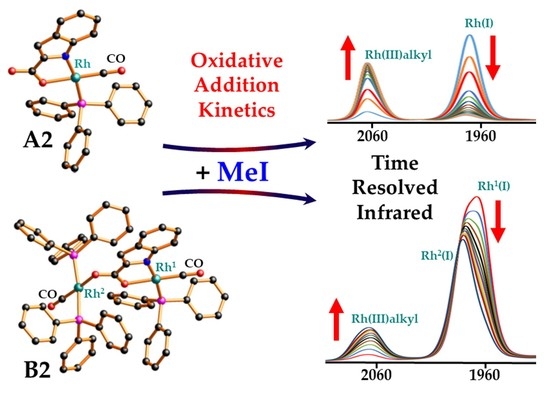
Structural Study of Model Rhodium(I) Carbonylation Catalysts Activated by Indole-2-/Indoline-2-Carboxylate Bidentate Ligands and Kinetics of Iodomethane Oxidative Addition
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Compounds

2.2. X-ray Crystallography
2.3. Spectroscopic Evaluation of the Oxidative Addition of Iodomethane to Rh(Indoli)(CO)(PPh3)] (A2) and [Rh1(Indol’)(CO)(PPh3)Rh2(CO)(PPh3)2] (B2)
2.3.1. Rh(Indoli)(CO)(PPh3)] (A2)
- IR Results
- 31P NMR study
2.3.2. [Rh1(Indol’)(CO)(PPh3)Rh2(CO)(PPh3)2] (B2)
- IR Study
- NMR study
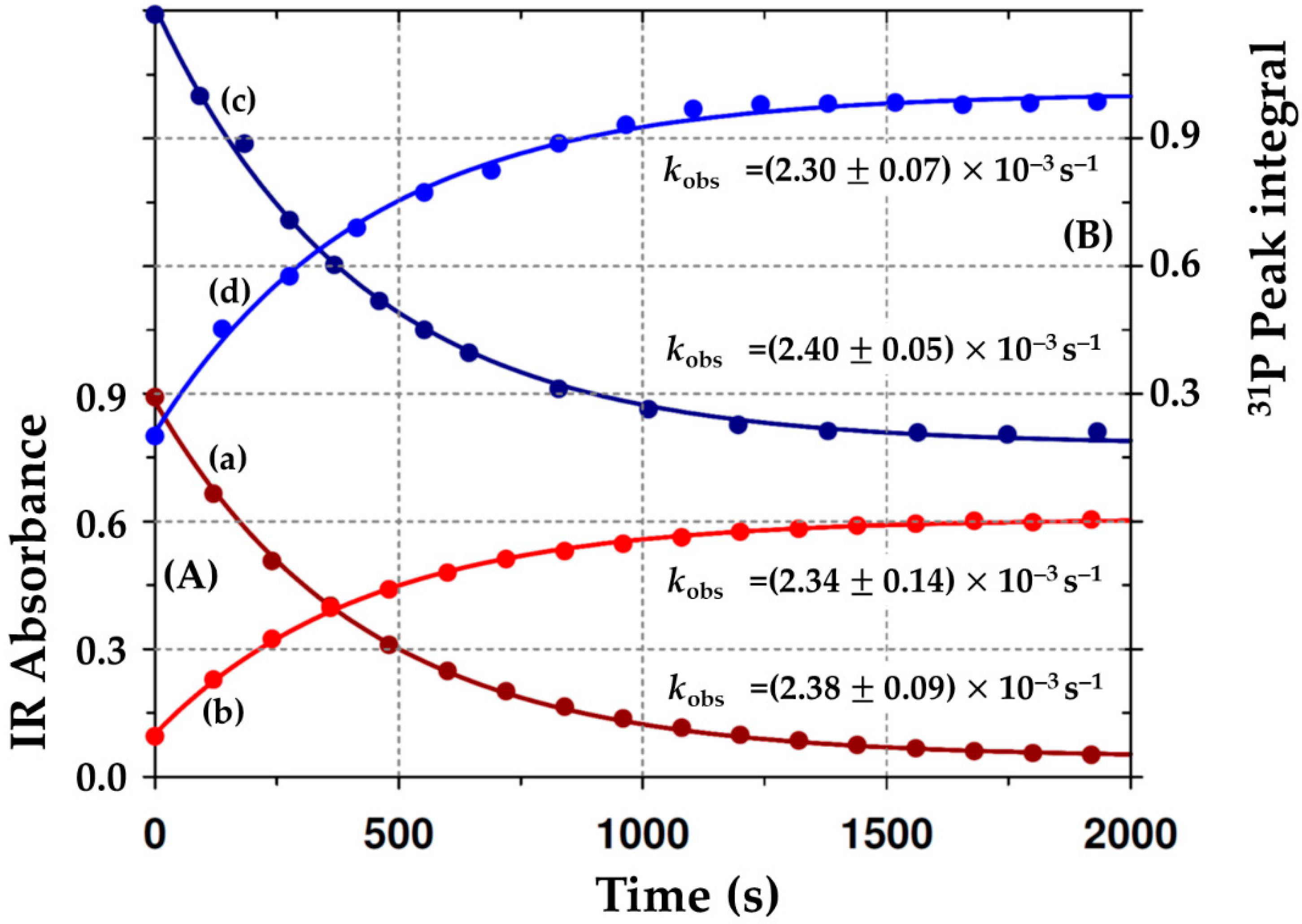
2.3.3. Summary of the Oxidative Addition of Iodomethane to Rh(Indoli)(CO)(PPh3)] (2A) and [Rh1(Indol’)(CO)(PPh3)Rh2(CO)(PPh3)2] (B2) as Monitored by Different Spectroscopies
- Rh(Indoli)(CO)(PPh3)] (A2)
| Species | IR Data | 31P{1H} NMR Data | |||
|---|---|---|---|---|---|
| νCO (cm−1) | kobs (s−1) | δ (ppm) | 1J(Rh-P) (Hz) | kobs (s–1) | |
| A2(a,b) | |||||
| Rh(I) | 1967 | (2.38 ± 0.09) × 10−3 (c) | 41.7 | 169 | (2.40 ± 0.05) × 10–3 (c) |
| RhIII-alkyl | 2054 | (2.34 ± 0.14) × 10−3 (c) | 30.3 | 124 | (2.30 ± 0.07) × 10–3 (c) |
| B2(d,e) | |||||
| Rh1(I) | 1967 | (5.56 ± 0.12) × 10−3 (f) | 41.0 | 150 | (5.50 ± 0.02) × 10–3 (f) |
| Rh2(I) | 1980 (d) | ” | 30.0 | 133 | ” |
| Rh1(III)-Alkyl | 2060 | (5.50 ± 0.04) × 10−3 (f) | 27.2 | 122 | (5.52 ± 0.02) × 10–3 (f) |
| B5 | 1968 | - | 29.0 | 126 | - |
- [Rh1(Indol’)(CO)(PPh3)Rh2(CO)(PPh3)2] (B2)

3. Discussion
3.1. Synthesis
3.2. X-ray Crystallography
3.2.1. Comparison of B2a Data with the Literature
3.2.2. Comparison of B5a Data with the Literature
