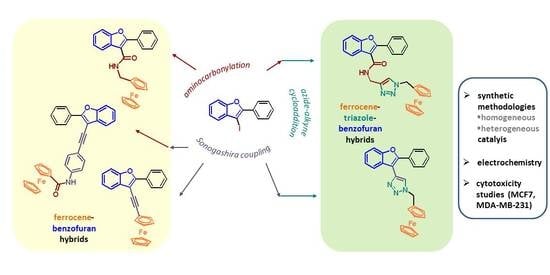
Synthesis of Novel Ferrocene-Benzofuran Hybrids via Palladium- and Copper-Catalyzed Reactions
Abstract
1. Introduction
2. Results and Discussion
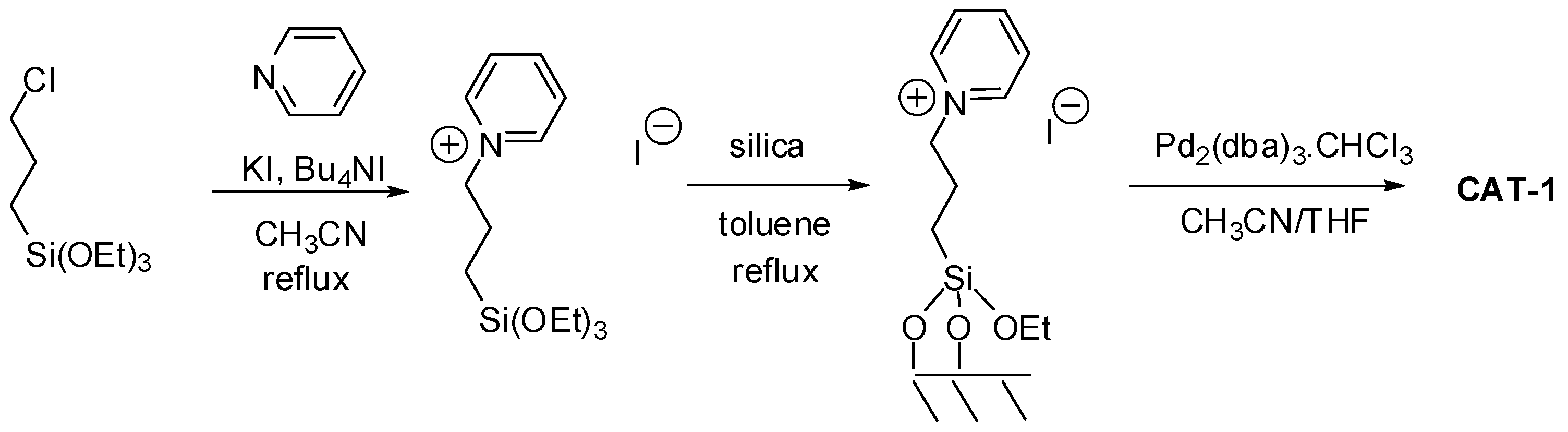
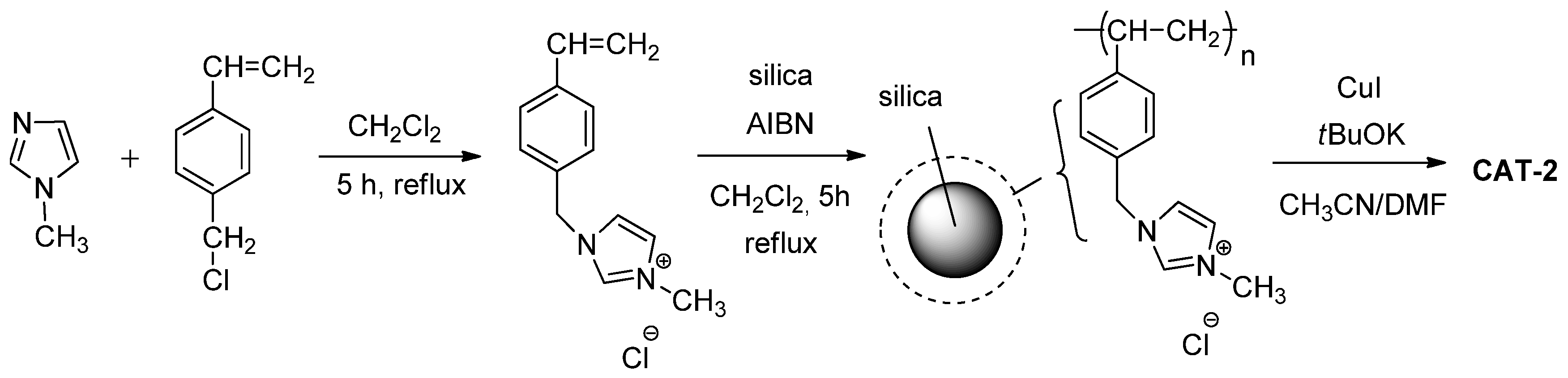
2.1. Preparation of the Heterogeneous Catalysts
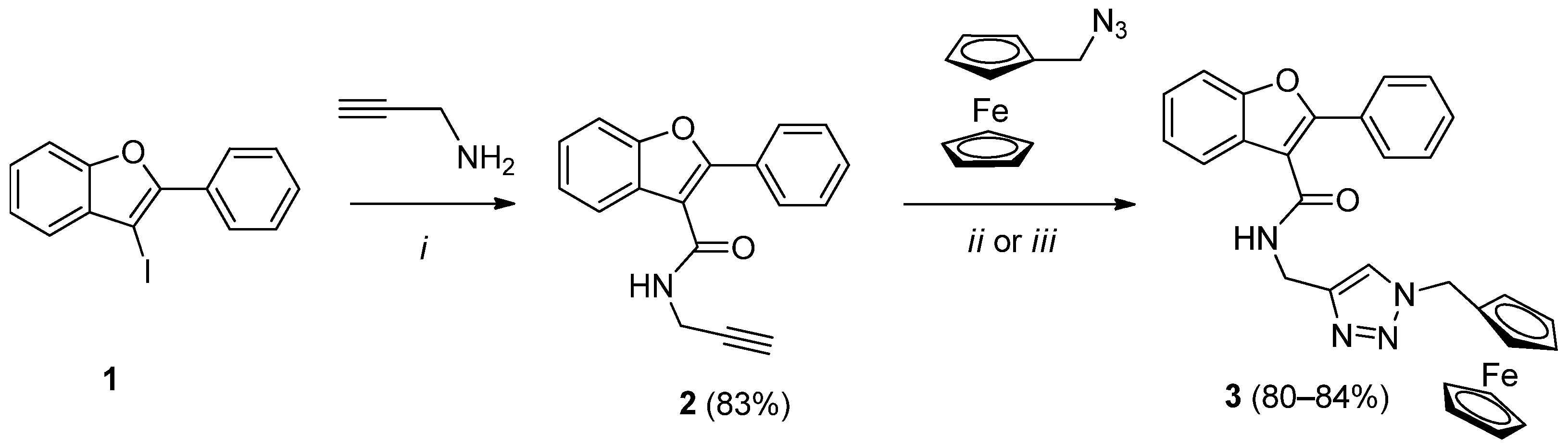
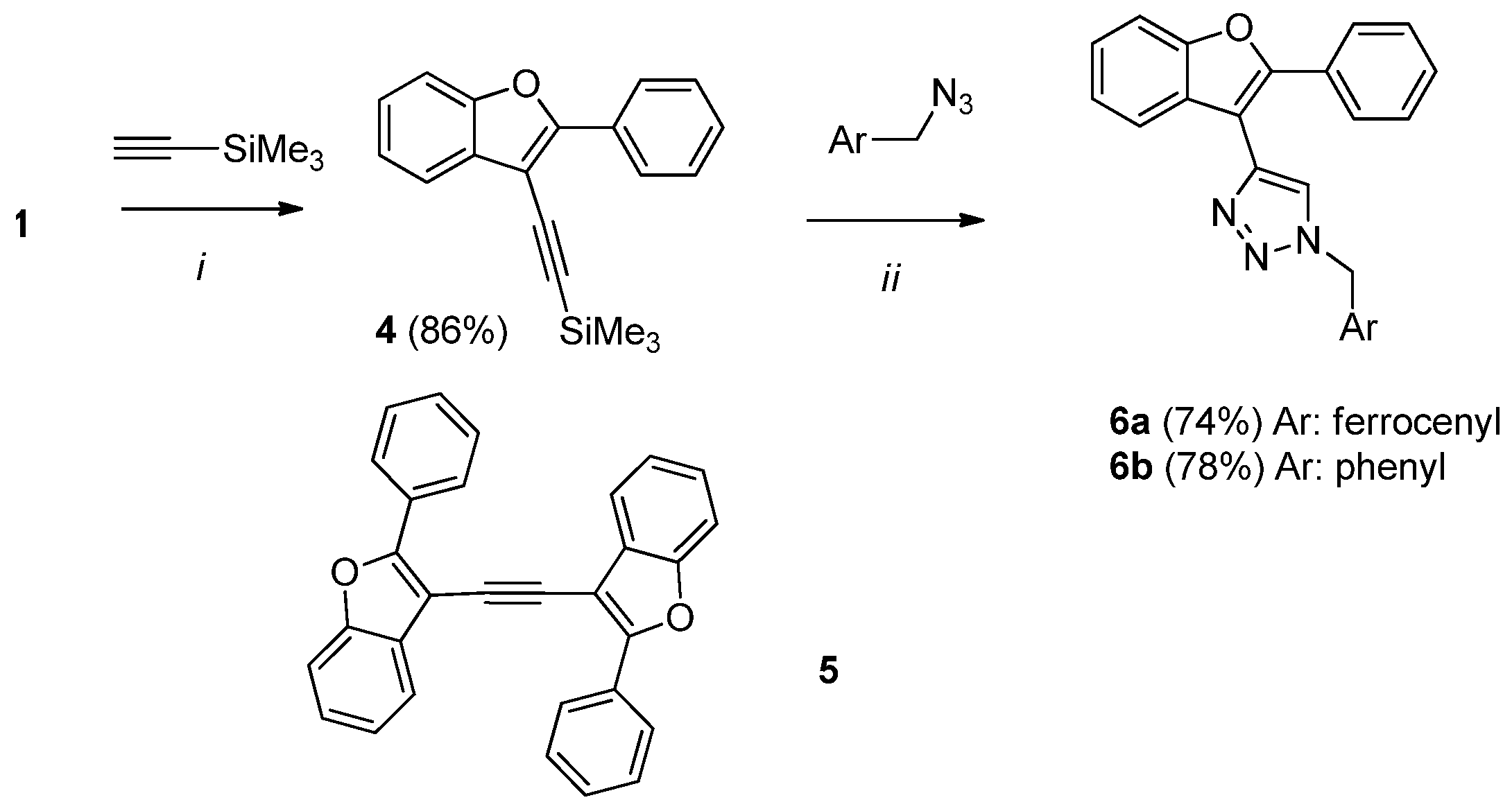
2.2. Synthesis of Ferrocene-Triazole-Benzofuran Hybrides
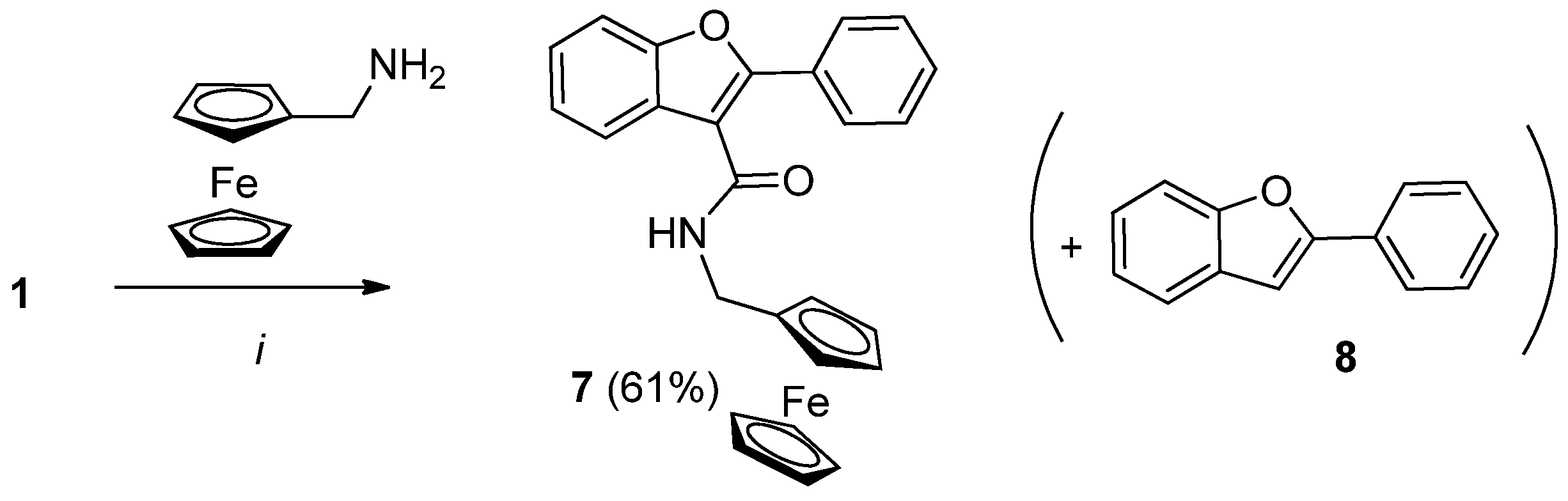
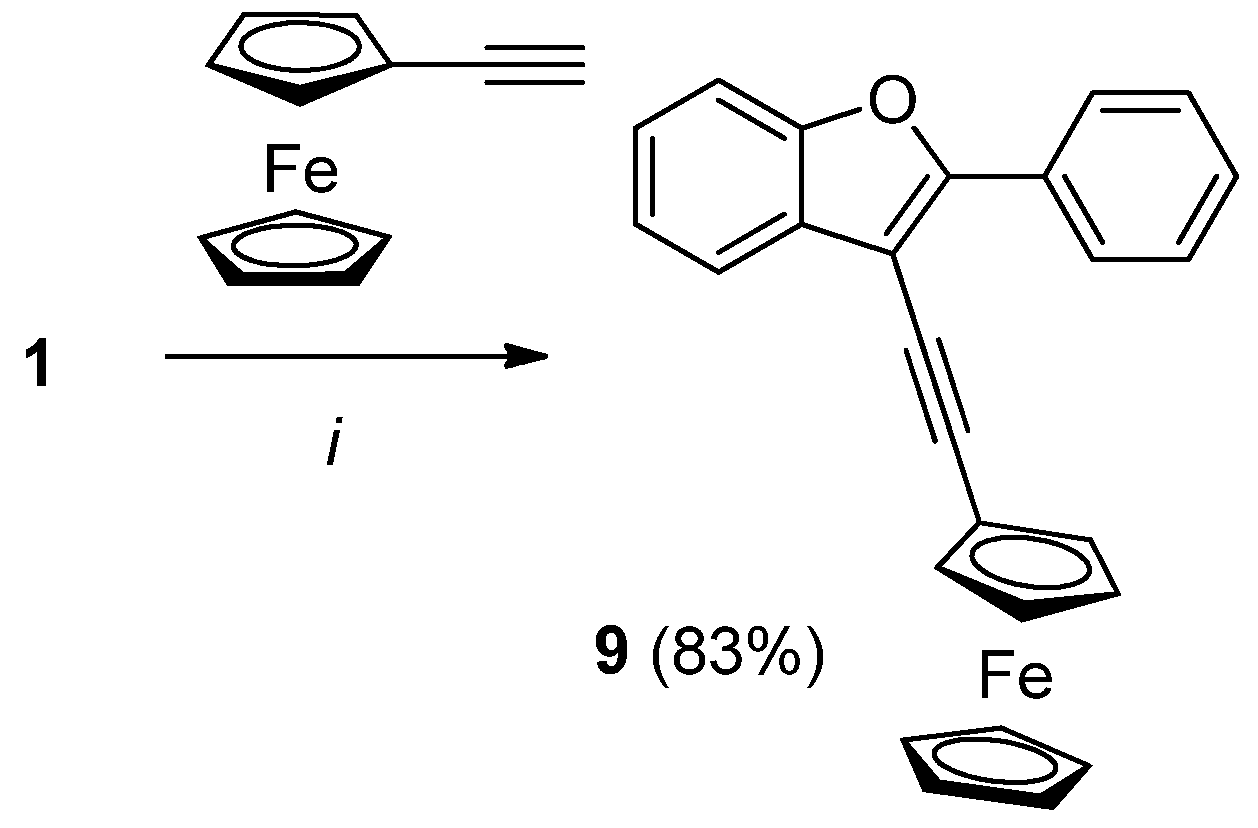
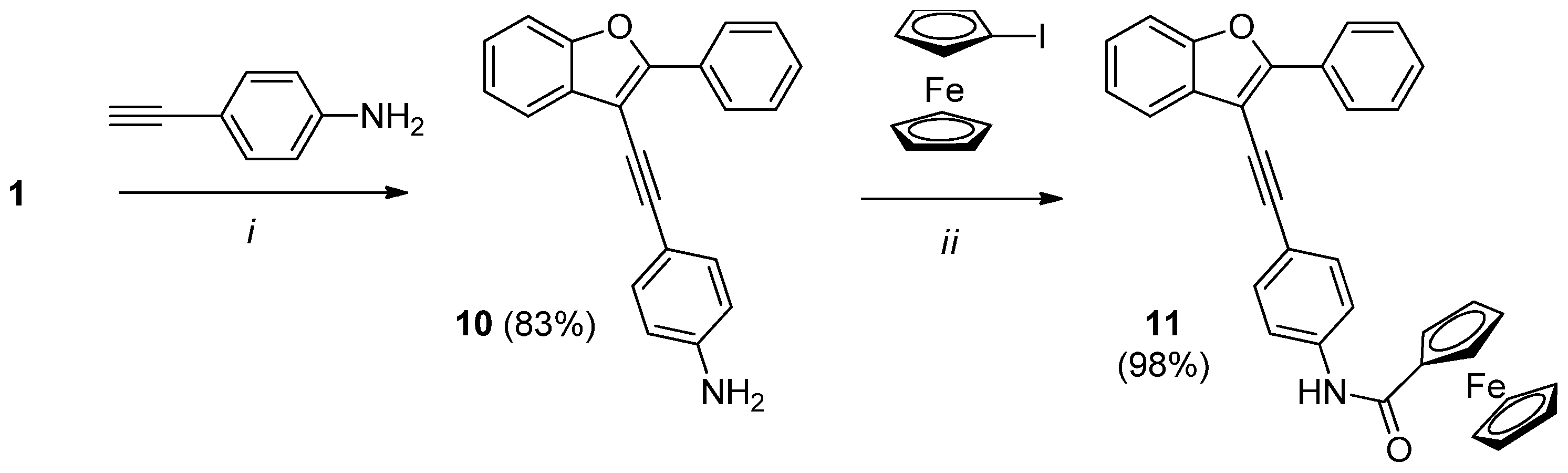
2.3. Synthesis of Ferrocene-Benzofuran Hybrides
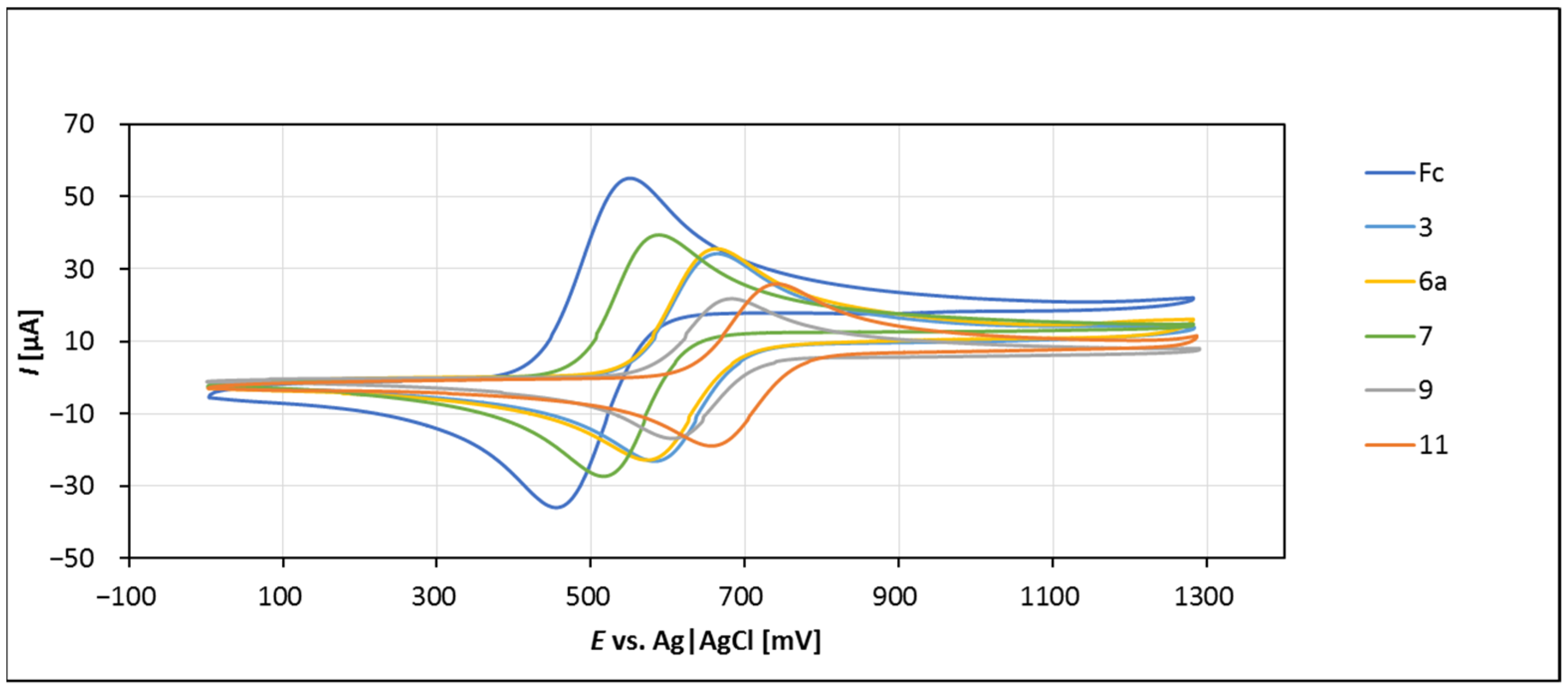
2.4. Electrochemistry
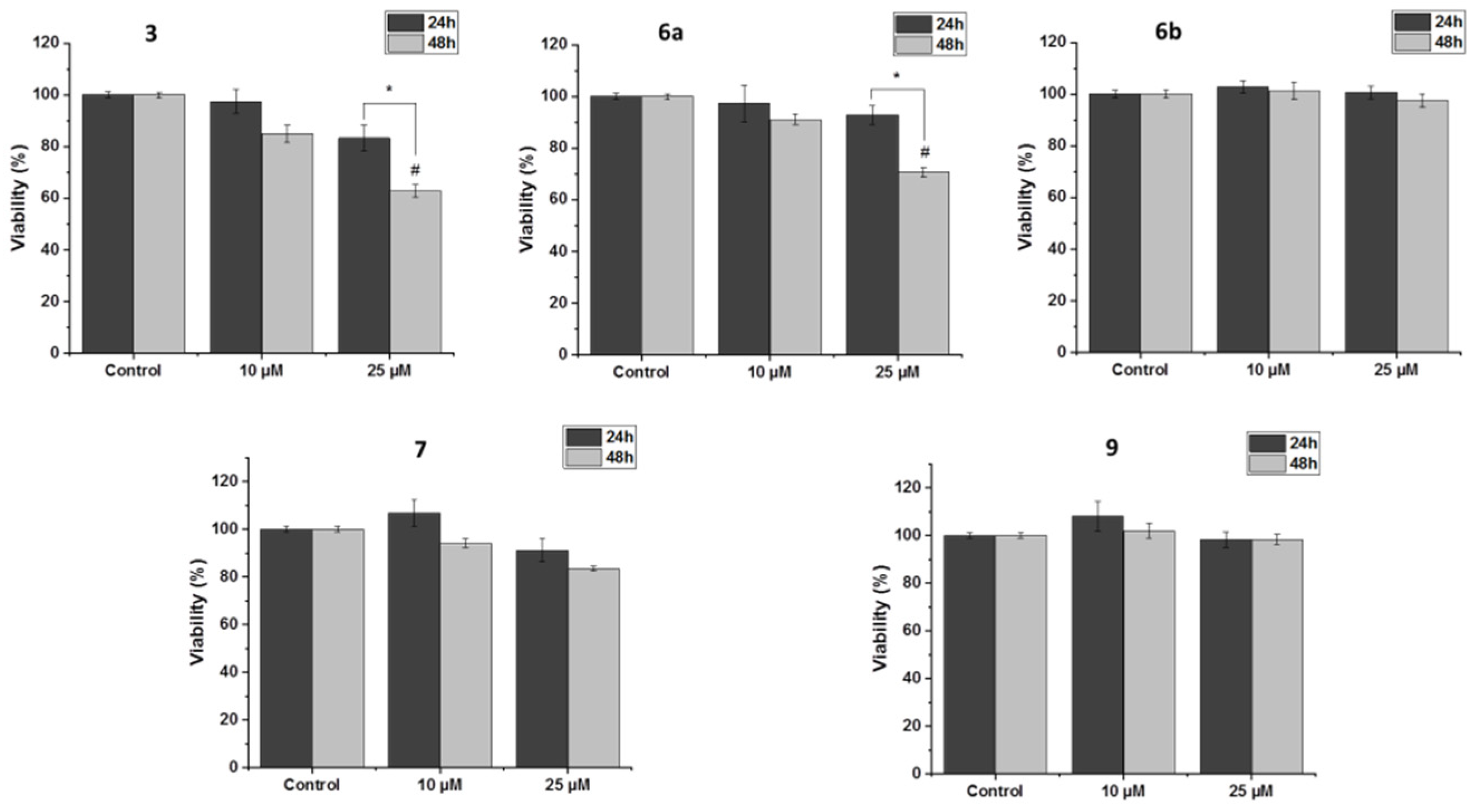
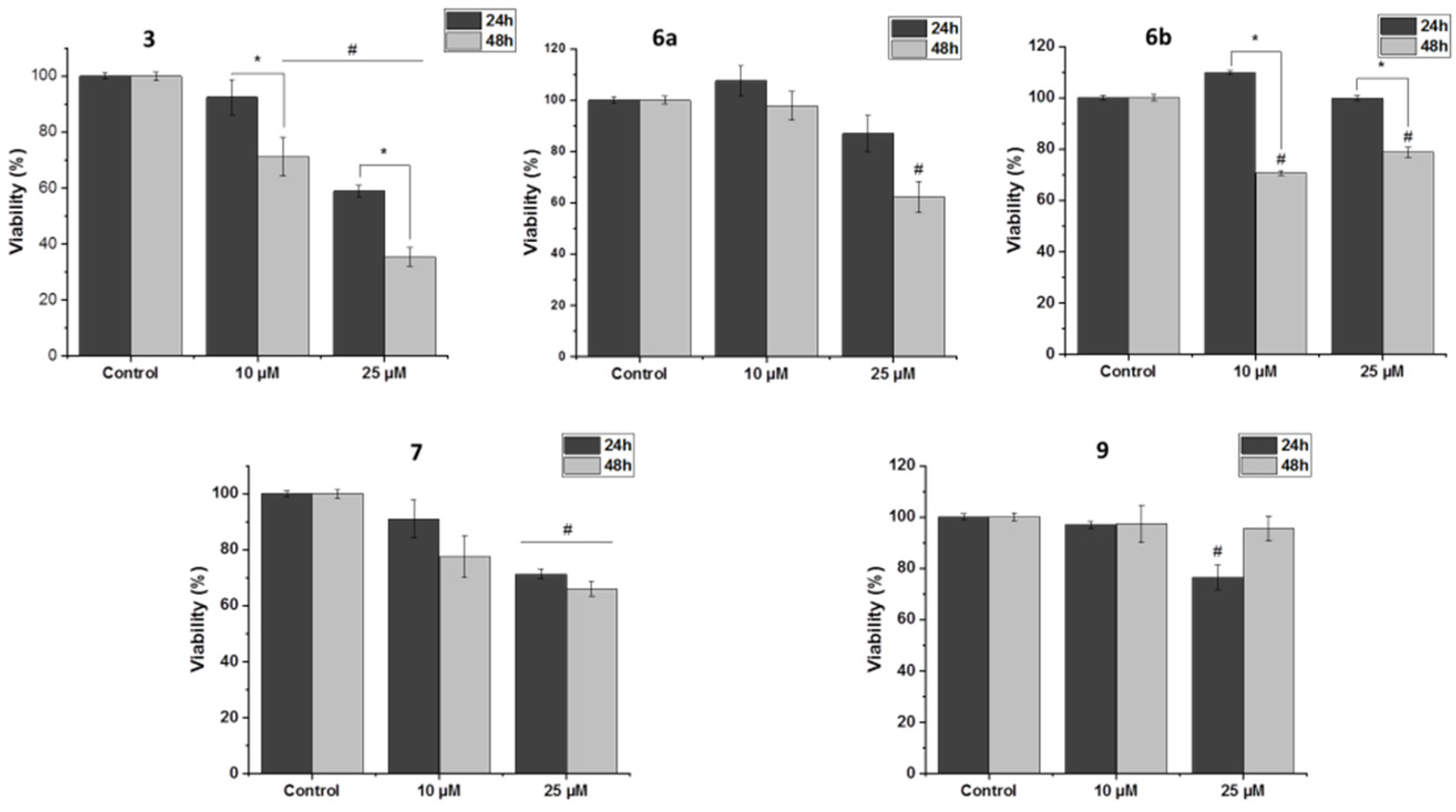
2.5. Biological Activity of Ferrocenyl Derivatives
3. Materials and Methods
3.1. General Information
3.2. Synthetic Procedures
3.2.1. Synthesis of 2-Phenyl-N-(prop-2-yn-1-yl)benzofuran-3-carboxamide (2)
3.2.2. Synthesis of N-((1-Ferrocenylmethyl-1H-1,2,3-triazol-4-yl)methyl)-2-phenylbenzofuran-3-carboxamide (3)
3.2.3. Synthesis of Trimethyl((2-Phenylbenzofuran-3-yl)ethynyl)silane (4)
3.2.4. Synthesis of 1-Ferrocenylmethyl-4-(2-phenylbenzofuran-3-yl)-1H-1,2,3-triazole (6a) and 1-Phenylmethyl-4-(2-phenylbenzofuran-3-yl)-1H-1,2,3-triazole (6b)
3.2.5. Synthesis of N-Ferrocenylmethyl-2-phenylbenzofuran-3-carboxamide (7)
3.2.6. Synthesis of 3-Ferrocenylethynyl-2-phenylbenzofuran (9)
3.2.7. Synthesis of N-(4-((2-Phenylbenzofuran-3-yl)ethynyl)phenyl)ferrocenecarboxamide (11)
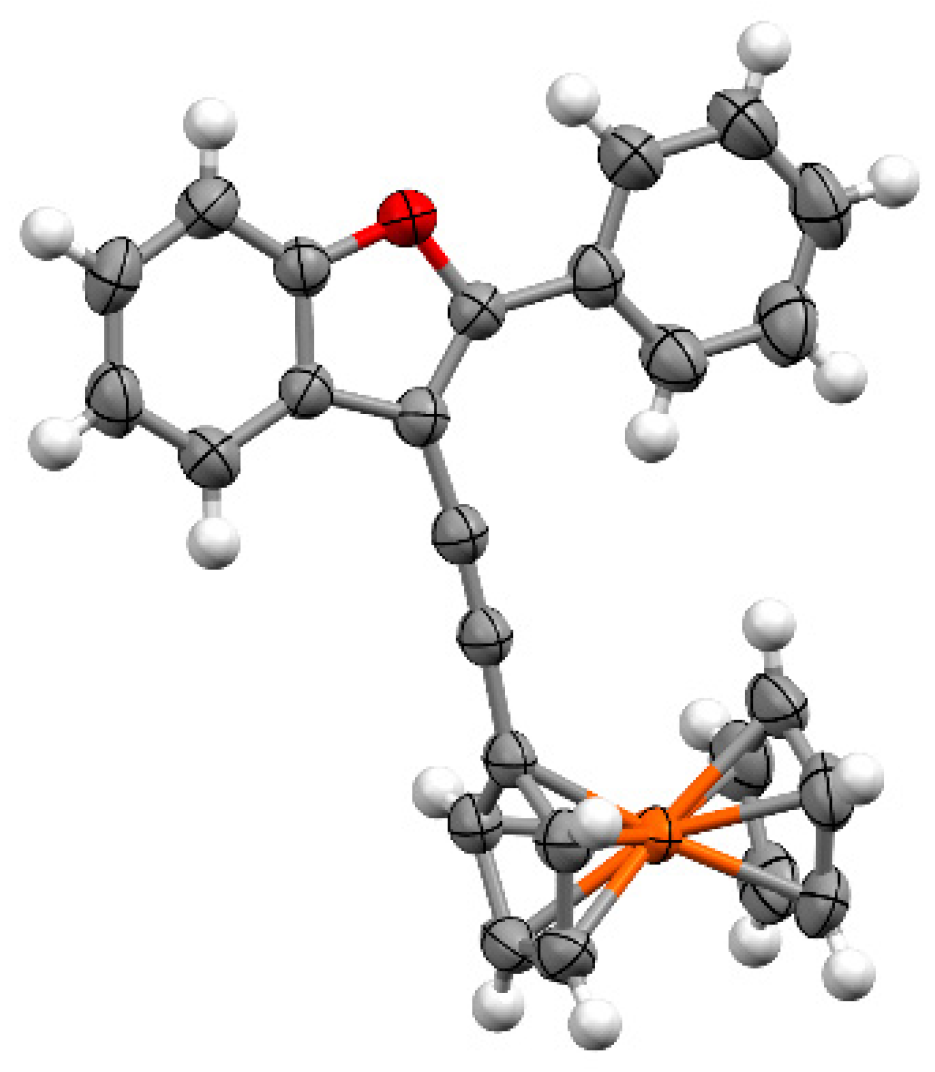
3.3. Crystallography
3.4. Electrochemical Measurements
3.5. Biological Activity
3.5.1. Cell Cultures
