A One-Dimensional Cu(I) Coordination Polymer with Optical Sensing of Oxygen and Temperature
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of [Cu2I2(TPP)2(BPB)]·CH3OH (1·g)
2.2. Crystal Structures of 1 and 1·g
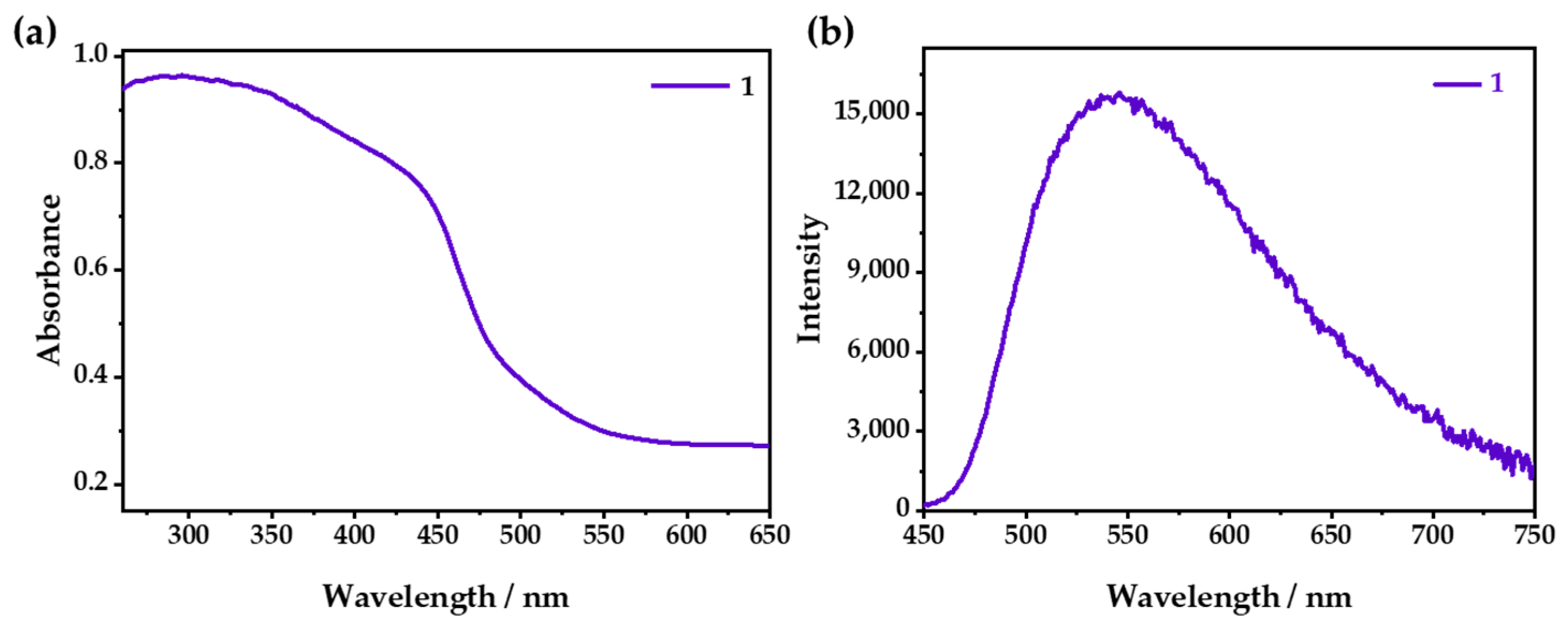
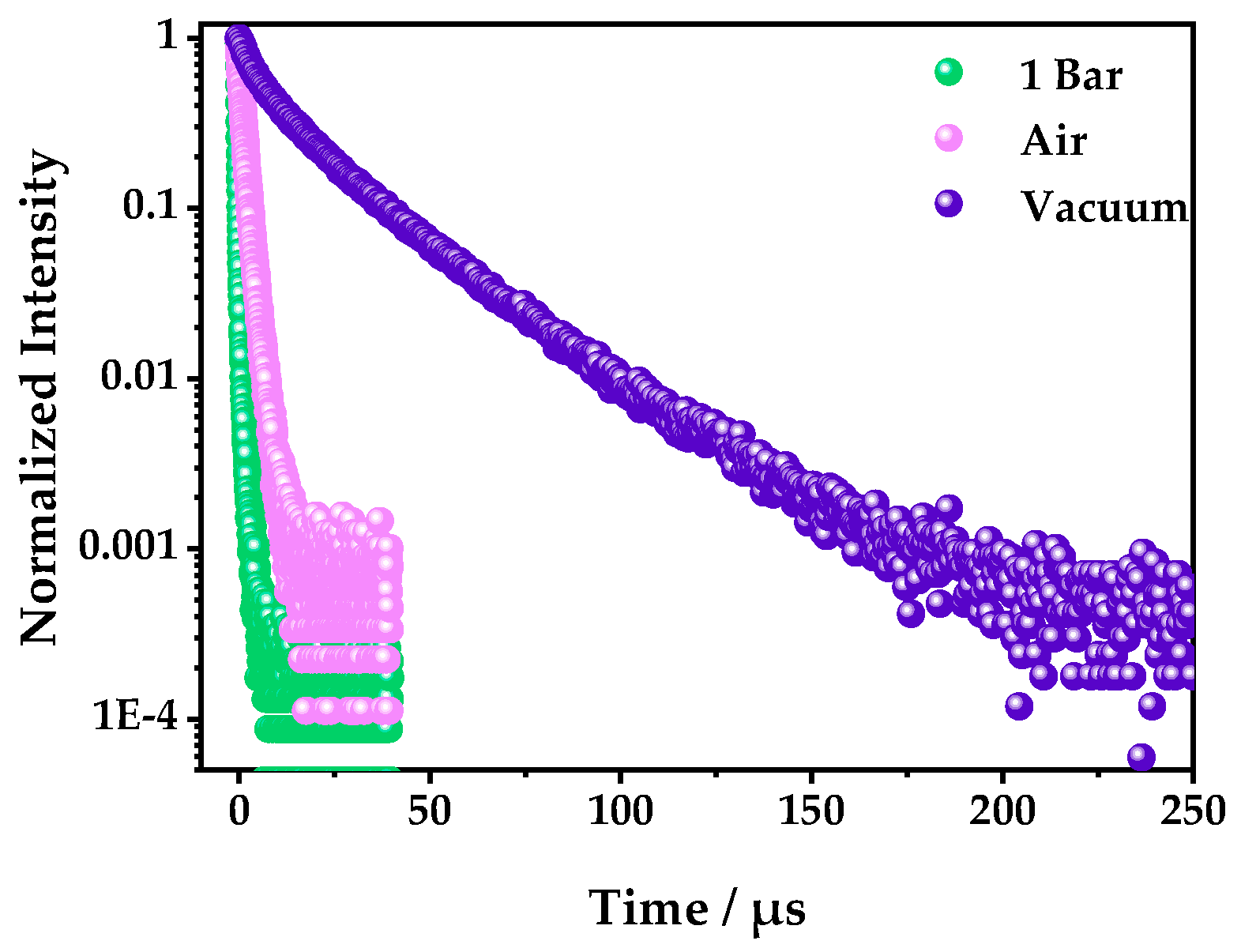
2.3. Optical O2-Sensing Properties
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Y.; Wu, H.; Cao, W.; Cui, Y.; Qian, G. Luminescent Metal–Organic Frameworks for White LEDs. Adv. Opt. Mater. 2020, 9, 2001817. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.F.; Lv, Y.K. Luminescent switch sensors for the detection of biomolecules based on metal-organic frameworks. Analyst 2018, 143, 4221–4229. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, G.; Giambastiani, G.; Rossin, A. Thiazole- and Thiadiazole-Based Metal–Organic Frameworks and Coordination Polymers for Luminescent Applications. Inorganics 2019, 7, 144. [Google Scholar] [CrossRef]
- Chen, L.; Dong, X.-B.; Liao, H.-Y.; Zhang, W.-J.; Mo, Z.-W.; Wang, H.-P.; Ye, J.-W.; Chen, X.-M. Long-Range Rigidity Induced Ultralong Cluster-Centered Phosphorescence. Chem. Mater. 2022, 34, 9182–9189. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [PubMed]
- Mills, A. Oxygen indicators and intelligent inks for packaging food. Chem. Soc. Rev. 2005, 34, 1003–1011. [Google Scholar] [CrossRef]
- Hei, X.; Zhu, K.; Carignan, G.; Teat, S.J.; Li, M.; Zhang, G.; Bonite, M.; Li, J. Solution-processable copper(I) iodide-based inorganic-organic hybrid semiconductors composed of both coordinate and ionic bonds. J. Solid State Chem. 2022, 314, 123427. [Google Scholar] [CrossRef]
- Ye, J.-W.; Li, X.-Y.; Zhou, H.-L.; Zhang, J.-P. Optimizing luminescence sensitivity and moisture stability of porous coordination frameworks by varying ligand side groups. Sci. China Chem. 2018, 62, 341–346. [Google Scholar] [CrossRef]
- Yang, T.; Dai, S.; Tan, H.; Zong, Y.; Liu, Y.; Chen, J.; Zhang, K.; Wu, P.; Zhang, S.; Xu, J.; et al. Mechanism of Photoluminescence in Ag Nanoclusters: Metal-Centered Emission versus Synergistic Effect in Ligand-Centered Emission. J. Phys. Chem. C 2019, 123, 18638–18645. [Google Scholar] [CrossRef]