3.3. Spectroscopic Study
3.3.1. [Rh(Indoli)(CO)(PPh3)] (A2)
3.3.2. [Rh1(Indol’)(CO)(PPh3)Rh2(CO)(PPh3)2] (B2)
- A fairly rapid oxidative addition equilibrium step causes the Rh1(I) fragment to form an oxidized RhIII-alkyl entity at the Rh1(N,O-Indol) part, i.e., [Rh1(N,O-Indol)(CO)(PPh3)(Me)(I)] (B3).
- This is usually followed by the much slower carbonyl insertion still at Rh1, i.e., the conversion of the corresponding Rh1(III)-alkyl fragment to a corresponding [Rh1(N,O-Idol)(COMe)-(PPh3)(I)] (B5) Rh1(III)-acyl species. However, this is not observed on the time scale utilized in the current study.
- The Rh2(I)-Vaska-type fragment, on the other hand, is cleaved off, and does not undergo appreciable oxidative addition, as shown in the literature [43]. Additional evidence of the effect wherein the Rh2(I) fragment does not undergo appreciable oxidative addition comes from the fact that the corresponding trans-[Rh(PPh3)2(I)(CO)] (B5) has been isolated from the final solution even after 1 week (see Section 4.3.7).
- There is some uncertainty as to exactly when the Rh2(I)-Vaska type fragment is cleaved off, since the product Rh(III)-alkyl (B3 in Scheme 2 and Scheme 3) with a signal at δ = 27.2 ppm (d, 1J(Rh-P) = 122 Hz) is close yet slightly different from the final signal of B5 at δ 28.9 ppm (d, 1J(Rh-P) = 126.8 Hz), although the integral value of the Rh(III)-alkyl is close to three equivalents of PPh3 (see the 31P NMR traces in Figure 6 pointing to an average, potentially dynamic equilibrium that might exist between the three different PPh3 sites in the ‘Rh(III)-alkyl’ (B3) species.
