Kinetic Control of Anion Stoichiometry in Hexagonal BaTiO3
Abstract
:1. Introduction
2. Experimental Section
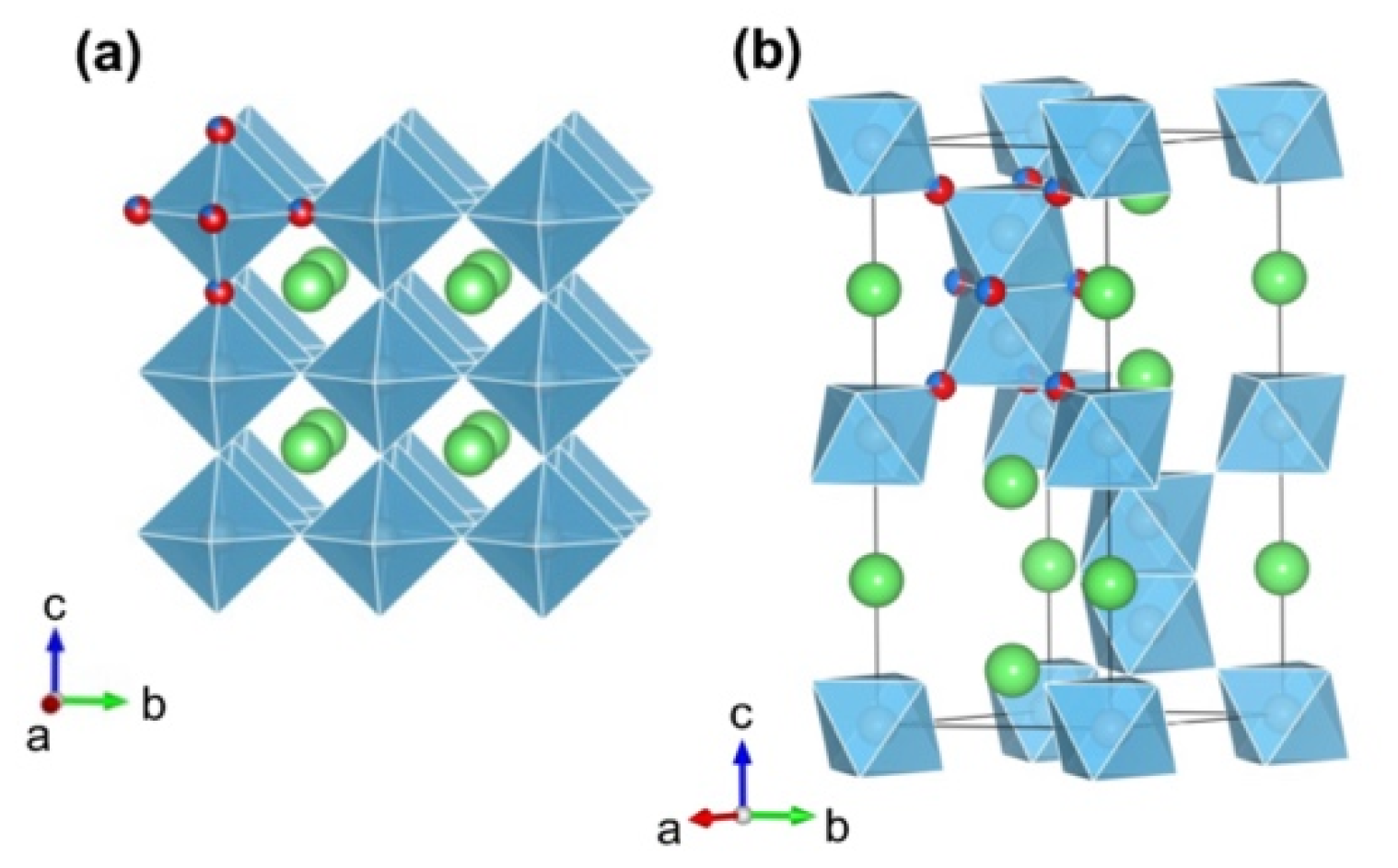
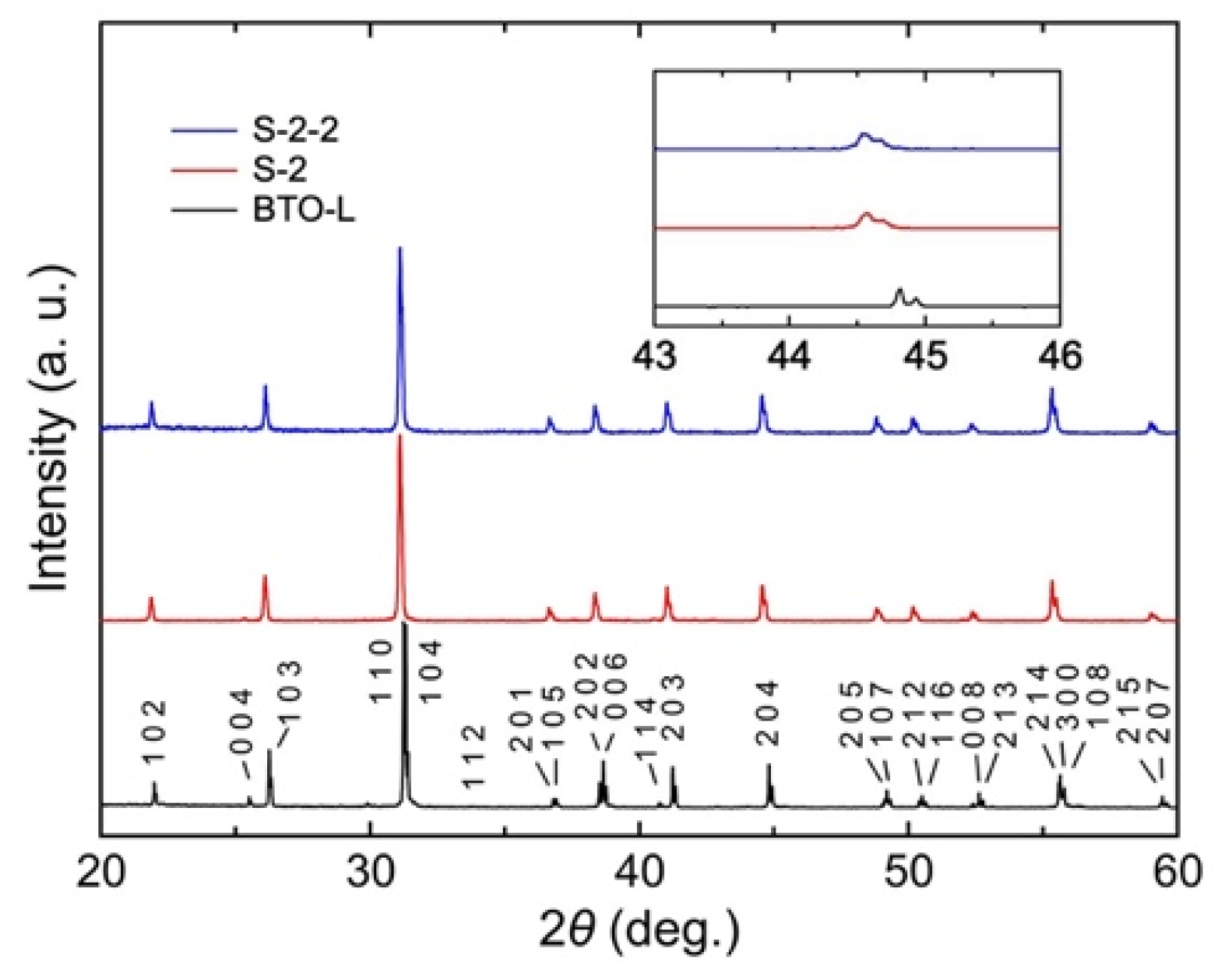
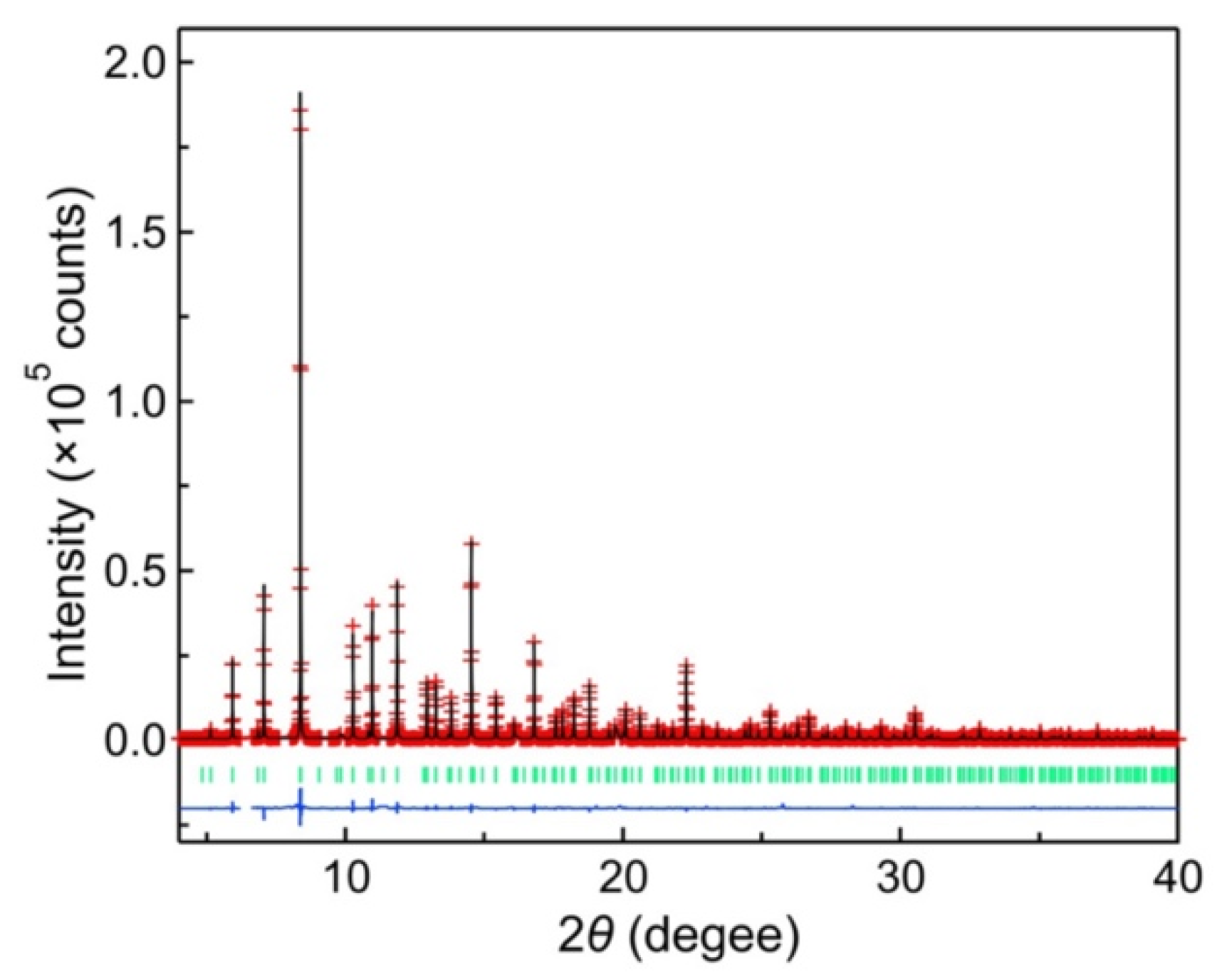
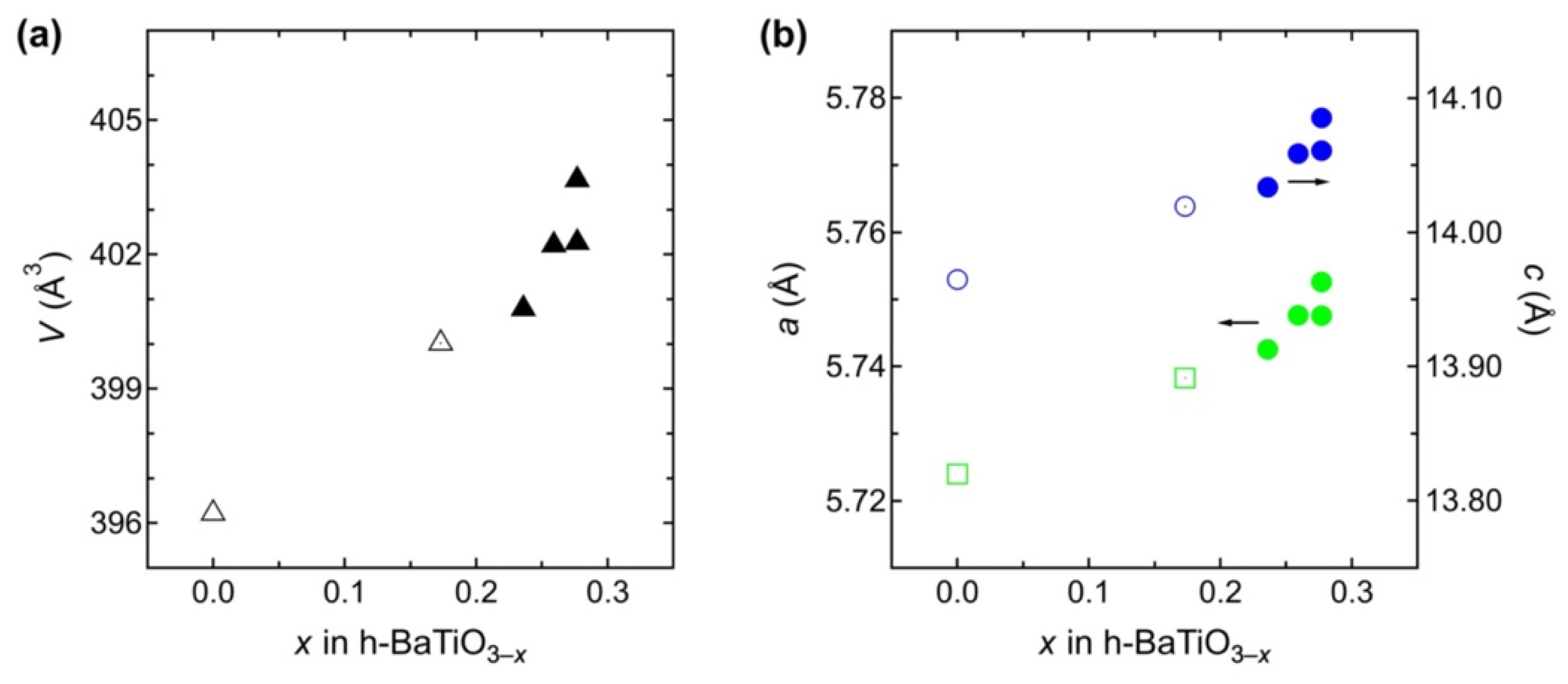
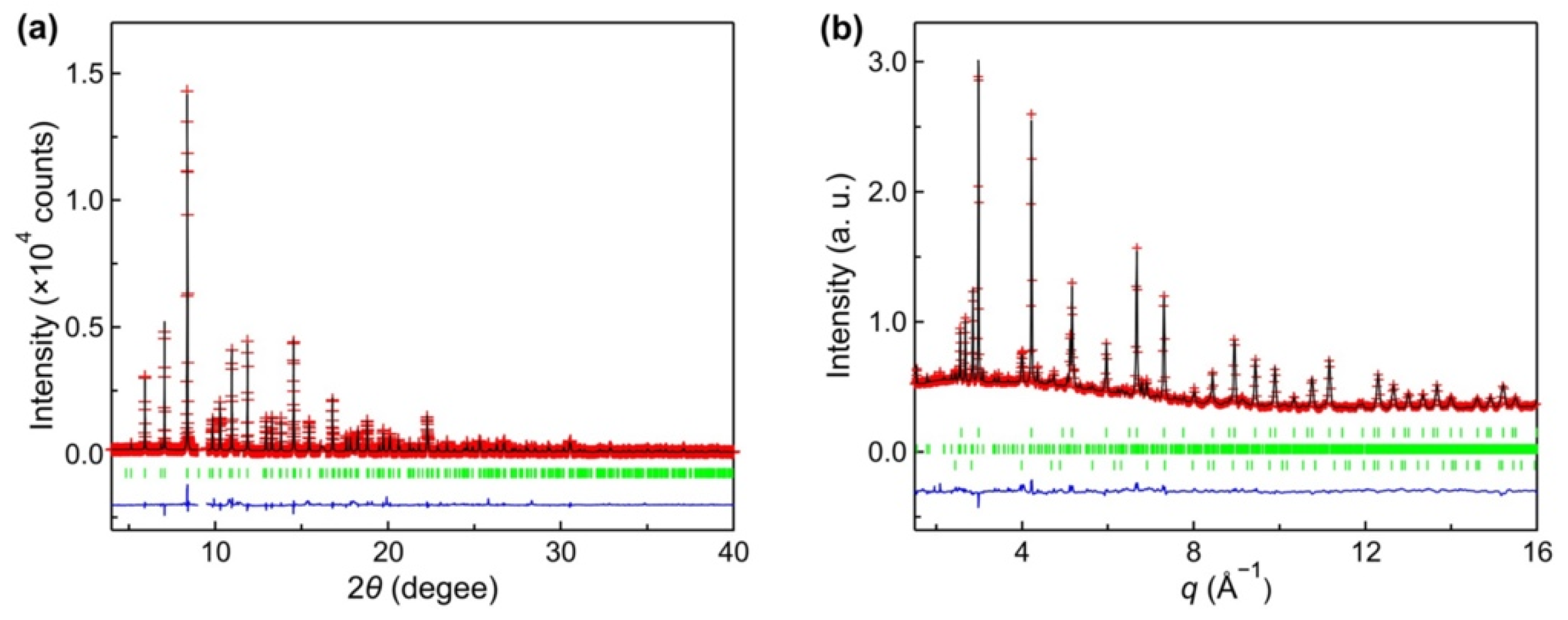
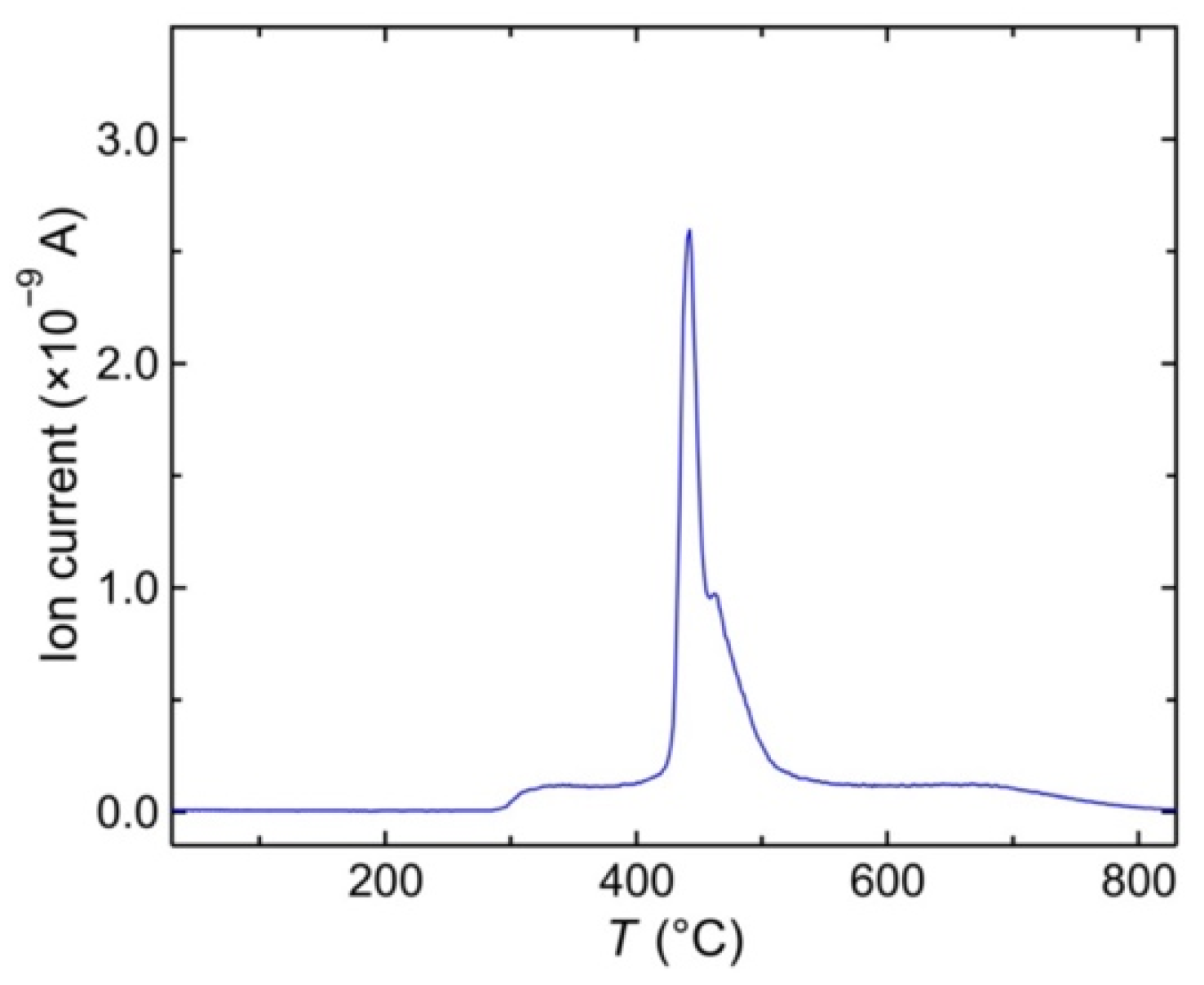
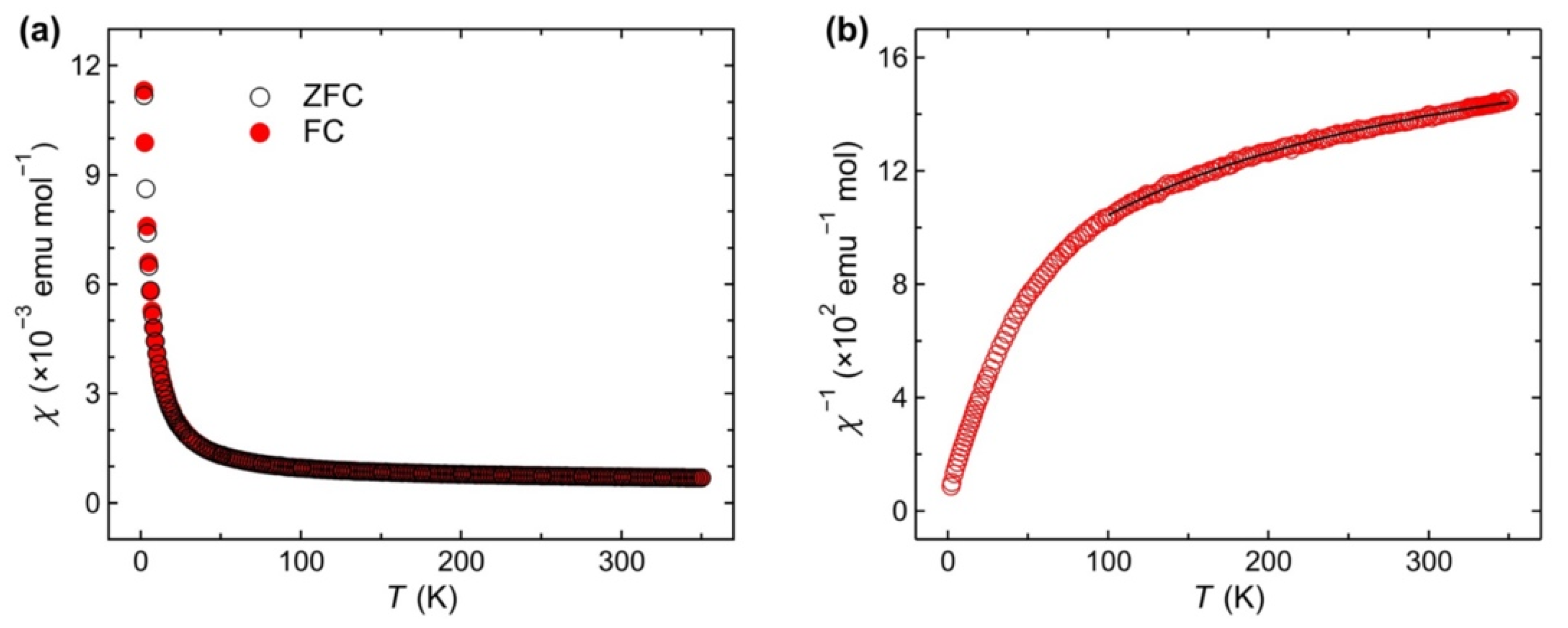
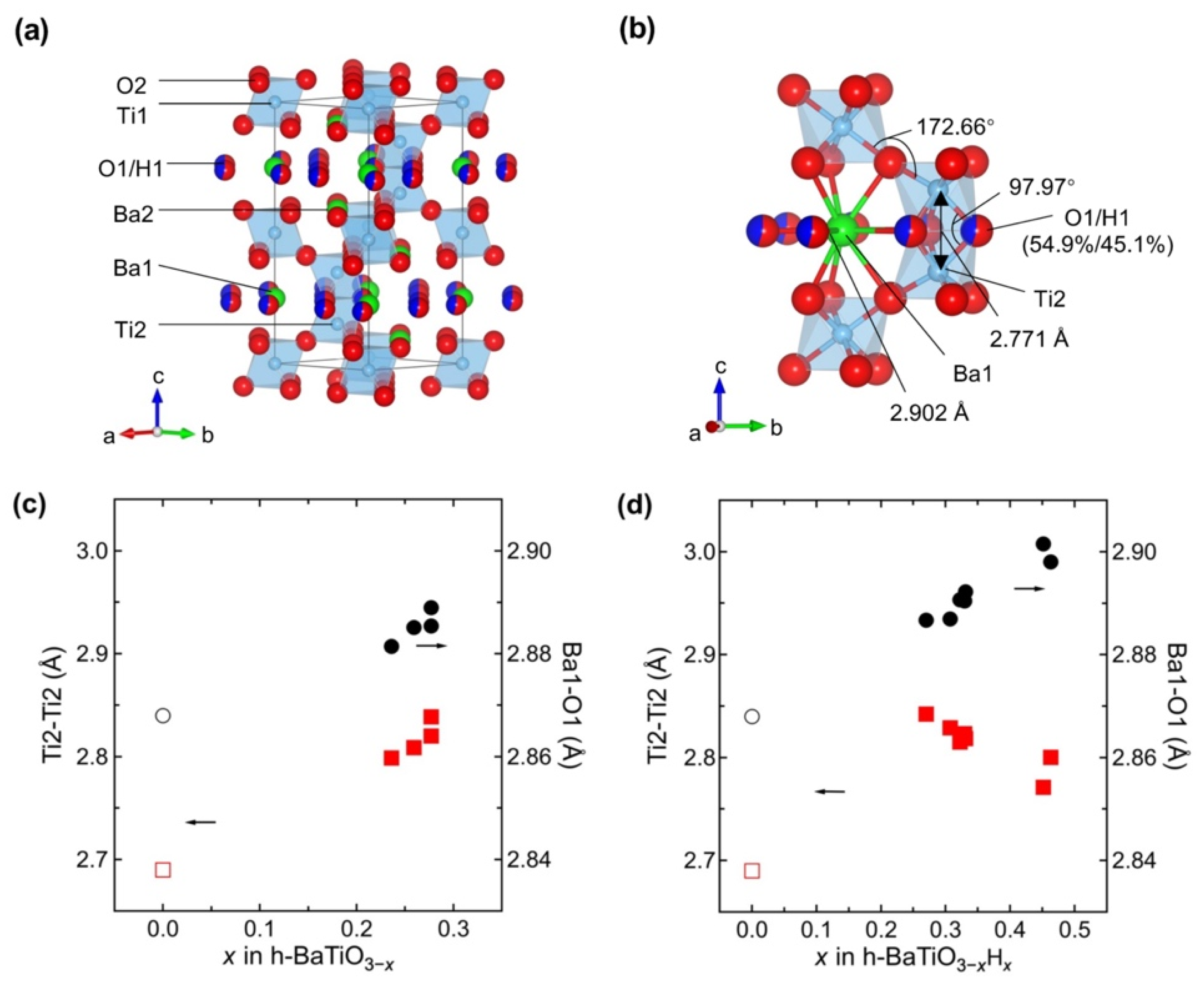
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kageyama, H.; Hayashi, K.; Maeda, K.; Attfield, J.P.; Hiroi, Z.; Rodonelli, J.M.; Poeppelmeier, K.R. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 2018, 9, 772. [Google Scholar] [CrossRef]
