Preparation and Real World Applications of Titania Composite Materials for Photocatalytic Surface, Air, and Water Purification: State of the Art
Abstract
1. Introduction
2. Band Gap Engineering
2.1. Metal Doping
2.2. Non-Metal Doping
3. Synthesis and Preparation
3.1. Dip and Spin Coating, Immersion, and Impregnation of Sol-Gel and TiO2 Suspensions
3.2. Spray and Electrospray Coating
3.3. Alternative Synthesis and Deposition Methods
4. Applications
4.1. Photocatalytic Air Purification
4.2. Self-Cleaning, Antifogging and Air/Water Purifying Building Materials and Coatings
4.3. Water Purification
5. Standardized Methods and Protocols
6. Conclusions and Outlook
- Band gap engineering can be successfully accomplished by doping (or modification) with metals or non-metals. The absorption of visible light is most often explained by LSPR enhancement or intergap states.
- In addition, the intrinsic photoactivity sensitively depends on the level of doping. In ideal cases, charge carrier recombination is lower due to better charge separation. However, if the concentration of the dopant is too high, the photoactivity often decreases as dopants become recombination centers.
- For the coating of glass, walls, ceilings, streets, and other large surfaces, spray coating is the method of choice. Reasons are that spray coating is less expensive than most of the other alternatives, it can be more easily scaled up, and it is more flexible with respect to materials and preparation conditions as compared, e.g., to dip or spin coating.
- For smaller objects, dip and spin coating as well as impregnation and immersion can become useful. The two former methods allow a very precise adjustment of the layer thickness.
- Other preparation methods such as CVD, PVD or sputtering are rarely applied in real applications, since they usually demand expensive equipment, well defined surface geometries, and they need special coating precursors.
- In many cases, P25 or nanoparticles that are synthesized via the sol-gel-route are used as titania source. For this purpose, the sol, water or, e.g., a paint are used as a carrier material.
- In a few cases, TiO2 is incorporated directly in the material. This is mainly the case with membranes and filters.
- The formation of anatase or rutile depends on the calcination temperature, time, and doping/modification technique.
- In the vast majority of published field or pilot scale tests, different building materials have been studied.
- Moreover, multiple tests have also been conducted in the field of air purifying systems. Here, some commercial systems are already available.
- In the field of water purification, industrial scale reactors are still rare. This is possibly because neither an efficient reactor system (fixed bed, flow through, coated concrete) nor a robust enough catalyst application technique (spray coating, dip coating, dispersion) could have been established for this purpose yet.
- Applications in medical fields such as implants are still niche applications. However, a number of studies could provide principal evidence that photocatalysts can effectively diminish the viral and bacterial load of air and surfaces in medical environments.
- Photocatalytic air purifiers are often used in combination with other technologies such as particle filters or plasma techniques.
- Photocatalytic air purification systems tested in realistic scenarios, such as coated walls and other surfaces in tunnels or on streets, very often exhibited a significant reduction in air purification efficiency as compared to laboratory scales. In some studies, there was even no effect at all verifiable. Reasons are probably the extremely high volume to catalyst surface ratio, the effect of temperature, relative humidity, other pollutants, the pollutant concentration, deactivation and ablation of titania, wind speed, contact as well as diffusion time. For air purifying purposes, the utilization of circulated air in combination with air purifiers is more promising to avoid some of the negative effects.
- Due to the low photocatalytic efficiency under visible light, most applications use artificial UV light from respective lamps. This is an additional cost factor and complicates the setting, but with energy-efficient UV-LED lamps it might be the best choice in many indoor and even outdoor applications.
- In general, many applications have been tested in pilot and especially in laboratory scale. However, field tests are very often missing to really evaluate the performance of the presented materials and systems. In the future, field studies should pay special attention to doped materials. These materials that could utilize a much larger part of the solar spectrum could become a show stopper for many outdoor applications.
- In most application studies, anatase is used as titania modification. However, there are hints that a combination of anatase and rutile layers or nanoparticles might be advantageous, probably due to enhanced charge carrier separation at the interface.
- Some studies focused on the long-term performance of the setups and the durability of the photocatalyst with regards to mechanical, optical, and catalytic properties under real conditions. However, it is consistently mentioned that more attention needs to be paid to this area in the future.
- In some studies, real pollutants such as NOx, VOC, bacteria or other organic molecules in real environments were used.
- For most projects, especially for the test of prototypes, model pollutants or dyes are usually tested.
- It became also obvious that spectroscopic or electrochemical characterization of the photocatalyst (system) to deduce information such as, e.g., charge transfer efficiency, formed radicals and intermediate products, surface properties, photoquantum yields, catalyst morphology, turnover rates etc. in realistic environments are rare or even non-existent.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CB | Conduction Band |
| CFU | Colony Forming Unit |
| COD | Chemical Oxygen Demand |
| CPC | Compound Parabolic Reactor |
| CVD | Chemical Vapor Deposition |
| LSPR | Localized Surface Plasmon Resonance |
| PVD | Physical Vapor Deposition |
| SWCNT | Single wall carbon nanotube |
| TMiP | titanium mesh sheet modified with tatania nanoparticles |
| TNT | Titania Nanotubes |
| TOBT | Tertoxybutyltitanate |
| TTIP | Titanium (IV) tetraisopropoxide |
| VB | Valence Band |
| VOC | Volatile Organic Compound |
References
- Birkett, M.; Dover, L.; Cherian Lukose, C.; Wasy Zia, A.; Tambuwala, M.M.; Serrano-Aroca, Á. Recent Advances in Metal-Based Antimicrobial Coatings for High-Touch Surfaces. Int. J. Mol. Sci. 2022, 23, 1162. [Google Scholar] [CrossRef] [PubMed]
- Mohite, V.S.; Darade, M.M.; Sharma, R.K.; Pawar, S.H. Nanoparticle Engineered Photocatalytic Paints: A Roadmap to Self-Sterilizing against the Spread of Communicable Diseases. Catalysts 2022, 12, 326. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Liu, T.; Chu, B. Cost-effective Polymer-based Membranes for Drinking Water Purification. Giant 2022, 10, 100099. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Van Vliet, M.T.; Jones, E.R.; Flörke, M.; Franssen, W.H.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global water scarcity including surface water quality and expansions of clean water technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Abedin, M.; Collins, A.E.; Habiba, U.; Shaw, R. Climate change, water scarcity, and health adaptation in southwestern coastal Bangladesh. Int. J. Disaster Risk Sci. 2019, 10, 28–42. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and prospects for wastewater treatment by UV and visible-light-active heterogeneous photocatalysis: A critical review. Heterog. Photocatal. 2020, 378, 225–264. [Google Scholar] [CrossRef]
- Negishi, N.; Chawengkijwanich, C.; Pimpha, N.; Larpkiattaworn, S.; Charinpanitkul, T. Performance verification of the photocatalytic solar water purification system for sterilization using actual drinking water in Thailand. J. Water Process. Eng. 2019, 31, 100835. [Google Scholar] [CrossRef]
- Marcelino, R.B.; Amorim, C.C.; Ratova, M.; Delfour-Peyrethon, B.; Kelly, P. Novel and versatile TiO2 thin films on PET for photocatalytic removal of contaminants of emerging concern from water. Chem. Eng. J. 2019, 370, 1251–1261. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, Q.; Mahmud, S.; Lu, J.; Zhang, G.; Huang, Z.; Wu, Q.; Zeng, Q.; Huang, Y.; Lei, H.; et al. Photocatalytic antifouling membrane with dense nano-TiO2 coating for efficient oil-in-water emulsion separation and self-cleaning. J. Membr. Sci. 2022, 645, 120204. [Google Scholar] [CrossRef]
- Cabrera-Reina, A.; Martínez-Piernas, A.B.; Bertakis, Y.; Xekoukoulotakis, N.P.; Agüera, A.; Pérez, J.A.S. TiO2 photocatalysis under natural solar radiation for the degradation of the carbapenem antibiotics imipenem and meropenem in aqueous solutions at pilot plant scale. Water Res. 2019, 166, 115037. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Márquez, J.J.; Palacios-Villarreal, C.; Manzano, M.; Blanco, E.; del Solar, M.R.; Levchuk, I. Photocatalytic degradation of pharmaceutically active compounds (PhACs) in urban wastewater treatment plants effluents under controlled and natural solar irradiation using immobilized TiO2. Sol. Energy 2020, 208, 480–492. [Google Scholar] [CrossRef]
- Hay, S.O.; Obee, T.; Luo, Z.; Jiang, T.; Meng, Y.; He, J.; Murphy, S.C.; Suib, S. The viability of photocatalysis for air purification. Molecules 2015, 20, 1319–1356. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Giampiccolo, A.; Tobaldi, D.M.; Leonardi, S.G.; Murdoch, B.J.; Seabra, M.P.; Ansell, M.P.; Neri, G.; Ball, R.J. Sol gel graphene/TiO2 nanoparticles for the photocatalytic-assisted sensing and abatement of NO2. Appl. Catal. Environ. 2019, 243, 183–194. [Google Scholar] [CrossRef]
- Gallus, M.; Ciuraru, R.; Mothes, F.; Akylas, V.; Barmpas, F.; Beeldens, A.; Bernard, F.; Boonen, E.; Boréave, A.; Cazaunau, M.; et al. Photocatalytic abatement results from a model street canyon. Environ. Sci. Pollut. Res. 2015, 22, 18185–18196. [Google Scholar] [CrossRef]
- Mahy, J.G.; Paez, C.A.; Hollevoet, J.; Courard, L.; Boonen, E.; Lambert, S.D. Durable photocatalytic thin coatings for road applications. Constr. Build. Mater. 2019, 215, 422–434. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, J.C.; Saianand, G.; Lee, K.P.; Sonar, P.; Dharmarajan, R.; Hou, Y.l.; Ann, K.Y.; Kannan, V.; Kim, W.J. Recent progress in the abatement of hazardous pollutants using photocatalytic TiO2-based building materials. Nanomaterials 2020, 10, 1854. [Google Scholar] [CrossRef]
- Verband der Mineralfarbenindustrie e.V. Photoactive Construction Materials. Available online: https://www.vdmi.de/en/products/applied-photocatalysis/product-range/photoactive-construction-materials/ (accessed on 3 August 2022).
- Kim, M.; Kim, H.; Park, J. Empirical NOx Removal Analysis of Photocatalytic Construction Materials at Real-Scale. Materials 2021, 14, 5717. [Google Scholar] [CrossRef]
- Linkous, C.A.; Carter, G.J.; Locuson, D.B.; Ouellette, A.J.; Slattery, D.K.; Smitha, L.A. Photocatalytic inhibition of algae growth using TiO2, WO3, and cocatalyst modifications. Environ. Sci. Technol. 2000, 34, 4754–4758. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Šuligoj, A.; Pliekhova, O.; Vodišek, N.; Mihelčič, M.; Surca, A.K.; Kunič, R.; Šubic, B.; Starman, J.; Ugovšek, A.; Lavrenčič Štangar, U. Field Test of Self-Cleaning Zr-Modified-TiO2-SiO2 Films on Glass with a Demonstration of Their Anti-Fogging Effect. Materials 2019, 12, 2196. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Ichihashi, E.; Nishida, N.; Machida, T.; Uchida, Y.; Hayashi, Y.; Morito, Y.; Fujishima, A. Field performance test of an air-cleaner with photocatalysis-plasma synergistic reactors for practical and long-term use. Molecules 2014, 19, 17424–17434. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.L.; Koziel, J.A. On-farm pilot-scale testing of black ultraviolet light and photocatalytic coating for mitigation of odor, odorous VOCs, and greenhouse gases. Chemosphere 2019, 221, 778–784. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Soutrel, I.; Petit, P.; Medimagh, K.; Wolbert, D. A study of pollution removal in exhaust gases from animal quartering centers by combining photocatalysis with surface discharge plasma: From pilot to industrial scale. Chem. Eng. Process. Process. Intensif. 2017, 111, 1–6. [Google Scholar] [CrossRef]
- Seiß, V.; Helbig, U.; Lösel, R.; Eichelbaum, M. Investigating and correlating photoelectrochemical, photocatalytic, and antimicrobial properties of TiO2 nanolayers. Sci. Rep. 2021, 11, 22200. [Google Scholar] [CrossRef]
- Villatte, G.; Massard, C.; Descamps, S.; Sibaud, Y.; Forestier, C.; Awitor, K.O. Photoactive TiO2 antibacterial coating on surgical external fixation pins for clinical application. Int. J. Nanomed. 2015, 10, 3367. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Sun, L.; Wang, X. A review on the application of photocatalytic materials on textiles. Text. Res. J. 2015, 85, 1104–1118. [Google Scholar] [CrossRef]
- Reid, M.; Whatley, V.; Spooner, E.; Nevill, A.M.; Cooper, M.; Ramsden, J.J.; Dancer, S.J. How does a photocatalytic antimicrobial coating affect environmental bioburden in hospitals? Infect. Control. Hosp. Epidemiol. 2018, 39, 398–404. [Google Scholar] [CrossRef]
- Verhoeven, J.W. Glossary of terms used in photochemistry. Pure Appl. Chem. 1996, 68, 2223. [Google Scholar] [CrossRef]
- Salomon, R.G. Homogeneous metal-catalysis in organic photochemistry. Tetrahedron 1983, 39, 485. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Suggested terms and definitions in photocatalysis and radiocatalysis. Int. J. Photoenergy 2002, 4, 91. [Google Scholar] [CrossRef]
- Wang, Y.H.; Rahman, K.H.; Wu, C.C.; Chen, K.C. A review on the pathways of the improved structural characteristics and photocatalytic performance of titanium dioxide (TiO2) thin films fabricated by the magnetron-sputtering technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.Q.; Al-Deyab, S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Luo, C.; Ren, X.; Dai, Z.; Zhang, Y.; Qi, X.; Pan, C. Present perspectives of advanced characterization techniques in TiO2-based photocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 23265–23286. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Do, H.H.; Tran, T.K.C.; Ung, T.D.T.; Dao, N.T.; Nguyen, D.D.; Trinh, T.H.; Hoang, T.D.; Le, T.L.; Tran, T.T.H. Controllable fabrication of photocatalytic TiO2 brookite thin film by 3D-printing approach for dyes decomposition. J. Water Process. Eng. 2021, 43, 102319. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic degradation of methylene blue over TiO2 pretreated with varying concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef]
- Gryparis, C.; Krasoudaki, T.; Maravelaki, P.N. Self-Cleaning Coatings for the Protection of Cementitious Materials: The Effect of Carbon Dot Content on the Enhancement of Catalytic Activity of TiO2. Coatings 2022, 12, 587. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Eichelbaum, M.; Seiß, V. Analysis of photoactive titanium dioxide layers: Common features and differences between photocatalysis and photoelectrochemistry. Wiley Anal. Sci. Mag. 2022, 3, 45–52. [Google Scholar]
- Marques, A.C.; Rojas-Hernandez, R.E.; Almeida, R.M. Optical spectroscopy methods for the characterization of sol—Gel materials. J. Sol-Gel Sci. Technol. 2021, 100, 1–43. [Google Scholar] [CrossRef]
- Ho, C.C.; Kang, F.; Chang, G.M.; You, S.J.; Wang, Y.F. Application of recycled lanthanum-doped TiO2 immobilized on commercial air filter for visible-light photocatalytic degradation of acetone and NO. Appl. Surf. Sci. 2019, 465, 31–40. [Google Scholar] [CrossRef]
- Milićević, N.; Novaković, M.; Potočnik, J.; Milović, M.; Rakočević, L.; Abazović, N.; Pjević, D. Influencing surface phenomena by Au diffusion in buffered TiO2-Au thin films: Effects of deposition and annealing processing. Surfaces Interfaces 2022, 30, 101811. [Google Scholar] [CrossRef]
- Fraunhofer. Sauber durch Sonnenkraft. Available online: https://www.light-and-surfaces.fraunhofer.de/en/Press-Events/Press-2018/Press-Release-1-8-2018.html (accessed on 6 August 2022).