3.4. Detailed Kinetic Study: Variation of [MeI] and Temperature
3.4.1. [Rh(Indoli)(CO)(PPh3)] (A2)
3.4.2. [Rh1(Indol’)(CO)(PPh3)Rh2(CO)(PPh3)2] (B2)
3.4.3. Kinetic Correlation of [Rh1(Indoli)(CO)(PPh3)] (A2) with [Rh1(Indol’)(CO)(PPh3)-Rh2(CO)(PPh3)2] (B2)
4. Materials and Methods
4.1. General Procedure and Instrumentation
4.2. Kinetic Measurements
4.3. Synthesis
4.3.1. Synthesis of Dicarbonyl(Indoline-2-Carboxylato-κO,N)rhodium(I), [Rh(Indoli)(CO)2] (A1)
4.3.2. Synthesis of carbonyl(indoline-2-caboxylato-κO,N)(triphenylphosphine-κP)-rhodium(I), [Rh(Indoli)(CO)(PPh3)] (A2)
4.3.3. In Situ Synthesis/Characterization of carbonyl(indoline-2-caboxylato-κO,N)(iodido)(methyl)(triphenylphosphine-κP)-rhodium(III)] [Rh(Indoli)(I)(Me)(CO)(PPh3)] (A3)
4.3.4. Synthesis of dicarbonyl(Indole-2-carboxylato-κO,N)rhodium(I), [Rh(Indol)(CO)2] (B1)
4.3.5. Synthesis of [Rh1(Indol’)(CO)(PPh3)Rh2(CO)(PPh3)2] (B2)
4.3.6. In Situ Synthesis/Characterization of carbonyl(Indole-2-carboxylato-κO,N)(iodido)(methyl)(triphenylphosphine-κP)rhodium(I), [Rh(Indol)(I)(Me)(CO)(PPh3)] (B3)
4.3.7. Synthesis of the Vaska-Type Complex trans-[Rh(CO)(I)(PPh3)2] (B5)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maitlis, P.M.; Haynes, A.; Sunley, G.J.; Howard, M.J. Methanol carbonylation revisited thirty years on. J. Chem. Soc. Dalton Trans. 1996, 2187–2196. [Google Scholar] [CrossRef]
- Bohnen, H.W.; Cornils, B. Hydroformylation of Alkenes: An Industrial View of the Status and Importance. Adv. Catal. 2002, 47, 1–64. [Google Scholar]
- van Leeuwen, P.W.N.M. Homogeneous Catalysis Understanding the Art; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 109–124. [Google Scholar]
- Roodt, A.; Visser, H.G.; Brink, A. Structure/reactivity relationships and mechanism from X-ray data and spectroscopic kinetic analysis. Cryst. Rev. 2011, 17, 241–280. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Crous, R.; Bennie, L.; Meij, A.M.; Blann, K.; Bezuidenhoudt, B.C.B.; Young, D.A.; Green, M.J.; Roodt, A. Borate Esters as Alternative Acid Promoters in the Palladium-Catalyzed Methoxycarbonylation of Ethylene. Angew. Chem. Int. Ed. 2007, 119, 2323–2325. [Google Scholar] [CrossRef]
- Jackman, K.M.K.; Liang, G.; Boyle, P.D.; Zimmerman, P.M.; Blacquiere, J.M. Changes in Ligand Coordination Mode Induce Bimetallic C-C Reductive Elimination. Dalton Trans. 2022, 51, 3977–3991. [Google Scholar] [CrossRef]
- Warsink, S.; Kotze, P.D.R.; van Rensburg, J.M.J.; Venter, J.A.; Otto, S.; Botha, E.; Roodt, A. Kinetic-Mechanistic and Solid-State Study of the Oxidative Addition and Migratory Insertion of Iodomethane to [Rhodium(S,O-BdiPT or N,O-ox)(CO)(PR1R2R3)] Complexes. Eur. J. Inorg. Chem. 2018, 2018, 3615–3625. [Google Scholar] [CrossRef]
- Roodt, A.; Otto, S.; Steyl, G. Structure and solution behaviour of rhodium(I) Vaska-type complexes for correlation of steric and electronic properties of tertiary phosphine ligands. Coord. Chem. Rev. 2003, 245, 121–137. [Google Scholar] [CrossRef]
- Basson, S.S.; Leipoldt, J.G.; Roodt, A.; Venter, J.A. Mechanism for the Oxidative Addition of Iodomethane to Carbonyl(N-hydroxy-Nitrosobenzenaminato-κ2O,O′)-triarylphosphine-rhodium(I) Complexes and Crystal Structure of [Rh(cupf)(CO)(CH)(I)(PPh3)]. Inorg. Chim. Acta 1987, 128, 31–37. [Google Scholar] [CrossRef]
