Synthesis, DFT and X-ray Studies of Trans CuCl2L2 with L Is (E)-(4-Chlorophenyl)-N-(3-phenyl-4H-1,2,4-triazol-4-yl)methanimine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 4-amino-5-aryl-3-mercapto-(4H)-1,2,4-triazole (3)
2.2. Preparation of the Schiff base 4-(4-chlorobenzylideneamino)-5-phenyl-2H-1,2,4-triazole-3(4H)-thione (5)
2.3. Crystal Structure of Trans-Copper Dichloride bis(E)-(4-chlorophenyl)-N-(3-phenyl-4H-1,2,4-triazol-4-yl)methanimine, CuL2Cl2 (6)
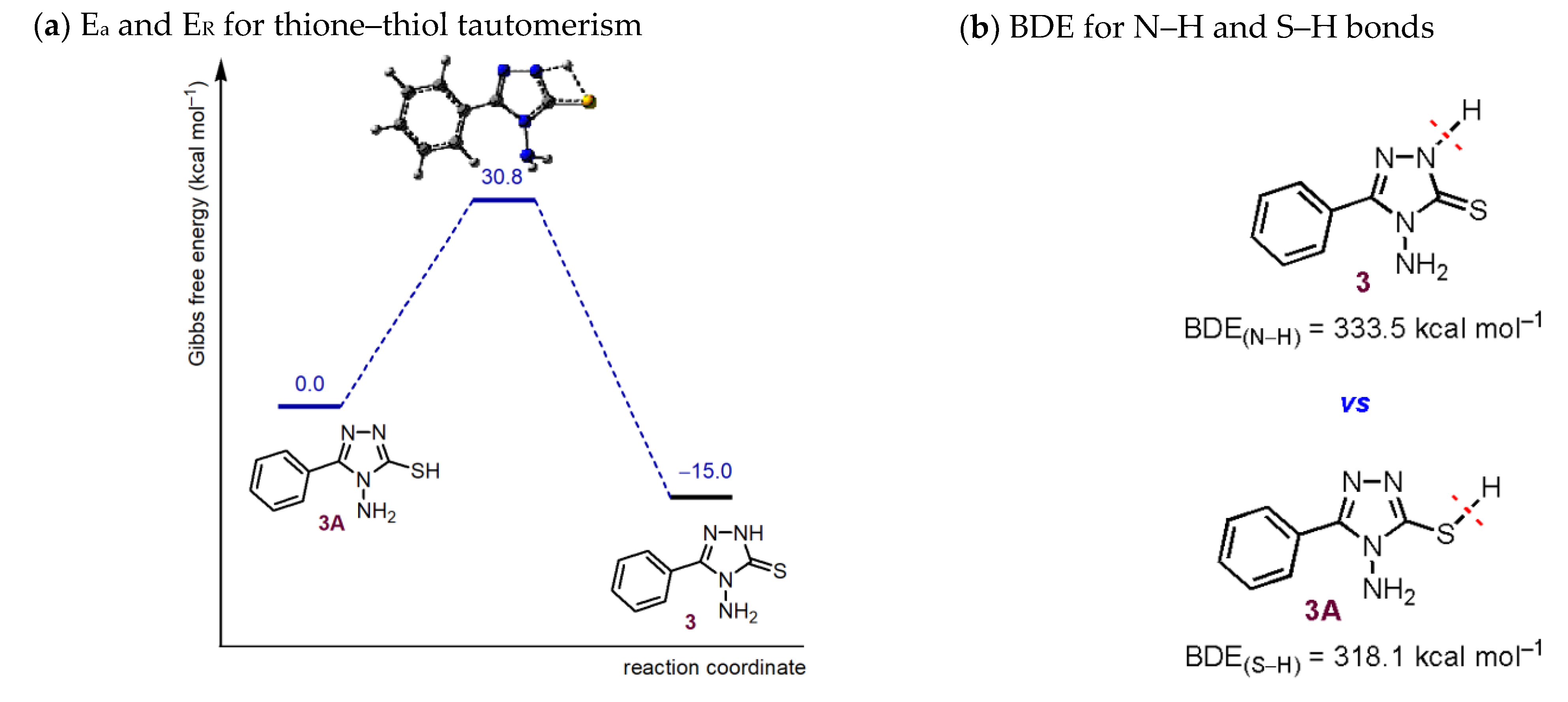
2.4. Computational Investigation
3. Experimental
3.1. X-ray Crystallography
3.2. Computational Method
3.3. Synthesis of Potassium Benzdithiocarbazinate (2)
3.4. Synthesis of 4-amino-3-mercapto-5-phenyl-(4H)-1,2,4-triazole (3)
3.5. Synthesis of 4-(4-chlorobenzylideneamino)-5-phenyl-2H-1,2,4-triazole-3(4H)-thione (5)
3.6. Preparation of Trans-Copper Dichloride bis(E)-(4-chlorophenyl)-N-(3-phenyl-4H-1,2,4-triazol-4-yl)methanimine, CuL2Cl2 (6)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Omar, M.E.A.M.; Aboulwafa, M.O. Synthesis and in vitro antimicrobial and antifungal properties of some novel 1,3,4-thiadiazole and s-triazolo[3,4-b][1,3,4]thiadiazole derivatives. J. Heterocycl. Chem. 1986, 23, 1339–1341. [Google Scholar] [CrossRef]
- Ding, Q.; Lei, X.; Jin, J.; Zhang, L.; Du, H.; Zhang, H. Synthesis and structure of novel 1,2,4-triazole derivatives containing the 2,4-dinitrophenylthio group. Int. J. Chem. Res. 2009, 2, 114–119. [Google Scholar] [CrossRef]
- Heeres, J.; Backx, L.J.; Van Custen, J. Antimycotic azoles 7. Synthesis and antifungal properties of a series of novel triazol-3-ones. J. Med. Chem. 1984, 27, 894–900. [Google Scholar] [CrossRef]
- Al-Masoudi, I.A.; Al-Soud, Y.A.; Al-Salihi, N.J.; Al-Masoudi, N.A. 1,2,4-Triazoles: Synthetic approaches and pharmacological importance. (Review). Chem. Heterocyl. Compd. 2006, 42, 1377–1403. [Google Scholar] [CrossRef]
- Raman, N.; Joseph, J.; Kumar, S.M.; Sujatha, S.; Sahayaraj, K. Insecticidal activity of the schiff-base derived from anthranilic acid and acetoacetanilide and its copper complex on Spodoptera litura (Fab.). J. Biopestic. 2008, 1, 206–209. [Google Scholar]
- Buvaylo, E.A.; Nesterova, O.V.; Goreshnik, E.A.; Vyshniakova, H.V.; Petrusenko, S.R.; Nesterov, D.S. Supramolecular Diversity, Theoretical Investigation and Antibacterial Activity of Cu, Co and Cd Complexes Based on the Tridentate N,N,O-Schiff Base Ligand Formed In Situ. Molecules 2022, 27, 8233. [Google Scholar] [CrossRef]
- Olar, R.; Badea, M.; Chifiriuc, M.C. Metal Complexes-A Promising Approach to Target Biofilm Associated Infections. Molecules 2022, 27, 758. [Google Scholar] [CrossRef]
- Naito, Y.; Akahoshi, F.; Takeda, S.; Okada, T.; Kajii, M.; Nishimura, H.; Sugiura, M.; Fukaya, C.; Kagitani, Y. Synthesis and pharmacological activity of triazole derivatives inhibiting eosinophilia. J. Med. Chem. 1996, 39, 3019–3029. [Google Scholar] [CrossRef]
