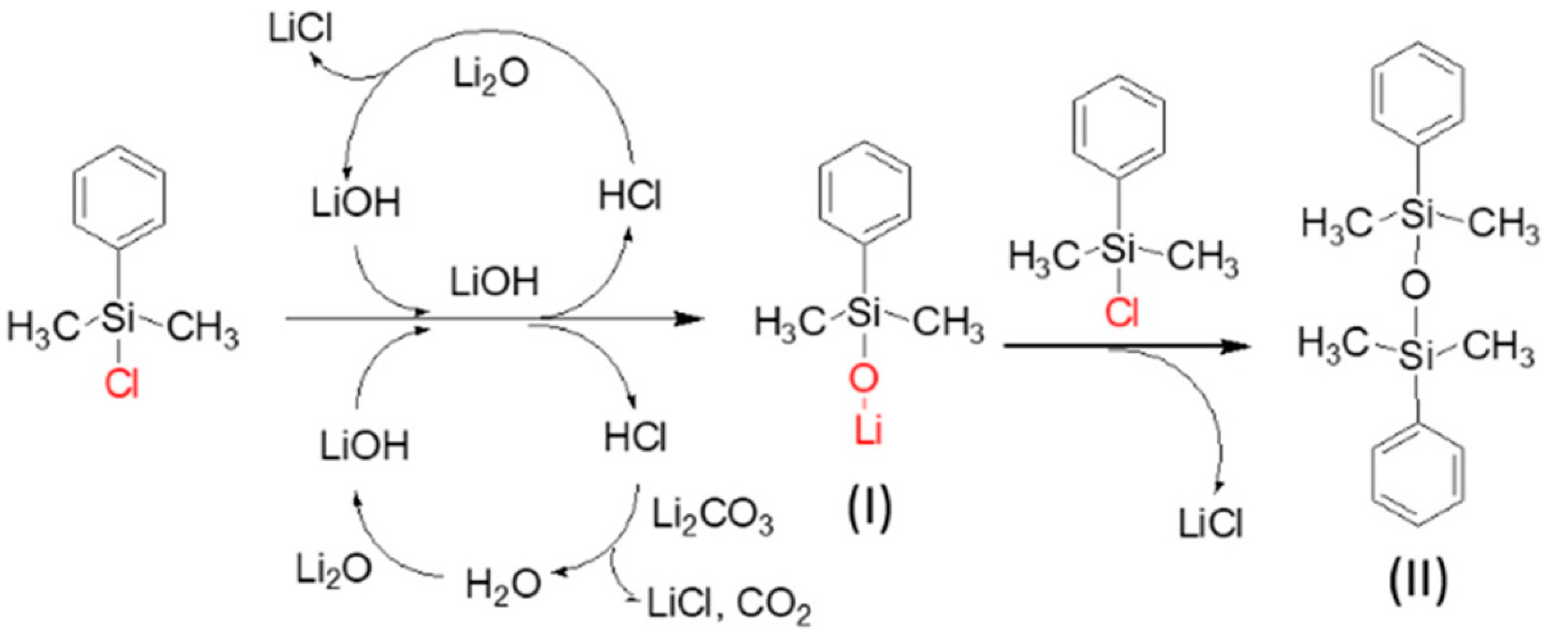
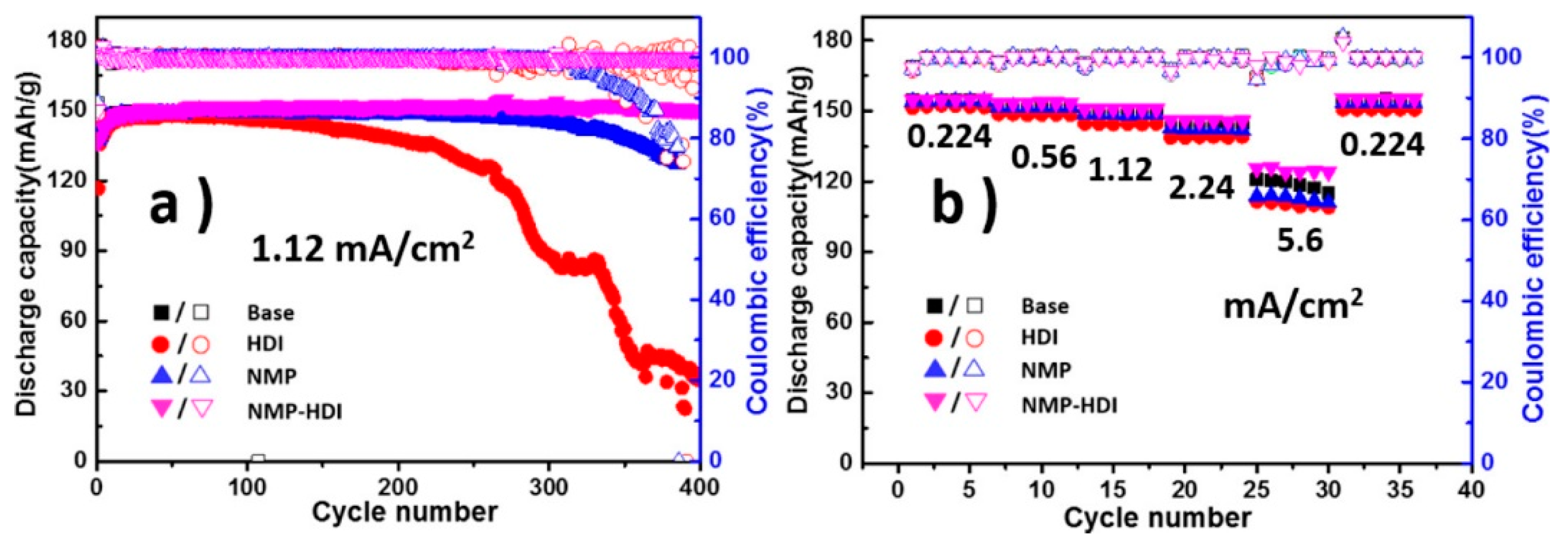


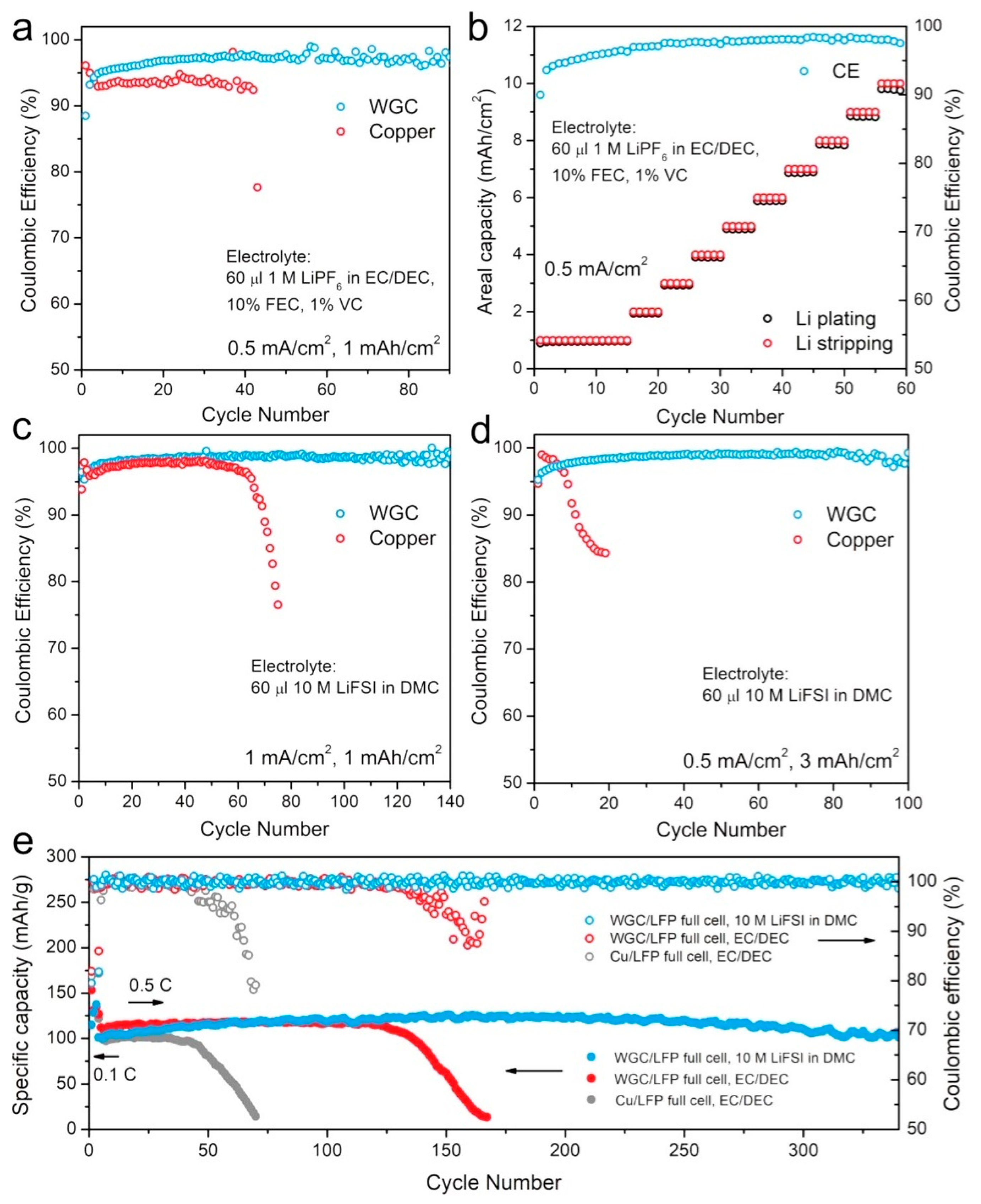

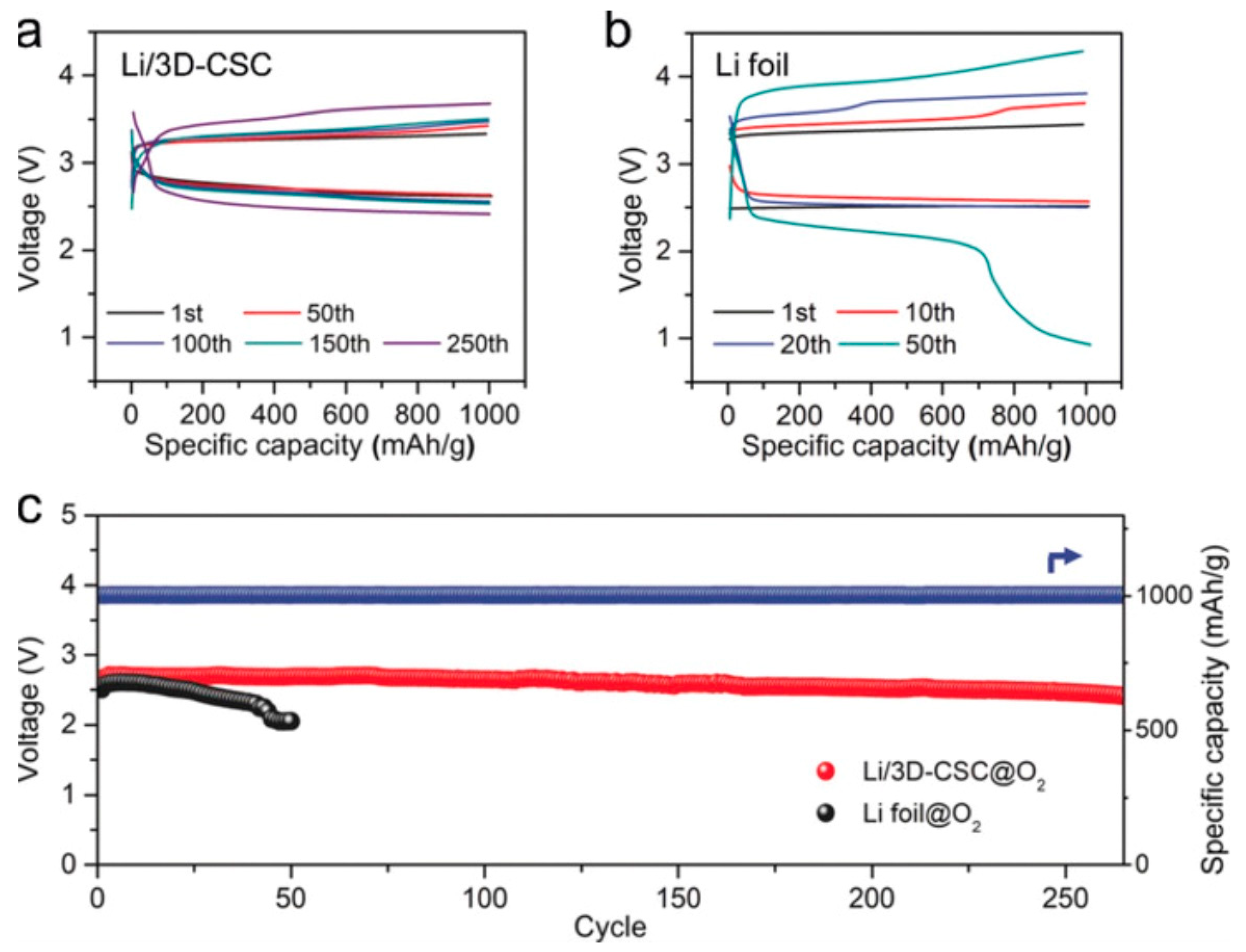

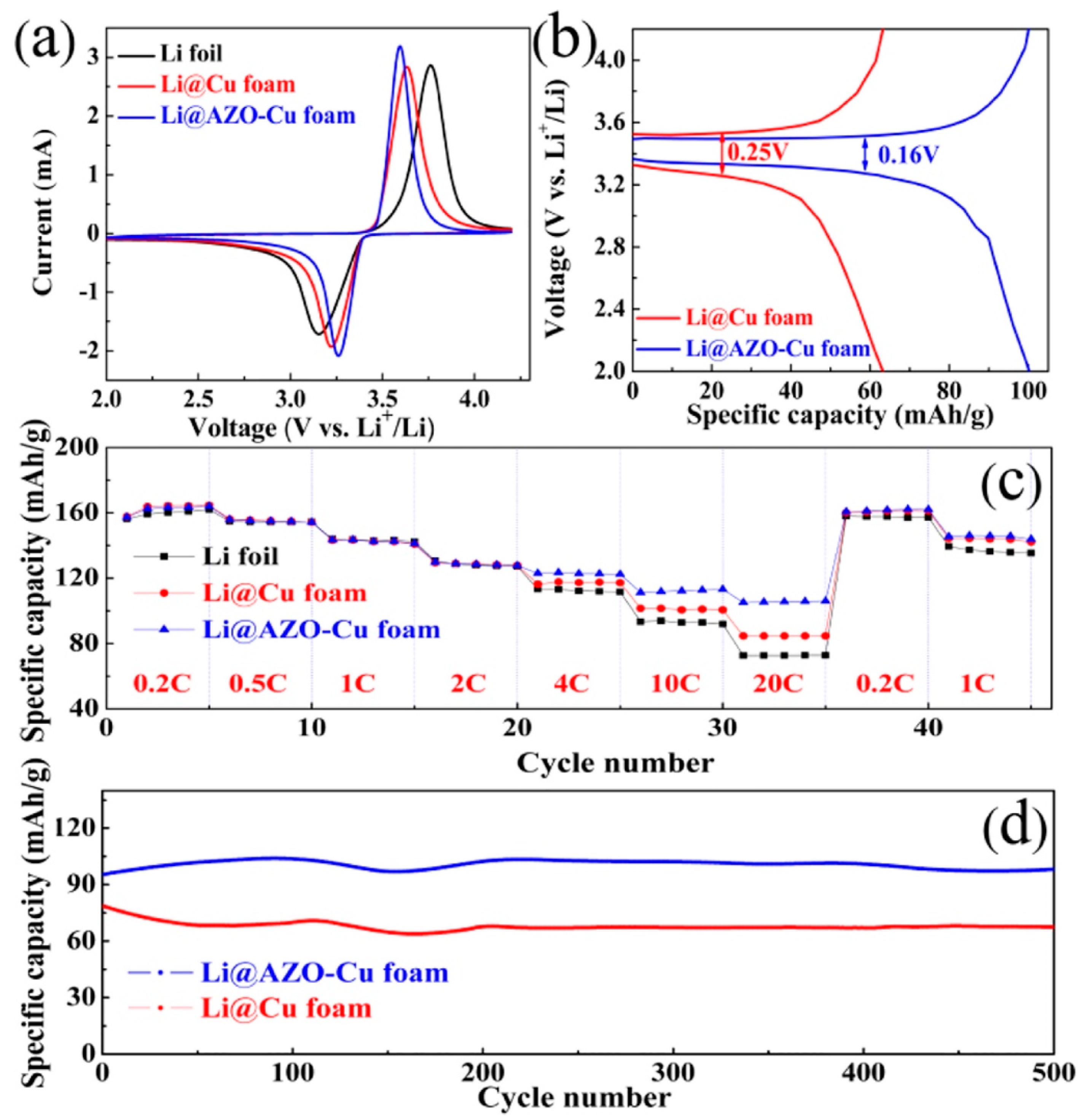
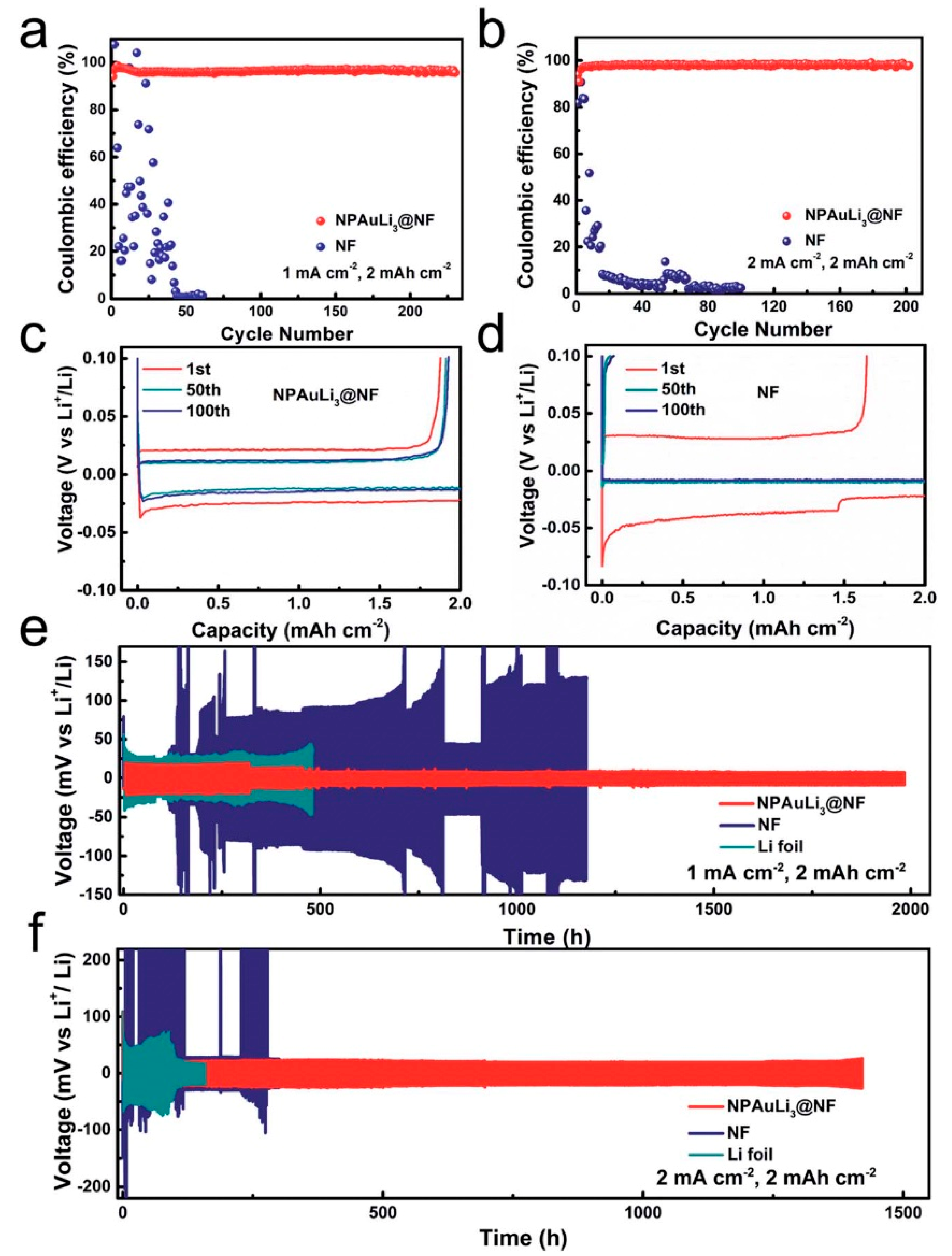
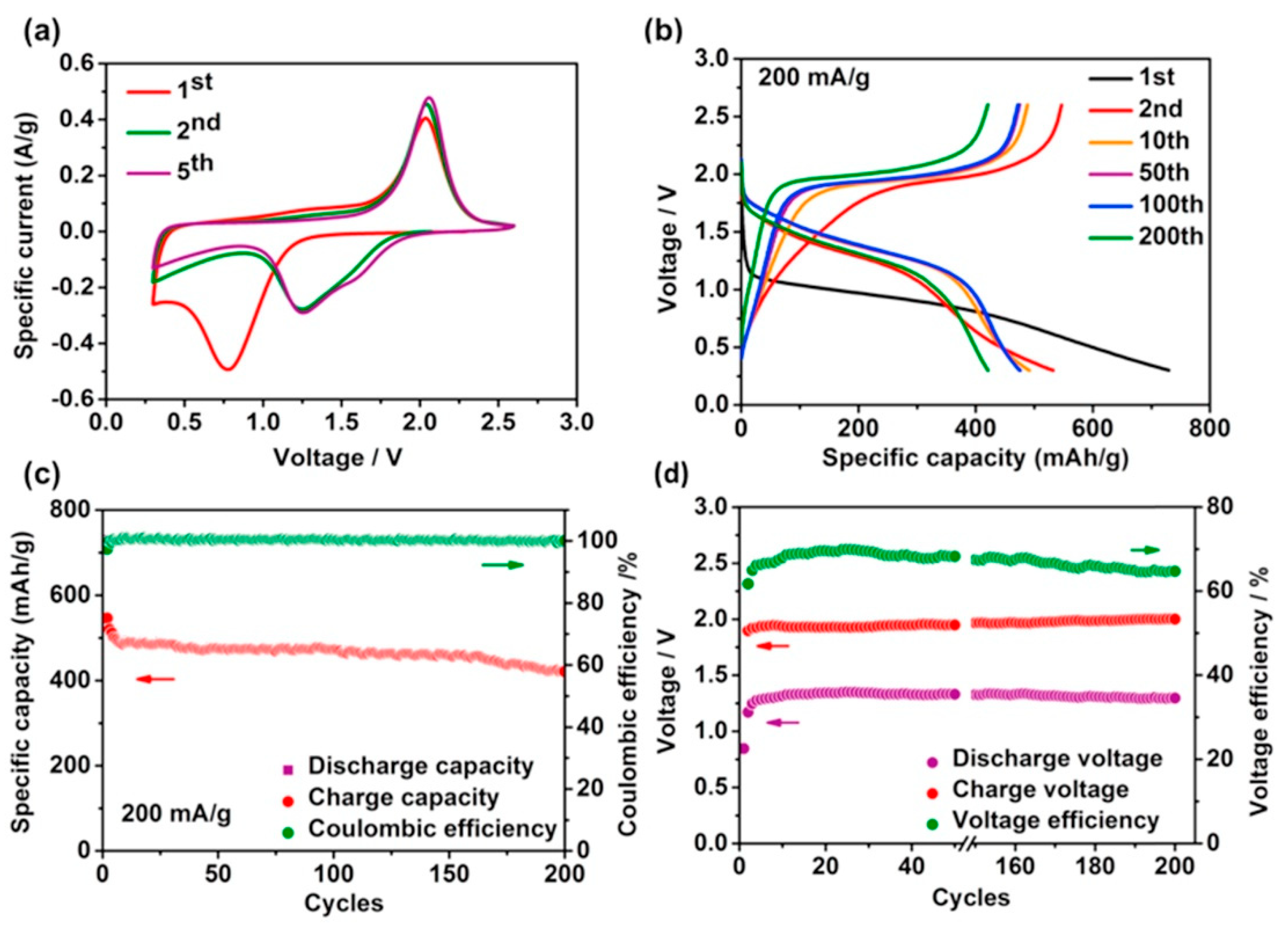
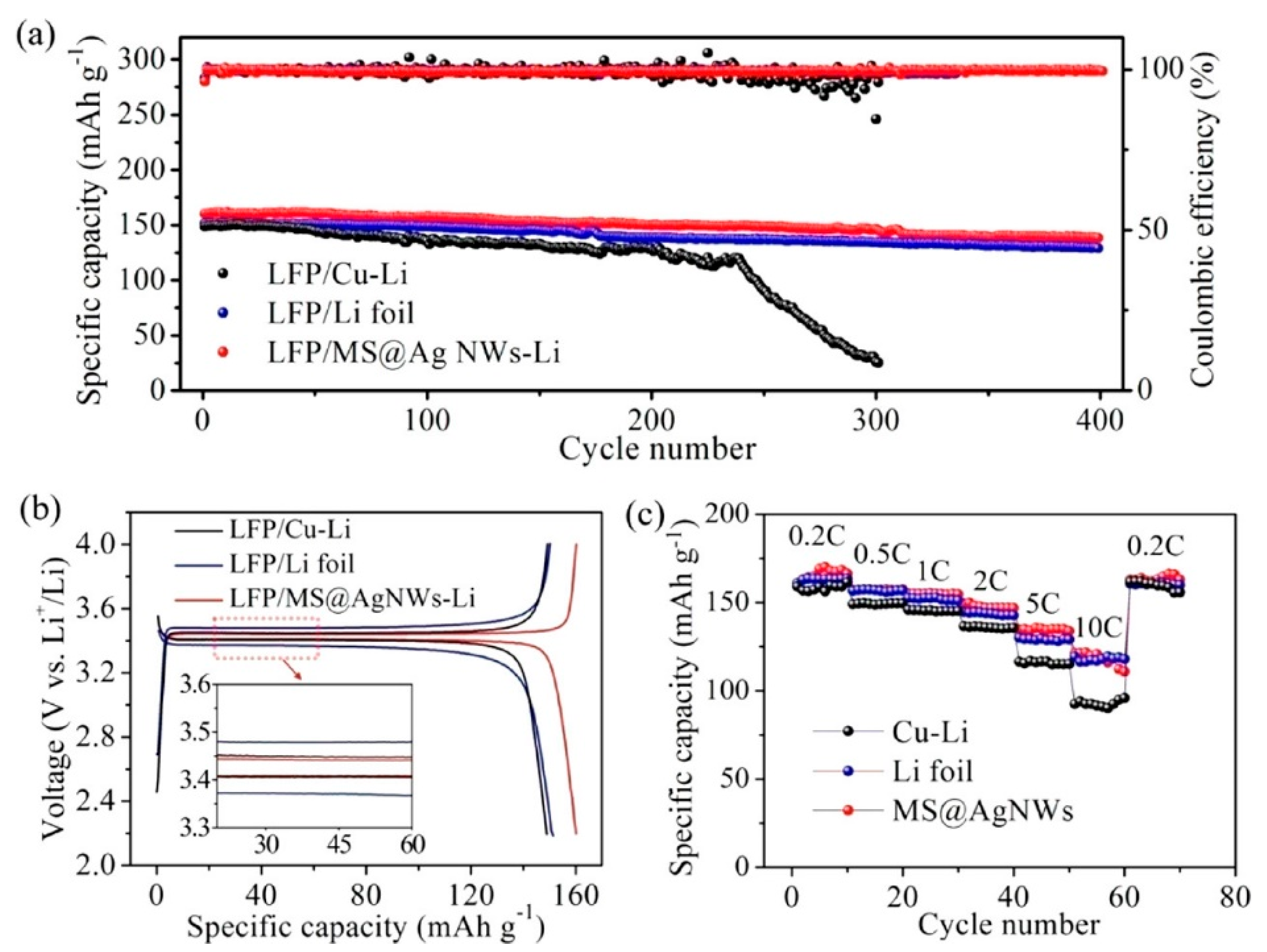

| System | Remedy | Current Density | Specific Capacity | CE (%) | Lifespan | Ref. |
|---|---|---|---|---|---|---|
| Li||LiFePO4 | Synergy of FEC and LiNO3 | 0.5 mA cm−2 | 3.3 mAh cm−2 | 99.96 | 1000 | [18] |
| Li||LiFePO4 | Function of FSI− anion | 3.0 mA cm−2 | 1.0 mAh cm−2 | 96 | 1000 | [32] |