- Venter, J.A.; Leipoldt, J.G.; van Eldik, R. Solvent, Temperature, and Pressure Dependence of the Oxidative Addition of Iodomethane to Complexes of the Type Rhl(β-diketonate)(CO)( PPh3). Inorg. Chem. 1991, 30, 2207–2209. [Google Scholar] [CrossRef]
- Elmakki, M.A.E.; Alexander, O.T.; Venter, G.J.S.; Venter, J.A.; Roodt, A. Synthesis and structural determination of [Rh(opo)(CO)(PR3)] complexes (opo− = 2-oxopyridin-1-olate) and in situ isomeric behavior from preliminary kinetic study of iodomethane oxidative addition. J. Coord. Chem. 2021, 74, 444–466. [Google Scholar] [CrossRef]
- Conradie, J.; Swarts, J.C. Oxidative Addition of CH3I and CO Migratory Insertion in a Series of Ferrocene-Containing Carbonyl Phosphine β-Diketonato Rhodium(I) Complexes. Organometallics 2009, 28, 1018–1026. [Google Scholar] [CrossRef]
- Conradie, J.; Lamprecht, G.J.; Roodt, A.; Swarts, J.C. Kinetic study of the oxidative addition reaction between methyl iodide and [Rh(FcCOCHCOCF3)(CO)(PPh3)]: Structure of [Rh(FcCOCHCOCF3)(CO)(PPh3)(CH3)(I)]. Polyhedron 2007, 26, 5075–5087. [Google Scholar] [CrossRef]
- Conradie, M.M.; Conradie, J. Methyl iodide oxidative addition to [Rh(acac)(CO)(PPh3)]: An experimental and theoretical study of the stereochemistry of the products and the reaction mechanism. Dalton Trans. 2011, 40, 8226–8237. [Google Scholar] [CrossRef]
- Steyn, G.J.J.; Roodt, A.; Poletaeva, I.; Varshavsky, Y.S. Structural correlation between Rh-P and Rh-C bond distances vs. 31P and 13C NMR parameters in monocarbonyl-phosphinerhodium(I) complexes: Crystal structure of (methyl 2-(amino)-1-cyclopentene-1-dithiocarboxylato-κN,κS)-carbonyl(triphenylphosphine) rhodium(I). J. Organomet. Chem. 1997, 536–537, 197–205. [Google Scholar]
- Varshavsky, Y.S.; Galding, M.R.; Cherkasova, T.G.; Podkorytov, I.S.; Nikol’skii, A.B.; Trzeciak, A.M.; Olejnik, Z.; Lis, T.; Zio´łkowski, J.J. 31P-NMR and X-ray studies of new rhodium(I) β-ketoiminato complexes Rh(R1C(O)CHC(NH)R2)(CO)(PZ3) where PZ3=PPh3, PCy3, P(OPh)3 or P(NC4H4)3. J. Organomet. Chem. 2001, 628, 195–210. [Google Scholar] [CrossRef]
- Warsink, S.; Fessha, F.G.; Purcell, W.; Venter, J.A. Synthesis and characterization of rhodium(I) 2-methylcupferrate complexes and their kinetic behaviour in iodomethane oxidative addition. J. Organomet. Chem. 2013, 726, 14–20. [Google Scholar] [CrossRef]
- Purcell, W.; Basson, S.S.; Leipoldt, J.G.; Roodt, A.; Preston, H. First structural confirmation of different geometrical isomers in the same crystal lattice: The crystal structure of benzoylacetonatocarbonyltriphenylphosphinerhodium(I). Inorg. Chim. Acta 1995, 234, 153–156. [Google Scholar] [CrossRef]
- McCleverty, J.A.; Wilkinson, G. 20. Tetracarbonyldichlorodirhodium. In Inorganic Syntheses; Angelici, R.J., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1990; Volume 28, pp. 84–86. [Google Scholar]
- Serp, P.; Hernandez, M.; Kalck, P. Dimethylformamide as a convenient CO source for the facile preparation of rhodium-, iridium- or rutheniumchlorocarbonyl complexes directly from RhCI3-3H2O, IrCI3-3H2O or RuCI3-3H2O. In Chimie des Surfaces et Catalysel/Surface Chemistry and Catalysis; Serie II; C.R. Acad. Sci.: Paris, France, 1999; pp. 267–272. [Google Scholar]
- Preston, H. Spesifieke Isomeervorming en Oksidatiewe Addisiegedrag van Rodium(I)-Tiolatokomplekse. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 1993. [Google Scholar]
