Field-Induced Single Molecule Magnetic Behavior of Mononuclear Cobalt(II) Schiff Base Complex Derived from 5-Bromo Vanillin
Abstract
:1. Introduction
2. Results and Discussion
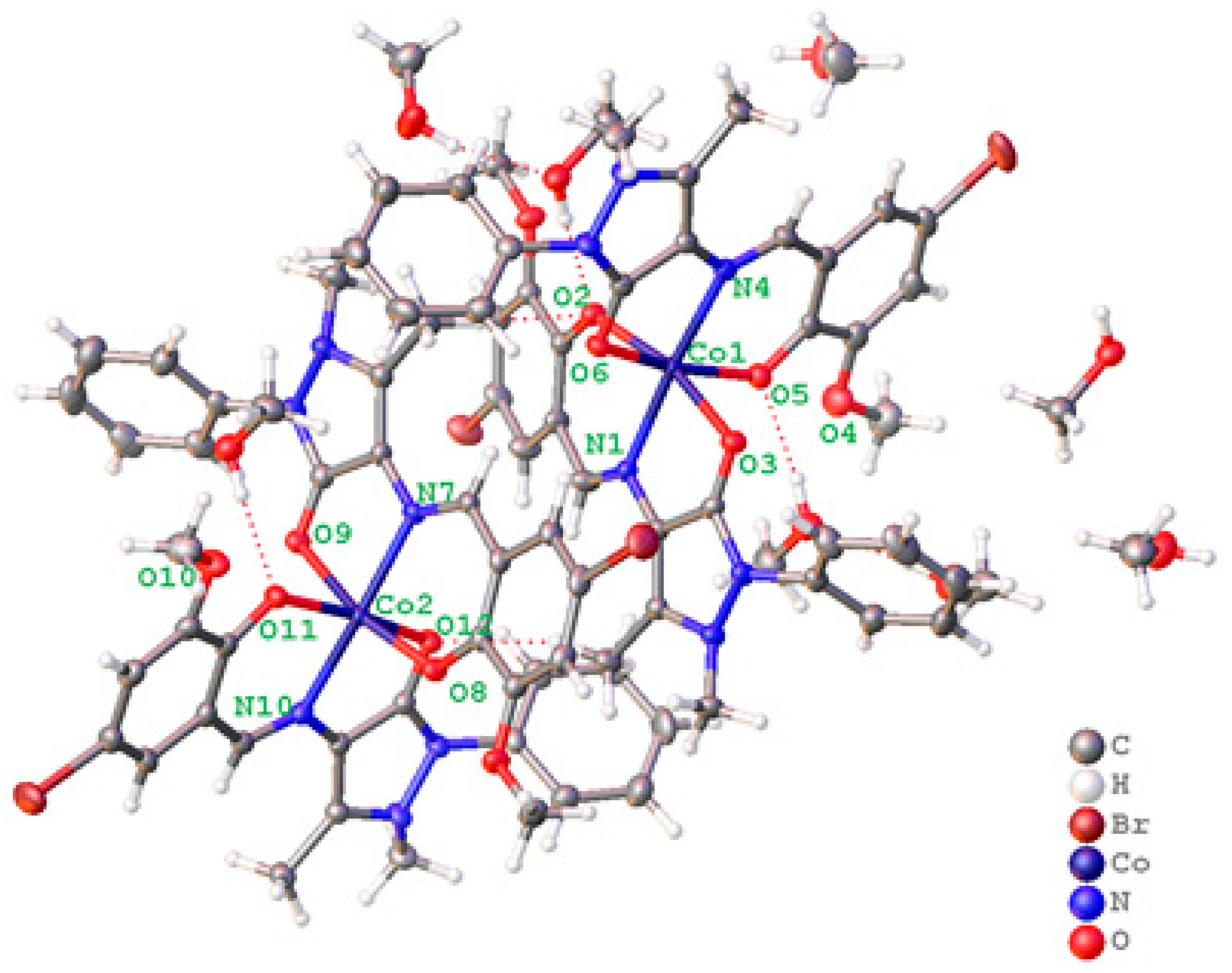
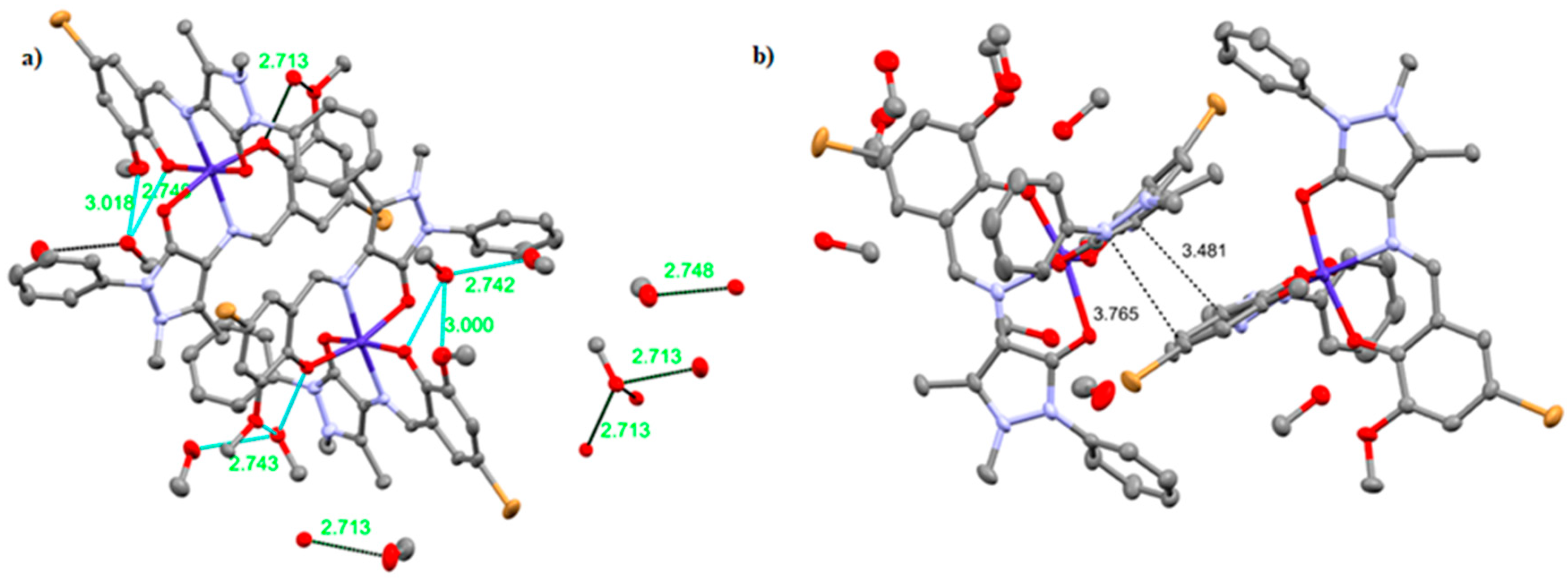
2.1. Crystal Structure Description
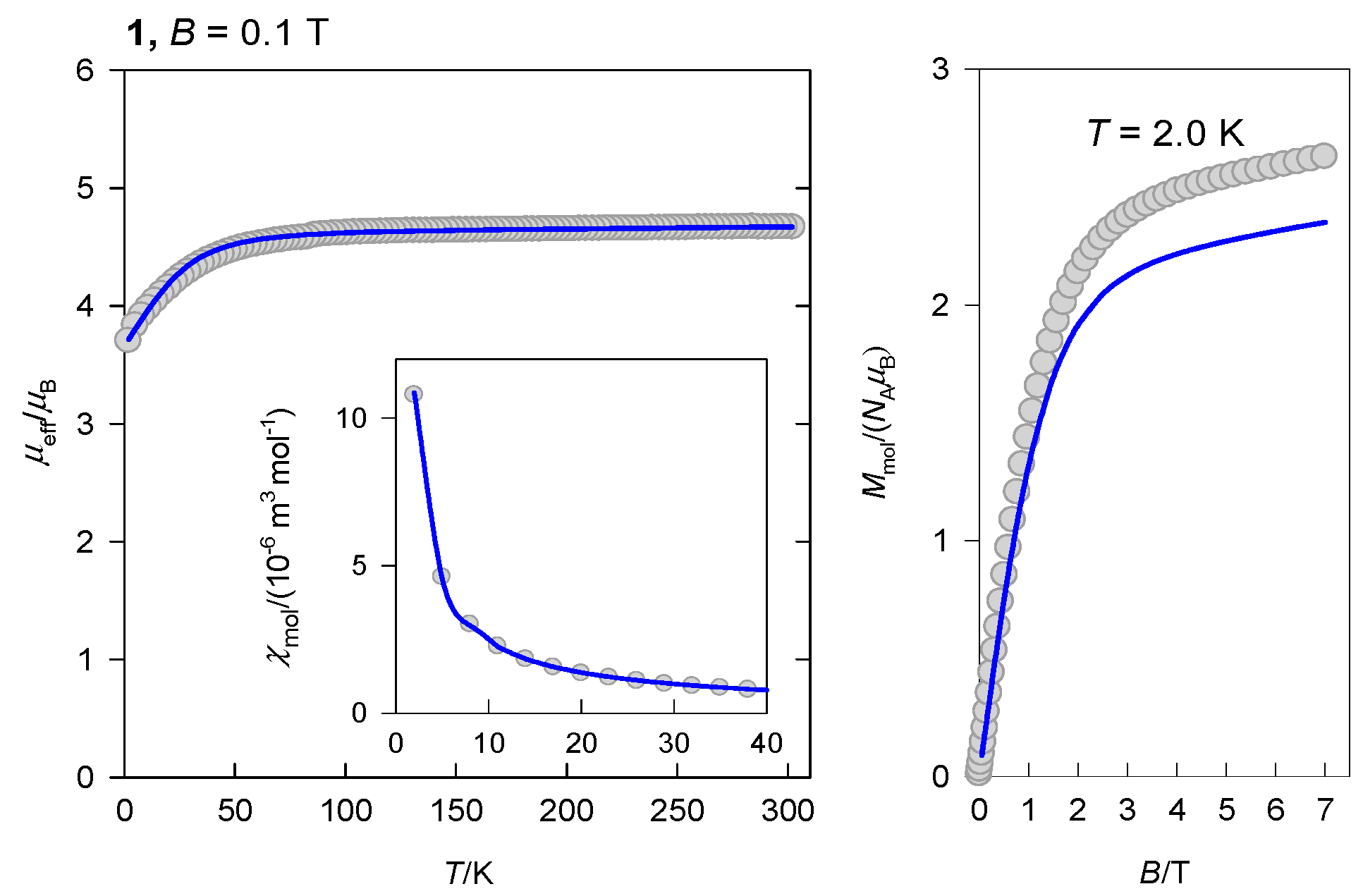
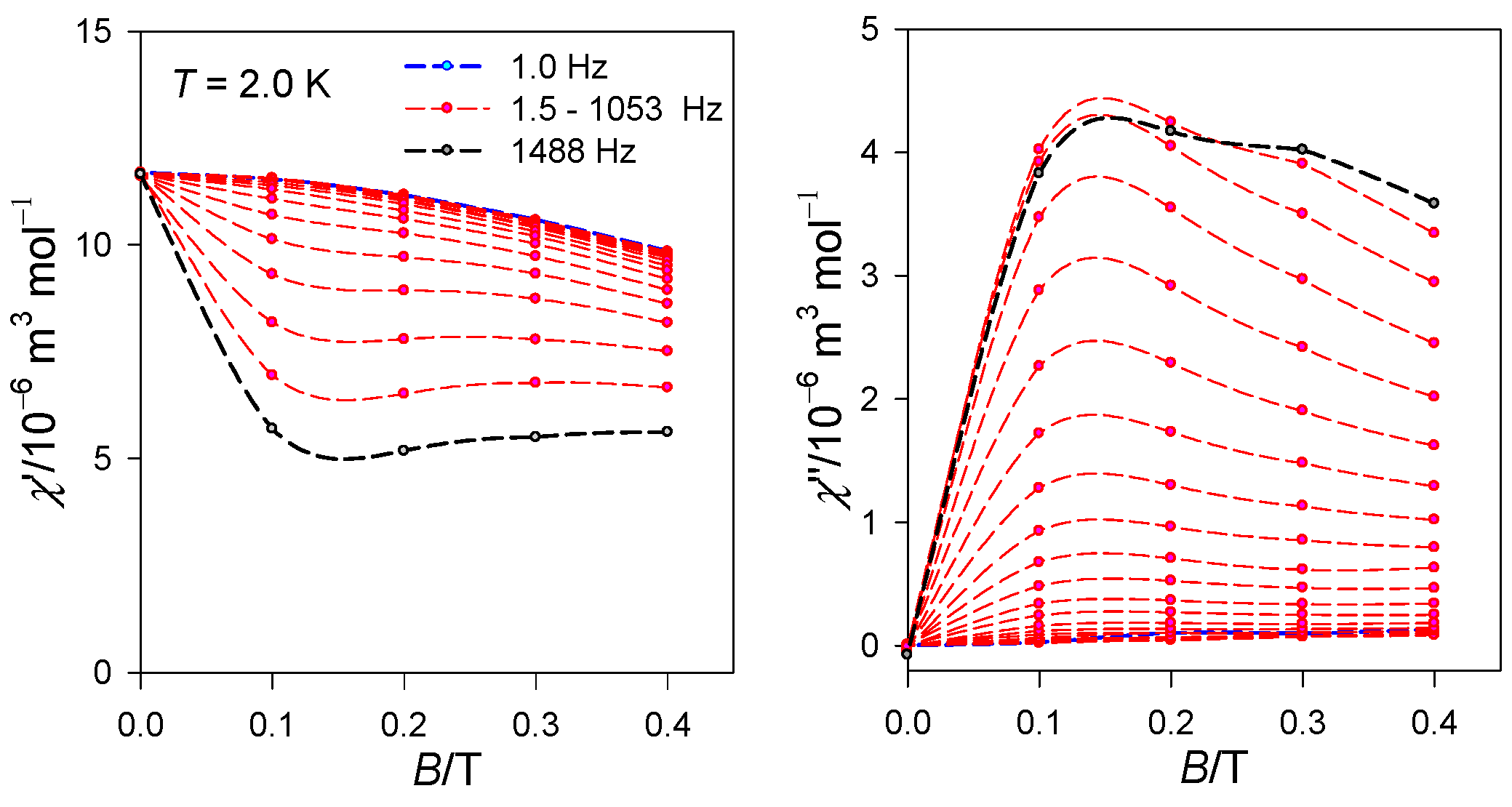
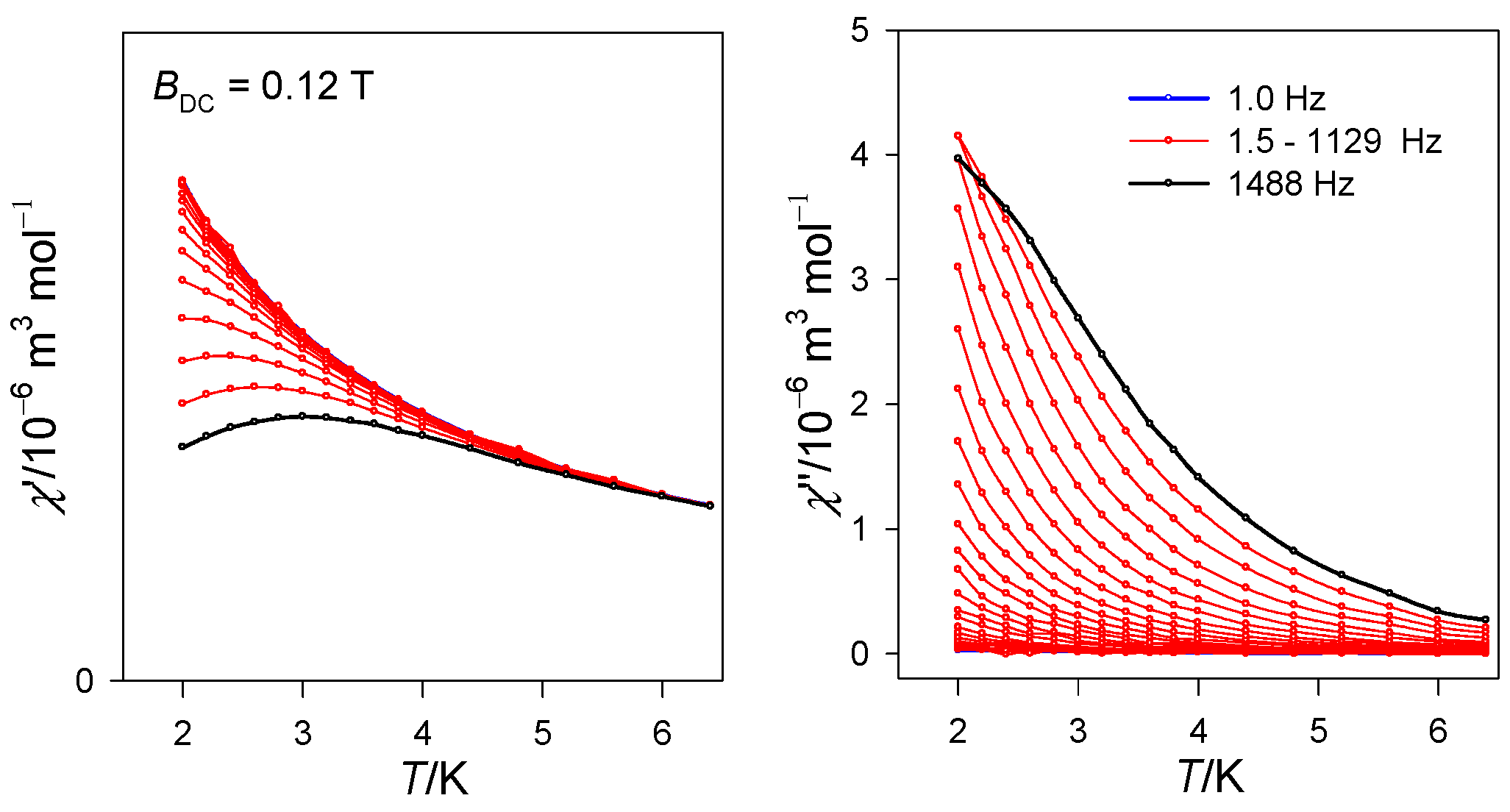
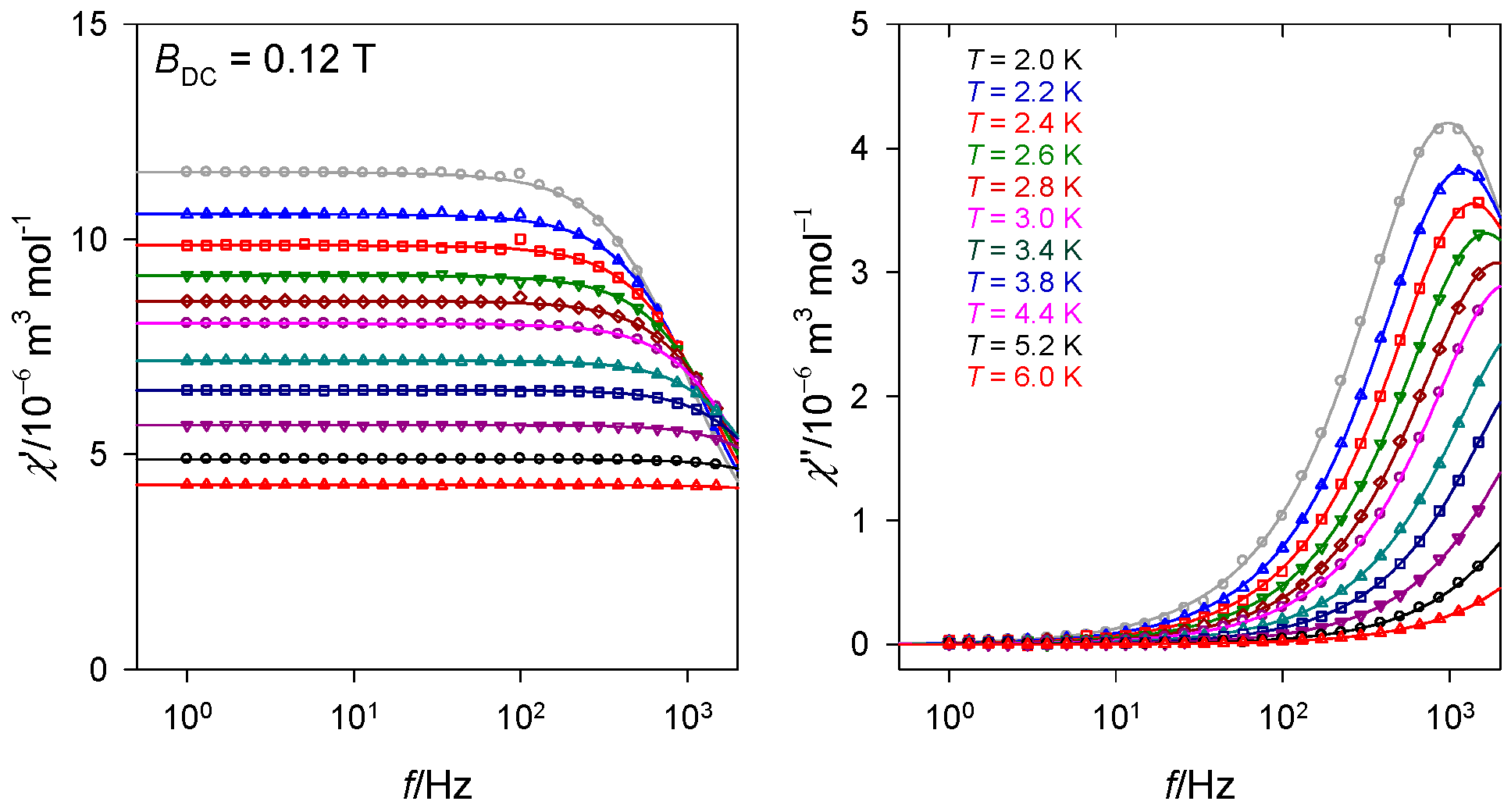
2.2. Magnetic Data
3. Materials and Methods
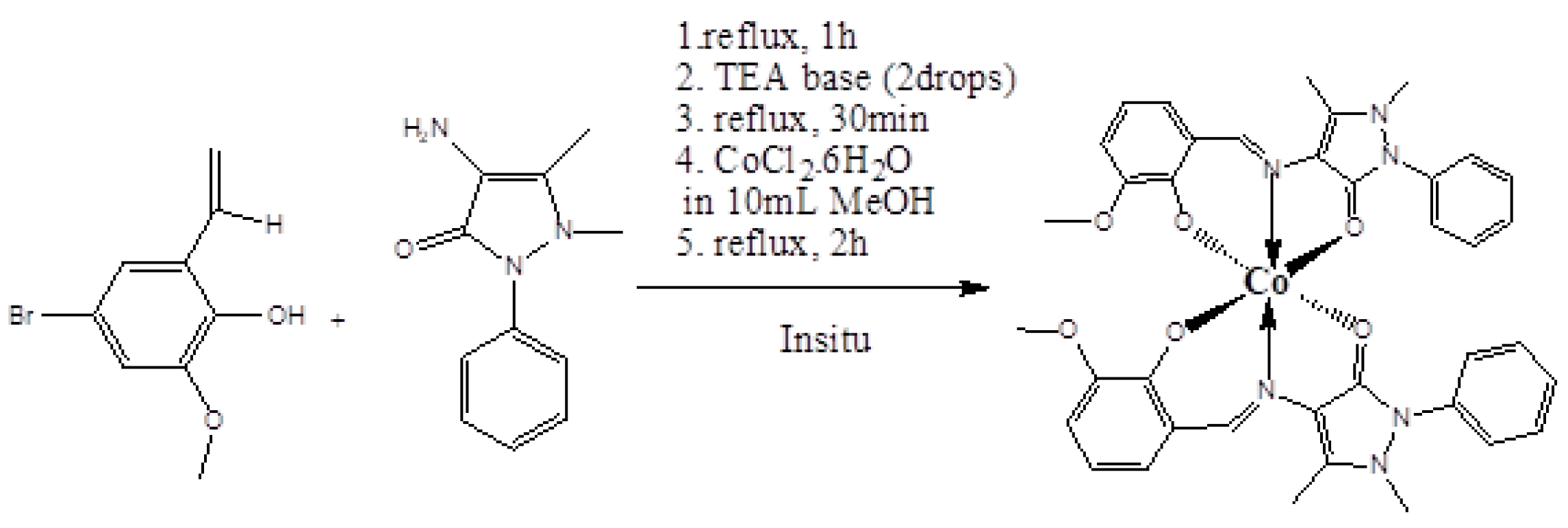
3.1. Synthesis Co(C19H17BrN3O3)2·4CH3OH (1)
3.2. Physical Measurements
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Affronte, M. Molecular Nanomagnets for Information Technologies. J. Mater. Chem. 2009, 19, 1731–1737. [Google Scholar] [CrossRef]
- Lumetti, S.; Candini, A.; Godfrin, C.; Balestro, F.; Wernsdorfer, W.; Klyatskaya, S.; Ruben, M.; Affronte, M. Single-Molecule Devices with Graphene Electrodes. Dalton Trans. 2016, 45, 16570–16574. [Google Scholar] [CrossRef]
- Shao, D.; Shi, L.; Wei, H.Y.; Wang, X.Y. Field-Induced Single-Ion Magnet Behaviour in Two New Cobalt(II) Coordination Polymers with 2,4,6-Tris(4-Pyridyl)-1,3,5-Triazine. Inorganics 2017, 5, 90. [Google Scholar] [CrossRef] [Green Version]
- Kar, P.; Biswas, R.; Drew, M.G.B.; Ida, Y.; Ghosh, A. Structure and Magnetic Properties of an Unprecedented Syn-Anti l-Nitrito-1kO:2kO’ Bridged Mn(III)-Salen Complex and Its Isoelectronic and Isostructural Formate Analogue. Dalton Trans. 2011, 40, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Kharwar, A.K.; Mondal, A.; Sarkar, A.; Rajaraman, G.; Konar, S. Modulation of Magnetic Anisotropy and Exchange Interaction in Phenoxide-Bridged Dinuclear Co(II) Complexes. Inorg. Chem. 2021, 60, 11948–11956. [Google Scholar] [CrossRef]
- Kazin, P.E.; Zykin, M.A.; Trusov, L.A.; Eliseev, A.A.; Magdysyuk, O.V.; Dinnebier, R.E.; Kremer, R.K.; Felser, C.; Jansen, M. A Co-Based Single-Molecule Magnet Confined in a Barium Phosphate Apatite Matrix with a High Energy Barrier for Magnetization Relaxation. Chem. Commun. 2017, 53, 5416–5419. [Google Scholar] [CrossRef] [Green Version]
- Milios, C.J.; Winpenny, R.E.P. Cluster-Based Single-Molecule Magnets. Struct. Bond. 2015, 164, 1–109. [Google Scholar] [CrossRef]