- Kamboj, V.K.; Verma, P.K.; Dhanda, A.; Ranjan, S. 1,2,4-triazole derivatives as potential scaffold for anticonvulsant activity. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 17–22. [Google Scholar] [CrossRef]
- Chiu, S.-H.L.; Huskey, S.-E.W. Species differences in N-glucuronidation: 1996 ASPET N-glucuronidation of xenobiotics symposium. Drug Metab. Dispos. 1998, 26, 838–847. [Google Scholar]
- Gomathinayagam, M.; Abdul Jaleel, C.; Lakshmanan, G.M.A.; Panneerselvam, R. Changes in carbohydrate metabolism by triazole growth regulators in cassava (Manihot esculenta Crantz); effects on tuber production and quality. C. R. Biol. 2007, 330, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Matela, G. Schiff Bases and Complexes: A Review on Anti-Cancer Activity. Anticancer Agents Med. Chem. 2020, 20, 1908–1917. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X.; Cheng, X.; Cao, Y.; Wang, D.; Li, Z.; Xu, W.; Pannecouque, C.; Witvrouw, M.; De Clercq, E. Synthesis of Novel Derivatives of 4-Amino-3-(2-Furyl)-5-Mercapto-1,2,4-Triazole as Potential HIV-1 NNRTIs. Molecules 2007, 12, 2003–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullicans, M.D.; Wilson, M.W.; Connor, D.T.; Kostlan, C.R.; Schrier, D.J.; Dyer, R.D. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-1,3,4-thiadiazoles, -1,3,4-oxadiazoles, and -1,2,4-triazoles as orally active, nonulcerogenic antiinflammatory agents. J. Med. Chem. 1993, 36, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Slivka, M.V.; Korol, N.I.; Fizer, M.M. Fused bicyclic 1,2,4-triazoles with one extra sulfur atom: Synthesis, properties, and biological activity. J. Heterocycl. Chem. 2020, 57, 3236–3254. [Google Scholar] [CrossRef]
- Kaur, R.; Ranjan, D.A.; Kumar, B.; Kumar, V. Recent Developments on 1,2,4-Triazole Nucleus in Anticancer Compounds: A Review. Anti-Cancer Agents Med. Chem. 2016, 16, 465–489. [Google Scholar] [CrossRef]
- Wright, G.D. Resisting resistance: New chemical strategies for battling superbugs. Chem. Biol. 2000, 7, R127–R132. [Google Scholar] [CrossRef] [Green Version]
- Travis, J.; Potempa, J. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. Biochim. Biophys. Acta 2000, 14, 35–50. [Google Scholar] [CrossRef]
- Smith, H.J.; Simons, C. Proteinase and Peptidase Inhibition: Recent Potential Targets for Drug Development; Taylor and Francis: London, UK, 2001. [Google Scholar]
- Rice, S.A.; Givskov, M.; Steinberg, P.; Kjelleberg, S.J. Bacterial Signals and Antagonists: The Interaction Between Bacteria and Higher Organisms. Mol. Microbiol. Biotechnol. 1999, 1, 23–31. [Google Scholar]
- Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase and Matrix Metalloproteinase Inhibitors: Sulfonylated Amino Acid Hydroxamates with MMP Inhibitory Properties Act as Efficient Inhibitors of CA Isozymes I, II, and IV, and N-Hydroxysulfonamides Inhibit Both These Zinc Enzymes. J. Med. Chem. 2000, 43, 3677–3687. [Google Scholar] [CrossRef]