- Roodt, A.; Leipoldt, J.G.; Helm, L.; Merbach, A.E. Equilibrium behavior and proton transfer kinetics of the dioxotetracyanometalate complexes of molybdenum(IV), tungsten(IV), technetium(V), and rhenium(V): Carbon-13 and Oxygen-17 NMR Study. Inorg. Chem. 1994, 33, 140–147. [Google Scholar] [CrossRef]
- Mokolokolo, P.P.; Brink, A.; Roodt, A.; Schutte-Smith, M. Subtle variation of stereo-electronic effects in rhodium(I) carbonyl Schiff base complexes and their iodomethane oxidative addition kinetics. J. Coord. Chem. 2020, 73, 2740–2762. [Google Scholar] [CrossRef]
- Otto, S. Structural and Reactivity Relationships in Platinum(II) and Rhodium(I) Complexes. Ph.D. Thesis, University of the Free State, Boemfontein, South Africa, 1999. [Google Scholar]
- Pretorius, C. Structural and Reactivity Study of Rhodium(I) Carbonyl Complexes as Model Nano Assemblies. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2015. [Google Scholar]
- Shestakova, E.P.; Cherkasova, T.G.; Osetrova, L.V.; Varshavsky, Y.S.; Leipoldt, J.G.; Roodt, A. NMR Study of the Octahedral Rhodium(III) Complexes formed by the Iodomethane Oxidative Addition to the Rhodium(I)-Diketonato bis-triphenylphosphine Complexes. Rhodium Express 1994, 7–8, 24–29. [Google Scholar]
- Damoense, L. Fundamental Aspects of Selected Rhodium Complexes in Homogeneous Catalytic Acetic Acid Production. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2001. [Google Scholar]
- Basson, S.S.; Leipoldt, J.G.; Nel, J.T. The Oxidative Addtion of Methyl Iodide to Betadiketonecarbonyltriphenylphosphine-rhodium(I) Complexes. Inorg. Chim. Acta 1984, 84, 167–172. [Google Scholar] [CrossRef]
- Goswami, K.; Singh, M.M. Di and Monocarbonyl Complexes of Rhodium(I) containing Singly Charged Bidentate Ligands. Transit. Met. Chem. 1980, 5, 83–85. [Google Scholar] [CrossRef]
- Singh, M.M.; Szafran, Z.; Pike, R.M. Microscale Chemistry and Green Chemistry: Complementary Pedagogies. J. Chem. Educ. 1999, 76, 1684–1686. [Google Scholar] [CrossRef]
- Goswami, K.; Singh, M.M. Few carbonyl oximato compounds of rhodium. J. Inorg. Nucl. Chem. 1977, 39, 1718–1719. [Google Scholar] [CrossRef]
- Kemp, G.; Roodt, A.; Purcell, W.; Koch, K.R. Unprecedented N,S,O co-ordination of the doubly deprotonated anion of N-benzoyl-N9-phenylthiourea (H2L2) bridging two rhodium(I) centres: Crystal structure of the acetone solvate of [Rh(nbnpt)(CO)(PPh3)Rh(CO)(PPh3)2].(CH3COCH3). J. Chem. Soc. Dalton Trans. 1997, 4481–4483. [Google Scholar] [CrossRef]
- Morzyk-Ociepa, B.; Michalska, D.; Pietraszko, A. Structures and vibrational spectra of Indole carboxylic acids. Part I. Indole-2-carboxylic acid. J. Mol. Struct. 2004, 688, 79–86. [Google Scholar] [CrossRef]
- Viossat, V.; Lemoine, P.; Dayan, E.; Dung, N.-H.; Viossat, B. Synthesis, crystal structure and IR spectroscopy of MnII(2-IC)2(NC)(DMSO) and [MnII(2-IC)2(phen)(H2O)]·DMA; (2-HIC, Indole-2-carboxylic acid; phen, 1,10-phenanthroline; NC, 2,9-dimethyl-1,10-phenanthroline; DMSO, dimethyl sulfoxide; DMA, dimethyl acetamide); catalysts for the disproportionation of hydrogen peroxide. Polyhedron 2003, 22, 1461–1470. [Google Scholar]
- Li, Y.X.; Zhang, B.S.; Zheng, M. Bis(2,2′-bipyridine-κ2N,N′)bis-(1H-Indole-2-carboxyl-ato- κ2O,O)cadmium-2,2′-bipyridine-water (1/0.5/2). Acta Crystallogr. Sect. E Struct. Rep. 2011, 67, m879–m880. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.H. (Ed.) Cambridge Structural Database (CSD), Version 5.38, November 2016 Update. In Acta Crystallographica; Wiley Online Library: Hoboken, NJ, USA, 2002; pp. 380–388. [Google Scholar]