- Chen, L.; Dong, X.B.; Mo, Z.W.; Wang, H.P.; Ye, J.W.; Zhang, K.; Chen, X.M. Efficient Restraint of Intra-Cluster Aggregation-Caused Quenching Effect Lighting Room-Temperature Photoluminescence. Adv. Opt. Mater. 2021, 9, 2100757. [Google Scholar] [CrossRef]
- Lou, X.Y.; Yang, Y.W. Pyridine-Conjugated Pillar[5]arene: From Molecular Crystals of Blue Luminescence to Red-Emissive Coordination Nanocrystals. J. Am. Chem. Soc. 2021, 143, 11976–11981. [Google Scholar] [CrossRef] [PubMed]
- Juvenal, F.; Fortin, D.; Harvey, P.D. A Platinum(II) Organometallic Building Block for the Design of Emissive Copper(I) and Silver(I) Coordination Polymers. Inorg. Chem. 2020, 59, 7117–7134. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Morgan, E.E.; Vishnoi, P.; Mao, L.; Teicher, S.M.L.; Wu, G.; Liu, Q.; Cheetham, A.K.; Seshadri, R. Tunable Luminescence in Hybrid Cu(I) and Ag(I) Iodides. Inorg. Chem. 2020, 59, 15487–15494. [Google Scholar] [CrossRef] [PubMed]
- Pander, P.; Sil, A.; Salthouse, R.J.; Harris, C.W.; Walden, M.T.; Yufit, D.S.; Williams, J.A.G.; Dias, F.B. Excimer or aggregate? Near infrared electro- and photoluminescence from multimolecular excited states of N^C^N-coordinated platinum(ii) complexes. J. Mater. Chem. C 2022, 10, 15084–15095. [Google Scholar] [CrossRef]
- Gao, X.; Ge, F.; Zheng, H. Improving the Stability and Visualizing the Structural Transformation of the Stimuli-Responsive Metal-Organic Frameworks (MOFs). Inorg. Chem. 2020, 59, 5093–5098. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Qin, J.S.; Huang, L.; Bose, R.; Pang, J.; Zhang, P.; Xiao, Z.; Tan, K.; Malko, A.V.; et al. Fluorescence Enhancement in the Solid State by Isolating Perylene Fluorophores in Metal-Organic Frameworks. ACS Appl. Mater. Inter. 2020, 12, 26727–26732. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X. Luminescent Organic-Inorganic Hybrid Metal Halides: An Emerging Class of Stimuli-Responsive Materials. Chemistry 2022, 28, e202200609. [Google Scholar]
- Dey, A.; Garai, A.; Gude, V.; Biradha, K. Thermochromic, Solvatochromic, and Piezochromic Cd(II) and Zn(II) Coordination Polymers: Detection of Small Molecules by Luminescence Switching from Blue to Green. Cryst. Growth Des. 2018, 18, 6070–6077. [Google Scholar] [CrossRef]