- Ochi, M.; Kuroki, K. Effective Interaction for Vanadium Oxyhydrides Srn+1VnO2n+1Hn (n = 1 and n → ∞): A Constrained-RPA Study. Phys. Rev. B Condens. Matter Mater. Phys. 2019, 99, 155143. [Google Scholar] [CrossRef] [Green Version]
- Tassel, C.; Goto, Y.; Kuno, Y.; Hester, J.; Green, M.; Kobayashi, Y.; Kageyama, H. Direct Synthesis of Chromium Perovskite Oxyhydride with a High Magnetic-Transition Temperature. Angew. Chem. Int. Ed. 2014, 53, 10377–10380. [Google Scholar] [CrossRef] [PubMed]
- Ubukata, H.; Takeiri, F.; Shitara, K.; Tassel, C.; Saito, T.; Kamiyama, T.; Broux, T.; Kuwabara, A.; Kobayashi, G.; Kageyama, H. Anion Ordering Enables Fast H– Conduction at Low Temperatures. Sci. Adv. 2021, 7, eabf7883/1-7. [Google Scholar] [CrossRef]
- Hayward, M.A.; Cussen, E.; Claridge, J.; Bieringer, M.; Rosseinsky, M.; Kiely, C.; Blundell, S.; Marshall, I.; Pratt, F. The hydride anion in an extended transition metal oxide array: LaSrCoO3H0.7. Science 2002, 295, 1882–1884. [Google Scholar] [CrossRef] [Green Version]
- Devonshire, A.F. CIX. Theory of barium titanate—Part II. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1951, 42, 1065–1079. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hernandez, O.J.; Sakaguchi, T.; Yajima, T.; Roisnel, T.; Tsujimoto, Y.; Morita, M.; Noda, Y.; Mogami, Y.; Kitada, A.; et al. An oxyhydride of BaTiO3 exhibiting hydride exchange and electronic conductivity. Nat. Mater. 2012, 11, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tang, Y.; Kageyama, T.; Yamashita, H.; Masuda, N.; Hosokawa, H.; Kageyama, H. Titanium-Based Hydrides as Heterogeneous Catalysts for Ammonia Synthesis. J. Am. Chem. Soc. 2017, 139, 18240–18246. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Kobayashi, Y.; Tassel, C.; Yamamoto, T.; Kageyama, H. Hydride-Enhanced CO2 Methanation: Water-Stable BaTiO2.4H0.6 as a New Support. Adv. Energy Mater. 2018, 8, 1800800. [Google Scholar] [CrossRef]
- Widerøe, K.; Fjellvag, H.; Norby, T.; Willy, F.; Rolf, P.; Berg, W. NdHO, a novel oxyhydride. J. Solid State Chemi. 2011, 184, 1890–1894. [Google Scholar] [CrossRef]
- Bang, J.; Matsuishi, S.; Hiraka, H.; Fujisaki, F.; Otomo, T.; Maki, S.; Yamaura, J.; Kumai, R.; Murakami, Y.; Hosono, H. Hydrogen Ordering and New Polymorph of Layered Perovskite Oxyhydrides: Sr2VO4−xHx. J. Am. Chem. Soc. 2014, 136, 7221–7224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tassel, C.; Goto, Y.; Watabe, D.; Tang, Y.; Lu, H.; Kuno, Y.; Takeiri, F.; Yamamoto, T.; Brown, C.M.; Hester, J.; et al. High-Pressure Synthesis of Manganese Oxyhydride with Partial Anion Order. Angew. Chem. Int. Ed. 2016, 55, 9667–9670. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Tassel, C.; Noda, Y.; Hernandez, O.; Pickard, C.J.; Green, M.A.; Sakaebe, H.; Taguchi, N.; Uchimoto, Y.; Kobayashi, Y.; et al. Pressure-Stabilized Cubic Perovskite Oxyhydride BaScO2H. Inorg. Chem. 2017, 56, 4840–4845. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Shitara, K.; Kitagawa, S.; Kuwabara, A.; Kuroe, M.; Ishida, K.; Ochi, M.; Kuroki, K.; Fujii, K.; Yashima, M.; et al. Selective Hydride Occupation in BaVO3−xHx (0.3 ≤ x ≤ 0.8) with Face- and Corner-Shared Octahedra. Chem. Mater. 