- Kemp, G.; Roodt, A.; Purcell, W. A New Crystalline Form of the Rhodium(I) Analogue of Vaska’s Complex: Crystal Structure of trans-Chlorocarbonyl-bis(triphenylphosphine)rhodium(I). Rhodium Express 1995, 12, 21–26. [Google Scholar]
- Basson, S.S.; Leipoldt, J.G.; Roodt, A. trans-Carbonyliodo bis(triphenyl-phosphine)rhodium(I). Acta Cryst. 1990, C46, 142–143. [Google Scholar]
- Botha, L.J.; Basson, S.S.; Leipoldt, J.G. The crystal structure of thioacetylacetonatocarbonyltriphenylphosphinerhodium(I). Inorg. Chim. Acta 1987, 126, 25–28. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Sizova, O.V.; Tunik, S.P.; Lough, A.; Poë, A.J. A Novel Five-Coordinate Rhodium(I) Complex. Eur. J. Inorg. Chem. 2005, 2005, 4516–4520. [Google Scholar] [CrossRef]
- Dilworth, J.R.; Morales, D.; Zheng, Y. Rhodium and iridium complexes with thiolate and tertiary phosphine ligands. The synthesis and structures of trans-[Ir(SC6H3Cl2-2,6)(CO)(PPh3)2], [Rh2(μ-SC6H3Pri3-2,4,6)2(CO)2(PPh3)2], [Rh2H2(μ-SC6H4PPh2-2)2(CO)2(PPh3)2][BF4]2, and [Rh2I6(MeSC6H4PPh2-2)2]. J. Chem. Soc. Dalton Trans. 2000, 17, 3007–3015. [Google Scholar] [CrossRef]
- Pruchnik, F.P.; Smoleñski, P.; Gałdecka, E.; Gałdecki, Z. New water-soluble rhodium(I) complexes containing 1-methyl-1-azonia-3,5-diaza-7-phosphaadamantane iodide. New J. Chem. 1998, 22, 1395–1398. [Google Scholar] [CrossRef]
- Otto, S.; Mzamane, S.N.; Roodt, A. Tuning rhodium(I) metal center accessibility in iodomethane oxidative addition to Vaska-type complexes by interchanging tertiary phosphine for arsine and stibine. In Legacy of Joseph Chatt; Winterton, N., Leigh, G., Eds.; Royal Society of Chemistry: Cambridge, UK, 2001; pp. 675–683. [Google Scholar]
- Schutte-Smith, M.; Roodt, A.; Visser, H.G. Ambient and high-pressure kinetic investigation of methanol substitution in fac-[Re(Trop)(CO)3(MeOH)] by different monodentate nucleophiles. Dalton Trans. 2019, 48, 9984–9997. [Google Scholar] [CrossRef]
- Otto, S.; Roodt, A.; Elding, L.I. Bridge-splitting kinetics, equilibria and structures of trans-bis-cyclooctene complexes of platinum(II). Dalton Trans. 2003, 2519–2525. [Google Scholar] [CrossRef]
- Bruker SAINT-Plus (Version 7.12) and SADABS, Bruker AXS Inc., Madison: Fitchburg, WI, USA, 2004.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND. Visual Crystal Structure Information System; Version 3.0c Crystal Impact; IUCr: Bonn, Germany, 2005. [Google Scholar]
- Scientist for Windows (32). Version 2.01: 1986-1995. Micromath Inc.: Salt Lake City, UT, USA.
- Romeo, R.; Arena, G.; Scolara, L.M.; Plutino, M.R. Ion-pair mechanism in square planar substitution. Reactivity of cationic platinum (II) complexes with negatively charged nucleophiles in solvents of high, medium and low polarity. Inorg. Chim. Acta 1995, 240, 81–92. [Google Scholar] [CrossRef]
- Purcell, W.; Roodt, A.; Basson, S.S.; Leipoldt, J.G. Kinetic study of the reaction between trans-tetracyanodioxorhenate(V) and Thiocyanate ions. Transit. Met. Chem. 1989, 14, 224–226. [Google Scholar] [CrossRef]
- Leipoldt, J.G.; Basson, S.S.; Roodt, A.; Potgieter, I.M. Kinetics and mechanism of fluoride ion substitution in trans-dioxotetracyanotungstate(IV) ions and the crystal structure of K3WOF(CN)4. S. Afr. J. Chem. 1986, 39, 179–183. [Google Scholar]