- Liu, Y.; Huang, J.; Feng, X.; Li, H. Thermal-sprayed photocatalytic coatings for biocidal applications: A review. J. Therm. Spray Technol. 2021, 30, 1–24. [Google Scholar] [CrossRef]
- Krakowiak, R.; Musial, J.; Bakun, P.; Spychała, M.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Koczorowski, T.; Sobotta, L.; Stanisz, B.; Goslinski, T. Titanium Dioxide-Based Photocatalysts for Degradation of Emerging Contaminants including Pharmaceutical Pollutants. Appl. Sci. 2021, 11, 8674. [Google Scholar] [CrossRef]
- Ghosh, S.; Patra, R.; Majumdar, D.; Sen, K. Developing scenario of titania-based building materials for environmental remediation. Int. J. Environ. Sci. Technol. 2021, 18, 2077–2102. [Google Scholar] [CrossRef]
- Oberdörster, G.; Stone, V.; Donaldson, K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology 2007, 1, 2–25. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of produced water with photocatalysis: Recent advances, affecting factors and future research prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Aruna, S.; Vismaya, A.; Balaji, N. Photocatalytic behavior of titania coatings fabricated by suspension and solution precursor plasma spray processes. Mater. Manuf. Process. 2021, 36, 868–875. [Google Scholar] [CrossRef]
- Dell’Edera, M.; Porto, C.L.; de Pasquale, I.; Petronella, F.; Curri, M.L.; Agostiano, A.; Comparelli, R. Photocatalytic TiO2-based coatings for environmental applications. Catal. Today 2021, 380, 62–83. [Google Scholar] [CrossRef]
- Angulo-Ibáñez, A.; Aranzabe, E.; Beobide, G.; Castillo, O.; Goitandia, A.M.; Pérez-Yáñez, S.; Villamayor, A. Slot-Die Process of a Sol–Gel Photocatalytic Porous Coating for Large-Area Fabrication of Functional Architectural Glass. Catalysts 2021, 11, 711. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent progress in defective TiO2 photocatalysts for energy and environmental applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent progress on titanium dioxide nanomaterials for photocatalytic applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709–6734. [Google Scholar] [CrossRef]
- Wu, M.C.; Lin, T.H.; Hsu, K.H.; Hsu, J.F. Photo-induced disinfection property and photocatalytic activity based on the synergistic catalytic technique of Ag doped TiO2 nanofibers. Appl. Surf. Sci. 2019, 484, 326–334. [Google Scholar] [CrossRef]
- Thunyasirinon, C.; Sribenjalux, P.; Supothina, S.; Chuaybamroong, P. Enhancement of air filter with TiO2 photocatalysis for mycobacterium tuberculosis removal. Aerosol Air Qual. Res. 2015, 15, 600–610. [Google Scholar] [CrossRef]
- Saqlain, S.; Cha, B.J.; Kim, S.Y.; Ahn, T.K.; Park, C.; Oh, J.M.; Jeong, E.C.; Seo, H.O.; Kim, Y.D. Visible light-responsive Fe-loaded TiO2 photocatalysts for total oxidation of acetaldehyde: Fundamental studies towards large-scale production and applications. Appl. Surf. Sci. 2020, 505, 144160. [Google Scholar] [CrossRef]
- Özkal, C.B.; Mantzavinos, D.; Meriç, S. Photocatalytic activity based-optimization of TTIP thin films for E. coli inactivation: Effect of Mn and Cu dopants. Catal. Today 2017, 280, 86–92. [Google Scholar] [CrossRef]
- Martínez-Montelongo, J.H.; Medina-Ramírez, I.E.; Romo-Lozano, Y.; Zapien, J.A. Development of a sustainable photocatalytic process for air purification. Chemosphere 2020, 257, 127236. [Google Scholar] [CrossRef]
