Palladium(II) and Platinum(II) Deprotonated Diaminocarbene Complexes Based on N-(2-Pyridyl)ureas with Oxadiazole Periphery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Complexes 3a–d
2.2. Structural Studies
2.3. Citotoxicity Assay
3. Materials and Methods
3.1. General
3.2. Synthesis and Characterization of Complexes 3a–d
3.3. Crystal Growth, Structure Solution and Refinement Details
3.4. Cytotoxicity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Czarnomysy, R.; Radomska, D.; Szewczyk, O.K.; Roszczenko, P.; Bielawski, K. Platinum and Palladium Complexes as Promising Sources for Antitumor Treatments. Int. J. Mol. Sci. 2021, 22, 8271. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Huq, F. Comprehensive review on tumour active palladium compounds and structure–activity relationships. Coord. Chem. Rev. 2016, 316, 36–67. [Google Scholar] [CrossRef]
- Zou, T.; Lok, C.-N.; Wan, P.-K.; Zhang, Z.-F.; Fung, S.-K.; Che, C.-M. Anticancer metal-N-heterocyclic carbene complexes of gold, platinum and palladium. Curr. Opin. Chem. Biol. 2018, 43, 30–36. [Google Scholar] [CrossRef]
- Dehand, J.; Pfeffer, M. Cyclometallated compounds. Coord. Chem. Rev. 1976, 18, 327–352. [Google Scholar] [CrossRef]
- Boyarskiy, V.P.; Bokach, N.A.; Luzyanin, K.V.; Kukushkin, V.Y. Metal-mediated and metal-catalyzed reactions of isocyanides. Chem. Rev. 2015, 115, 2698–2779. [Google Scholar] [CrossRef]
- Slaughter, L.M. “Covalent Self-Assembly” of Acyclic Diaminocarbene Ligands at Metal Centers. Comments Inorg. Chem. 2008, 29, 46–72. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Luzyanin, K.V. Reactivity of acyclic diaminocarbene ligands. Coord. Chem. Rev. 2019, 399, 213014. [Google Scholar] [CrossRef]
- Singh, C.; Prakasham, A.P.; Gangwar, M.K.; Ghosh, P. Binuclear Fused 5-membered Palladacycle and Palladium Complex of Amido-Functionalized N-heterocyclic Carbene Precatalysts for the One-Pot Tandem Hiyama Alkynylation/Cyclization Reactions. ChemistrySelect 2018, 3, 9361–9367. [Google Scholar] [CrossRef]
- Singh, C.; Prakasham, A.P.; Ghosh, P. Palladium Acyclic Diaminocarbene (ADC) Triflate Complexes as Effective Precatalysts for the Hiyama Alkynylation/Cyclization Reaction Yielding Benzofuran Compounds: Probing the Influence of the Triflate Co-Ligand in the One-Pot Tandem Reaction. ChemistrySelect 2019, 4, 329–336. [Google Scholar] [CrossRef]