3.5.2. Survival Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chand, K.; Rajeshwari; Hiremathad, A.; Singh, M.; Santos, M.A.; Keri, R.S. A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives. Pharmacol. Rep. 2017, 69, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, S.; Lv, Z.; Feng, L.; Wang, Y.; Zhang, F.; Bai, L.; Deng, J. Benzofuran derivatives and their anti-tubercular, anti-bacterial activities. Eur. J. Med. Chem. 2019, 162, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, D.; Zhou, W.; Zhang, X. Therapeutic Potential of Naturally Occurring Benzofuran Derivatives and Hybrids of Benzofurans with other Pharmacophores as Antibacterial Agents. Curr. Top. Med. Chem. 2022, 22, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Jalal, M.; Khodaei Hamed, A.S.; Kheirouri, S.; Tabrizi, F.P.F.; Kamari, N. Recent updates on anti-inflammatory and antimicrobial effects of furan natural derivatives. J. Inflamm. Res. 2020, 13, 451–463. [Google Scholar] [CrossRef]
- Farhat, J.; Alzyoud, L.; Alwahsh, M.; Al-Omari, B. Structure–activity relationship of benzofuran derivatives with potential anticancer activity. Cancers 2022, 14, 2196. [Google Scholar] [CrossRef]
- Cabrera-Pardo, J.R.; Fuentealba, J.; Gavilán, J.; Cajas, D.; Becerra, J.; Napiórkowska, M. Exploring the multi–target neuroprotective chemical space of benzofuran scaffolds: A new strategy in drug development for Alzheimer’s disease. Front. Pharmacol. 2020, 10, 1679. [Google Scholar] [CrossRef]
- Szoke-Kovacs, Z.; More, C.; Szoke-Kovacs, R.; Mathe, E.; Frecska, E. Selective inhibition of the serotonin transporter in the treatment of depression: Sertraline, fluoxetine and citalopram. Neuropsychopharmacol. Hung. 2020, 22, 4–15. [Google Scholar]
- Plenge, P.; Yang, D.; Salomon, K.; Laursen, L.; Kalenderoglou, I.E.; Newman, A.H.; Gouaux, E.; Coleman, J.A.; Loland, C.J. The antidepressant drug vilazodone is an allosteric inhibitor of the serotonin transporter. Nat. Commun. 2021, 12, 34417466. [Google Scholar] [CrossRef]