- Rechkemmer, Y.; Breitgoff, F.D.; Van Der Meer, M.; Atanasov, M.; Hakl, M.; Orlita, M.; Neugebauer, P.; Neese, F.; Sarkar, B.; Van Slageren, J. A Four-Coordinate Cobalt(II) Single-Ion Magnet with Coercivity and a Very High Energy Barrier. Nat. Commun. 2016, 7, 10467. [Google Scholar] [CrossRef] [Green Version]
- Campbell, V.E.; Tonelli, M.; Cimatti, I.; Moussy, J.B.; Tortech, L.; Dappe, Y.J.; Rivière, E.; Guillot, R.; Delprat, S.; Mattana, R.; et al. Engineering the Magnetic Coupling and Anisotropy at the Molecule-Magnetic Surface Interface in Molecular Spintronic Devices. Nat. Commun. 2016, 7, 13646. [Google Scholar] [CrossRef] [Green Version]
- Coronado, E. Molecular Magnetism: From Chemical Design to Spin Control in Molecules, Materials and Devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Shao, D.; Wang, X.Y. Development of Single-Molecule Magnets†. Chin. J. Chem. 2020, 38, 1005–1018. [Google Scholar] [CrossRef]
- Shen, Y.; Ito, H.; Zhang, H.; Yamochi, H.; Cosquer, G.; Herrmann, C.; Ina, T.; Yoshina, S.K.; Breedlove, B.K.; Otsuka, A.; et al. Emergence of Metallic Conduction and Cobalt(II)-Based Single-Molecule Magnetism in the Same Temperature Range. J. Am. Chem. Soc. 2021, 143, 4891–4895. [Google Scholar] [CrossRef] [PubMed]
- Novitchi, G.; Jiang, S.; Shova, S.; Rida, F.; Hlavička, I.; Orlita, M.; Wernsdorfer, W.; Hamze, R.; Martins, C.; Suaud, N.; et al. From Positive to Negative Zero-Field Splitting in a Series of Strongly Magnetically Anisotropic Mononuclear Metal Complexes. Inorg. Chem. 2017, 56, 14809–14822. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.X.; Chen, X.X.; Xiang, J.; Chen, Y.Z.; Jia, L.H.; Wang, B.W.; Cheng, S.C.; Zhou, X.; Leung, C.F.; Gao, S. Slow Magnetic Relaxation in a Series of Mononuclear 8-Coordinate Fe(II) and Co(II) Complexes. Inorg. Chem. 2018, 57, 3761–3774. [Google Scholar] [CrossRef]
- Dolai, M.; Mondal, A.; Liu, J.L.; Ali, M. Three Novel Mononuclear Mn(III)-Based Magnetic Materials with Square Pyramidal: Versus Octahedral Geometries. New J. Chem. 2017, 41, 10890–10898. [Google Scholar] [CrossRef]
- Luo, Q.C.; Zheng, Y.Z. Methods and Models of Theoretical Calculation for Single-Molecule Magnets. Magnetochemistry 2021, 7, 107. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Telser, J.; Long, J.R. Slow Magnetic Relaxation in the Tetrahedral Cobalt(II) Complexes [Co(EPh)4]2- (EO, S, Se). Polyhedron 2013, 64, 209–217. [Google Scholar] [CrossRef]