- Singh, C.; Prakasham, A.P.; Gangwar, M.K.; Butcher, R.J.; Ghosh, P. One-pot tandem hiyama alkynylation/cyclizations by palladium(II) acyclic diaminocarbene (ADC) complexes yielding biologically relevant benzofuran scaffolds. ACS Omega 2018, 3, 1740–1756. [Google Scholar] [CrossRef]
- Boyarskiy, V.P.; Luzyanin, K.V.; Kukushkin, V.Y. Acyclic diaminocarbenes (ADCs) as a promising alternative to N-heterocyclic carbenes (NHCs) in transition metal catalyzed organic transformations. Coord. Chem. Rev. 2012, 256, 2029–2056. [Google Scholar] [CrossRef]
- Mikhaylov, V.; Sorokoumov, V.; Liakhov, D.; Tskhovrebov, A.; Balova, I. Polystyrene-Supported Acyclic Diaminocarbene Palladium Complexes in Sonogashira Cross-Coupling: Stability vs. Catalytic Activity. Catalysts 2018, 8, 141. [Google Scholar] [CrossRef] [Green Version]
- Mikhaylov, V.N.; Sorokoumov, V.N.; Korvinson, K.A.; Novikov, A.S.; Balova, I.A. Synthesis and Simple Immobilization of Palladium(II) Acyclic Diaminocarbene Complexes on Polystyrene Support as Efficient Catalysts for Sonogashira and Suzuki–Miyaura Cross-Coupling. Organometallics 2016, 35, 1684–1697. [Google Scholar] [CrossRef]
- Gee, J.C.; Fuller, B.A.; Lockett, H.-M.; Sedghi, G.; Robertson, C.M.; Luzyanin, K.V. Visible light accelerated hydrosilylation of alkynes using platinum–[acyclic diaminocarbene] photocatalysts. Chem. Commun. 2018, 54, 9450–9453. [Google Scholar] [CrossRef]
- Dobrynin, M.V.; Kasatkina, S.O.; Baykov, S.V.; Savko, P.Y.; Antonov, N.S.; Mikherdov, A.S.; Boyarskiy, V.P.; Islamova, R.M. Deprotonated diaminocarbene platinum complexes for thermoresponsive luminescent silicone materials: Both catalysts and luminophores. Dalt. Trans. 2021, 50, 14994–14999. [Google Scholar] [CrossRef]
- Dobrynin, M.V.; Kasatkina, S.O.; Baykov, S.V.; Savko, P.Y.; Antonov, N.S.; Mikherdov, A.S.; Boyarskiy, V.P.; Islamova, R.M. Cyclometallated Platinum(II) Complexes for Obtaining Phenyl-Containing Silicone Rubbers via Catalytic Hydrosilylation Reaction. Russ. J. Gen. Chem. 2022, 92, 79–84. [Google Scholar] [CrossRef]