- Vamos, M.; Hohnloser, S.H. Amiodarone and dronedarone: An update. Trends Cardiovasc. Med. 2016, 26, 597–602. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, X.; Jing, L.; Wu, g.; Kang, D.; Liu, X.; Zhan, P. Recent applications of click chemistry in drug discovery. Expert Opin. Drug. Discov. 2019, 14, 779–789. [Google Scholar] [CrossRef]
- Kaur, J.; Saxena, M.; Rishi, N. An overview of recent advances in biomedical applications of click chemistry. Bioconjugate Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Gondru, R.; Kanugala, S.; Raj, S.; Kumar, C.G.; Pasupuleti, M.; Banothu, J.; Bavantula, R. 1,2,3-triazole-thiazole hybrids: Synthesis, in vitro antimicrobial activity and antibiofilm studies. Bioorg. Med. Chem. Lett. 2021, 33, 127746. [Google Scholar] [CrossRef] [PubMed]
- Pokhodylo, N.; Shyyka, O.; Matiychuk, V. Synthesis of 1,2,3-triazole derivatives and evaluation of their anticancer activity. Sci. Pharm. 2013, 81, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Laamari, Y.; Oubella, A.; Bimoussa, A.; El Mansouri, A.E.; Ketatni, E.M.; Mentre, O.; Auhmani, A. Design, hemiysnthesis, crystal structure and anticancer activity of 1, 2, 3-triazoles derivatives of totarol. Bioorg. Chem. 2021, 115, 105165. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Y.; Gao, S.; Dong, X.; Xiao, T.; Jiang, Y. Palladium-catalyzed selective ortho C–H alkoxylation at 4-aryl of 1, 4-disubstituted 1, 2, 3-triazoles. Tetrahedron 2020, 76, 130985. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, D.; Agarwal, D.; Gupta, R.D.; Tilak, R.; Awasthi, S.K.; Agarwal, A. Synthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2016, 113, 34–49. [Google Scholar] [CrossRef]
- Sun, L.; Huang, T.; Dick, A.; Meuser, M.E.; Zalloum, W.A.; Chen, C.H.; Liu, X. Design, synthesis and structure-activity relationships of 4-phenyl-1H-1, 2, 3-triazole phenylalanine derivatives as novel HIV-1 capsid inhibitors with promising antiviral activities. Eur. J. Med. Chem. 2020, 190, 112085. [Google Scholar] [CrossRef]