- Thakur, I.; Verma, A.; Örmeci, B. Inactivation of bacteria present in secondary municipal wastewater effluent using the hybrid effect of Fe–TiO2 catalyst. J. Clean. Prod. 2022, 352, 131575. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Oros-Ruiz, S.; Zanella, R.; Prado, B. Photocatalytic degradation of trimethoprim by metallic nanoparticles supported on TiO2-P25. J. Hazard. Mater. 2013, 263, 28–35. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Prati, L.; Canton, P.; Selli, E. Effects of gold nanoparticles deposition on the photocatalytic activity of titanium dioxide under visible light. Phys. Chem. Chem. Phys. 2009, 11, 7171–7180. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Choi, H.; Chen, W.T.; Kamat, P.V. Know thy nano neighbor. Plasmonic versus electron charging effects of metal nanoparticles in dye-sensitized solar cells. ACS Nano 2012, 6, 4418–4427. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.t.; Guan, Z.s.; Cao, Y.a.; Yao, J.n. Effects of zinc (II) and iron (III) doping of titania films on their photoreactivity to decompose rhodamine B. J. Mater. Res. 2001, 16, 2928–2933. [Google Scholar] [CrossRef]
- Tong, T.; Zhang, J.; Tian, B.; Chen, F.; He, D. Preparation of Fe3+-doped TiO2 catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation. J. Hazard. Mater. 2008, 155, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Appasamy, J.S.; Kurnia, J.C.; Assadi, M.K. Synthesis and evaluation of nitrogen-doped titanium dioxide/single walled carbon nanotube-based hydrophilic self-cleaning coating layer for solar photovoltaic panel surface. Sol. Energy 2020, 196, 80–91. [Google Scholar] [CrossRef]
- Fan, W.; Chan, K.Y.; Zhang, C.; Leung, M.K. Advanced Solar Photocatalytic Asphalt for Removal of Vehicular NOx. Energy Procedia 2017, 143, 811–816. [Google Scholar] [CrossRef]
- Jiang, Q.; Hou, J.; Liu, J.; Fu, Y.; Liu, Y. Visible Photocatalysis of a Building Glass Coated with N-F-TiO2/rGO. In Proceedings of the 11th International Symposium on Heating, Ventilation and Air Conditioning (ISHVAC 2019), Harbin, China, 12–15 July 2019; Wang, Z., Zhu, Y., Wang, F., Wang, P., Shen, C., Liu, J., Eds.; Springer: Singapore, 2020; pp. 181–190. [Google Scholar] [CrossRef]
- Dineshram, R.; Subasri, R.; Somaraju, K.; Jayaraj, K.; Vedaprakash, L.; Ratnam, K.; Joshi, S.; Venkatesan, R. Biofouling studies on nanoparticle-based metal oxide coatings on glass coupons exposed to marine environment. Colloids Surf. Biointerfaces 2009, 74, 75–83. [Google Scholar] [CrossRef]
- Krumdieck, S.; Miya, S.; Lee, D.; Davies-Talwar, S.; Bishop, C. Titania-based photocatalytic coatings on stainless steel hospital fixtures. Phys. Status Solidi 2015, 12, 1028–1035. [Google Scholar] [CrossRef]
- Villardi de Oliveira, C.; Petitbois, J.; Fay, F.; Sanchette, F.; Schuster, F.; Alhussein, A.; Chaix-Pluchery, O.; Deschanvres, J.L.; Jiménez, C. Marine antibiofouling properties of TiO2 and Ti-Cu-O films deposited by aerosol-assisted chemical vapor deposition. Coatings 2020, 10, 779. [Google Scholar] [CrossRef]
- Dalanta, F.; Kusworo, T.D.; Aryanti, N. Synthesis, characterization, and performance evaluation of UV light-driven Co-TiO2@ SiO2 based photocatalytic nanohybrid polysulfone membrane for effective treatment of petroleum refinery wastewater. Appl. Catal. Environ. 2022, 316, 121576. [Google Scholar] [CrossRef]
- Horváth, E.; Gabathuler, J.; Bourdiec, G.; Vidal-Revel, E.; Benthem Muñiz, M.; Gaal, M.; Grandjean, D.; Breider, F.; Rossi, L.; Sienkiewicz, A.; et al. Solar water purification with photocatalytic nanocomposite filter based on TiO2 nanowires and carbon nanotubes. NPJ Clean Water 2022, 5, 10. [Google Scholar] [CrossRef]
- Delnavaz, M.; Mahdian, S.S.; Boshagh, M.A. Economical and operational optimization of the enhanced titania photocatalytic activity by plasmonic effect for the treatment of real petrochemical wastewater. Pet. Sci. Technol. 2022, 40, 2525–2544. [Google Scholar] [CrossRef]