- Chay, R.S.; Rocha, B.G.M.; Pombeiro, A.J.L.; Kukushkin, V.Y.; Luzyanin, K.V. Platinum Complexes with Chelating Acyclic Aminocarbene Ligands Work as Catalysts for Hydrosilylation of Alkynes. ACS Omega 2018, 3, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Barbazanges, M.; Fensterbank, L. Chiral Acyclic Diaminocarbene Complexes: A New Opportunity for Gold Asymmetric Catalysis. ChemCatChem 2012, 4, 1065–1066. [Google Scholar] [CrossRef]
- Handa, S.; Slaughter, L.M. Enantioselective Alkynylbenzaldehyde Cyclizations Catalyzed by Chiral Gold(I) Acyclic Diaminocarbene Complexes Containing Weak Au-Arene Interactions. Angew. Chem. Int. Ed. 2012, 51, 2912–2915. [Google Scholar] [CrossRef] [PubMed]
- Ruch, A.A.; Ellison, M.C.; Nguyen, J.K.; Kong, F.; Handa, S.; Nesterov, V.N.; Slaughter, L.M. Highly Sterically Encumbered Gold Acyclic Diaminocarbene Complexes: Overriding Electronic Control in Regiodivergent Gold Catalysis. Organometallics 2021, 40, 1416–1433. [Google Scholar] [CrossRef]
- Eremina, A.A.; Kinzhalov, M.A.; Katlenok, E.A.; Smirnov, A.S.; Andrusenko, E.V.; Pidko, E.A.; Suslonov, V.V.; Luzyanin, K.V. Phosphorescent Iridium(III) Complexes with Acyclic Diaminocarbene Ligands as Chemosensors for Mercury. Inorg. Chem. 2020, 59, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Mikherdov, A.S.; Kinzhalov, M.A.; Novikov, A.S.; Boyarskiy, V.P.; Boyarskaya, I.A.; Dar’in, D.V.; Starova, G.L.; Kukushkin, V.Y. Difference in Energy between Two Distinct Types of Chalcogen Bonds Drives Regioisomerization of Binuclear (Diaminocarbene)Pd II Complexes. J. Am. Chem. Soc. 2016, 138, 14129–14137. [Google Scholar] [CrossRef] [PubMed]
- Kinzhalov, M.A.; Grachova, E.V.; Luzyanin, K.V. Tuning the luminescence of transition metal complexes with acyclic diaminocarbene ligands. Inorg. Chem. Front. 2022, 9, 417–439. [Google Scholar] [CrossRef]
- Serebryanskaya, T.V.; Kinzhalov, M.A.; Bakulev, V.; Alekseev, G.; Andreeva, A.; Gushchin, P.V.; Protas, A.V.; Smirnov, A.S.; Panikorovskii, T.L.; Lippmann, P.; et al. Water soluble palladium(II) and platinum(II) acyclic diaminocarbene complexes: Solution behavior, DNA binding, and antiproliferative activity. New J. Chem. 2020, 44, 5762–5773. [Google Scholar] [CrossRef]