| Li||NMC333 | Transient HCE layer | C/3, 1C | 1.5 mAh cm−2 | 99.5 | 500 | [39] |
| Li||NCM622 | Use of TTE as counter solvent | 2C | 140 mAh g−1 | 99.4 | 200 | [52] |
| Li||NCM | LiTFSI–LiBOB dual salt | 1.75 mA cm−2 | 1.75 mAh cm−2 | 99 | 500 | [62] |
| Li||Li | Solvated IL electrolyte | 5 mA cm−2 | 12 mAh cm−2 | 99.98 | - | [44] |
| Li||NCA | SEI-forming additive | 0.5C | 186 mAh g−1 | 99.5 | 300 | [74] |
| Li||NCM811 | HFE antisolvent additive | 0.5C | 200 mAh g−1 | 99 | 200 | [76] |
| Li||LiFePO4 | Acrylic-containing additive | 1C | 200 mAh g−1 | 99.5 | 300 | [80] |
| Li||NCM532 | Polymer-inorganic SEI | 2 mA cm−2 | 3.4 mAh cm−2 | 99.1 | 200 | [100] |
| System | Remedy | Current Density | Specific Capacity | CE (%) | Lifespan | Ref. |
|---|---|---|---|---|---|---|
| Li||Cu | SEI tailoring in diluted SIL | 5 mA cm−2 | 12 mAh cm−2 | 99.98 | 100 | [44] |
| Li||LiFePO4 | Zigzag-porous SiO2 layer | 2C | 95.1 mAh g−1 | >99.0 | 300 | [113] |
| Li||LiFePO4 | Li3PO4 coating | 0.5C | 150 mAh g−1 | - | 200 | [114] |
| Li||Li4Ti5O12 | Nanolayer deposited by ALD | 8C | 152 mAh g−1 | - | 100 | [124] |
| Li||NCM532 | Cu-based SEI with CuF2 | 0.5C | 50 mAh g−1 | 96.3 | 500 | [126] |
| Li||Cu | Garnet protection | 0.5 mA cm−2 | 0.5 mAh cm−2 | 97.9 | 220 | [131] |
| Li||LiFePO4 | Tetraethoxylane treated Li | 0.5C | 103 mAh g−1 | 98.6 | 500 | [137] |