- Porley, V.; Chatzisymeon, E.; Meikap, B.C.; Ghosal, S.; Robertson, N. Field testing of low-cost titania-based photocatalysts for enhanced solar disinfection (SODIS) in rural India. Environ. Sci. Water Res. Technol. 2020, 6, 809–816. [Google Scholar] [CrossRef]
- Faccani, L.; Ortelli, S.; Blosi, M.; Costa, A.L. Ceramized Fabrics and Their Integration in a Semi-Pilot Plant for the Photodegradation of Water Pollutants. Catalysts 2021, 11, 1418. [Google Scholar] [CrossRef]
- Thuy, N.T.; May, D.T.; Thao, D.N.P.; Thuy, V.T.T.; Thanh, D.V.; Thanh, N.T.; Huy, N.N. Field study of visitors’ behavior in incense burning and its induced air pollution assessment and treatment. Environ. Sci. Pollut. Res. 2022, 29, 45933–45946. [Google Scholar] [CrossRef]
- Yu, H.; Song, L.; Hao, Y.; Lu, N.; Quan, X.; Chen, S.; Zhang, Y.; Feng, Y. Fabrication of pilot-scale photocatalytic disinfection device by installing TiO2 coated helical support into UV annular reactor for strengthening sterilization. Chem. Eng. J. 2016, 283, 1506–1513. [Google Scholar] [CrossRef]
- Chen, M.; Baglee, D.; Chu, J.W.; Du, D.; Guo, X. Photocatalytic oxidation of NOx under visible light on asphalt pavement surface. J. Mater. Civ. Eng. 2017, 29, 1. [Google Scholar] [CrossRef]
- Mahy, J.G.; Wolfs, C.; Vreuls, C.; Drot, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Hermans, S.; Lambert, S.D. Advanced oxidation processes for waste water treatment: From laboratory-scale model water to on-site real waste water. Environ. Technol. 2021, 42, 3974–3986. [Google Scholar] [CrossRef]
- Maggos, T.; Binas, V.; Siaperas, V.; Terzopoulos, A.; Panagopoulos, P.; Kiriakidis, G. A promising technological approach to improve indoor air quality. Appl. Sci. 2019, 9, 4837. [Google Scholar] [CrossRef]
- Monteiro, R.A.; Rodrigues-Silva, C.; Lopes, F.V.; Silva, A.M.; Boaventura, R.A.; Vilar, V.J. Evaluation of a solar/UV annular pilot scale reactor for 24 h continuous photocatalytic oxidation of n-decane. Chem. Eng. J. 2015, 280, 409–416. [Google Scholar] [CrossRef]
- Abou Saoud, W.; Kane, A.; le Cann, P.; Gérard, A.; Lamaa, L.; Peruchon, L.; Brochier, C.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. Innovative photocatalytic reactor for the degradation of VOCs and microorganism under simulated indoor air conditions: Cu-Ag/TiO2-based optical fibers at a pilot scale. Chem. Eng. J. 2021, 411, 128622. [Google Scholar] [CrossRef]
- Schnabel, T.; Dutschke, M.; Schuetz, F.; Hauser, F.; Springer, C. Photocatalytic air purification of polycyclic aromatic hydrocarbons: Application of a flow-through reactor, kinetic studies and degradation pathways. J. Photochem. Photobiol. Chem. 2022, 430, 113993. [Google Scholar] [CrossRef]
- Deepracha, S.; Atfane, L.; Ayral, A.; Ogawa, M. Simple and efficient method for functionalizing photocatalytic ceramic membranes and assessment of its applicability for wastewater treatment in up-scalable membrane reactors. Sep. Purif. Technol. 2021, 262, 118307. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Monteiro, R.A.; Dezotti, M.; Silva, A.M.; Pinto, E.; Boaventura, R.A.; Vilar, V.J. A facile method to prepare translucent anatase thin films in monolithic structures for gas stream purification. Environ. Sci. Pollut. Res. 2018, 25, 27796–27807. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, N.; Katsaros, F.; Kontos, A.; Romanos, G.E.; Dionysiou, D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Zhao, J.H.; Chen, W.; Zhao, Y.; Liu, C.; Liu, R. UV/TiO2 photocatalytic disinfection of carbon-bacteria complexes in activated carbon-filtered water: Laboratory and pilot-scale investigation. J. Environ. Sci. Health Part A 2015, 50, 1274–1281. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Ho, W. Efficient photocatalytic degradation of NO by ceramic foam air filters coated with mesoporous TiO2 thin films. Chin. J. Catal. 2015, 36, 2109–2118. [Google Scholar] [CrossRef]