- El-Khatib, F.; Cahier, B.; Shao, F.; López-Jordà, M.; Guillot, R.; Rivière, E.; Hafez, H.; Saad, Z.; Girerd, J.J.; Guihéry, N.; et al. Design and Magnetic Properties of a Mononuclear Co(II) Single Molecule Magnet and Its Antiferromagnetically Coupled Binuclear Derivative. Inorg. Chem. 2017, 56, 4601–4608. [Google Scholar] [CrossRef]
- Gomez-Coca, S.; Cremades, E.; Aliaga-Alcalde, N.; Ruiz, E. Mononuclear Single-Molecule Magnets: Tailoring the Magnetic Anisotropy of First-Row Transition-Metal Complexes. J. Am. Chem. Soc. 2013, 135, 7010–7018. [Google Scholar] [CrossRef]
- Gebrezgiabher, M.; Bayeh, Y.; Gebretsadik, T. Lanthanide-Based Single-Molecule Magnets Derived from Schiff Base Ligands of Salicylaldehyde Derivatives. Inorganics 2020, 8, 66. [Google Scholar] [CrossRef]
- Villa-Pérez, C.; Oyarzabal, I.; Echeverría, G.A.; Valencia-Uribe, G.C.; Seco, J.M.; Soria, D.B. Single-Ion Magnets Based on Mononuclear Cobalt (II) Complexes with Sulfadiazine. Eur. J. Inorg. Chem. 2016, 29, 4835–4841. [Google Scholar] [CrossRef] [Green Version]
- Senthil Kumar, K.; Bayeh, Y.; Gebretsadik, T.; Elemo, F.; Gebrezgiabher, M.; Thomas, M.; Ruben, M. Spin-Crossover in Iron(II)-Schiff Base Complexes. Dalton Trans. 2019, 48, 15321–15337. [Google Scholar] [CrossRef] [PubMed]
- Zadrozny, J.M.; Xiao, D.J.; Atanasov, M.; Long, G.J.; Grandjean, F.; Neese, F.; Long, J.R. Magnetic Blocking in a Linear Iron(I) Complex. Nat. Chem. 2013, 5, 577–581. [Google Scholar] [CrossRef]
- Fataftah, M.S.; Zadrozny, J.M.; Rogers, D.M.; Freedman, D.E. A Mononuclear Transition Metal Single-Molecule Magnet in a Nuclear Spin-Free Ligand Environment. Inorg. Chem. 2014, 53, 10716–10721. [Google Scholar] [CrossRef]
- Frost, J.M.; Harriman, K.L.M.; Murugesu, M. The Rise of 3-d Single-Ion Magnets in Molecular Magnetism: Towards Materials from Molecules? Chem. Sci. 2016, 7, 2470–2491. [Google Scholar] [CrossRef] [Green Version]
- Mondal, A.K.; Sundararajan, M.; Konar, S. A New Series of Tetrahedral Co(II) Complexes [CoLX2] (X = NCS, Cl, Br, I) Manifesting Single-Ion Magnet Features. Dalton Trans. 2018, 47, 3745–3754. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Hao, X.; Dou, Y.; Zhou, Z.; Yang, L.; Liu, H.; Li, D.; Dong, Y.; Kong, L.; Zhang, D. Various Structural Types of Cyanide-Bridged FeIII–MnIII Bimetallic Coordination Polymers (CPs) and Polynuclear Clusters Based-on A New mer-Tricyanoiron(III)Building Block: Synthesis, Crystal Structures, and Magnetic Properties. Polymers 2019, 11, 1585. [Google Scholar] [CrossRef] [Green Version]