| Li||S | Si-based surface modification | 6.69 mA cm−2 | 600 mAh g−1 | - | 100 | [138] |
| Li||NCM811 | Mo6S8/carbon composite | 1C | CR of 63% | 99.6 | 200 | [144] |
| Li||LiFePO4 | Polymeric protective film | 1C | 132.7 mAh g−1 | ~100 | 300 | [146] |
| Li||LiFePO4 | Poly(dimethylsiloxane) film | 1 mA cm−2 | 142 mAh g−1 | 99.5 | 50 | [155] |
| Li||LiFePO4 | PPE anionic polymerization | 0.5C | 151 mAh g−1 | 99.6 | 500 | [160] |
| Li||NCM | Ester polar groups interaction | 2.5 mA cm−2 | 121 mAh g−1 | - | 100 | [161] |
| System | Remedy | Current Density | Specific Capacity | CE (%) | Lifespan | Ref. |
|---|---|---|---|---|---|---|
| Li||Cu | Lithiophilic sites in graphene | 1 mA cm−2 | 1.0 mAh cm−2 | 98 | 200 | [178] |
| Li||Cu | N-doped carbon nanospheres | 1 mA cm−2 | 1.0 mAh cm−2 | 99.25 | 500 | [196] |
| Li||NGCF | N-doped graphitic carbon | 3 mA cm−2 | 10 mAh cm−2 | 99.6 | 300 | [206] |
| Ag-NCNS/Li | Doping with nanoparticles | 0.5 mA cm−2 | 1.0 mAh cm−2 | 98 | 200 | [208] |
| Li||LiFePO4 | Graphene skeleton | 0.2C | 138 mAh g−1 | 99.5 | 200 | [211] |
| Li-WGC||LiFePO4 | Wrinkled graphene cage | 0.5C | 100 mAh g−1 | 99.9 | 375 | [216] |
| Li-Gr||Li-Gr | CNF-stabilized Gr aerogel film | 2 mA cm−2 | 10 mAh cm−2 | >99.0 | 70 | [225] |
| Li||LiFePO4 | 3D SiO2/CNF composite skeleton | 1C | 117 mAh g−1 | 99.7 | 1000 | [228] |