- Assadi, A.A.; Palau, J.; Bouzaza, A.; Penya-Roja, J.; Martinez-Soriac, V.; Wolbert, D. Abatement of 3-methylbutanal and trimethylamine with combined plasma and photocatalysis in a continuous planar reactor. J. Photochem. Photobiol. Chem. 2014, 282, 1–8. [Google Scholar] [CrossRef]
- Zadi, T.; Assadi, A.A.; Nasrallah, N.; Bouallouche, R.; Tri, P.N.; Bouzaza, A.; Azizi, M.M.; Maachi, R.; Wolbert, D. Treatment of hospital indoor air by a hybrid system of combined plasma with photocatalysis: Case of trichloromethane. Chem. Eng. J. 2018, 349, 276–286. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Wu, H.Y.; Bai, H. Photocatalytic reduction of NO2 and CO2 using molybdenum-doped titania nanotubes. Chem. Eng. J. 2015, 269, 60–66. [Google Scholar] [CrossRef]
- Boonen, E.; Akylas, V.; Barmpas, F.; Boréave, A.; Bottalico, L.; Cazaunau, M.; Chen, H.; Daële, V.; de Marco, T.; Doussin, J.; et al. Construction of a photocatalytic de-polluting field site in the Leopold II tunnel in Brussels. J. Environ. Manag. 2015, 155, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Gallus, M.; Akylas, V.; Barmpas, F.; Beeldens, A.; Boonen, E.; Boréave, A.; Cazaunau, M.; Chen, H.; Daële, V.; Doussin, J.; et al. Photocatalytic de-pollution in the Leopold II tunnel in Brussels: NOx abatement results. Build. Environ. 2015, 84, 125–133. [Google Scholar] [CrossRef]
- Fernández-Pampillón, J.; Palacios, M.; Núñez, L.; Pujadas, M.; Sanchez, B.; Santiago, J.L.; Martilli, A. NOx depolluting performance of photocatalytic materials in an urban area–Part I: Monitoring ambient impact. Atmos. Environ. 2021, 251, 118190. [Google Scholar] [CrossRef]
- Mahy, J.G.; Wolfs, C.; Mertes, A.; Vreuls, C.; Drot, S.; Smeets, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Lambert, S.D. Advanced photocatalytic oxidation processes for micropollutant elimination from municipal and industrial water. J. Environ. Manag. 2019, 250, 109561. [Google Scholar] [CrossRef]
- Pal, S.; Laera, A.M.; Licciulli, A.; Catalano, M.; Taurino, A. Biphase TiO2 microspheres with enhanced photocatalytic activity. Ind. Eng. Chem. Res. 2014, 53, 7931–7938. [Google Scholar] [CrossRef]
- Lettieri, M.; Colangiuli, D.; Masieri, M.; Calia, A. Field performances of nanosized TiO2 coated limestone for a self-cleaning building surface in an urban environment. Build. Environ. 2019, 147, 506–516. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Masieri, M.; Pal, S.; Licciulli, A.; Arima, V. Limestones coated with photocatalytic TiO2 to enhance building surface with self-cleaning and depolluting abilities. J. Clean. Prod. 2017, 165, 1036–1047. [Google Scholar] [CrossRef]
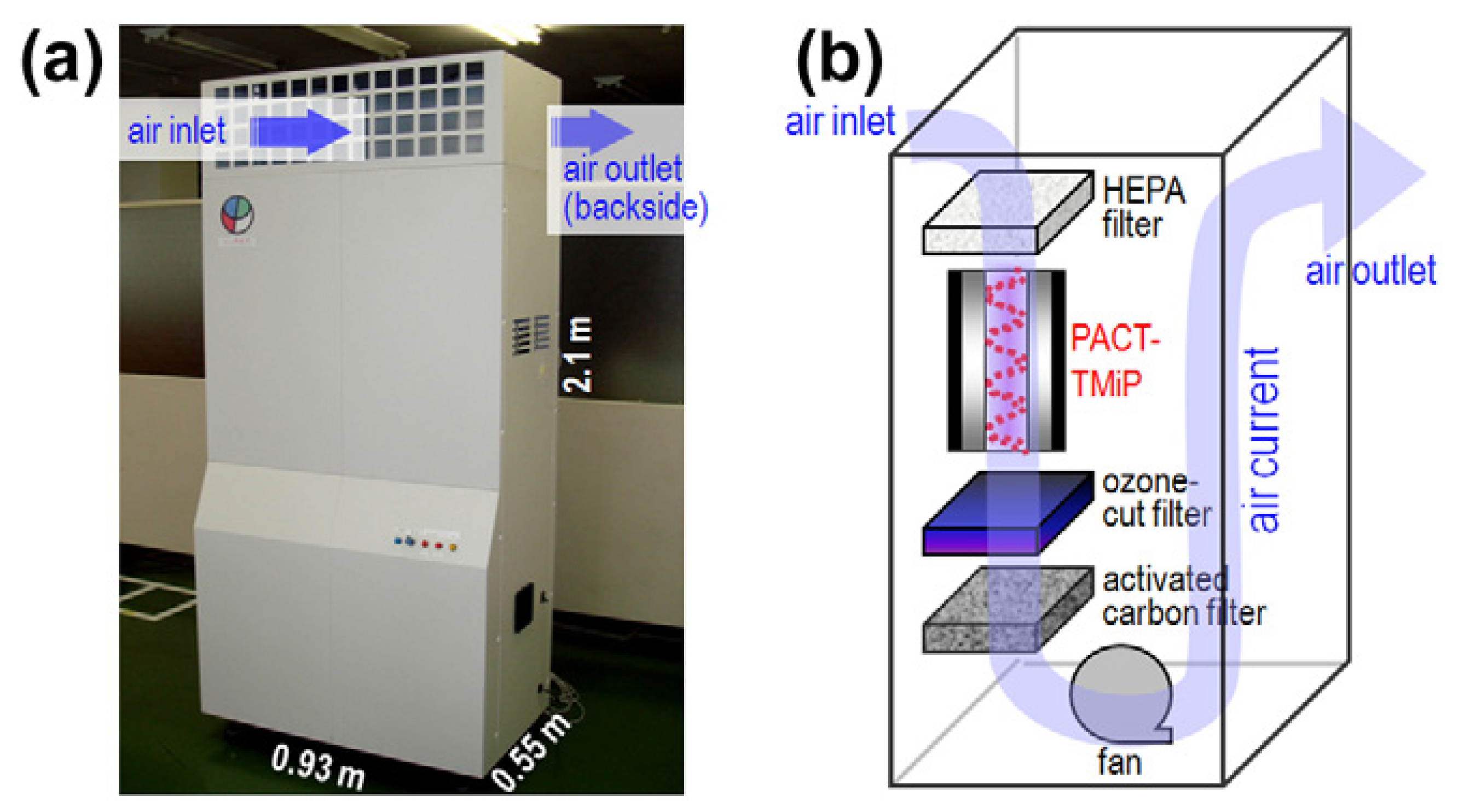
- Ochiai, T.; Hoshi, T.; Slimen, H.; Nakata, K.; Murakami, T.; Tatejima, H.; Koide, Y.; Houas, A.; Horie, T.; Morito, Y.; et al. Fabrication of a TiO2 nanoparticles impregnated titanium mesh filter and its application for environmental purification. Catal. Sci. Technol. 2011, 1, 1324–1327. [Google Scholar] [CrossRef]
- Saran, S.; Arunkumar, P.; Devipriya, S. Disinfection of roof harvested rainwater for potable purpose using pilot-scale solar photocatalytic fixed bed tubular reactor. Water Sci. Technol. Water Supply 2018, 18, 49–59. [Google Scholar] [CrossRef]