- Battigelli, A.; Almeida, B.; Shukla, A. Recent Advances in Bioorthogonal Click Chemistry for Biomedical Applications. Bioconjugate Chem. 2022, 33, 263–271. [Google Scholar] [CrossRef]
- Sodhi, R.K.; Paul, S. Metal Complexes in medicine: An overview and update from drug design perspective. Cancer Ther. Oncol. Int. J. 2019, 14, 25–32. [Google Scholar] [CrossRef]
- Chellan, P.; Sadler, P.J. Enhancing the Activity of drugs by conjugation to organometallic fragments. Chem. Eur. J. 2020, 26, 8676–8688. [Google Scholar] [CrossRef]
- Yousuf, I.; Bashir, M.; Arjmand, F.; Tabassum, S. Advancement of metal compounds as therapeutic and diagnostic metallodrugs: Current frontiers and future perspectives. Coord. Chem. Rev. 2021, 445, 214104. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef]
- Top, S.; Tang, J.; Vessieres, A.; Carrez, D.; Provot, C.; Jaouen, G. Ferrocenyl hydroxytamoxifen: A prototype for a new range of oestradiol receptor site-directed cytotoxics. Chem. Commun. 1996, 8, 955–956. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Rückle, T.; Djeriou, E.; Cantalloube, C.; Ter-Minassian, D.; Baker, M.; O’Rourke, P.; Griffin, P.; Marquart, L.; van Huijsduijnen, R.H.; et al. A Phase II pilot trial to evaluate safety and efficacy of ferroquine against early Plasmodium falciparum in an induced blood-stage malaria infection study. Malar. J. 2016, 15, 469. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.C.; Pilli, R.A.; Vargas, M.D.; Violante, F.A.; Garden, S.J.; Pinto, A.C. Synthesis of 1-ferrocenyl-2-aryl (heteroaryl) acetylenes and 2-ferrocenylindole derivatives via the Sonogashira–Heck–Cassar reaction. Tetrahedron 2002, 58, 4487–4492. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.Q. Modification by ferrocene: An approach to enhance antioxidant ability of ailanthoidol to protect DNA. Biochimie 2012, 94, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Franz, K.J. Application of inorganic chemistry for non-cancer therapeutics. Dalton Trans. 2012, 41, 6333–6334. [Google Scholar] [CrossRef]