- Hay, M.A.; Sarkar, A.; Marriott, K.E.R.; Wilson, C.; Rajaraman, G.; Murrie, M. Investigation of the Magnetic Anisotropy in a Series of Trigonal Bipyramidal Mn(Ii) Complexes. Dalton Trans. 2019, 48, 15480–15486. [Google Scholar] [CrossRef] [Green Version]
- Perlepe, P.S.; Maniaki, D.; Pilichos, E.; Katsoulakou, E.; Perlepes, S.P. Smart Ligands for Efficient 3d-, 4d- and 5d-Metal Single-Molecule Magnets and Single-Ion Magnets. Inorganics 2020, 8, 39. [Google Scholar] [CrossRef]
- Huang, X.C.; Zhou, C.; Shao, D.; Wang, X.Y. Field-Induced Slow Magnetic Relaxation in Cobalt(II) Compounds with Pentagonal Bipyramid Geometry. Inorg. Chem. 2014, 53, 12671–12673. [Google Scholar] [CrossRef]
- Gebrezgiabher, M.; Schlittenhardt, S.; Rajnák, C.; Sergawie, A.; Ruben, M.; Thomas, M.; Boča, R. A Tetranuclear Dysprosium Schiff Base Complex Showing Slow Relaxation of Magnetization. Inorganics 2022, 10, 66. [Google Scholar] [CrossRef]
- Mondal, A.K.; Goswami, T.; Misra, A.; Konar, S. Probing the Effects of Ligand Field and Coordination Geometry on Magnetic Anisotropy of Pentacoordinate Cobalt(II) Single-Ion Magnets. Inorg. Chem. 2017, 56, 6870–6878. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zhang, S.L.; Shi, L.; Zhang, Y.Q.; Wang, X.Y. Probing the Effect of Axial Ligands on Easy-Plane Anisotropy of Pentagonal-Bipyramidal Cobalt(II) Single-Ion Magnets. Inorg. Chem. 2016, 55, 10859–10869. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Luca, O.R.; Vieru, V.; Shiddiq, M.; Korobkov, I.; Gorelsky, S.I.; Takase, M.K.; Chibotaru, L.F.; Hill, S.; Crabtree, R.H.; et al. Influence of the Ligand Field on Slow Magnetization Relaxation versus Spin Crossover in Mononuclear Cobalt Complexes. Angew. Chem.-Int. Ed. 2013, 52, 11290–11293. [Google Scholar] [CrossRef]
- Peng, Y.; Mereacre, V.; Anson, C.E.; Zhang, Y.; Bodenstein, T.; Fink, K.; Powell, A.K. Field-Induced Co(II) Single-Ion Magnets with Mer-Directing Ligands but Ambiguous Coordination Geometry. Inorg. Chem. 2017, 56, 6056–6066. [Google Scholar] [CrossRef]
- Chen, L.; Dulaney, H.A.; Wilkins, B.O.; Farmer, S.; Zhang, Y.; Fronczek, F.R.; Jurss, J.W. High-Spin Enforcement in First-Row Metal Complexes of a Constrained Polyaromatic Ligand: Synthesis, Structure, and Properties. New J. Chem. 2018, 42, 18667–18677. [Google Scholar] [CrossRef]
- Li, G.L.; Sato, O. A Compressed Octahedral Cobalt(II) Complex in the Crystal Structure of Diaqua[6,6′-Sulfanediylbis(2,2′-Bipyridine)]Cobalt(II) Dinitrate Li Guo-Ling. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 993–995. [Google Scholar] [CrossRef] [Green Version]