- Saran, S.; Kamalraj, G.; Arunkumar, P.; Devipriya, S. Pilot scale thin film plate reactors for the photocatalytic treatment of sugar refinery wastewater. Environ. Sci. Pollut. Res. 2016, 23, 17730–17741. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Bai, W.; Han, Z.; Yano, A.; Thakur, A.; Georgieva, A.; Tolley, K.; Navarro, J.; Koopman, B.; Moudgil, B. Contaminant-activated visible light photocatalysis. Sci. Rep. 2018, 8, 1894. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Haupt, M.; Klostermann, H.; Kim, H.S.; Eichler, M.; Peetsch, A.; Scheideler, L.; Doering, C.; Oehr, C.; Wendel, H.; et al. Multifunctional nature of UV-irradiated nanocrystalline anatase thin films for biomedical applications. Acta Biomater. 2010, 6, 4566–4577. [Google Scholar] [CrossRef]
- Ochiai, T.; Hayashi, Y.; Ito, M.; Nakata, K.; Murakami, T.; Morito, Y.; Fujishima, A. An effective method for a separation of smoking area by using novel photocatalysis-plasma synergistic air-cleaner. Chem. Eng. J. 2012, 209, 313–317. [Google Scholar] [CrossRef]
- Colangiuli, D.; Lettieri, M.; Masieri, M.; Calia, A. Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. Sci. Total. Environ. 2019, 650, 2919–2930. [Google Scholar] [CrossRef]
- Saft, F.; Heymer, H.; Adler, J.; Faßauer, B. Photocatalytic Wastewater Treatment with Functionalized Cellular Ceramics; Annual Report 14/15; Fraunhofer Institute for Ceramic Technologies and Systems IKTS: Hermsdorf, Germany, 2014; Volume 1, p. 77. [Google Scholar]
- Lee, M.; Li, P.; Koziel, J.A.; Ahn, H.; Wi, J.; Chen, B.; Meiirkhanuly, Z.; Banik, C.; Jenks, W.S. Pilot-scale testing of UV-A light treatment for mitigation of NH3, H2S, GHGs, VOCs, odor, and O3 inside the poultry barn. Front. Chem. 2020, 8, 613. [Google Scholar] [CrossRef]
- Maggos, T.; Bartzis, J.; Liakou, M.; Gobin, C. Photocatalytic degradation of NOx gases using TiO2-containing paint: A real scale study. J. Hazard. Mater. 2007, 146, 668–673. [Google Scholar] [CrossRef]
- Fan, W.; Chan, K.Y.; Zhang, C.; Zhang, K.; Ning, Z.; Leung, M.K. Solar photocatalytic asphalt for removal of vehicular NOx: A feasibility study. Appl. Energy 2018, 225, 535–541. [Google Scholar] [CrossRef]
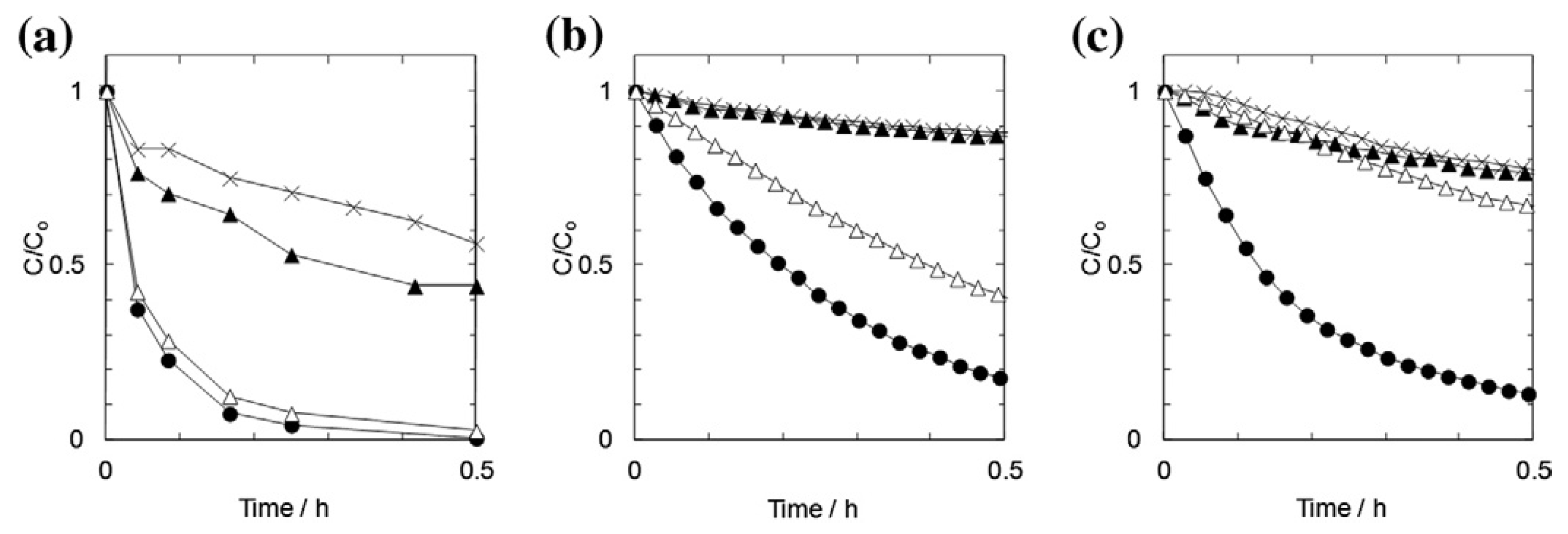
- Zhao, Y.K.; Sung, W.P.; Tsai, T.T.; Wang, H.J. Application of nanoscale silver-doped titanium dioxide as photocatalyst for indoor airborne bacteria control: A feasibility study in medical nursing institutions. J. Air Waste Manag. Assoc. 2010, 60, 337–345. [Google Scholar] [CrossRef]
- Sung, W.; Tsai, T.; Wu, M.; Wang, H.; Surampalli, R. Removal of indoor airborne bacteria by nano-Ag/TiO 2 as photocatalyst: Feasibility study in museum and nursing institutions. J. Environ. Eng. 2011, 137, 163–170. [Google Scholar] [CrossRef]
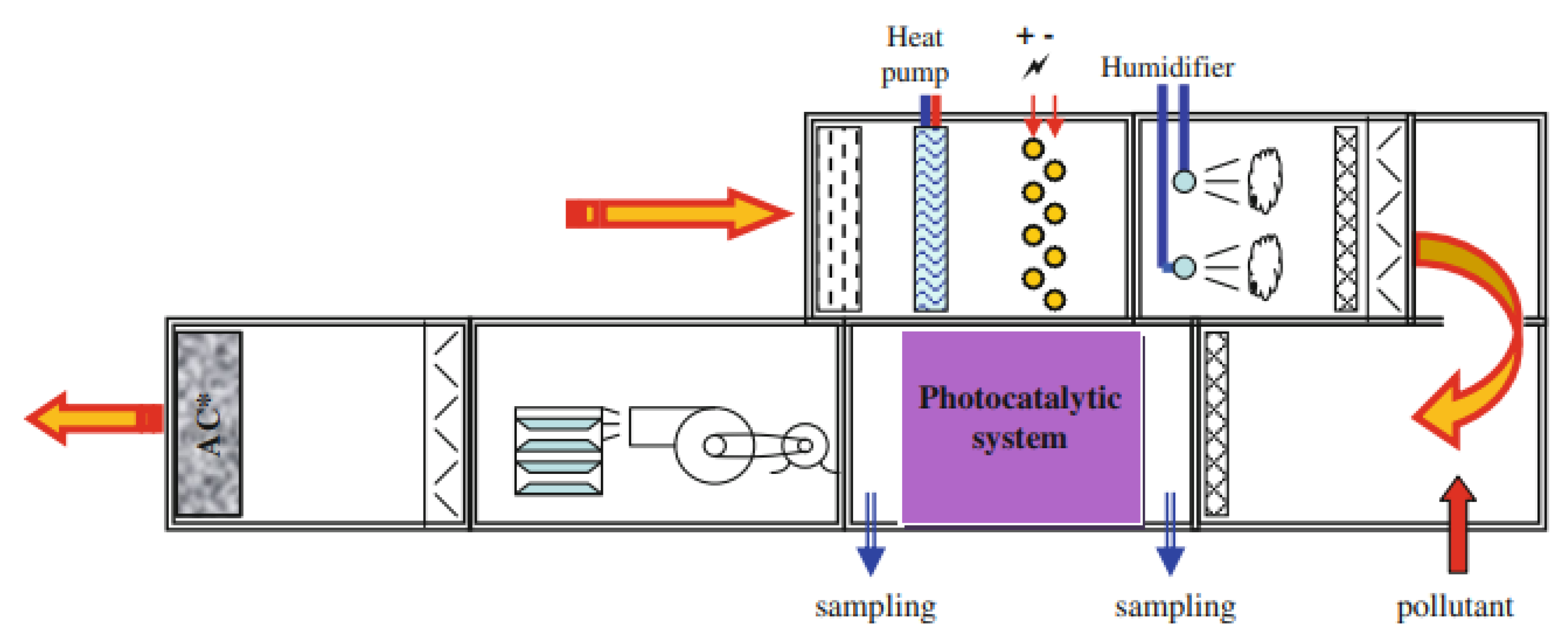
- Blondeau, P.; Abadie, M.O.; Durand, A.; Kaluzny, P.; Parat, S.; Ginestet, A.; Pugnet, D.; Tourreilles, C.; Duforestel, T. Experimental characterization of the removal efficiency and energy effectiveness of central air cleaners. Energy Built Environ. 2021, 2, 1–12. [Google Scholar] [CrossRef]
- Ehm, C.; Frohmüller, M.O.; Flassak, T.; Stephan, D. On-site reduction of nitrogen oxides at an emission hotspot using actively vented photocatalytic reactors in a highway tunnel. SN Appl. Sci. 2022, 4, 153. [Google Scholar] [CrossRef]
- Gharaibeh, A.; Smith, R.H.; Conway, M.J. Reducing spread of infections with a Photocatalytic reactor—Potential applications in control of hospital Staphylococcus aureus and Clostridioides difficile infections and inactivation of RNA viruses. Infect. Dis. Rep. 2021, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Özkal, C.B.; Venieri, D.; Gounaki, I.; Meric, S. Assessment of thin-film photocatalysis inactivation of different bacterial indicators and effect on their antibiotic resistance profile. Appl. Catal. Environ. 2019, 244, 612–619. [Google Scholar] [CrossRef]