2018, 30, 1566–1574. [Google Scholar] [CrossRef]
- Higashi, K.; Ochi, M.; Nambu, Y.; Yamamoto, T.; Murakami, T.; Yamashina, N.; Tassel, C.; Matsumoto, Y.; Takatsu, H.; Brown, M.C.; et al. Enhanced Magnetic Interaction by Face-Shared Hydride Anions in 6H-BaCrO2H. Inorg. Chem. 2021, 60, 11957–11963. [Google Scholar] [CrossRef]
- Recnik, A.; Kolar, D. Exaggerated Growth of Hexagonal Barium Titanate under Reducing Sintering conditions. J. Am. Ceram. Soc. 1996, 79, 1015–1018. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-Dimensional Visualization in Powder Diffraction. Solid State Phenom. 2007, 130, 15. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Oishi, R.; Yonemura, M.; Nishimaki, Y.; Torii, S.; Hoshikawa, A.; Ishigaki, T.; Morishima, T.; Mori, K.; Kamiyama, T. Rietveld analysis software for J-PARC. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 600, 94–96. [Google Scholar] [CrossRef]
- Oishi, R.; Yonemura, M.; Morishima, T.; Hoshikawa, A.; Torii, S.; Ishigaki, T.; Kamiyama, T. Application of matrix decomposition algorithms for singular matrices to the Pawley method in Z-Rietveld. J. Appl. Crystallogr. 2012, 45, 299–308. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter Mater. Phys. 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togo, A. SPGLIB: A C Library for Finding and Handling Crystal Symmetries. Available online: https://atztogo.github.io/spglib/ (accessed on 6 December 2017).
