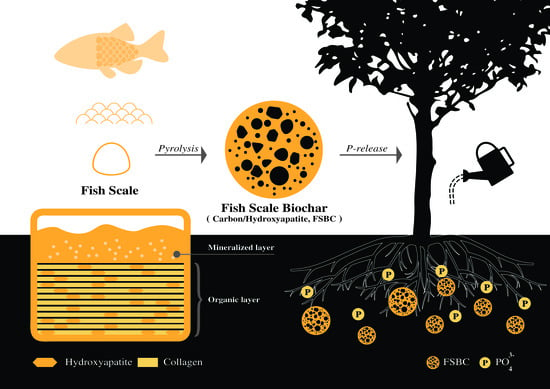
Dissolution Performance of Carbon/Hydroxyapatite Nanocomposite Prepared from Fish Scales
Abstract
:1. Introduction
2. Results and Discussion
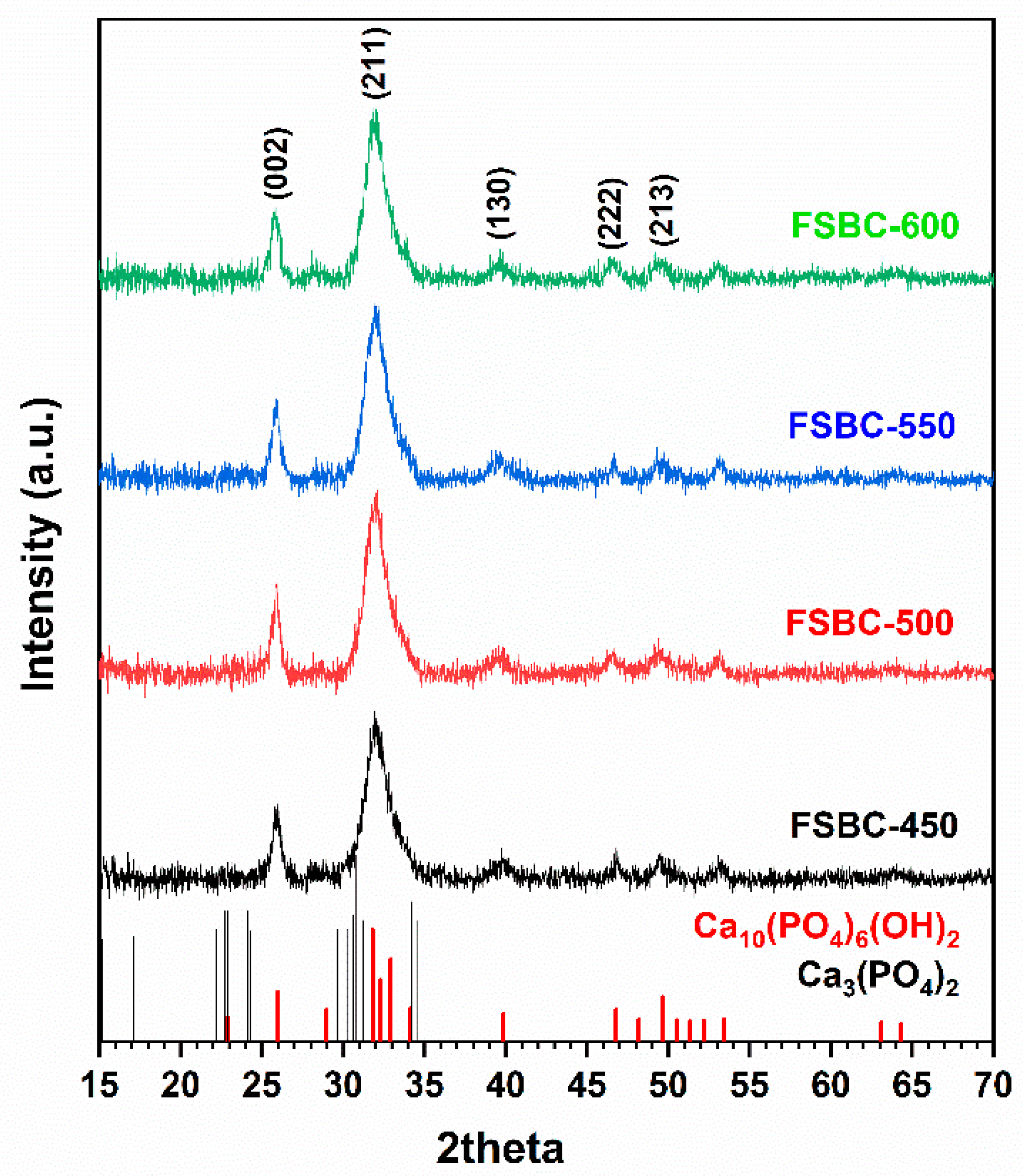
2.1. Elemental and Phase Composition of the Fish Scale Biochars
2.2. Dissolution of FSBC Materials in Citric Acid
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Fish Scale Biochar (FSBC)
3.3. Characterization of the Fish Scale Biochar
3.4. Methods to Calculate Crystallinity Index (CI) and Calculate Crystallite Size (L) Using XRD
3.5. Dissolution Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Zhu, D.; Ortega, C.F.; Motamedi, R.; Szewciw, L.; Vernerey, F.; Barthelat, F. Structure and mechanical performance of a “modern” fish scale. Adv. Eng. Mater. 2012, 14, B185–B194. [Google Scholar] [CrossRef]
- Qin, D.; Bi, S.; You, X.; Wang, M.; Cong, X.; Yuan, C.; Yu, M.; Cheng, X.; Chen, X.-G. Development and application of fish scale wastes as versatile natural biomaterials. Chem. Eng. J. 2022, 428, 131102. [Google Scholar] [CrossRef]
- Mohd Pu’Ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modolon, H.B.; Inocente, J.; Bernardin, A.M.; Montedo, O.R.K.; Arcaro, S. Nanostructured biological hydroxyapatite from Tilapia bone: A pathway to control crystallite size and crystallinity. Ceram. Int. 2021, 47, 27685–27693. [Google Scholar] [CrossRef]
- Ayala-Barajas, D.; Gonzalez-Velez, V.; Velez-Tirado, M.; Aguilar-Pliego, J. Hydroxyapatite extraction from fish scales of Tilapia. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar] [CrossRef]
- Uddin, M.H.; Matsumoto, T.; Ishihara, S.; Nakahira, A.; Okazaki, M.; Sohmura, T. Apatite Containing Aspartic Acid for Selective Protein Loading. J. Dent. Res. 2010, 89, 488–492. [Google Scholar] [CrossRef]
- Maghsoodi, M.R.; Ghodszad, L.; Lajayer, B.A. Dilemma of hydroxyapatite nanoparticles as phosphorus fertilizer: Potentials, challenges and effects on plants. Environ. Technol. Innov. 2020, 19, 100869. [Google Scholar] [CrossRef]
- Kongsri, S.; Janpradit, K.; Buapa, K.; Techawongstien, S.; Chanthai, S. Nanocrystalline hydroxyapatite from fish scale waste: Preparation, characterization and application for selenium adsorption in aqueous solution. Chem. Eng. J. 2013, 215–216, 522–532. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Shanmugavel, M.; Manikandan, E.; Nguyen-Tri, P.; Brindhadevi, K.; Pugazhendhi, A. Facile synthesis and characterization of hydroxyapatite from fish bones: Photocatalytic degradation of industrial dyes (crystal violet and Congo red). Prog. Org. Coat. 2020, 148, 105890. [Google Scholar] [CrossRef]