- Madhu, N.T.; Tang, J.K.; Hewitt, I.J.; Clérac, R.; Wernsdorfer, W.; Van Slageren, J.; Anson, C.E.; Powell, A.K. What Makes a Single Molecule Magnet? Polyhedron 2005, 24, 2864–2869. [Google Scholar] [CrossRef]
- Goswami, S.; Mondal, A.K.; Konar, S. Nanoscopic Molecular Magnets. Inorg. Chem. Front. 2015, 2, 687–712. [Google Scholar] [CrossRef]
- Bu, X.H.; Morishita, H.; Tanaka, K.; Biradha, K.; Furusho, S.; Shionoya, M. A Spontaneously Resolved Chiral Molecular Box: A Cyclic Tetranuclear Zn(II) Complex with DPTZ (DPTZ = 3,6-Di-2-Pyridyl-1,2,4,5-Tetrazine). Chem. Commun. 2000, 971–972. [Google Scholar] [CrossRef]
- Xie, L.; Wei, Y.; Wang, Y.; Hou, H.; Fan, Y.; Zhu, Y. 2D and 3D Binuclear Cobalt Supramolecular Complexes: Synthesis and Crystal Structures. J. Mol. Struct. 2004, 692, 201–207. [Google Scholar] [CrossRef]
- Nikam, R.; Rayaprol, S.; Mukherjee, S.; Kaushik, S.D.; Goyal, P.S.; Babu, P.D.; Radha, S.; Siruguri, V. Structure and Magnetic Properties of Mn Doped α-Fe2O3. Phys. B Condens. Matter 2019, 574, 411663. [Google Scholar] [CrossRef]
- Moseley, I.P.; Lin, C.Y.; Zee, D.Z.; Zadrozny, J.M. Synthesis and Magnetic Characterization of a Dinuclear Complex of Low-Coordinate Iron(II). Polyhedron 2020, 175, 114171. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.A.; Sarkar, A.; Craig, G.A.; Bhaskaran, L.; Nehrkorn, J.; Ozerov, M.; Marriott, K.E.R.; Wilson, C.; Rajaraman, G.; Hill, S.; et al. In-Depth Investigation of Large Axial Magnetic Anisotropy in Monometallic 3d Complexes Using Frequency Domain Magnetic Resonance and: Ab Initio Methods: A Study of Trigonal Bipyramidal Co(Ii). Chem. Sci. 2019, 10, 6354–6361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, A.K.; Mondal, A.; Konar, S. Field Induced Single Ion Magnetic Behaviour in Square-Pyramidal Cobalt(II) Complexes with Easy-Plane Magnetic Anisotropy. Magnetochemistry 2019, 5, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Murrie, M. Cobalt(Ii) Single-Molecule Magnets. Chem. Soc. Rev. 2010, 39, 1986–1995. [Google Scholar] [CrossRef]
- Shao, F.; Cahier, B.; Wang, Y.T.; Yang, F.L.; Rivière, E.; Guillot, R.; Guihéry, N.; Tong, J.P.; Mallah, T. Magnetic Relaxation Studies on Trigonal Bipyramidal Cobalt(II) Complexes. Chem.-Asian J. 2020, 15, 391–397. [Google Scholar] [CrossRef]