- Devkota, J.; Kim, K.J.; Ohodnicki, P.R.; Culp, J.T.; Greve, D.W.; Lekse, J.W. Zeolitic imidazolate framework-coated acoustic sensors for room temperature detection of carbon dioxide and methane. Nanoscale 2018, 10, 8075–8087. [Google Scholar] [CrossRef] [PubMed]
- Artem’ev, A.V.; Davydova, M.P.; Berezin, A.S.; Samsonenko, D.G. Synthesis and Thermochromic Luminescence of Ag(I) Complexes Based on 4,6-Bis(diphenylphosphino)-Pyrimidine. Inorganics 2020, 8, 46. [Google Scholar] [CrossRef]
- Cappuccino, C.; Farinella, F.; Braga, D.; Maini, L. Mechanochemistry, an Easy Technique to Boost the Synthesis of CuI Pyrazine Coordination Polymers. Cryst. Growth Des. 2019, 19, 4395–4403. [Google Scholar] [CrossRef]
- Ki, W.; Hei, X.; Yi, H.T.; Liu, W.; Teat, S.J.; Li, M.; Fang, Y.; Podzorov, V.; Garfunkel, E.; Li, J. Two-Dimensional Copper Iodide-Based Inorganic–Organic Hybrid Semiconductors: Synthesis, Structures, and Optical and Transport Properties. Chem. Mater. 2021, 33, 5317–5325. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Q.; Li, X.; Miao, C.; Hou, Q.; Ai, S. A Cu(i)–I coordination polymer fluorescent chemosensor with amino-rich sites for nitro aromatic compound (NAC) detection in water. CrystEngComm 2020, 22, 5690–5697. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Davydova, M.P.; Hei, X.; Rakhmanova, M.I.; Samsonenko, D.G.; Bagryanskaya, I.Y.; Brylev, K.A.; Fedin, V.P.; Chen, J.-S.; Cotlet, M.; et al. Family of Robust and Strongly Luminescent CuI-Based Hybrid Networks Made of Ionic and Dative Bonds. Chem. Mater. 2020, 32, 10708–10718. [Google Scholar] [CrossRef]
- Li, R.-Z.; Li, D.; Huang, X.-C.; Qi, Z.-Y.; Chen, X.-M. A photoluminescent polymeric chain complex: Synthesis and structure of [(PPh3)2Cu2(μ-I)2(μ-4,4′-bpy)]n. Inorg. Chem. Commun. 2003, 6, 1017–1019. [Google Scholar] [CrossRef]
- Agarwal, R.A. One Dimensional Coordination Polymer of Zn(II) for Developing Multifunctional Nanoparticles. Sci. Rep. 2017, 7, 13212. [Google Scholar] [CrossRef]
- Vizuet, J.P.; Howlett, T.S.; Lewis, A.L.; Chroust, Z.D.; McCandless, G.T.; Balkus, K.J., Jr. Transition from a 1D Coordination Polymer to a Mixed-Linker Layered MOF. Inorg. Chem. 2019, 58, 5031–5041. [Google Scholar] [CrossRef]