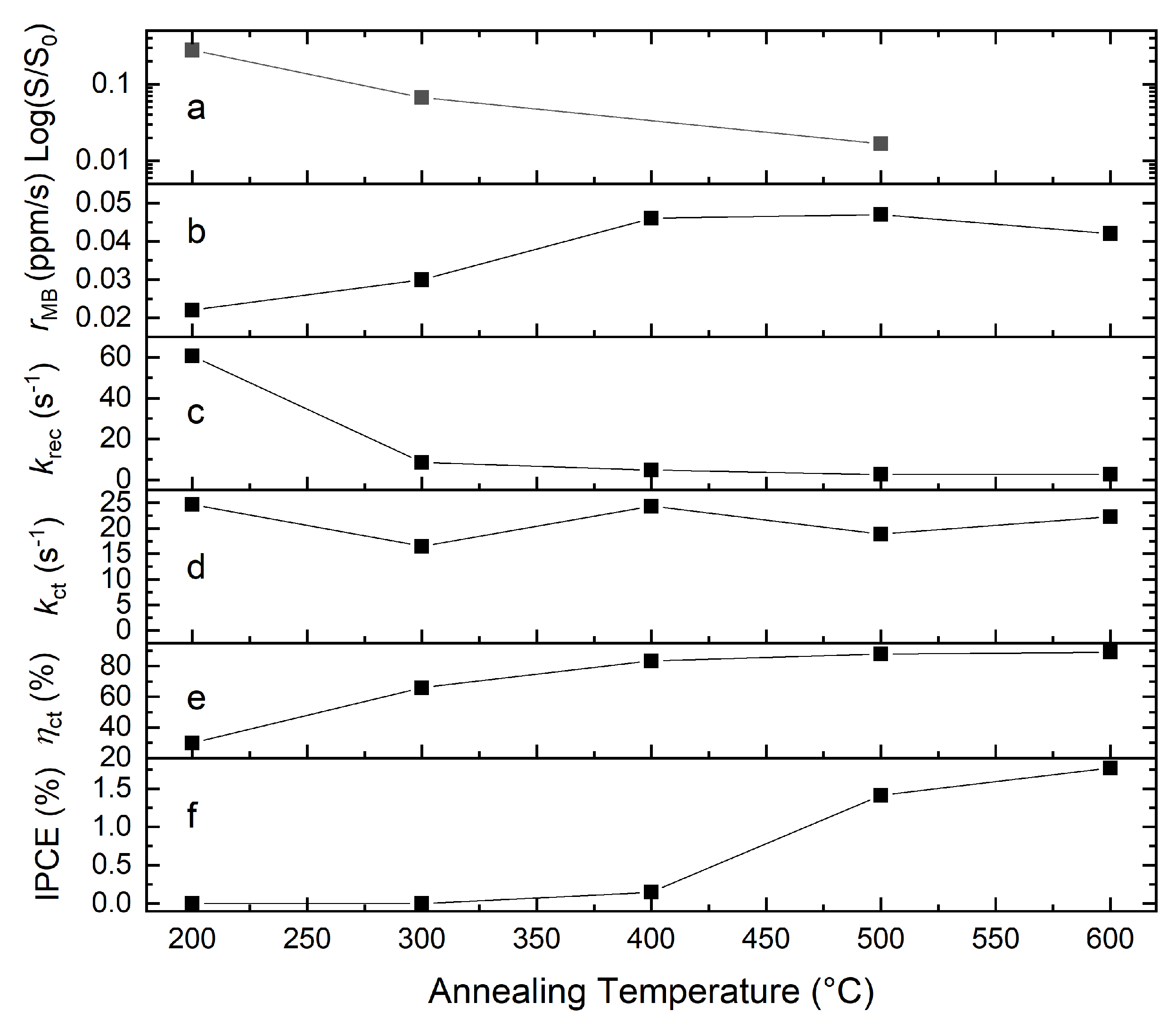
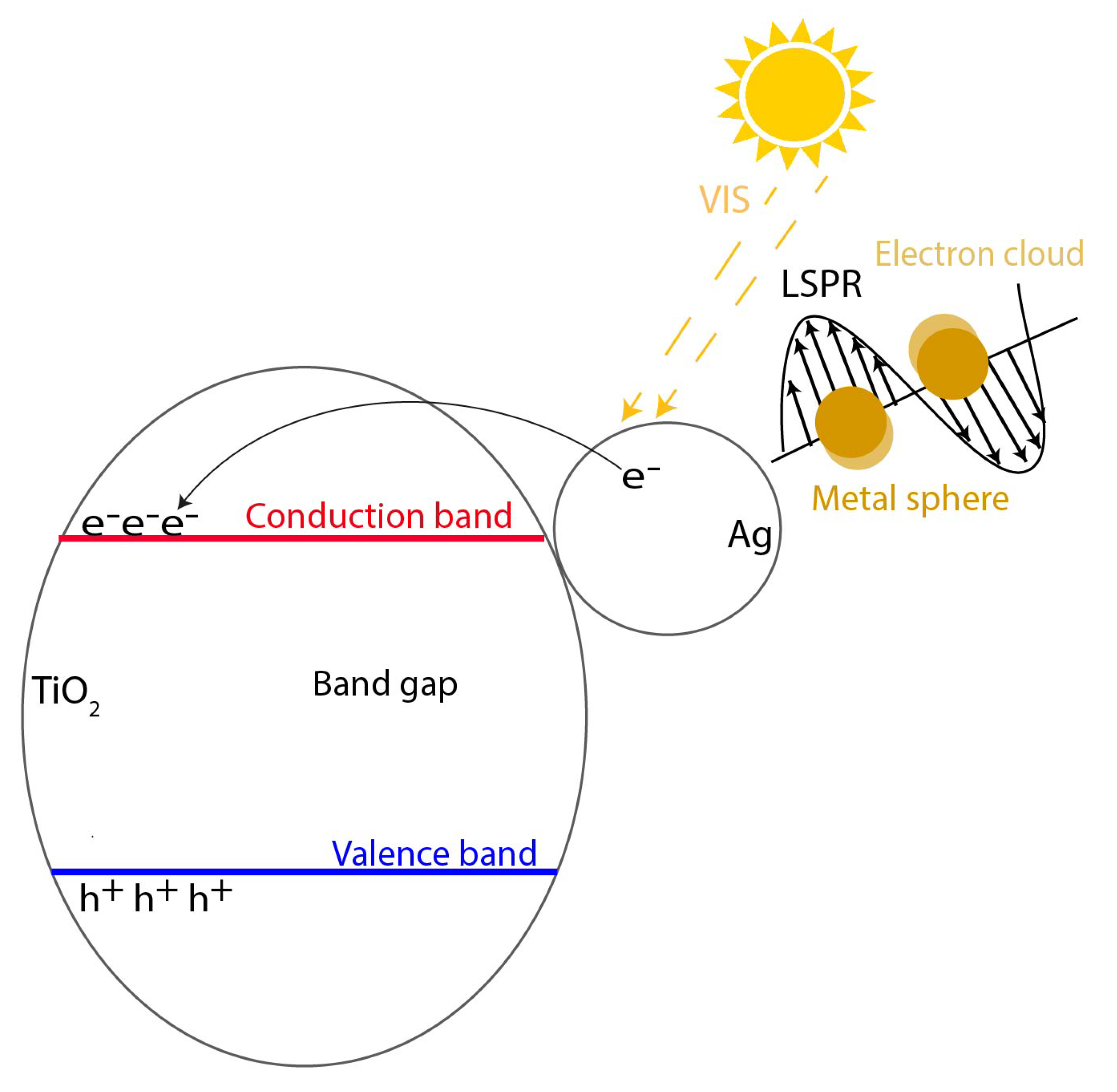
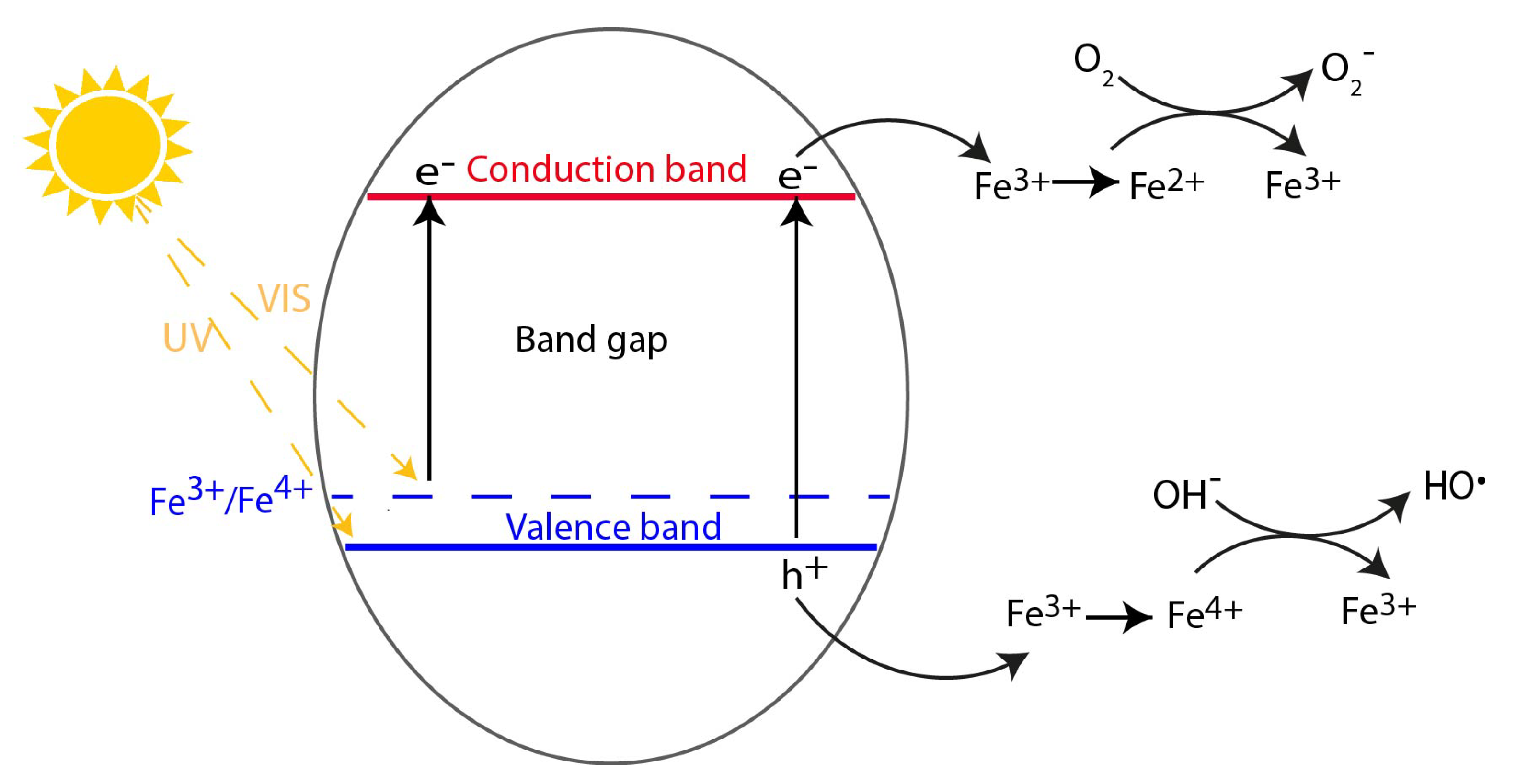

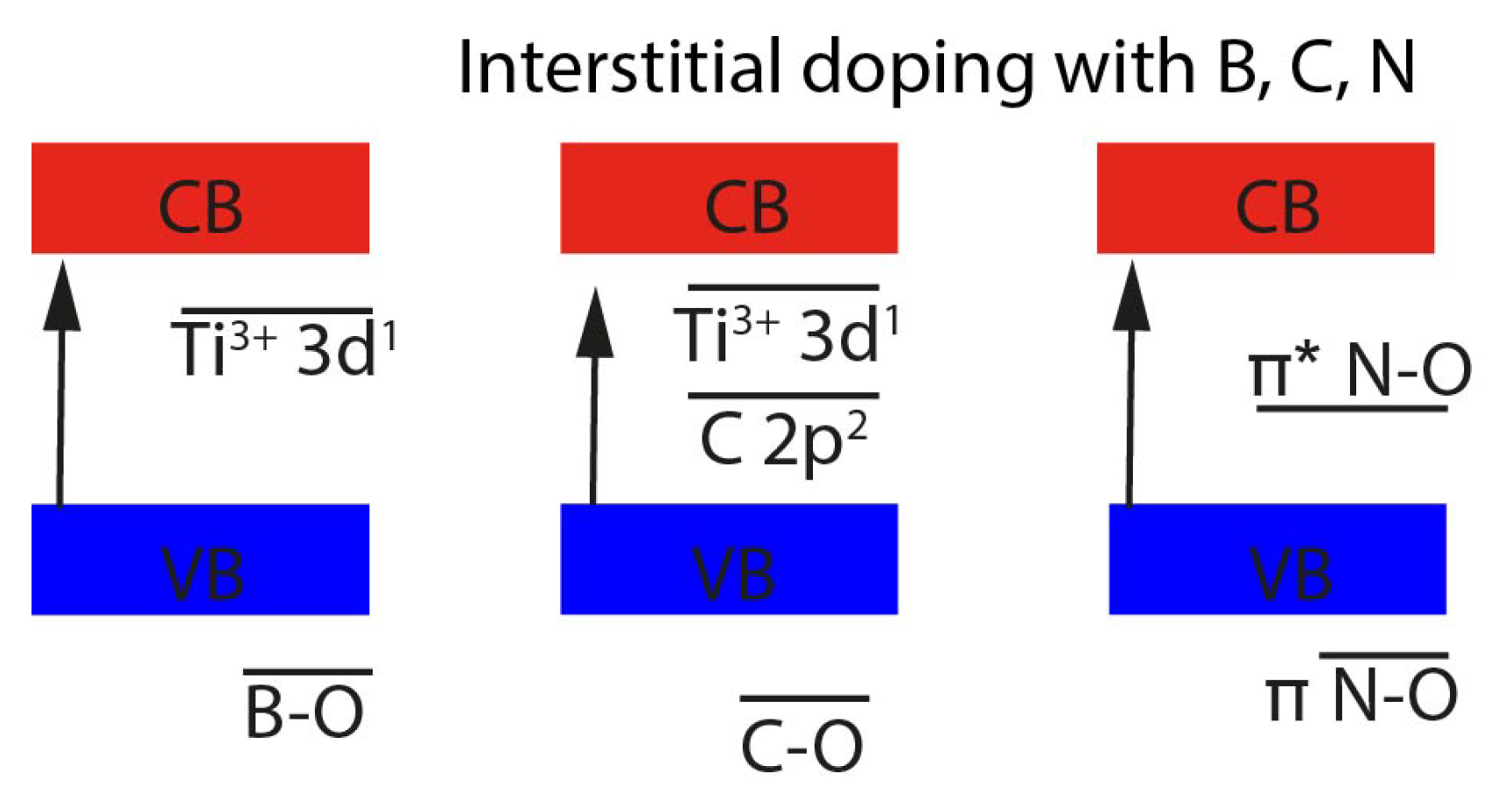
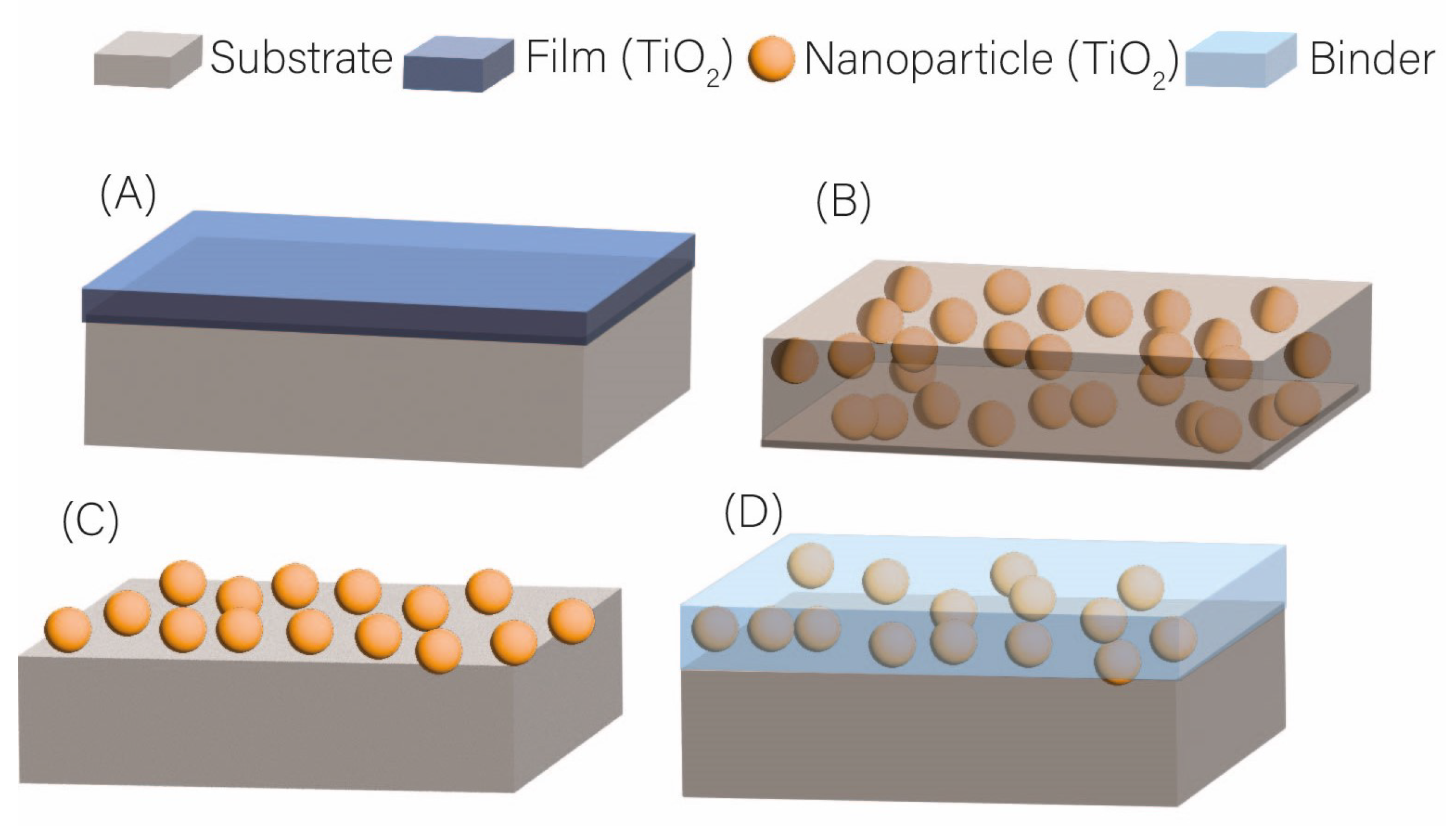
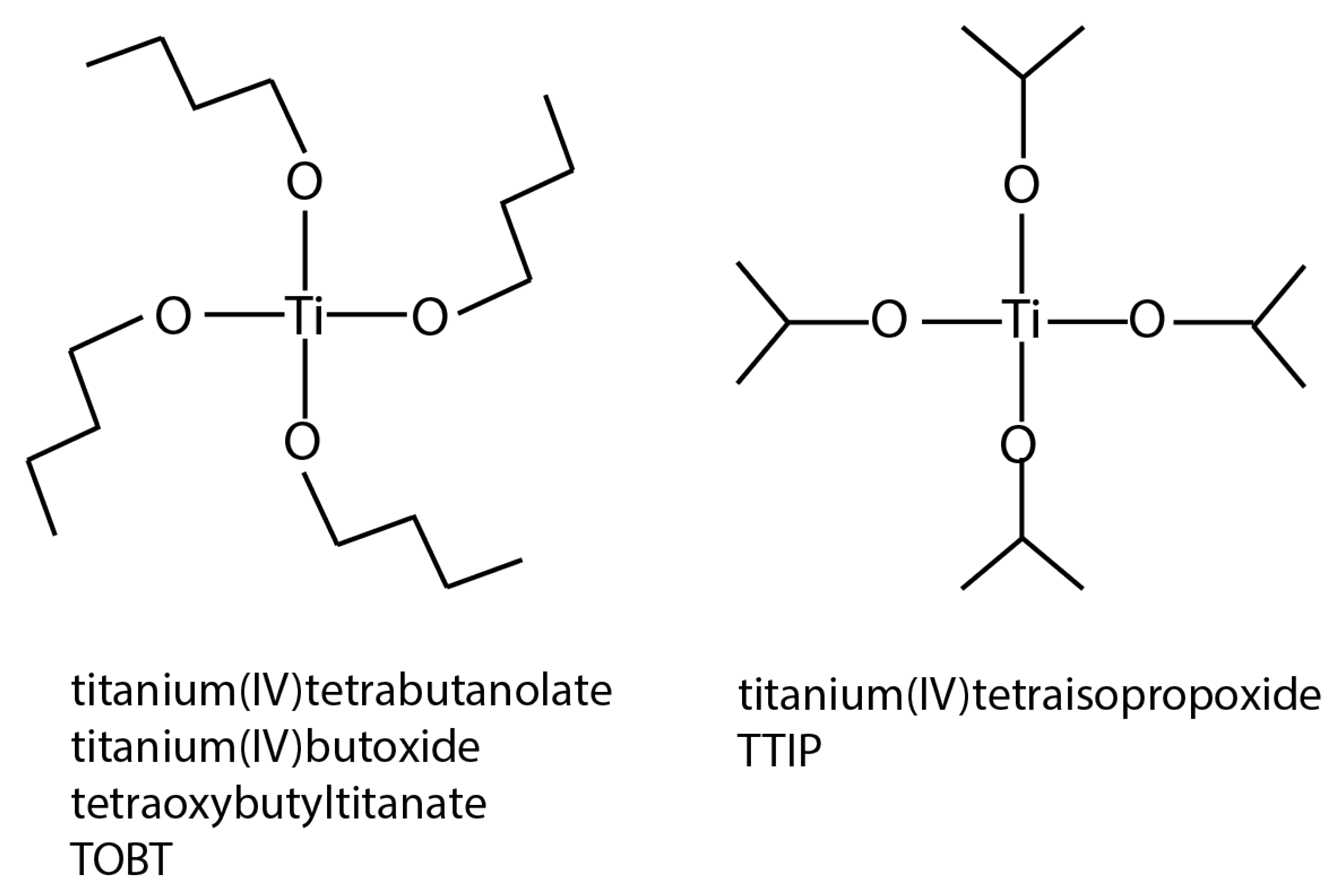
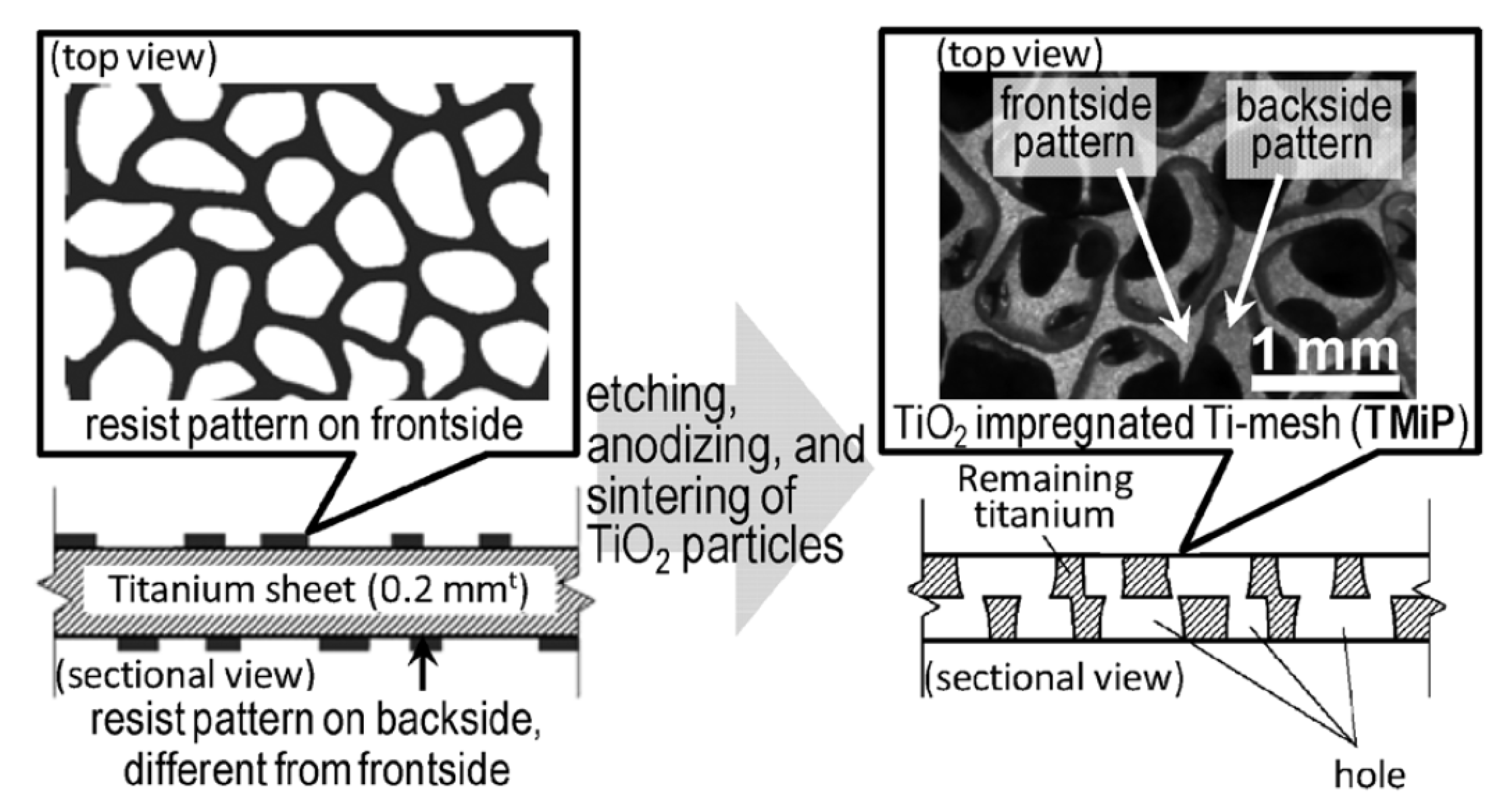
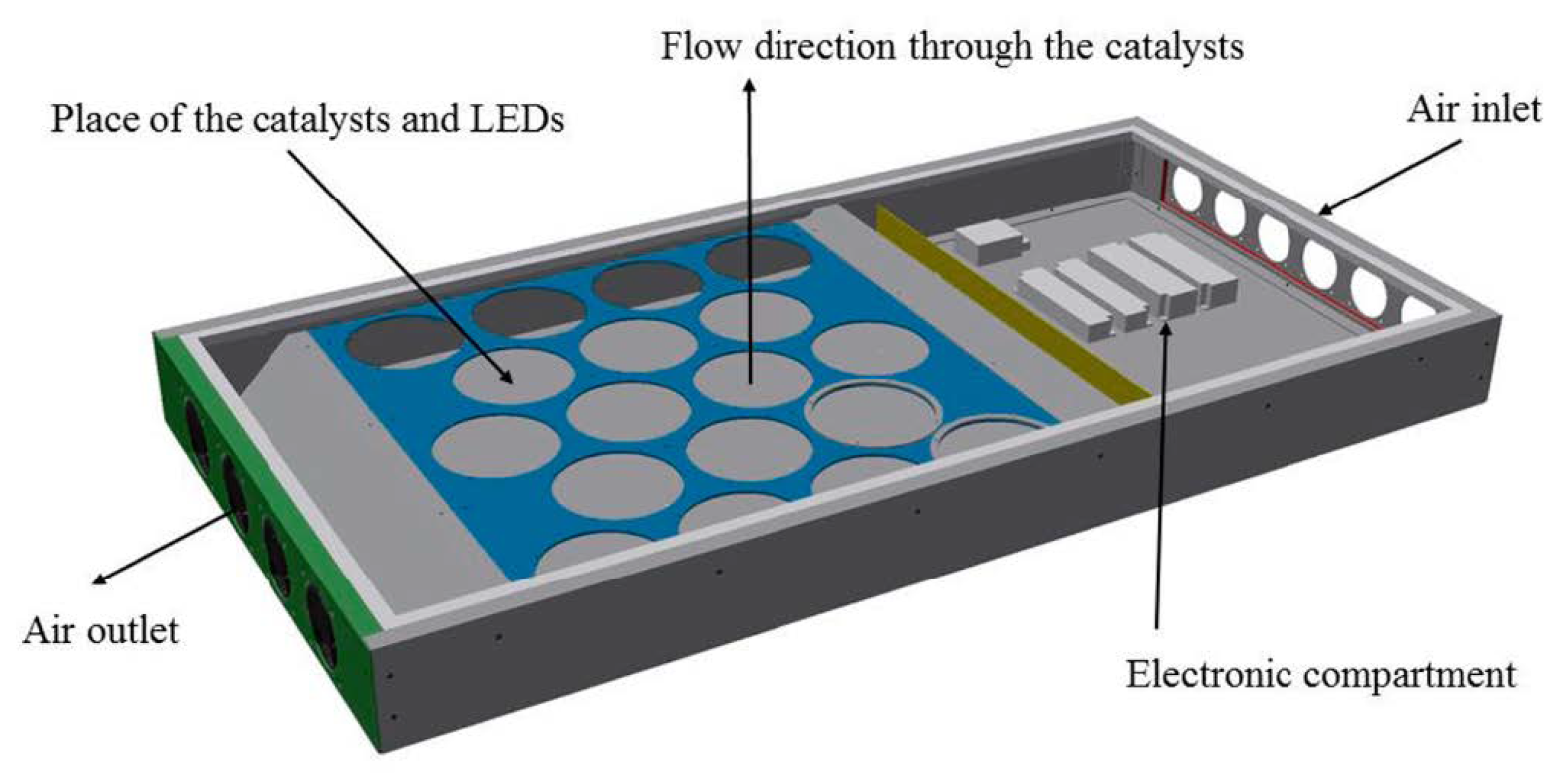
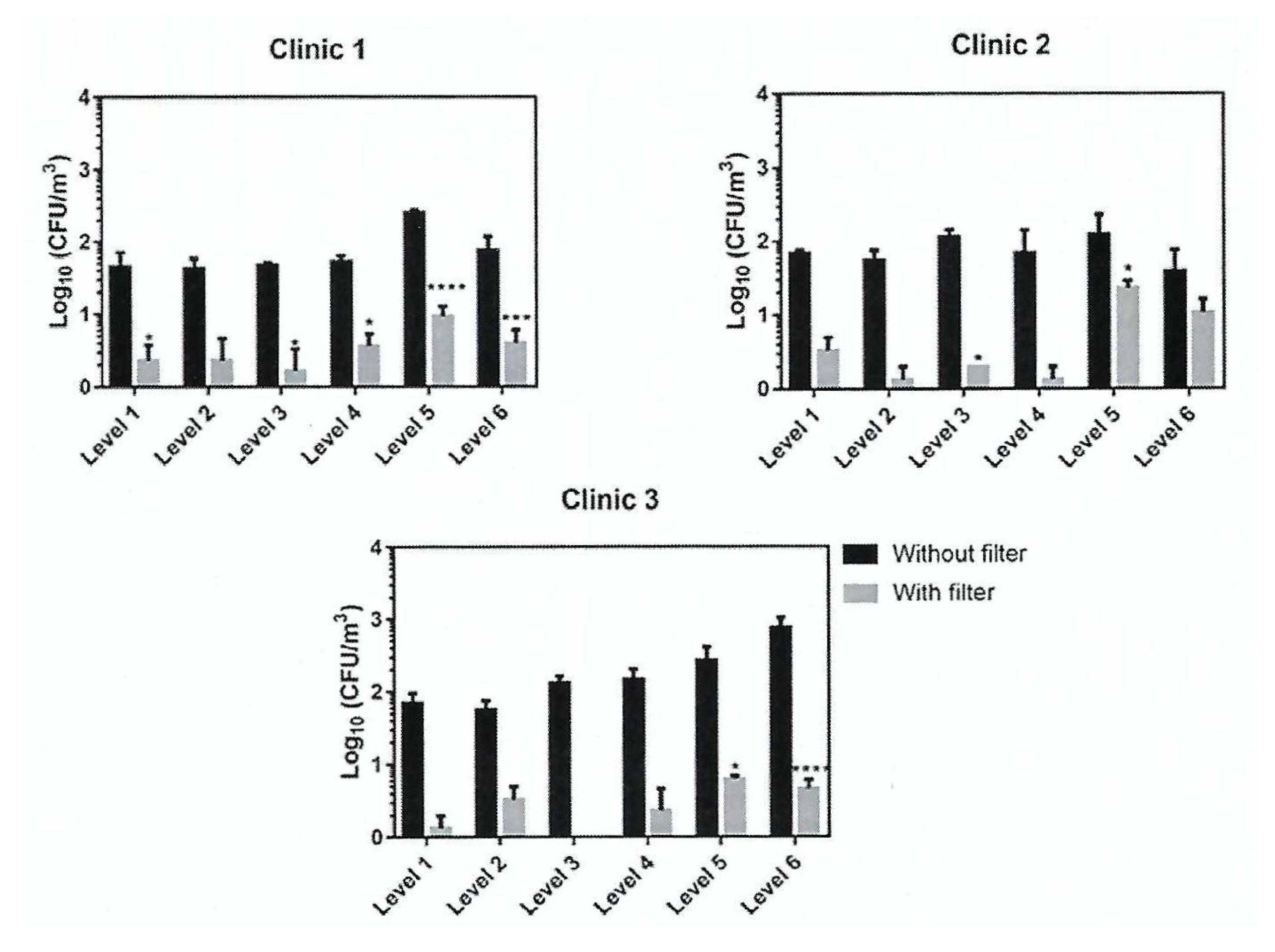

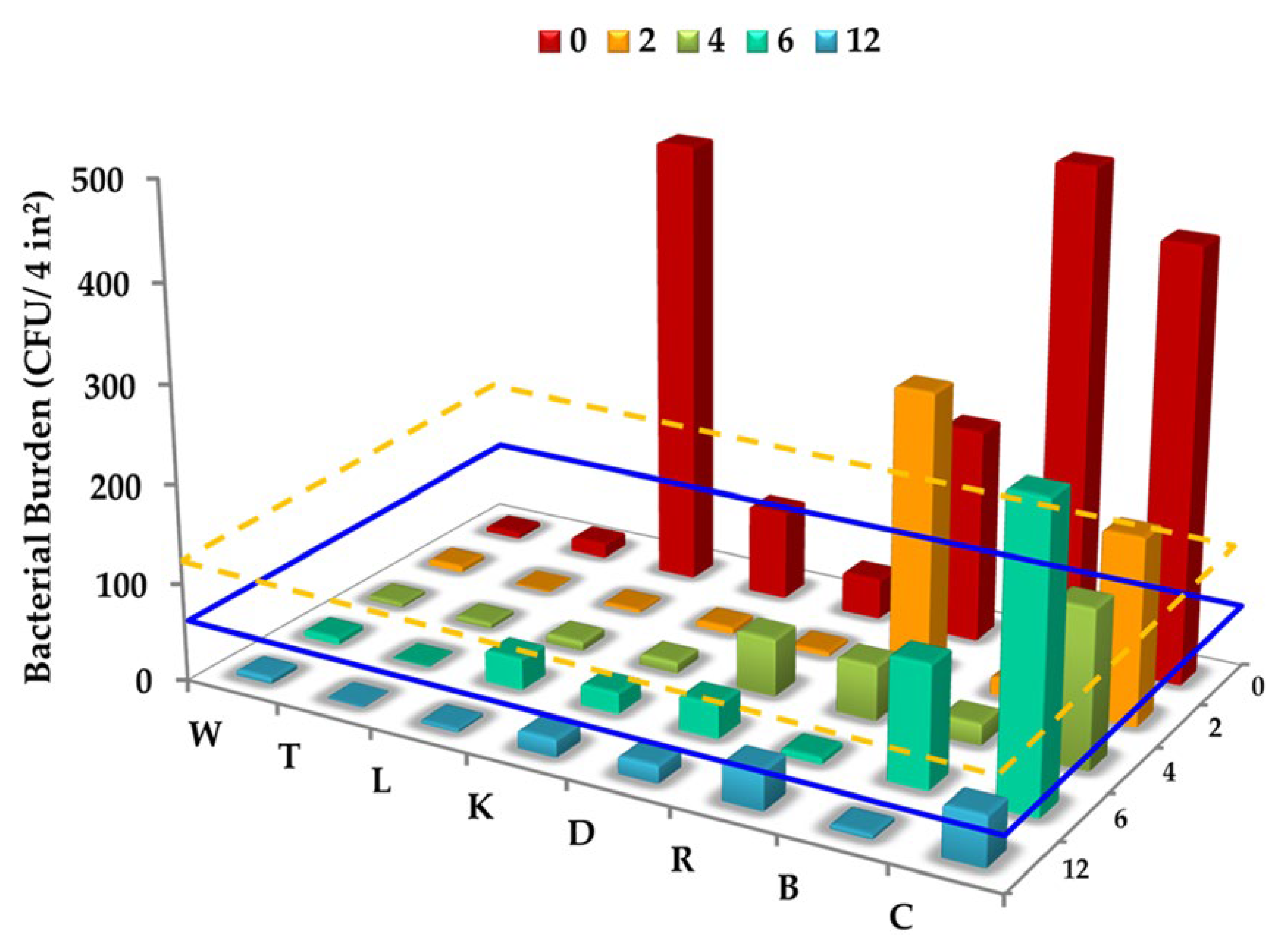


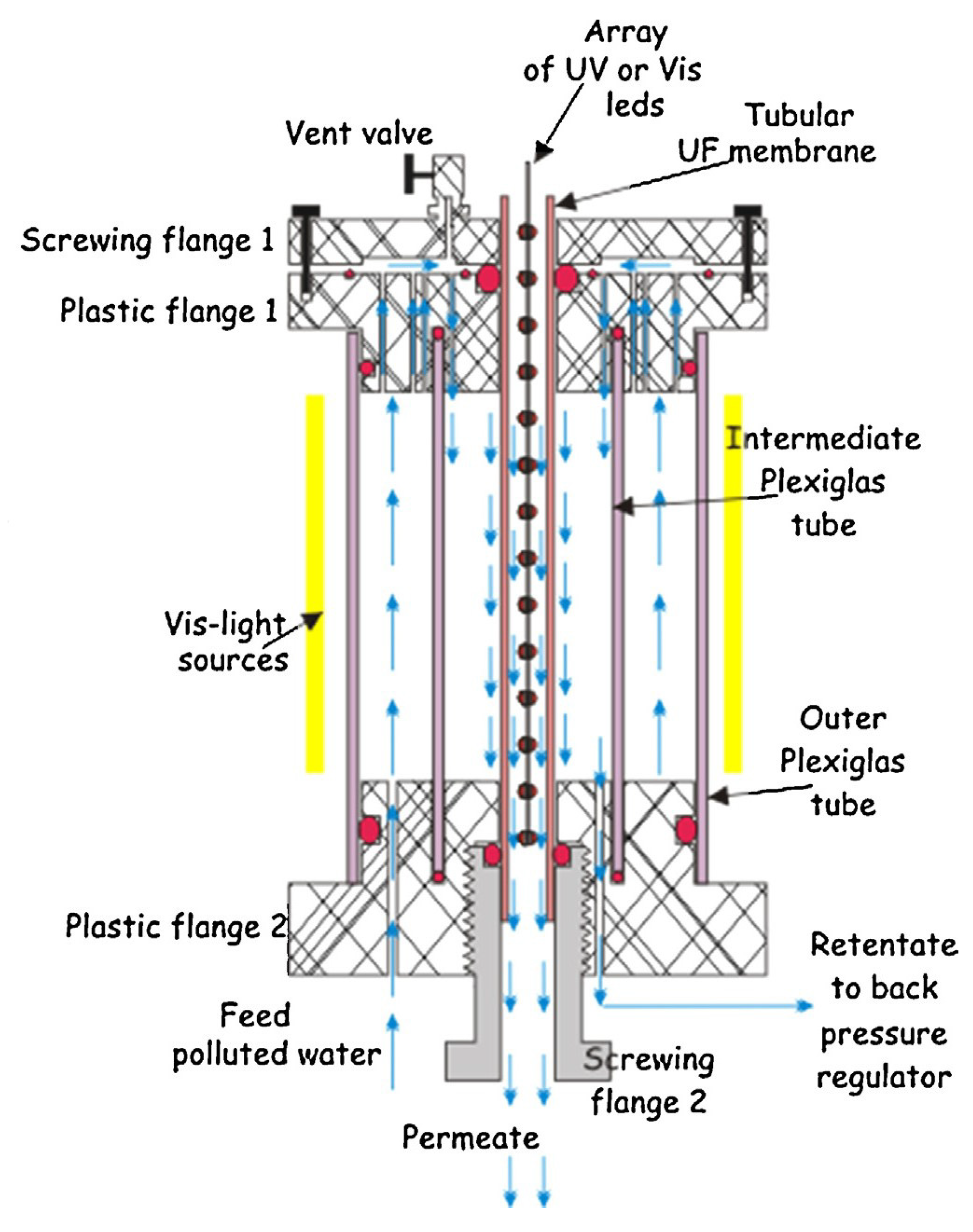


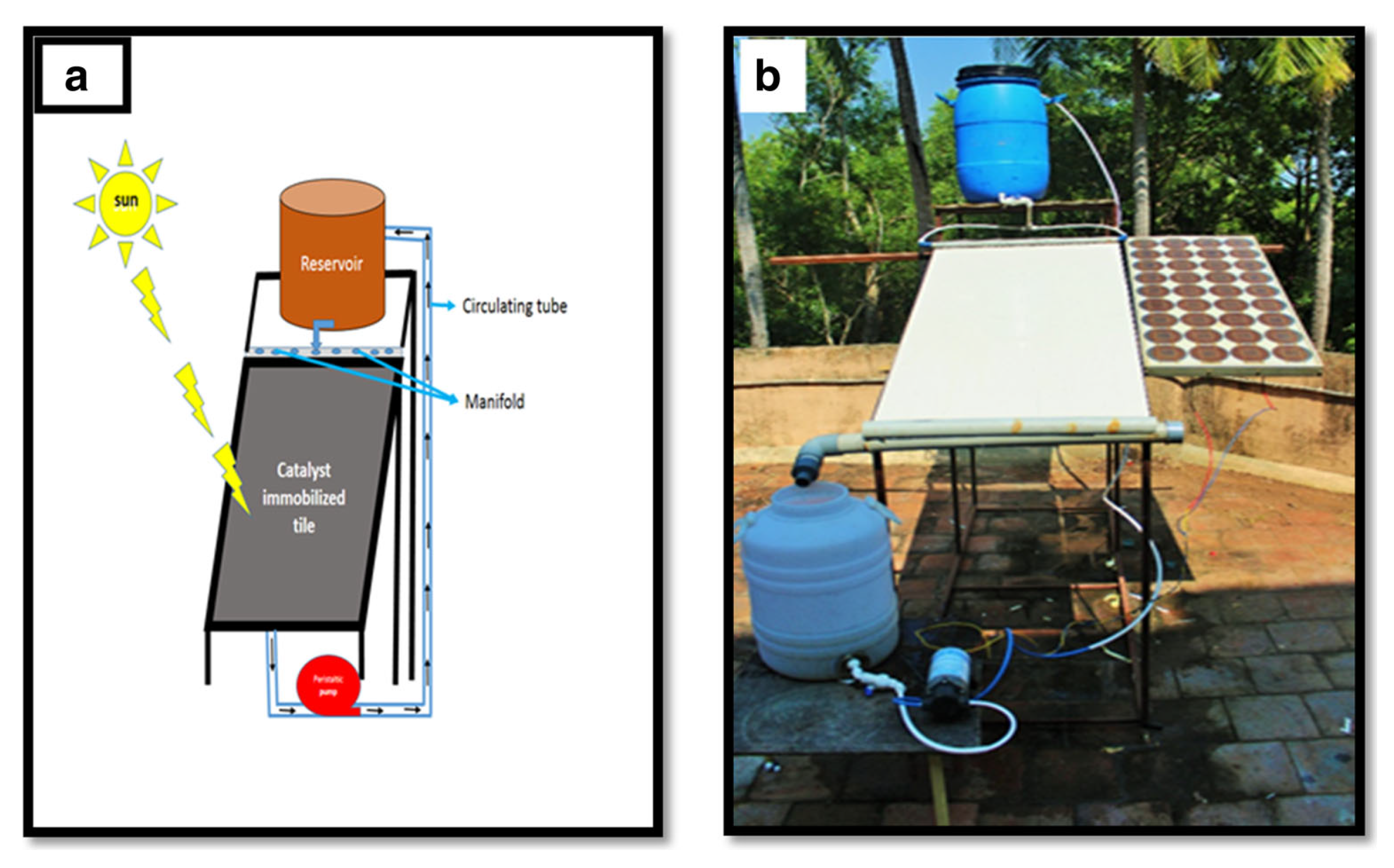

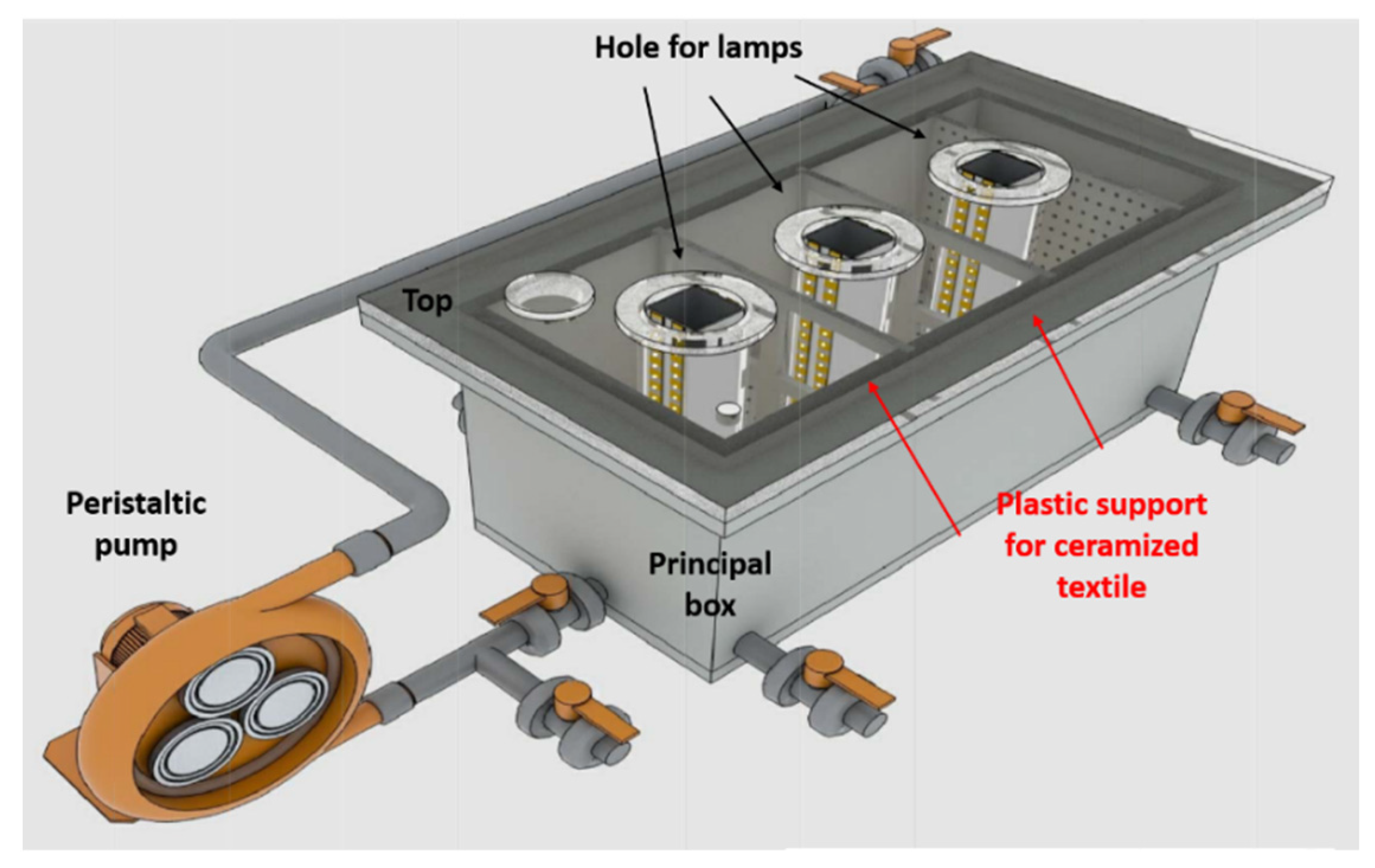

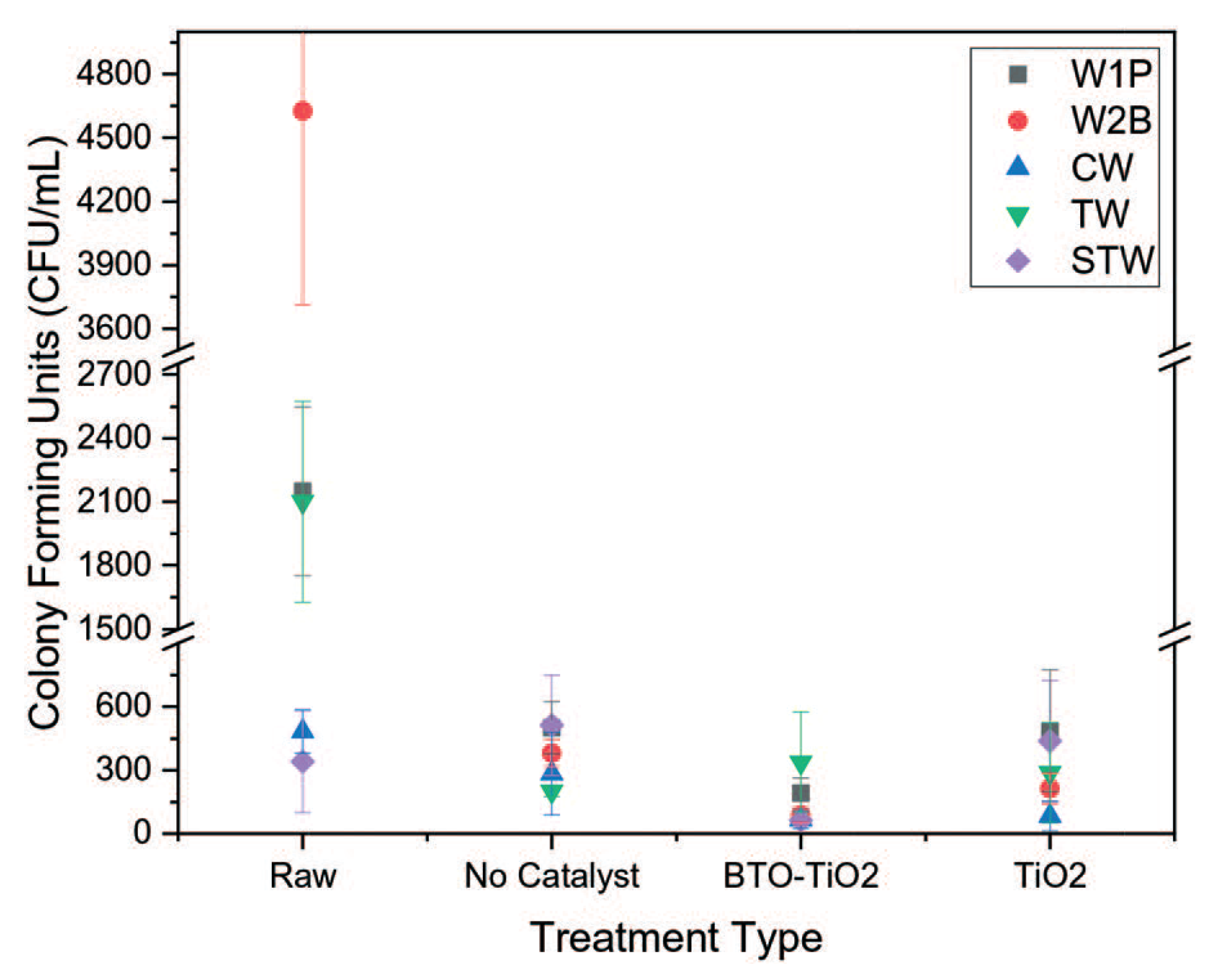

| Photocatalyst | Titania Synthesis Route | Modification | Deposition Method | Substrate | Later Application | References |
|---|---|---|---|---|---|---|
| TiO2 | Italcementi, TX-Active® and TX-Boosted® | - | spray coating | wall | air purification | [16,106,107] |
| TiO2 | titanium and sol | anatase and rutile (minimal amount) | anodization and dip coating | titanium | air purification | [24,113,118] |
| TiO2 | PureTiTM | - | - | fiber-reinforced plastic | air purification | [25] |
| TiO2 | P25 | anatase (80%) and rutile (20%) | impregnation | glass fiber tissue | air purification | [26,103] |
| TiO2 | - | anatase | spin coating | glass | antimacrofouling | [80] |
| TiO2 | precursor | anatase and rutile (17%) | CVD | stainless steel | antimicrobial | [81] |
| TiO2 | heating titanate | anatase | doctor blading | matrix with carbon nanotubes | water purification | [84] |
| TiO2 | sol-gel | anatase and rutile (16%) | dip-pad | ceramized fabrics | water purification | [87] |
| TiO2 | P25 | (anatase) | spray coating | SiO2@stainless steel | water prufication | [89] |
| TiO2 | P25 and PC500 | anatase and rutile (<20%) | dip coating | Cellulose acetate monolithic | air purification | [93] |
| TiO2 | - | - | spray coating | fibre glass | air purification | [95] |
| TiO2 | P25 | anatase (80%) and rutile (20%) | dip coating | porous alumina membrane | water purification | [96] |
| TiO2 | sol-gel | anatase | dip coating | cellulose acetate monolith | air purification | [97] |
| TiO2 | sol-gel | anatase | dip coating | glass | water purification | [99] |
| TiO2 | sol-gel | anatase | dip coating | porous ceramic foam filters | air purification | [102] |
| TiO2 | PC500 | (anatase) | impregnation | glass fiber tissues | air purification | [104] |
| TiO2 | - | - | spray coating | bituminous pavement | air purification | [108] |
| TiO2 | sol-gel | - | spray coating | limestone | self-cleaning | [111] |
| TiO2 | - | - | brush | limestone | self-cleaning | [119] |
| TiO2 | - | anatase | PVD | silicon wafer stripes and capillary tubes | medical application | [117] |
| TiO2 | TiO2-suspension | - | immersion | cellular ceramics | water purification | [120] |
| TiO2 | PureTiTM | - | spray coating | fiber-reinforced plastic | air purification | [121] |
| TiO2 | TiO2 containing paint | - | - | wall | air purification | [122] |
| Photocatalyst | Titania Synthesis Route | Modification | Deposition Method | Substrate | Later Application | References |
|---|---|---|---|---|---|---|
| Zr-TiO2 | sol-gel | anatase | spray coating | glass | self-cleaning and antifogging window glass | [23] |
| Ag-TiO2 | sol-gel with P25 | anatase | spray coating | stainless steel | water purification | [91,109] |
| Mn-TiO2 | precursor | anatase | dispersion | wall | air purification | [92] |
| N-TiO2 | sol-gel | - | spray coating | asphalt | air purification | [90] |
| C-TiO2 | sol-gel | anatase | brushed | cementeous mortar | self-cleaning | [42] |