- Zhuoma, B.; Li, Y.; Gao, W. First synthesis of 2-ferrocenoyl-3-methylbenzofuran derivatives. J. Chem. Res. 2015, 39, 82–85. [Google Scholar] [CrossRef]
- Li, Y.; Zhuoma, B.; Gao, W. A Novel synthesis of 2-ferrocenoyl-substituted iodobenzofurans. J. Heterocycl. Chem. 2017, 54, 764–768. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, H.; Tian, Y.; Guo, M.; Su, X.; Guo, C. Design, synthesis and antifungal activity of novel benzofuran-triazole hybrids. Molecules 2016, 21, 732. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, V.; Kishore Kumar, A.; Shankar, B.; Anil Kumar, A.; Krishna, T.M.; Lincoln, C.A.; Pochampalli, J. Synthesis and antimicrobial activity of some novel benzofuran based 1, 2, 3-triazoles. Russ. J. Gen. Chem. 2017, 87, 322–330. [Google Scholar] [CrossRef]
- Qi, Z.Y.; Hao, S.Y.; Tian, H.Z.; Bian, H.L.; Hui, L.; Chen, S.W. Synthesis and biological evaluation of 1-(benzofuran-3-yl)-4-(3, 4, 5-trimethoxyphenyl)-1H-1,2,3-triazole derivatives as tubulin polymerization inhibitors. Bioorg. Chem. 2020, 94, 103392. [Google Scholar] [CrossRef] [PubMed]
- Urbán, B.; Papp, M.; Skoda-Földes, R. Carbonylation of aryl halides in the presence of heterogeneous catalysts. Curr. Green Chem. 2019, 6, 78–95. [Google Scholar] [CrossRef]
- McCarthy, S.; Braddock, D.C.; Wilton-Ely, J.D.E.T. Strategies for sustainable palladium catalysis. Coord. Chem. Rev. 2021, 442, 213925. [Google Scholar] [CrossRef]
- Mandoli, A. Recent advances in recoverable systems for the copper-catalyzed azide-alkyne cycloaddition reaction (CuAAC). Molecules 2016, 21, 1174. [Google Scholar] [CrossRef]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, homogeneous and heterogeneous catalysts, and novel developments and applications. Macromol. Rapid Commun. 2020, 41, 1900359. [Google Scholar] [CrossRef]