- Andrzejewski, M.; Katrusiak, A. Piezochromic Porous Metal-Organic Framework. J. Phys. Chem. Lett. 2017, 8, 279–284. [Google Scholar] [CrossRef]
- Bai, L.; Bose, P.; Gao, Q.; Li, Y.; Ganguly, R.; Zhao, Y. Halogen-Assisted Piezochromic Supramolecular Assemblies for Versatile Haptic Memory. J. Am. Chem. Soc. 2017, 139, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, Q.; Yang, M.; Shao, B.; Ouyang, R.; Guo, N. Thermal Quenching Mechanism of Metal-Metal Charge Transfer State Transition Luminescence Based on Double-Band-Gap Modulation. Inorg. Chem. 2022, 61, 9823–9831. [Google Scholar] [CrossRef]
- Carstea, E.M.; Baker, A.; Bieroza, M.; Reynolds, D.M.; Bridgeman, J. Characterisation of dissolved organic matter fluorescence properties by PARAFAC analysis and thermal quenching. Water Res. 2014, 61, 152–161. [Google Scholar] [CrossRef]
- Liao, W.-M.; Li, X.-N.; Zeng, Q.; Zhong, Y.-H.; Yin, Y.-G.; He, J. Enantiomerism, diastereomerism and thermochromism in two Cu7I4 cluster-based coordination polymers. J. Mater. Chem. C 2019, 7, 15136–15140. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Excited State Energy Transfer in Metal-Organic Frameworks. Adv. Mater. 2021, 33, e2005819. [Google Scholar] [CrossRef] [PubMed]
- Artem’ev, A.V.; Baranov, A.Y.; Rakhmanova, M.I.; Malysheva, S.F.; Samsonenko, D.G. Copper(i) halide polymers derived from tris[2-(pyridin-2-yl)ethyl]phosphine: Halogen-tunable colorful luminescence spanning from deep blue to green. N. J. Chem. 2020, 44, 6916–6922. [Google Scholar] [CrossRef]
- López, J.; Murillo, M.; Lifante-Pedrola, G.; Cantelar, E.; Gonzalez-Platas, J.; Rodríguez-Mendoza, U.R.; Amo-Ochoa, P. Multi-stimulus semiconductor Cu(i)–I-pyrimidine coordination polymer with thermo- and mechanochromic sensing. CrystEngComm 2022, 24, 341–349. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhou, D.D.; He, C.T.; Liao, P.Q.; Cheng, X.N.; Xu, Y.T.; Ye, J.W.; Zhang, J.P.; Chen, X.M. Flexible, Luminescent Metal-Organic Frameworks Showing Synergistic Solid-Solution Effects on Porosity and Sensitivity. Angew. Chem. Int. Ed. 2016, 55, 16021–16025. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Qi, X.-L.; Lin, R.-B.; Cheng, X.-N.; Liao, P.-Q.; Zhang, J.-P.; Chen, X.-M. Porous Cu(I) Triazolate Framework and Derived Hybrid Membrane with Exceptionally High Sensing Efficiency for Gaseous Oxygen. Adv. Funct. Mater. 2014, 24, 5866–5872. [Google Scholar] [CrossRef]