- Drabent, K.; Bialoska, A.; Ciunik, Z. New porous crystals of Cu(I) complexes with Schiff-base-containing triazole ligands. Inorg. Chem. Commun. 2004, 7, 224–227. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Slepukhin, P.A.; Burgart, Y.V.; Malysheva, N.N.; Kozitsina, A.N.; Ivanova, A.V.; Bogomyakov, A.S.; Saloutin, V.I. Dinuclear copper(ii) complex with novel N,N’,N’’,O-tetradentate Schiff base ligand containing trifluoromethylpyrazole and hydrazone moieties. Mendeleev Commun. 2018, 28, 202–204. [Google Scholar] [CrossRef]
- Naik, A.D.; Annigeri, S.M.; Gangadharmath, U.B.; Ravankar, V.K.; Mahale, V.B.; Reddy, V.K. Anchoring mercapto-triazoles on dicarbonyl backbone to assemble novel binucleating, acyclic SNONS compartmental ligands. Indian J. Chem. 2002, 41A, 2046–2053. [Google Scholar]
- Mazzoni, R.; Roncaglia, F.; Rigamonti, L. When the Metal Makes the Difference: Template Syntheses of Tridentate and Tetradentate Salen-Type Schiff Base Ligands and Related Complexes. Crystals 2021, 11, 483. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Pervez, H.; Khan, K.M.; Supuran, C.T. Organometallic-based antibacterial and antifungal compounds: Transition metal complexes of 1,1’-diacetylferrocene-derived thiocarbohydrazone, carbohydrazone, thiosemicarbazone and semicarbazone. J. Enz. Inhib. Med. Chem. 2005, 20, 81–88. [Google Scholar] [CrossRef]
- Palmer, M.H.; Christen, D. An ab initio study of the structure, tautomerism and molecular properties of the C- and N-amino-1,2,4-triazoles. J. Mol. Struct. 2004, 705, 177–187. [Google Scholar] [CrossRef]
- Singh, K.; Barwa, M.S.; Tyagi, P. Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with bidentate Schiff bases derived by heterocyclic ketone. Eur. J. Med. Chem. 2006, 41, 147–153. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Scozzafava, A.; Supuran, C.T. Unsymmetrical 1,1′-disubstituted Ferrocenes: Synthesis of Co(ii), Cu(ii), Ni(ii) and Zn(ii) Chelates of Ferrocenyl -1-thiadiazolo-1′-tetrazole, -1-thiadiazolo-1′-triazole and -1-tetrazolo-1′-triazole with Antimicrobial Properties. J. Enz. Inhib. Med. Chem. 2002, 17, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Klingele, M.H.; Brooker, S. The coordination chemistry of 4-substituted 3,5-di(2-pyridyl)-4H-1,2,4-triazoles and related ligands. Coord. Chem. Rev. 2003, 241, 119–132. [Google Scholar] [CrossRef]
- Arion, V.B.; Reisner, E.; Fremuth, M.; Jokupec, M.A.; Keppler, B.K.; Kukushkin, V.Y.; Pombeiro, A.J.L. Synthesis, X-ray Diffraction Structures, Spectroscopic Properties, and in vitro Antitumor Activity of Isomeric (1H-1,2,4-Triazole)Ru(III) Complexes. Inorg. Chem. 2003, 42, 6024–6031. [Google Scholar] [CrossRef]