- López, O.; Padrón, J.M. Iridium- and palladium-based catalysts in the pharmaceutical industry. Catalysts 2022, 12, 164. [Google Scholar] [CrossRef]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery: Review. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Gómez, C.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Solid-supported palladium catalysts in Sonogashira reactions: Recent developments. Catalysts 2018, 8, 202. [Google Scholar] [CrossRef]
- Adamcsik, B.; Nagy, E.; Urbán, B.; Szabó, P.; Skoda-Földes, R. Palladium nanoparticles on a pyridinium supported ionic liquid phase: A recyclable and low-leaching palladium catalyst for aminocarbonylation reactions. RSC Adv. 2020, 10, 23988–23998. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Nagymihály, Z.; Kollár, L.; Fonyó, M.; Skoda-Földes, R. Synthesis of 3-aryl- and 3-alkynyl benzofurans in the presence of a supported palladium catalyst. Synthesis 2022. [Google Scholar] [CrossRef]
- Fehér, K.; Nagy, E.; Szabó, P.; Juzsakova, T.; Srankó, D.; Gömöry, Á.; Kollár, L.; Skoda-Földes, R. Heterogeneous azide–alkyne cycloaddition in the presence of a copper catalyst supported on an ionic liquid polymer/silica hybrid material. Appl. Organomet. Chem. 2018, 32, e4343. [Google Scholar] [CrossRef]
- Fehér, K.; Balogh, J.; Csók, Z.; Kégl, T.; Kollár, L.; Skoda-Földes, R. Synthesis of ferrocene-labeled steroids via copper-catalyzed azide–alkyne cycloaddition. Reactivity difference between 2β-, 6β- and 16β-azido-androstanes. Steroids 2012, 77, 738–744. [Google Scholar] [CrossRef]
- Lasányi, D.; Mészáros, Á.; Novák, Z.; Tolnai, G.L. Catalytic Activation of trimethylsilylacetylenes: A one-pot route to unsymmetrical acetylenes and heterocycles. J. Org. Chem. 2018, 83, 8281–8291. [Google Scholar] [CrossRef]
- Shultz, D.A.; Gwaltney, K.P.; Lee, H. A modified procedure for Sonogashira couplings: Synthesis and characterization of a bisporphyrin, 1,1-bis[zinc(II)5′-ethynyl-10′,15′,20′-trimesitylporphyrinyl]methylenecyclohexane. J. Org. Chem. 1998, 63, 4034–4038. [Google Scholar] [CrossRef]