- Wang, L.; Maxisch, T.; Ceder, G. Oxidation energies of transition metal oxides within the GGA U framework. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 73, 195107. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Hautier, G.; Ong, S.P.; Moore, C.J.; Fischer, C.C.; Persson, K.A.; Ceder, G. Formation enthalpies by mixing GGA and GGA U calculations. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 84, 045115. [Google Scholar] [CrossRef] [Green Version]
- Dudarev, S.; Botton, G.; Savrasov, S.; Humphreys, C.; Sutton, A. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA U study. Phys. Rev. B Condens. Matter Mater. Phys. 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Akimoto, J.; Gotoh, Y.; Oosawa, Y. Refinement of hexagonal BaTiO3. Acta. Crystallogr. Sect. C 1994, 50, 160. [Google Scholar] [CrossRef]
- Yajima, T.; Kitada, A.; Kobayashi, Y.; Sakaguchi, T.; Bouilly, G.; Kasahara, S.; Terashima, T.; Takano, M.; Kageyama, H. Epitaxial Thin Films of ATiO3−xHx (A = Ba, Sr, Ca) with Metallic Conductivity. J. Am. Chem. Soc. 2012, 134, 8782–8785. [Google Scholar] [CrossRef]
- Sinclair, D.; Skakle, J.S.; Morrison, F.; Smith, R.; Beales, T. Structure and electrical properties of oxygen-deficient hexagonal BaTiO3. J. Mater. Chem. 1999, 9, 1327–1331. [Google Scholar] [CrossRef]
- Takeiri, F.; Aidzu, K.; Yajima, T.; Matsui, T.; Yamamoto, T.; Kobayashi, Y.; Hester, J.; Kageyama, H. Promoted Hydride/Oxide Exchange in SrTiO3 by Introduction of Anion Vacancy via Aliovalent Cation Substitution. Inorg. Chem. 2017, 56, 13035–13040. [Google Scholar] [CrossRef]
- Ueda, Y.; Kobayashi, Y.; Itoh, M. Tailoring a new oxyhydride, CaVO3−xHx, with ordered hydride anions. J. Solid State Chem. 2022, 305, 122685. [Google Scholar] [CrossRef]
- Mikita, R.; Aharen, T.; Yamamoto, T.; Takeiri, F.; Tang, Y.; Yoshimune, W.; Fujita, K.; Yoshida, S.; Tanaka, K.; Batuk, D.; et al. Topochemical Nitridation with Anion Vacancy-Assisted N3−/O2− Exchange. J. Am. Chem. Soc. 2016, 138, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Ogasawara, K.; Nakao, T.; Sasase, M.; Kitana, M.; Hosono, H. Hexagonal BaTi3−xHx Oxyhydride as a Water-Durable Catalyst Support for Chemoselective Hydrogenation. J. Am. Chem. Soc. 2022, 144, 6453–6464. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kageyama, K.; Yang, Y.; Kageyama, T.; Murayama, K.; Shitara, K.; Saito, T.; Ubukata, H.; Tassel, C.; Kuwabara, A.; Kageyama, H. Kinetic Control of Anion Stoichiometry in Hexagonal BaTiO3. Inorganics 2022, 10, 73. https://doi.org/10.3390/inorganics10060073
Kageyama K, Yang Y, Kageyama T, Murayama K, Shitara K, Saito T, Ubukata H, Tassel C, Kuwabara A, Kageyama H. Kinetic Control of Anion Stoichiometry in Hexagonal BaTiO3. Inorganics. 2022; 10(6):73. https://doi.org/10.3390/inorganics10060073
Chicago/Turabian StyleKageyama, Keisuke, Yang Yang, Toki Kageyama, Kantaro Murayama, Kazuki Shitara, Takashi Saito, Hiroki Ubukata, Cédric Tassel, Akihide Kuwabara, and Hiroshi Kageyama. 2022. "Kinetic Control of Anion Stoichiometry in Hexagonal BaTiO3" Inorganics 10, no. 6: 73. https://doi.org/10.3390/inorganics10060073
APA StyleKageyama, K., Yang, Y., Kageyama, T., Murayama, K., Shitara, K., Saito, T., Ubukata, H., Tassel, C., Kuwabara, A., & Kageyama, H. (2022). Kinetic Control of Anion Stoichiometry in Hexagonal BaTiO3. Inorganics, 10(6), 73. https://doi.org/10.3390/inorganics10060073