- Mudhafar, M.; Zainol, I.; Alsailawi, H.A.; Jaafar, C.N.A. Synthesis and characterization of fish scales of hydroxyapatite/collagen–silver nanoparticles composites for the applications of bone filler. J. Korean Ceram. Soc. 2022, 59, 229–239. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Ji, S.; Zhang, L.; Cao, W.; Wang, H.; Wang, S. Preparation and application of hydroxyapatite extracted from fish scale waste using deep eutectic solvents. J. Food Sci. 2021, 47, 9366–9372. [Google Scholar] [CrossRef]
- Dou, S.; Ke, X.-X.; Shao, Z.-D.; Zhong, L.-B.; Zhao, Q.-B.; Zheng, Y.-M. Fish scale-based biochar with defined pore size and ultrahigh specific surface area for highly efficient adsorption of ciprofloxacin. Chemosphere 2022, 287, 131962. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Liu, Y.; Cheng, X.; Gu, P.; Chen, Q.; Zhang, Z. Temperature-tuned fish-scale biochar with two-dimensional homogeneous porous structure: A promising uranium extractant. Appl. Surf. Sci. 2022, 591, 153136. [Google Scholar] [CrossRef]
- Módenes, A.N.; Bazarin, G.; Borba, C.E.; Locatelli, P.P.P.; Borsato, F.P.; Pagno, V.; Pedrini, R.; Trigueros, D.E.G.; Espinoza-Quiñones, F.R.; Scheufele, F.B. Tetracycline adsorption by tilapia fish bone-based biochar: Mass transfer assessment and fixed-bed data prediction by hybrid statistical-phenomenological modeling. J. Clean. Prod. 2021, 279, 123775. [Google Scholar] [CrossRef]
- Khandare, D.; Tembhurkar, A.; Mukherjee, S. Adsorptive Removal of Fluoride from Water Using Non-Conventional Adsorbents; advances in civil engineering and infrastructural development; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Achieng’, G.O.; Kowenje, C.O.; Lalah, J.O.; Ojwach, S.O. Synthesis and characterization of FSB@Fe3O4 composites and application in removal of indigo carmine dye from industrial wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 54876–54890. [Google Scholar] [CrossRef]
- Shikhaliyev, K.; Hameed, B.; Okoye, P. Utilization of biochars as sustainable catalysts for upgrading of glycerol from biodiesel production. J. Environ. Chem. Eng. 2021, 9, 104768. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Lehmann, J.; Solomon, D. Recycling slaughterhouse waste into fertilizer: How do pyrolysis temperature and biomass additions affect phosphorus availability and chemistry? J. Sci. Food Agric. 2015, 95, 281–288. [Google Scholar] [CrossRef]