- Gómez-Coca, S.; Urtizberea, A.; Cremades, E.; Alonso, P.J.; Camón, A.; Ruiz, E.; Luis, F. Origin of Slow Magnetic Relaxation in Kramers Ions with Non-Uniaxial Anisotropy. Nat. Commun. 2014, 5, 4300. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Rajaraman, G. Probing the Origin of Magnetic Anisotropy in a Dinuclear {Mn IIICuII} Single-Molecule Magnet: The Role of Exchange Anisotropy. Chem.-A Eur. J. 2014, 20, 5214–5218. [Google Scholar] [CrossRef]
- Vallejo, J.; Castro, I.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; De Munno, G.; Wernsdorfer, W.; Pardo, E. Field-Induced Slow Magnetic Relaxation in a Six-Coordinate Mononuclear Cobalt(II) Complex with a Positive Anisotropy. J. Am. Chem. Soc. 2012, 134, 15704–15707. [Google Scholar] [CrossRef]
- Vallejo, J.; Viciano-Chumillas, M.; Lloret, F.; Julve, M.; Castro, I.; Krzystek, J.; Ozerov, M.; Armentano, D.; De Munno, G.; Cano, J. Coligand Effects on the Field-Induced Double Slow Magnetic Relaxation in Six-Coordinate Cobalt(II) Single-Ion Magnets (SIMs) with Positive Magnetic Anisotropy. Inorg. Chem. 2019, 58, 15726–15740. [Google Scholar] [CrossRef]
- Świtlicka, A.; Palion-Gazda, J.; Machura, B.; Cano, J.; Lloret, F.; Julve, M. Field-Induced Slow Magnetic Relaxation in Pseudooctahedral Cobalt(Ii) Complexes with Positive Axial and Large Rhombic Anisotropy. Dalton Trans. 2019, 48, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Pilichos, E.; Font-Bardia, M.; Cano, J.; Escuer, A.; Mayans, J. Slow Magnetic Relaxation for Cobalt (II) Complexes in Axial Bipyramidal Environment: An S = ½ Spin Case. Dalton Trans. 2022, 51, 8986–8993. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Deng, Y.F.; Li, T.; Xiong, J.; Wang, B.W.; Zheng, Z.; Zhang, Y.Z. Construction and Magnetic Study of a Trigonal-Prismatic Cobalt(II) Single-Ion Magnet. Inorg. Chem. 2018, 57, 14047–14051. [Google Scholar] [CrossRef] [PubMed]
- Fürmeyer, F.; Münzberg, D.; Carrella, L.M.; Rentschler, E. First Cobalt(II) Spin Crossover Compound with N4S2-Donorset. Molecules 2020, 25, 855. [Google Scholar] [CrossRef] [Green Version]
- Pankratova, Y.A.; Nelyubina, Y.V.; Novikov, V.V.; Pavlov, A.A. High-Spin Cobalt(II) Complex with Record-Breaking Anisotropy of the Magnetic Susceptibility According to Paramagnetic NMR Spectroscopy Data. Russ. J. Coord. Chem. Koord. Khimiya 2021, 47, 10–16. [Google Scholar] [CrossRef]