- Martínez-Junquera, M.; Lalinde, E.; Moreno, M.T.; Alfaro-Arnedo, E.; López, I.P.; Larráyoz, I.M.; Pichel, J.G. Luminescent cyclometalated platinum(II) complexes with acyclic diaminocarbene ligands: Structural, photophysical and biological properties. Dalt. Trans. 2021, 50, 4539–4554. [Google Scholar] [CrossRef]
- Rassadin, V.A.; Zimin, D.P.; Raskil’dina, G.Z.; Ivanov, A.Y.; Boyarskiy, V.P.; Zlotskii, S.S.; Kukushkin, V.Y. Solvent- and halide-free synthesis of pyridine-2-yl substituted ureas through facile C–H functionalization of pyridine N-oxides. Green Chem. 2016, 18, 6630–6636. [Google Scholar] [CrossRef] [Green Version]
- Geyl, K.K.; Baykov, S.V.; Kasatkina, S.O.; Savko, P.Y.; Boyarskiy, V.P. Reaction of coordinated isocyanides with substituted N-(2-pyridyl)ureas as a route to new cyclometallated Pd(II) complexes. J. Organomet. Chem. 2022, 980–981, 122518. [Google Scholar] [CrossRef]
- Krasavin, M.; Shetnev, A.; Sharonova, T.; Baykov, S.; Kalinin, S.; Nocentini, A.; Sharoyko, V.; Poli, G.; Tuccinardi, T.; Presnukhina, S.; et al. Continued exploration of 1,2,4-oxadiazole periphery for carbonic anhydrase-targeting primary arene sulfonamides: Discovery of subnanomolar inhibitors of membrane-bound hCA IX isoform that selectively kill cancer cells in hypoxic environment. Eur. J. Med. Chem. 2019, 164, 92–105. [Google Scholar] [CrossRef]
- Shetnev, A.; Baykov, S.; Kalinin, S.; Belova, A.; Sharoyko, V.; Rozhkov, A.; Zelenkov, L.; Tarasenko, M.; Sadykov, E.; Korsakov, M.; et al. 1,2,4-Oxadiazole/2-Imidazoline Hybrids: Multi-target-directed Compounds for the Treatment of Infectious Diseases and Cancer. Int. J. Mol. Sci. 2019, 20, 1699. [Google Scholar] [CrossRef] [Green Version]
- Glomb, T.; Szymankiewicz, K.; Świątek, P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules 2018, 23, 3361. [Google Scholar] [CrossRef] [Green Version]
- Benassi, A.; Doria, F.; Pirota, V. Groundbreaking Anticancer Activity of Highly Diversified Oxadiazole Scaffolds. Int. J. Mol. Sci. 2020, 21, 8692. [Google Scholar] [CrossRef]