- Schütze, E.; Gypser, S.; Freese, D. Kinetics of Phosphorus Release from Vivianite, Hydroxyapatite, and Bone Char Influenced by Organic and Inorganic Compounds. Soil Syst. 2020, 4, 15. [Google Scholar] [CrossRef]
- Zimmer, D.; Panten, K.; Frank, M.; Springer, A.; Leinweber, P. Sulfur-Enriched Bone Char as Alternative P Fertilizer: Spectroscopic, Wet Chemical, and Yield Response Evaluation. Agriculture 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Chan, W.G.; Hajaligol, M.R. Product compositions from pyrolysis of some aliphatic α-amino acids. J. Anal. Appl. Pyrolysis 2006, 75, 69–81. [Google Scholar] [CrossRef]
- Choi, S.-S.; Ko, J.-E. Analysis of cyclic pyrolysis products formed from amino acid monomer. J. Chromatogr. A 2011, 1218, 8443–8455. [Google Scholar] [CrossRef]
- Gopi, D.; Kanimozhi, K.; Bhuvaneshwari, N.; Indira, J.; Kavitha, L. Novel banana peel pectin mediated green route for the synthesis of hydroxyapatite nanoparticles and their spectral characterization. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.; Guo, Y.; Feng, X.; Wang, M.; Li, P.; Gao, Y.; Yang, X.; Jiang, T. Are different crystallinity-index-calculating methods of hydroxyapatite efficient and consistent? New J. Chem. 2017, 41, 5723–5731. [Google Scholar] [CrossRef]
- Huang, Z.L.; Liu, G.Y.; He, Y.; Yi, Z.Z.; Guo, J.M. Interaction between Hydroxyapatite and Collagen. Adv. Mater. Res. Trans. Tech. Publ. 2012, 412, 384–387. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Simultaneous fabrication of carbon nanodots and hydroxyapatite nanoparticles from fish scale for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2022, 117, 111313. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xie, Y.; Jaisi, D.P.; Jin, Y. Effects of low-molecular-weight organic acids on the dissolution of hydroxyapatite nanoparticles. Environ. Sci. Nano 2016, 3, 768–779. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Darvell, B.W. Effect of carbonate on hydroxyapatite solubility. Cryst. Growth Des. 2010, 10, 845–850. [Google Scholar] [CrossRef]
- Leventouri, T.; Chakoumakos, B.; Moghaddam, H.; Perdikatsis, V. Powder neutron diffraction studies of a carbonate fluorapatite. J. Mater. Res. 2000, 15, 511–517. [Google Scholar] [CrossRef]
- Bano, N.; Jikan, S.S.; Basri, H.; Adzila, S.; Zago, D.M. XRD and FTIR study of A&B type carbonated hydroxyapatite extracted from bovine bone. AIP Conf. Proc. 2019, 2068, 020100. [Google Scholar] [CrossRef]
- Grunenwald, A.; Keyser, C.; Sautereau, A.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Mahadevaiah, U.; Kumar, M.S.Y.; Galil, M.S.A.; Suresha, M.S.; Sathish, M.A.; Nagendrappa, G. A Simple Spectrophotometric Determination of Phosphate in Sugarcane Juices, Water and Detergent Samples. J. Chem. 2007, 4, 467–473. [Google Scholar] [CrossRef]