| La-TiO2 | sol-gel | anatase and rutile (minimal amount) | impregnation | porous ceramic air filter | air purification | [46] |
| Au-TiO2 | titanium | anatase | PVD | silicon wafers and microscopic glass | - | [47] |
| Ag-TiO2 | P25 | - | dip coating | glass fiber | air purification | [62] |
| Fe-TiO2 | P25 | rutile | pouring | stainless steel | air purification | [63] |
| Ag-TiO2-Cu2+ | sol-gel | anatase | impregnation | perlite | air purification | [65] |
| N-TiO2/SWCNT | sol-gel | anatase | spin coating | glass | self-cleaning | [77] |
| N-F-TiO2 | sol-gel | anatase | vaccum rotary coating | glass sheet | building glass | [79] |
| Ti-Cu-O | precursor | anatase | CVD | single crystal and boroaluminosilicate | antibiofouling | [82] |
| Co-TiO2 | sol-gel | anatase | induced phase separation | matrix with SiO2 | water purification | [83] |
| Bi-TiO2 | sol-gel with P25 | - | immersion | glass spheres | water purification | [86] |
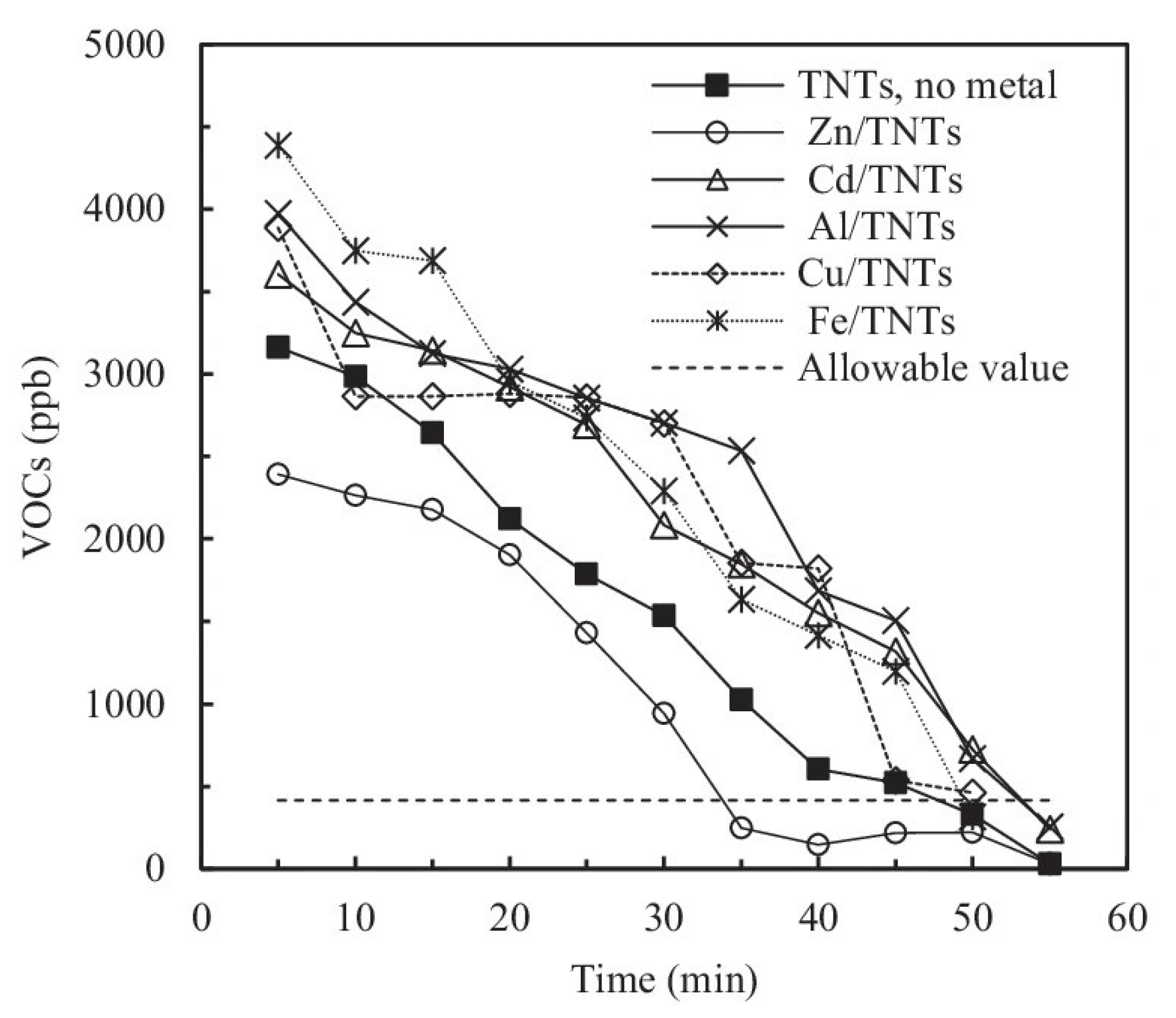
| Al/Zn/Cd/Cu/Fe-TiO2 | P25 | - | impregnation | fiber glass | air purification | [88] |
| Cu-Ag-TiO2 | P25 | anatase | dip coating | optical fiber | air purification | [94] |
| N-TiO2 | sol-gel | anatase | immersion | ultrafiltration membrane | water purification | [98] |
| Ag-TiO2 | sol-gel | rutile | - | Pyrex glass | water purification | [114] |
| Ag-TiO2 | P25 | anatase | brush coating | ceramic | water purification | [115] |
| TiO2/polyhydroxy fullerenes | - | anatase | - | surface | antimicrobial | [116] |
| C-TiO2 | - | anatase | spray | bituminous asphalt | air purification | [123] |
| Irradiation | Photocatalyst | Degradation Rate (%)/min |
|---|---|---|
| UV-A light | TC0 | 45.5/60 |
| UV-A light | TC25 | 96.2/60 |
| UV-A light | TC50 | 90.9/60 |
| UV-A light | TC62.5 | 62.9/60 |
| UV-A light | TC75 | 46.1./60 |
| Visible light | TC0 | 20.8/120 |
| Visible light | TC25 | 91.4/120 |
| Visible light | TC50 | 92.0/120 |
| Visible light | TC62.5 | 56.9/120 |
| Visible light | TC75 | 48.7/120 |
| Solar light | TC0 | 48.1/60 |
| Solar light | TC25 | 96.0/30 |
| Solar light | TC50 | 90.2/30 |
| Solar light | TC62.5 | 78.2/60 |
| Solar light | TC75 | 71.0/60 |
| Solar light | TAu | 70.6/60 |
| Irradiation Light | Coating | Photocatalytic Efficiency % |
|---|---|---|
| Visible | TACR | 49 |
| Visible | TiO2:SiO2 | 51 |
| UV | TACR | 64 |
| UV | TiO2:SiO2 | 60 |
| Fabric | Photocatalytic Efficiency % (UV LED) | Photocatalytic Efficiency % (Visible LED) |
|---|---|---|
| SP | 49 | 64 |
| SC | 64 | 54 |
| SM | 65 | 61 |
| C | 67 | 57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seiß, V.; Thiel, S.; Eichelbaum, M. Preparation and Real World Applications of Titania Composite Materials for Photocatalytic Surface, Air, and Water Purification: State of the Art. Inorganics 2022, 10, 139. https://doi.org/10.3390/inorganics10090139
Seiß V, Thiel S, Eichelbaum M. Preparation and Real World Applications of Titania Composite Materials for Photocatalytic Surface, Air, and Water Purification: State of the Art. Inorganics. 2022; 10(9):139. https://doi.org/10.3390/inorganics10090139
Chicago/Turabian StyleSeiß, Volker, Susanne Thiel, and Maik Eichelbaum. 2022. "Preparation and Real World Applications of Titania Composite Materials for Photocatalytic Surface, Air, and Water Purification: State of the Art" Inorganics 10, no. 9: 139. https://doi.org/10.3390/inorganics10090139
APA StyleSeiß, V., Thiel, S., & Eichelbaum, M. (2022). Preparation and Real World Applications of Titania Composite Materials for Photocatalytic Surface, Air, and Water Purification: State of the Art. Inorganics, 10(9), 139. https://doi.org/10.3390/inorganics10090139