- Kapoor, G.; Bhutani, R.; Pathak, D.P.; Chauhan, G.; Kant, R.; Grover, P.; Nagarajan, K.; Siddiqui, S.A. Current Advancement in the Oxadiazole-Based Scaffolds as Anticancer Agents. Polycycl. Aromat. Compd. 2022, 42, 4183–4215. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Abbas, N. Oxadiazoles as Privileged Motifs for Promising Anticancer Leads: Recent Advances and Future Prospects. Arch. Pharm. 2014, 347, 1–20. [Google Scholar] [CrossRef]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Shahar Yar, M. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef]
- Desai, N.; Monapara, J.; Jethawa, A.; Khedkar, V.; Shingate, B. Oxadiazole: A highly versatile scaffold in drug discovery. Arch. Pharm. 2022, 355, 2200123. [Google Scholar] [CrossRef]
- Bajaj, S.; Asati, V.; Singh, J.; Roy, P.P. 1,3,4-Oxadiazoles: An emerging scaffold to target growth factors, enzymes and kinases as anticancer agents. Eur. J. Med. Chem. 2015, 97, 124–141. [Google Scholar] [CrossRef]
- Salassa, G.; Terenzi, A. Metal Complexes of Oxadiazole Ligands: An Overview. Int. J. Mol. Sci. 2019, 20, 3483. [Google Scholar] [CrossRef] [Green Version]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in Medicinal Chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef]
- Flipo, M.; Desroses, M.; Lecat-Guillet, N.; Villemagne, B.; Blondiaux, N.; Leroux, F.; Piveteau, C.; Mathys, V.; Flament, M.-P.; Siepmann, J.; et al. Ethionamide Boosters. 2. Combining Bioisosteric Replacement and Structure-Based Drug Design to Solve Pharmacokinetic Issues in a Series of Potent 1,2,4-Oxadiazole EthR Inhibitors. J. Med. Chem. 2012, 55, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.A.; Ding, D.; Meisel, J.E.; Janardhanan, J.; Spink, E.; Peng, Z.; Qian, Y.; Yamaguchi, T.; Testero, S.A.; O’Daniel, P.I.; et al. Structure–Activity Relationship for the Oxadiazole Class of Antibacterials. ACS Med. Chem. Lett. 2020, 11, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.J.; Macdonald, S.J.F.; Peace, S.; Pickett, S.D.; Luscombe, C.N. The developability of heteroaromatic and heteroaliphatic rings—do some have a better pedigree as potential drug molecules than others? Medchemcomm 2012, 3, 1062. [Google Scholar] [CrossRef]