- Friscourt, F.; Boons, G.J. One-pot three-step synthesis of 1, 2, 3-triazoles by copper-catalyzed cycloaddition of azides with alkynes formed by a Sonogashira cross-coupling and desilylation. Org. Lett. 2010, 12, 4936–4939. [Google Scholar] [CrossRef]
- Cuevas, F.; Oliva, A.I.; Pericas, M.A. Direct copper (I)-catalyzed cycloaddition of organic azides with TMS-protected alkynes. Synlett 2010, 2010, 1873–1877. [Google Scholar] [CrossRef]
- Stefani, H.A.; Amaral, M.F.; Manarin, F.; Ando, R.A.; Silva, N.C.; Juaristi, E. Functionalization of 2-(S)-isopropyl-5-iodo-pyrimidin-4-ones through Cu (I)-mediated 1, 3-dipolar azide–alkyne cycloadditions. Tetrahedron Lett. 2011, 52, 6883–6886. [Google Scholar] [CrossRef]
- Carmona-Negrón, J.A.; Santana, A.; Rheingold, A.L.; Meléndez, E. Synthesis, structure, docking and cytotoxic studies of ferrocene–hormone conjugates for hormone dependent breast cancer application. Dalton Trans. 2019, 48, 5952–5964. [Google Scholar] [CrossRef]
- Casas-Solvas, J.M.; Vargas-Berenguel, A.; Capitán-Vallvey, L.F.; Santoyo-González, F. Convenient methods for the synthesis of ferrocene–carbohydrate conjugates. Org. Lett. 2004, 6, 3687–3690. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, X.; He, Z.; Jin, Y.; Gong, L.; Mi, A. Reduction of azides to amines or amides with zinc and ammonium chloride as reducing agent. Synth. Commun. 2002, 32, 3279–3284. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis PRO; Version 1.171.40.67a; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2019. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B.J. ShelXle: A Qt graphical user interface for SHELXL. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
| Entry | Catalyst | Solvent | Base | Temp. (°C) | R.Time (h) | Conv. (%) c | Ratio of 4/5 c |