- Leipoldt, J.G.; Van Eldik, R.; Basson, S.S.; Roodt, A. Kinetics and mechanism of the reaction between trans-dioxotetracyanotungstate (IV) and azide in aqueous solution. Inorg. Chem. 1986, 25, 4639–4642. [Google Scholar] [CrossRef]
- Smit, J.P.; Purcell, W.; Roodt, A.; Leipoldt, J.G. Kinetics of the substitution reaction between aquaoxotetracyanomolybdate (IV) and cyanide/hydrogen cyanide. Polyhedron 1993, 12, 2271–2277. [Google Scholar] [CrossRef]
- Muller, A.J.; Otto, S.; Roodt, A. Rapid phosphorus (III) ligand evaluation utilising potassium selenocyanate. Dalton Trans. 2008, 650–657. [Google Scholar] [CrossRef]
- Atkins, P.; Paula, J.D. Physical Chemistry, 8th ed.; Oxford University Press: Oxford, UK, 2006; pp. 880–883. [Google Scholar]









| Identification Code | B2a (a) | B5a |
|---|---|---|
| Empirical formula | C65H50NO4P3 Rh2 | C43H42IO2P2Rh |
| Formula weight | 632.93 | 898.51 |
| Temperature (K) | 100(2) | 100(2) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system, space group | Triclinic, P | Monoclinic, P21/c |
| Unit cell dimensions | ||
| a (Å) | 14.097(2) | 11.974(5) |
| b (Å) | 14.280(2) | 20.289(5) |
| c (Å) | 17.942(3) | 8.335(5) |
| α (°) | 93.451(6) | 90.000(5) |
| β (°) | 112.987(5) | 98.311(5) |
| γ (°) | 115.164(6) | 90.000(5) |
| Volume (Å3) | 2896.4(8) | 2003.6(2) |
| Z | 2 | 2 |
| Densitycalc (g cm–3) | 1.451 | 1.489 |
| μ (mm−1) | 0.705 | 1.313 |
| F(000) | 1292 | 904 |
| Crystal size (mm3) | 0.196 × 0.240 × 0.414 | 0.163 × 0.189 × 0.471 |
| Theta range (°) | 2.381 to 28.00 | 3.183 to 28.00 |
| Index ranges | −14 ≤ h ≤ 14 | −15 ≤ h ≤ 15 |
| −14 ≤ k ≤ 14 | −26 ≤ k ≤ 26 | |
| −17 ≤ l ≤ 17 | −8 ≤ l ≤ 11 |
| Bond Lengths (Å) | |||||
|---|---|---|---|---|---|
| Rh(1)-C(01) | 1.784(5) | P(2)-C(41) | 1.826(5) | O(2)-C(1) | 1.268(6) |
| Rh(1)-N(1) | 2.065(4) | P(2)-C(61) | 1.835(5) | N(1)-C(2) | 1.384(7) |
| Rh(1)-O(1) | 2.094(3) | P(3)-C(81) | 1.827(5) | C(2)-C(1) | 1.453(7) |
| Rh(1)-P(1) | 2.2626(14) | P(1)-C(21) | 1.837(5) | C(3)-C(2) | 1.373(7) |
| Rh(2)-C(02) | 1.800(6) | P(2)-C(51) | 1.822(5) | C(4)-C(3) | 1.413(7) |
| Rh(2)-O(2) | 2.080(3) | O(01)-C(01) | 1.162(6) | C(4)-C(5) | 1.411(8) |
| Rh(2)-P(2) | 2.3355(14) | O(02)-C(02) | 1.149(6) | C(6)-C(5) | 1.367(8) |
| Rh(2)-P(3) | 2.3415(14) | O(1)-C(1) | 1.280(6) | C(7)-C(6) | 1.406(8) |
| P(1)-C(11) | 1.828(5) | C(9)-N(1) | 1.370(7) | C(8)-C(7) | 1.386(8) |
| P(1)-C(31) | 1.832(5) | C(9)-C(8) | 1.408(7) | C(9)-C(4) | 1.431(7) |
| Rh(2)-H96 | 2.786(1) | N(1)--O(1) | 2.654(1) | H(14)-H(94) | 16.09(3) |
| Bond Angles (°) | |||||
| C(01)-Rh(1)-N(1) | 98.0(2) | N(1)-Rh(1)-P(1) | 171.10(12) | C(7)-C(8)-C(9) | 117.5(5) |
| C(01)-Rh(1)-O(1) | 176.1(2) | C(11)-P(1)-Rh(1) | 116.46(17) | C(3)-C(2)-N(1) | 112.4(4) |
| N(1)-Rh(1)-O(1) | 79.32(15) | N(1)-Rh(1)-O(1) | 79.32(15) | C(3)-C(2)-C(1) | 132.0(4) |
| C(01)-Rh(1)-P(1) | 90.07(17) | C(21)-P(1)-Rh(1) | 119.01(17) | N(1)-C(2)-C(1) | 115.6(4) |
| N(1)-Rh(1)-P(1) | 171.10(12) | C(1)-O(2)-Rh(2) | 120.0(3) | C(8)-C(7)-C(6) | 122.0(5) |
| O(1)-Rh(1)-P(1) | 92.41(10) | C(1)-O(1)-Rh(1) | 114.4(3) | C(5)-C(6)-C(7) | 121.2(5) |
| C(02)-Rh(2)-O(2) | 174.9(2) | N(1)-C(9)-C(8) | 129.6(5) | C(6)-C(5)-C(4) | 118.8(5) |
| C(02)-Rh(2)-P(2) | 91.37(16) | N(1)-C(9)-C(4) | 109.8(4) | O(2)-C(1)-O(1) | 122.4(4) |