- Bokach, N.A.; Khripoun, A.V.; Kukushkin, V.Y.; Haukka, M.; Pombeiro, A.J.L. A Route to 1,2,4-Oxadiazoles and Their Complexes via Platinum-Mediated 1,3-Dipolar Cycloaddition of Nitrile Oxides to Organonitriles. Inorg. Chem. 2003, 42, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Bokach, N.A.; Kukushkin, V.Y.; Haukka, M.; Pombeiro, A.J.L. Synthesis of (1,2,4-Oxadiazole)palladium(II) Complexes by [2 + 3] Cycloaddition of Nitrile Oxides to Organonitriles in the Presence of PdCl2. Eur. J. Inorg. Chem. 2005, 2005, 845–853. [Google Scholar] [CrossRef]
- Geyl, K.; Baykov, S.; Tarasenko, M.; Zelenkov, L.E.; Matveevskaya, V.; Boyarskiy, V.P. Convenient entry to N-pyridinylureas with pharmaceutically privileged oxadiazole substituents via the acid-catalyzed C H activation of N-oxides. Tetrahedron. Lett. 2019, 60, 151108. [Google Scholar] [CrossRef]
- Baykov, S.; Mikherdov, A.; Novikov, A.; Geyl, K.; Tarasenko, M.; Gureev, M.; Boyarskiy, V. π–π Noncovalent Interaction Involving 1,2,4- and 1,3,4-Oxadiazole Systems: The Combined Experimental, Theoretical, and Database Study. Molecules 2021, 26, 5672. [Google Scholar] [CrossRef]
- Bossi, A.; Rausch, A.F.; Leitl, M.J.; Czerwieniec, R.; Whited, M.T.; Djurovich, P.I.; Yersin, H.; Thompson, M.E. Photophysical Properties of Cyclometalated Pt(II) Complexes: Counterintuitive Blue Shift in Emission with an Expanded Ligand π System. Inorg. Chem. 2013, 52, 12403–12415. [Google Scholar] [CrossRef]
- Chassot, L.; Von Zelewsky, A. Cyclometalated complexes of platinum(II): Homoleptic compounds with aromatic C,N ligands. Inorg. Chem. 1987, 26, 2814–2818. [Google Scholar] [CrossRef]
- Geyl, K.K.; Baykov, S.V.; Kalinin, S.A.; Bunev, A.S.; Troshina, M.A.; Sharonova, T.V.; Skripkin, M.Y.; Kasatkina, S.O.; Presnukhina, S.I.; Shetnev, A.A.; et al. Synthesis, Structure, and Antiproliferative Action of 2-Pyridyl Urea-Based Cu(II) Complexes. Biomedicines 2022, 10, 461. [Google Scholar] [CrossRef]
- Matveevskaya, V.V.; Pavlov, D.I.; Sukhikh, T.S.; Gushchin, A.L.; Ivanov, A.Y.; Tennikova, T.B.; Sharoyko, V.V.; Baykov, S.V.; Benassi, E.; Potapov, A.S. Arene–Ruthenium(II) Complexes Containing 11H-Indeno [1,2-b]quinoxalin-11-one Derivatives and Tryptanthrin-6-oxime: Synthesis, Characterization, Cytotoxicity, and Catalytic Transfer Hydrogenation of Aryl Ketones. ACS Omega 2020, 5, 11167–11179. [Google Scholar] [CrossRef]