| Elemental Composition (Weight %) | |||||
|---|---|---|---|---|---|
| Element | FSBC-450 | FSBC-500 | FSBC-550 | FSBC-600 | |
| Composition analyzed by EA | C | 25.6 | 23.47 | 23.38 | 22.56 |
| H | 2.77 | 2.16 | 1.88 | 1.67 | |
| N | 5.95 | 4.52 | 4.31 | 4.21 | |
| Others | 65.68 | 69.85 | 70.42 | 71.55 | |
| Composition analyzed by EDXRF | Ca | 54.90 | 56.97 | 56.78 | 57.40 |
| P | 17.49 | 17.62 | 17.90 | 18.11 | |
| C * | 25.60 * | 23.47 * | 23.38 * | 22.56 * | |
| Mg | 0.54 | 0.52 | 0.55 | 0.53 | |
| Sr | 0.34 | 0.38 | 0.38 | 0.38 | |
| Si | 0.37 | 0.34 | 0.34 | 0.34 | |
| Cl | 0.32 | 0.31 | 0.31 | 0.30 | |
| K | 0.20 | 0.14 | 0.14 | 0.14 | |
| Mn | 0.11 | 0.13 | 0.12 | 0.12 | |
| S | 0.05 | 0.04 | 0.03 | 0.03 | |
| Fe | 0.04 | 0.04 | 0.04 | 0.03 | |
| Zn | 0.03 | 0.03 | 0.03 | 0.03 | |
| Sample ID | Crystal Size (nm) | Crystallinity Index | Specific Surface Area (m2 g−1) | rC/P |
|---|---|---|---|---|
| FSBC-450 | 16.88 | 0.12660 | 73.61 | 0.25 |
| FSBC-500 | 16.91 | 0.12749 | 95.54 | 0.22 |
| FSBC-550 | 17.82 | 0.14902 | 100.27 | 0.17 |
| FSBC-600 | 13.21 | 0.060730 | 111.16 | 0.077 |
| Sample ID | Initial P Content (mg g−1 FSBC) * | P Release (mg g−1) ** | |
|---|---|---|---|
| 1 Day | 5 Days | ||
| FSBC-450 | 175 | 0.311 ± 0.12 | 1.01 ± 0.07 |
| FSBC-500 | 176 | 0.314 ± 0.17 | 2.39 ± 0.08 |
| FSBC-550 | 179 | 0.371 ± 0.08 | 2.30 ± 0.21 |
| FSBC-600 | 180 | 0.332 ± 0.10 | 2.36 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sittitut, U.; Jettanasen, J.; Supothina, S.; Rattanakam, R. Dissolution Performance of Carbon/Hydroxyapatite Nanocomposite Prepared from Fish Scales. Inorganics 2022, 10, 242. https://doi.org/10.3390/inorganics10120242
Sittitut U, Jettanasen J, Supothina S, Rattanakam R. Dissolution Performance of Carbon/Hydroxyapatite Nanocomposite Prepared from Fish Scales. Inorganics. 2022; 10(12):242. https://doi.org/10.3390/inorganics10120242
Chicago/Turabian StyleSittitut, Umaporn, Junya Jettanasen, Sitthisuntorn Supothina, and Ramida Rattanakam. 2022. "Dissolution Performance of Carbon/Hydroxyapatite Nanocomposite Prepared from Fish Scales" Inorganics 10, no. 12: 242. https://doi.org/10.3390/inorganics10120242
APA StyleSittitut, U., Jettanasen, J., Supothina, S., & Rattanakam, R. (2022). Dissolution Performance of Carbon/Hydroxyapatite Nanocomposite Prepared from Fish Scales. Inorganics, 10(12), 242. https://doi.org/10.3390/inorganics10120242