|---|---|---|---|---|---|---|---|
| 1 a | CAT-1 | DMF | Et3N | 80 | 2 | 5 | 5/0 |
| 2 a | CAT-1 | DMF | Et3N | 100 | 2 | 7 | 7/0 |
| 3 a | CAT-1 | DMF | KOAc | 80 | 2 | 20 | 4/16 |
| 4 a | CAT-1 | DMF | KOAc | 100 | 2 | 100 | 0/100 |
| 5 b | CAT-1 | Et3N | Et3N | rt | 2 | 20 | 20/0 |
| 6 a | CAT-1 | Et3N | Et3N | rt | 24 | 60 | 60/0 |
| 7 a | PdCl2(PPh3)2 | Et3N | Et3N | rt | 2 | 100 | 100/0 |
| Compound | D (cm2 s−1) × 106 | E1/2 (mV) b | Erel (mV) c |
|---|---|---|---|
| Fc | 11.70 | 503 | 0 |
| 3 | 4.22 | 624 | 159.4 |
| 6a | 4.30 | 618 | 169.0 |
| 7 | 8.34 | 553 | 121.1 |
| 9 | 2.05 | 645 | 164.2 |
| 11 | 3.62 | 698 | 224.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, E.; Váradi, M.; Nagymihály, Z.; Kollár, L.; Kovács, K.; Andreidesz, K.; Gömöry, Á.; Tumanov, N.; Wouters, J.; Skoda-Földes, R. Synthesis of Novel Ferrocene-Benzofuran Hybrids via Palladium- and Copper-Catalyzed Reactions. Inorganics 2022, 10, 205. https://doi.org/10.3390/inorganics10110205
Nagy E, Váradi M, Nagymihály Z, Kollár L, Kovács K, Andreidesz K, Gömöry Á, Tumanov N, Wouters J, Skoda-Földes R. Synthesis of Novel Ferrocene-Benzofuran Hybrids via Palladium- and Copper-Catalyzed Reactions. Inorganics. 2022; 10(11):205. https://doi.org/10.3390/inorganics10110205
Chicago/Turabian StyleNagy, Enikő, Márk Váradi, Zoltán Nagymihály, László Kollár, Krisztina Kovács, Kitti Andreidesz, Ágnes Gömöry, Nikolay Tumanov, Johan Wouters, and Rita Skoda-Földes. 2022. "Synthesis of Novel Ferrocene-Benzofuran Hybrids via Palladium- and Copper-Catalyzed Reactions" Inorganics 10, no. 11: 205. https://doi.org/10.3390/inorganics10110205
APA StyleNagy, E., Váradi, M., Nagymihály, Z., Kollár, L., Kovács, K., Andreidesz, K., Gömöry, Á., Tumanov, N., Wouters, J., & Skoda-Földes, R. (2022). Synthesis of Novel Ferrocene-Benzofuran Hybrids via Palladium- and Copper-Catalyzed Reactions. Inorganics, 10(11), 205. https://doi.org/10.3390/inorganics10110205