- Boyarskii, V.P.; Mikherdov, A.S.; Baikov, S.V.; Savko, P.Y.; Suezov, R.V.; Trifonov, R.E. Diaminocarbene Complexes of Palladium(II) Containing 2-Aminooxazole and 2-Aminothiazole Heterocyclic Ligands as Potential Antitumor Agents. Pharm. Chem. J. 2021, 55, 130–132. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dai, C.-H.; Li, J.; Chen, P.; Jiang, H.-G.; Wu, M.; Chen, Y.-C. RNA interferences targeting the Fanconi anemia/BRCA pathway upstream genes reverse cisplatin resistance in drug-resistant lung cancer cells. J. Biomed. Sci. 2015, 22, 77. [Google Scholar] [CrossRef]
- Savić, A.; Gligorijević, N.; Aranđelović, S.; Dojčinović, B.; Kaczmarek, A.M.; Radulović, S.; Van Deun, R.; Van Hecke, K. Antitumor activity of organoruthenium complexes with chelate aromatic ligands, derived from 1,10-phenantroline: Synthesis and biological activity. J. Inorg. Biochem. 2020, 202, 110869. [Google Scholar] [CrossRef]
- Potapova, O.; Haghighi, A.; Bost, F.; Liu, C.; Birrer, M.J.; Gjerset, R.; Mercola, D. The Jun Kinase/Stress-activated Protein Kinase Pathway Functions to Regulate DNA Repair and Inhibition of the Pathway Sensitizes Tumor Cells to Cisplatin. J. Biol. Chem. 1997, 272, 14041–14044. [Google Scholar] [CrossRef] [Green Version]
- Mikherdov, A.S.; Orekhova, Y.A.; Boyarskii, V.P. Formation of Homo- and Heteronuclear Platinum(II) and Palladium(II) Carbene Complexes in the Reactions of Coordinated Isocyanides with Aminothiazaheterocycles. Russ. J. Gen. Chem. 2018, 88, 2119–2124. [Google Scholar] [CrossRef]
- Luzyanin, K.V.; Pombeiro, A.J.L.; Haukka, M.; Kukushkin, V.Y. Coupling between 3-Iminoisoindolin-1-ones and Complexed Isonitriles as a Metal-Mediated Route to a Novel Type of Palladium and Platinum Iminocarbene Species. Organometallics 2008, 27, 5379–5389. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlis Pro. Data Collection and Processing Software for Agilent X-ray Diffractometers; Aglient Technologies: Yarnton, UK, 2013. [Google Scholar]

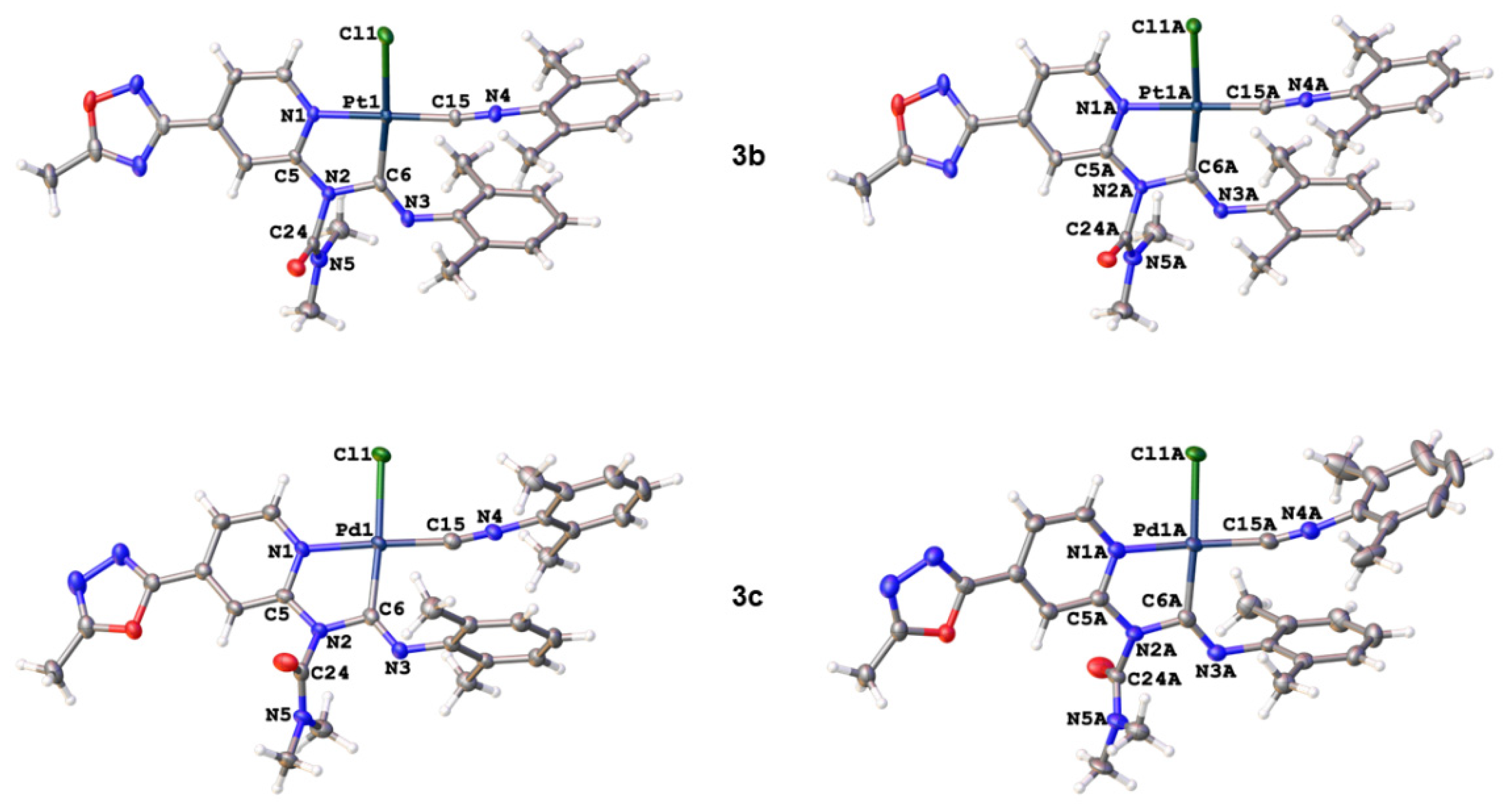
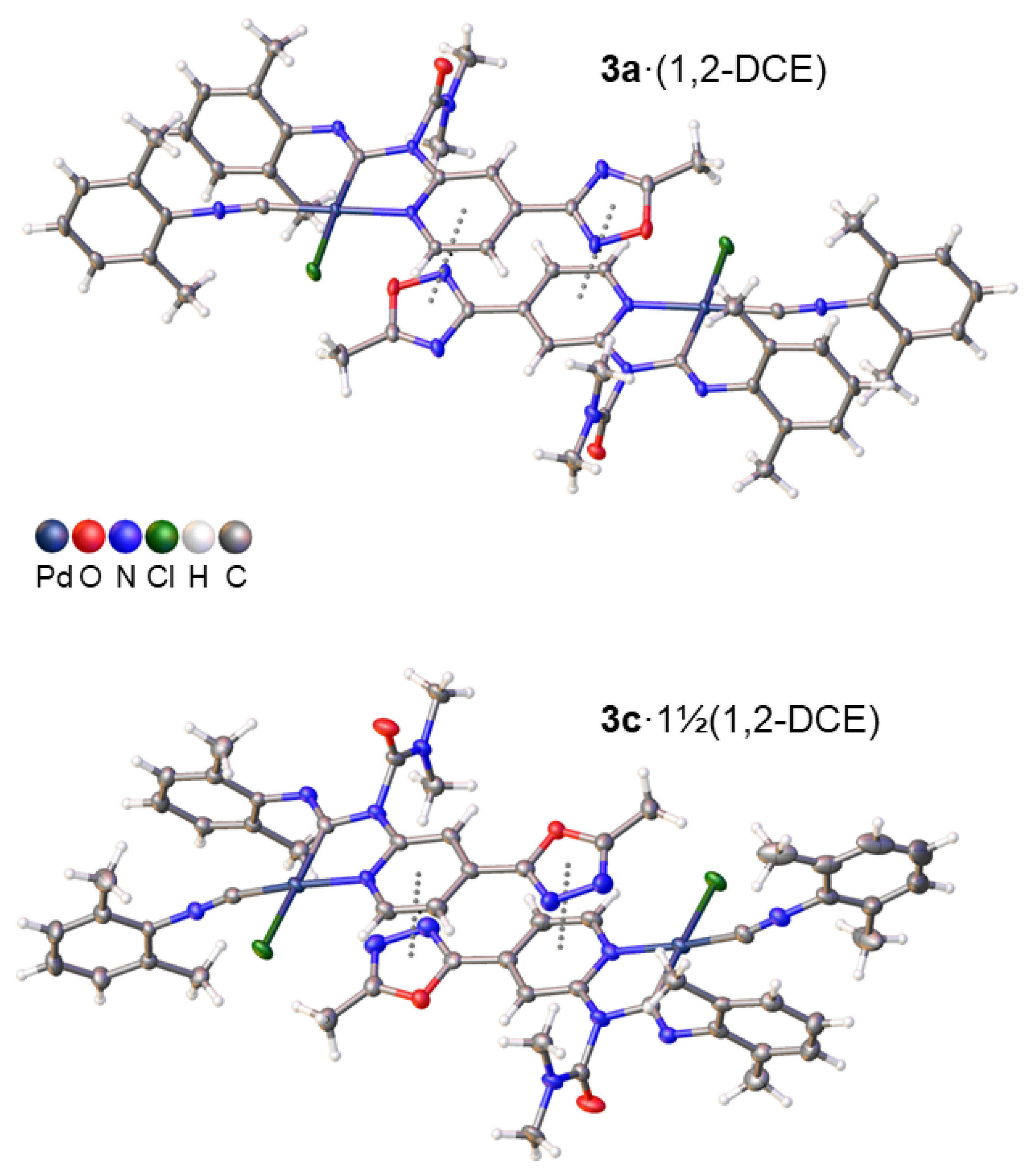
| Parameter | 3a·1,2-DCE | 3b·1,2-DCE | 3b·1,2-DCEA | 3c·1½(1,2-DCE) | 3c·1½(1,2-DCE)A |
|---|---|---|---|---|---|
| M1–Cl1, Å | 2.3912(5) | 2.3895(13) | 2.3821(12) | 2.3856(11) | 2.3866(11) |
| M1–N1, Å | 2.0420(17) | 2.036(4) | 2.040(4) | 2.053(4) | 2.043(4) |
| M1–C6, Å | 2.015(2) | 2.005(5) | 2.012(5) | 2.006(4) | 2.005(5) |