| O(2)-Rh(2)-P(2) | 88.66(10) | O(01)-C(01)-Rh(1) | 177.0(5) | O(2)-C(1)-C(2) | 120.0(4) |
| C(02)-Rh(2)-P(3) | 86.54(16) | C(9)-N(1)-Rh(1) | 141.7(3) | O(1)-C(1)-C(2) | 117.6(4) |
| O(2)-Rh(2)-P(3) | 92.71(10) | C(2)-N(1)-Rh(1) | 112.3(3) | C(2)-C(3)-C(4) | 106.0(4) |
| P(2)-Rh(2)-P(3) | 171.80(5) | O(02)-C(02)-Rh(2) | 177.3(5) | C(9)-N(1)-C(2) | 105.5(4) |
| C(02)-Rh(2)-O(2) | 174.9(2) | C(5)-C(4)-C(9) | 119.8(5) | C(8)-C(9)-C(4) | 120.6(5) |
| C(01)-Rh(1)-O(1) | 176.1(2) | C(3)-C(4)-C(9) | 106.3(4) | C(5)-C(4)-C(3) | 134.0(5) |
| Bond Lengths (Å) | |||
|---|---|---|---|
| I(1)-Rh(1) | 2.7103(7) | P(1)-C(11) | 1.820(3) |
| Rh(1)-C(01) | 1.725(5) | P(1)-C(31) | 1.822(3) |
| Rh(1)-P(1) | 2.3178(9) | P(1)-C(21) | 1.828(3) |
| Rh(1)-C(01) #1 | 1.725(5) | O(01)-C(01) | 0.995(5) |
| Bond Angles (°) | |||
| I(1)-C(01)-Rh(1) | 169.6(6) | C(11)-P(1)-Rh(1) | 110.67(9) |
| C(01)-Rh(1)-P(1) #1 | 94.01(18) | C(21)-P(1)-Rh(1) | 119.53(9) |
| C(01) #1-Rh(1)-P(1) #1 | 85.99(18) | C(31)-P(1)-Rh(1) | 113.53(9) |
| C(01)-Rh(1)-P(1) | 85.99(18) | P(1) #1-Rh(1)-I(1) #1 | 87.60(3) |
| C(01) #1-Rh(1)-I(1) | 176.2(2) | C(31)-P(1)-C(11) | 107.50(12) |
| P(1) #1-Rh(1)-I(1) | 92.40(3) | C(31)-P(1)-C(21) | 101.23(13) |
| P(1)-Rh(1)-I(1) | 87.59(3) | C(11)-P(1)-C(21) | 103.20(13) |
| Rh(Indoli)(CO)(PPh3)] (A2) (a,b) | Activation Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Temp (°C) | 102 k1 (M−1 s−1) | 104 k−1 (s−1) | K1(c) (M−1) | ΔH≠ (kJ mol−1) | ΔS≠ (J K−1 mol−1) | ΔG≠ (d) (kJ mol−1) | % ΔS≠ to ΔG≠ (d) |
| 4.5 | 0.83 ± 0.01 | 2.5 ± 0.4 | 33 ± 5 | 20 ± 2 (e) | −210 ± 6 (e) | 83 ± 3 | 76 |
| 14.7 | 1.18 ± 0.04 | 3 ± 1 | 39 ± 13 | ||||
| 26.1 | 1.71 ± 0.01 | 5.8 ± 0.5 | 29 ± 2 | 21 ± 1 (f) | −209 ± 4 (f) | 83 ± 2 | 75 |
| [Rh1(Indol’)(CO)(PPh3)Rh2(CO)(PPh3)2] (B2) (g,h) | |||||||
| 7.1 | 1.70 ± 0.04 | 5 ± 3 | 66 ± 2 (e) | −40 ± 6 (e) | 78 ± 3 | 15 | |
| 14.5 | 3.41 ± 0.01 | 0 | >100 (i) | ||||
| 24.8 | 8.6 ± 0.01 | 5 ± 5 | 73.0 ± 1.2 (f) | −21 ± 4 (f) | 79 ± 2 | 8 | |
| 37.1 | 30.8 ± 0.07 | 0 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmakki, M.A.E.; Alexander, O.T.; Venter, G.J.S.; Venter, J.A.; Roodt, A. Structural Study of Model Rhodium(I) Carbonylation Catalysts Activated by Indole-2-/Indoline-2-Carboxylate Bidentate Ligands and Kinetics of Iodomethane Oxidative Addition. Inorganics 2022, 10, 251. https://doi.org/10.3390/inorganics10120251
Elmakki MAE, Alexander OT, Venter GJS, Venter JA, Roodt A. Structural Study of Model Rhodium(I) Carbonylation Catalysts Activated by Indole-2-/Indoline-2-Carboxylate Bidentate Ligands and Kinetics of Iodomethane Oxidative Addition. Inorganics. 2022; 10(12):251. https://doi.org/10.3390/inorganics10120251
Chicago/Turabian StyleElmakki, Mohammed A. E., Orbett Teboho Alexander, Gertruida J. S. Venter, Johan Andries Venter, and Andreas Roodt. 2022. "Structural Study of Model Rhodium(I) Carbonylation Catalysts Activated by Indole-2-/Indoline-2-Carboxylate Bidentate Ligands and Kinetics of Iodomethane Oxidative Addition" Inorganics 10, no. 12: 251. https://doi.org/10.3390/inorganics10120251
APA StyleElmakki, M. A. E., Alexander, O. T., Venter, G. J. S., Venter, J. A., & Roodt, A. (2022). Structural Study of Model Rhodium(I) Carbonylation Catalysts Activated by Indole-2-/Indoline-2-Carboxylate Bidentate Ligands and Kinetics of Iodomethane Oxidative Addition. Inorganics, 10(12), 251. https://doi.org/10.3390/inorganics10120251