- Jia, H.R.; Li, J.; Sun, Y.X.; Guo, J.Q.; Yu, B.; Wen, N.; Xu, L. Two Supramolecular Cobalt(II) Complexes: Syntheses, Crystal Structures, Spectroscopic Behaviors, and Counter Anion Effects. Crystals 2017, 7, 247. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.; Nashre-ul-Islam, S.M.; Saha, U.; Frontera, A.; Bhattacharyya, M.K. Cu(II) and Co(II) Coordination Solids Involving Unconventional Parallel Nitrile(π)‒nitrile(π) and Energetically Significant Cooperative Hydrogen Bonding Interactions: Experimental and Theoretical Studies. J. Mol. Struct. 2019, 1195, 733–743. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.G.; Xie, L.M.; Yu, L.; Shi, J.M. π-π Stacking, Hydrogen Bonding and Anti-Ferromagnetic Coupling Mechanism on a Mononuclear Cu(II) Complex. J. Coord. Chem. 2011, 64, 1456–1468. [Google Scholar] [CrossRef]
- Tang, J.; Costa, J.S.; Smulders, S.; Molnár, G.; Bousseksou, A.; Teat, S.J.; Li, Y.; Van Albada, G.A.; Gamez, P.; Reedijk, J. Two-Step Spin-Transition Iron(III) Compound with a Wide [High Spin-Low Spin] Plateau. Inorg. Chem. 2009, 48, 2128–2135. [Google Scholar] [CrossRef]
- Wheeler, S.E. Understanding Substituent Effects in Noncovalent Interactions Involving Aromatic Rings. Acc. Chem. Res. 2013, 46, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Oshita, H.; Suzuki, T.; Kawashima, K.; Abe, H.; Tani, F.; Mori, S.; Yajima, T.; Shimazaki, Y. The Effect of π-π Stacking Interaction of the Indole Ring with the Coordinated Phenoxyl Radical in a Nickel(II)-Salen Type Complex. Comparison with the Corresponding Cu(II) Complex. Dalton Trans. 2019, 48, 12060–12069. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Bridonneau, N.; Poneti, G.; Tesi, L.; Sorace, L.; Pinkowicz, D.; Jover, J.; Ruiz, E.; Sessoli, R.; Cornia, A. A Pseudo-Octahedral Cobalt(II) Complex with Bispyrazolylpyridine Ligands Acting as a Zero-Field Single-Molecule Magnet with Easy Axis Anisotropy. Chem.-A Eur. J. 2018, 24, 8857–8868. [Google Scholar] [CrossRef]
- Comba, P.; Kerscher, M.; Lawrance, G.A.; Martin, B.; Wadepohl, H. Stable Five- and Six-Coordinate Cobalt (III) Complexes with a Pentadentate Bispidine Ligand. Angew. Chem. Int. Ed. 2008, 47, 4740–4743. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Oyarzabal, I.; Vallejo, J.; Cano, J.; Colacio, E.; Bauza, A.; Frontera, A.; Kirillov, A.M.; Drew, M.G.B.; Das, S. Two Polymorphic Forms of a Six-Coordinate Mononuclear Cobalt(II) Complex with Easy-Plane Anisotropy: Structural Features, Theoretical Calculations, and Field-Induced Slow Relaxation of the Magnetization. Inorg. Chem. 2016, 55, 8502–8513. [Google Scholar] [CrossRef]