| Li||Li | CNF coated by Li-Nafion layer | 1 mA cm−2 | 2.0 mAh cm−2 | 94.9 | 900 | [237] |
| Li||Cu | Flexible semi-tubular carbon film | 0.25 mA cm−2 | 1.0 mAh cm−2 | 99.5 | 180 | [242] |
| Li||Li | 3D interconnected CNTs on CC | 1 mA cm−2 | 1.0 mAh cm−2 | 99 | 500 | [245] |
| Li||Li | Covalently connected graphite | 10 mA cm−2 | 10 mAh cm−2 | ~97.0 | 100 | [246] |
| Li||LiFePO4 | Li–CNT–AB composite skeleton | 1.25 mA cm−2 | 130 mAh g−1 | 98.7 | 700 | [250] |
| Li||Li | MOF with Zn particles | 2 mA cm−2 | 1.0 mAh cm−2 | 99 | 200 | [258] |
| Li||LiFePO4 | Cu–Zn alloy skeleton | 0.5C | 136 mAh g−1 | 98.6 | 200 | [273] |
| Li||Li | Br-doped Gr film on Cu foam | 2 mA cm−2 | 2.0 mAh cm−2 | 98.8 | 300 | [286] |
| Li||LiFePO4 | AuLi3 sheet-modified Ni foam | 5C | 1.0 mAh cm−2 | 99.8 | 1000 | [290] |
| Li||Li | Porous LixSi–Li2O matrix | 1 mA cm−2 | 1.0 mAh cm−2 | - | 100 | [138] |
| Li||LiFePO4 | Entrapment in Li7B6 framework | 0.5C | 140 mAh g−1 | - | 200 | [298] |
| Li/CuNW||LFP | Cu NW phosphidation gradient | 0.5C | 140 mAh g−1 | 98.8 | 300 | [313] |
| Li||Li | Ti3C2/rGO aerogel scaffolds | 10 mA cm−2 | 1.0 mAh cm−2 | ~90.0 | 150 | [321] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauger, A.; Julien, C.M. Remedies to Avoid Failure Mechanisms of Lithium-Metal Anode in Li-Ion Batteries. Inorganics 2022, 10, 5. https://doi.org/10.3390/inorganics10010005
Mauger A, Julien CM. Remedies to Avoid Failure Mechanisms of Lithium-Metal Anode in Li-Ion Batteries. Inorganics. 2022; 10(1):5. https://doi.org/10.3390/inorganics10010005
Chicago/Turabian StyleMauger, Alain, and Christian M. Julien. 2022. "Remedies to Avoid Failure Mechanisms of Lithium-Metal Anode in Li-Ion Batteries" Inorganics 10, no. 1: 5. https://doi.org/10.3390/inorganics10010005
APA StyleMauger, A., & Julien, C. M. (2022). Remedies to Avoid Failure Mechanisms of Lithium-Metal Anode in Li-Ion Batteries. Inorganics, 10(1), 5. https://doi.org/10.3390/inorganics10010005