- Filatov, M.A.; Baluschev, S.; Landfester, K. Protection of densely populated excited triplet state ensembles against deactivation by molecular oxygen. Chem. Soc. Rev. 2016, 45, 4668–4689. [Google Scholar] [CrossRef]
- Li, M.; Cheng, Z.; Wang, X.; Yu, Z.; Zhou, M.; Miao, H.; Zhaxi, W.; Huang, W.; Ma, X.; Chen, Q.; et al. Negative/Zero Thermal Quenching of Luminescence via Electronic Structural Transition in Copper-Iodide Cluster-Based Coordination Networks. J. Phys. Chem. Lett. 2021, 12, 8237–8245. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wen, M.-T.; Shen, M.-L.; Miao, W.-N.; He, T.-T.; Xu, L. A new 3D cadmium coordination polymer containing 3-amino-1H-1,2,4-triazole: Synthesis, structure, and property. Inorg. Chem. Commun. 2018, 88, 38–41. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, S.; Samanta, P.N.; Xie, Y.; Li, J.; Wang, X.; Devashis, M.; Gu, X.; Wang, Y.; Huang, W.; et al. Negative thermal quenching of photoluminescence in a copper-organic framework emitter. Chem. Commun. 2020, 56, 12057–12060. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-T.; Li, C.-H.; Zhou, W.-Q.; Huang, J.-T.; Ye, J.-W.; Chen, L. A One-Dimensional Cu(I) Coordination Polymer with Optical Sensing of Oxygen and Temperature. Inorganics 2022, 10, 253. https://doi.org/10.3390/inorganics10120253
Chen W-T, Li C-H, Zhou W-Q, Huang J-T, Ye J-W, Chen L. A One-Dimensional Cu(I) Coordination Polymer with Optical Sensing of Oxygen and Temperature. Inorganics. 2022; 10(12):253. https://doi.org/10.3390/inorganics10120253
Chicago/Turabian StyleChen, Wan-Tao, Chen-Hui Li, Wan-Qing Zhou, Jing-Tao Huang, Jia-Wen Ye, and Ling Chen. 2022. "A One-Dimensional Cu(I) Coordination Polymer with Optical Sensing of Oxygen and Temperature" Inorganics 10, no. 12: 253. https://doi.org/10.3390/inorganics10120253
APA StyleChen, W.-T., Li, C.-H., Zhou, W.-Q., Huang, J.-T., Ye, J.-W., & Chen, L. (2022). A One-Dimensional Cu(I) Coordination Polymer with Optical Sensing of Oxygen and Temperature. Inorganics, 10(12), 253. https://doi.org/10.3390/inorganics10120253