- Lloret, F.; Julve, M.; Cano, J.; Ruiz-García, R.; Pardo, E. Magnetic Properties of Six-Coordinated High-Spin Cobalt (II) Complexes: Theoretical Background and Its Application. Inorg. Chim. Acta 2008, 361, 3432–3445. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Gómez-Coca, S.; Brown, A.J.; Saber, M.R.; Zhang, X.; Dunbar, K.R. Trigonal Antiprismatic Co(II) Single Molecule Magnets with Large Uniaxial Anisotropies: Importance of Raman and Tunneling Mechanisms. Chem. Sci. 2016, 7, 6519–6527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.B.; Jing, Z.Y.; Li, M.M.; Yin, L.; Gao, Y.D.; Yu, F.; Hu, T.P.; Wang, Z.; Song, Y. Important Role of Intermolecular Interaction in Cobalt(II) Single-Ion Magnet from Single Slow Relaxation to Double Slow Relaxation. Inorg. Chem. 2018, 57, 10761–10767. [Google Scholar] [CrossRef]
- Chahine, A.Y.; Phonsri, W.; Murray, K.S.; Turner, D.R.; Batten, S.R. Coordination Polymers of a Bis-Isophthalate Bridging Ligand with Single Molecule Magnet Behaviour of the CoII Analogue. Dalton Trans. 2020, 49, 5241–5249. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elemo, F.; Schlittenhardt, S.; Sani, T.; Rajnák, C.; Linert, W.; Boča, R.; Thomas, M.; Ruben, M. Field-Induced Single Molecule Magnetic Behavior of Mononuclear Cobalt(II) Schiff Base Complex Derived from 5-Bromo Vanillin. Inorganics 2022, 10, 105. https://doi.org/10.3390/inorganics10080105
Elemo F, Schlittenhardt S, Sani T, Rajnák C, Linert W, Boča R, Thomas M, Ruben M. Field-Induced Single Molecule Magnetic Behavior of Mononuclear Cobalt(II) Schiff Base Complex Derived from 5-Bromo Vanillin. Inorganics. 2022; 10(8):105. https://doi.org/10.3390/inorganics10080105
Chicago/Turabian StyleElemo, Fikre, Sören Schlittenhardt, Taju Sani, Cyril Rajnák, Wolfgang Linert, Roman Boča, Madhu Thomas, and Mario Ruben. 2022. "Field-Induced Single Molecule Magnetic Behavior of Mononuclear Cobalt(II) Schiff Base Complex Derived from 5-Bromo Vanillin" Inorganics 10, no. 8: 105. https://doi.org/10.3390/inorganics10080105
APA StyleElemo, F., Schlittenhardt, S., Sani, T., Rajnák, C., Linert, W., Boča, R., Thomas, M., & Ruben, M. (2022). Field-Induced Single Molecule Magnetic Behavior of Mononuclear Cobalt(II) Schiff Base Complex Derived from 5-Bromo Vanillin. Inorganics, 10(8), 105. https://doi.org/10.3390/inorganics10080105