| Pd1–C15, Å | 1.958(2) | 1.928(5) | 1.914(5) | 1.954(4) | 1.967(5) |
| C6–N2, Å | 1.428(3) | 1.441(6) | 1.420(6) | 1.421(5) | 1.426(6) |
| C6–N3, Å | 1.251(3) | 1.261(7) | 1.265(7) | 1.259(6) | 1.259(6) |
| C24–N2, Å | 1.462(3) | 1.461(6) | 1.456(6) | 1.465(5) | 1.463(5) |
| C24–N5, Å | 1.326(3) | 1.338(7) | 1.332(7) | 1.327(6) | 1.331(6) |
| C6–Pd1–N1, ° | 81.26(8) | 81.48(18) | 81.21(18) | 81.76(16) | 81.85(17) |
| N2–C6–N3, ° | 115.52(19) | 113.6(4) | 114.1(4) | 115.3(4) | 115.4(4) |
| Compound | IC50, μM | ||
|---|---|---|---|
| A549 | PANC-1 | T98G | |
| 3a | 98.0 ± 9.8 | 85.1 ± 8.5 | >100 |
| 3b | 64.6 ± 6.0 | 95.0 ± 9.5 | 100.0 ± 10.0 |
| 3c | 32.2 ± 3.0 | 10.3 ± 1.5 | 34.7 ± 3.5 |
| 3d | 47.1 ± 5.0 | 40.6 ± 4.6 | 39.4 ± 3.9 |
| Cisplatin | 4.97 ± 0.32 [54] | 16.44 ± 1.56 [55] | 140 ± 13 [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geyl, K.K.; Baykova, S.O.; Andoskin, P.A.; Sharoyko, V.V.; Eliseeva, A.A.; Baykov, S.V.; Semenov, K.N.; Boyarskiy, V.P. Palladium(II) and Platinum(II) Deprotonated Diaminocarbene Complexes Based on N-(2-Pyridyl)ureas with Oxadiazole Periphery. Inorganics 2022, 10, 247. https://doi.org/10.3390/inorganics10120247
Geyl KK, Baykova SO, Andoskin PA, Sharoyko VV, Eliseeva AA, Baykov SV, Semenov KN, Boyarskiy VP. Palladium(II) and Platinum(II) Deprotonated Diaminocarbene Complexes Based on N-(2-Pyridyl)ureas with Oxadiazole Periphery. Inorganics. 2022; 10(12):247. https://doi.org/10.3390/inorganics10120247
Chicago/Turabian StyleGeyl, Kirill K., Svetlana O. Baykova, Pavel A. Andoskin, Vladimir V. Sharoyko, Anastasiya A. Eliseeva, Sergey V. Baykov, Konstantin N. Semenov, and Vadim P. Boyarskiy. 2022. "Palladium(II) and Platinum(II) Deprotonated Diaminocarbene Complexes Based on N-(2-Pyridyl)ureas with Oxadiazole Periphery" Inorganics 10, no. 12: 247. https://doi.org/10.3390/inorganics10120247
APA StyleGeyl, K. K., Baykova, S. O., Andoskin, P. A., Sharoyko, V. V., Eliseeva, A. A., Baykov, S. V., Semenov, K. N., & Boyarskiy, V. P. (2022). Palladium(II) and Platinum(II) Deprotonated Diaminocarbene Complexes Based on N-(2-Pyridyl)ureas with Oxadiazole Periphery. Inorganics, 10(12), 247. https://doi.org/10.3390/inorganics10120247