- El-Masry, A.H.; Fahmy, H.H.; Abdelwahed, S.H.A. Synthesis and antimicrobial activity of some new benzimidazole derivatives. Molecules 2000, 5, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Pandeya, S.N.; Sriram, D.; Nath, G.; De Clereq, E. Synthesis and antimicrobial activity of Schiff and Mannich bases of isatin and its derivatives with pyrimidine. IL Farmaco 1999, 54, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Singh, W.M.; Dash, B.C. Synthesis of some new Schiff bases containing thiazole and oxazole nuclei and their fungicidal activity. Pesticides 1988, 22, 33–37. [Google Scholar]
- Desai, S.B.; Desai, P.B.; Desai, K.R. Synthesis of some Schiff bases, thiazolidinones and azetidinones derived from 2, 6-diaminobenzo [1, 2-d: 4, 5-d’] bisthiazole and their anticancer activities. Hetrocycl. Commun. 2001, 7, 83–90. [Google Scholar] [CrossRef]
- Pathak, P.; Jolly, V.S.; Sharma, K.P. Synthesis and Biological Activities of Some New Substituted Arylazo Schiff Bases. Orient. J. Chem. 2000, 16, 161–162. [Google Scholar]
- Samadhiya, S.; Halve, A. Synthetic Utility of Schiff Bases as Potential Herbicidal Agents. Orient. J. Chem. 2001, 17, 119–122. [Google Scholar]
- Karegoudar, P.; Karthikeyan, M.S.; Prasad, D.J.; Mahalinga, M.; Holla, B.S.; Kumari, N.S. Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 261–267. [Google Scholar] [CrossRef]
- Jubie, S.; Sikdar, P.; Antony, S.; Kalirajan, R.; Gowramma, B.; Gomathy, S.; Elango, K. Synthesis and biological evaluation of some Schiff bases of [4-(amino)-5-phenyl-4H-1,2,4-Triazole-3-Thiol]. Pak. J. Pharm. Sci. 2011, 24, 109–112. [Google Scholar]
- El Ashry, E.S.H.; Kassem, A.A.; Abdel-Hamid, H.; Louis, F.F.; Khattab, S.A.N.; Aouad, M.R. Synthesis of 4-amino-5-(3-chlorobenzo[b]thien-2-yl)-3-mercapto-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles and triazolo[3,4,b][1,3,4]thiadiazines under classical and microwave conditions. Arkivoc 2006, (14), 119–132. [Google Scholar]
- Mange, Y.J.; Isloor, A.M.; Malladi, S.; Isloor, S. Synthesis and antimicrobial activities of some novel 1,2,4-triazole derivatives. Arab J. Chem. 2013, 6, 177–181. [Google Scholar]
- Zhou, S.; Zhang, L.; Jin, J.; Zhang, A.; Lei, X.; Lin, J.; He, J.; Zhang, H. Synthesis and Biological Activities of Some Novel Triazolothiadiazines and Schiff Bases Derived from 1,2,4-Triazole. Phosphorus Sulfur Silicon 2007, 182, 419–432. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xu, W.F.; Wu, J.D. Synthesis of 4-Amino-5-furyl-2-yl-4H-1, 2, 4-triazole-3-thiol derivatives as a Novel Class of Endothelin (ET) Receptor Antagonists. Chin. Chem. Lett. 2003, 14, 790–793. [Google Scholar]
- Kabbani, A.T.; Zaworotko, M.J.; Abourahma, H.; Baily Walsh, R.D.; Hammud, H.H. Supramolecular Structure of Tetrakis-μ-[4- Chloro-3-nitrobenzoato)bis(methanol)dicopper(II)]. J. Chem. Crystallogr. 2004, 34, 749–756. [Google Scholar] [CrossRef]
- Zaworotko, M.J.; Hammud, H.H.; Kravtsov, V.C. The co-crystal of iron(II) complex hydrate with hydroxybenzoic acid: [Fe(Phen)3]Cl(p-hydroxybenzoate).2(p-hydroxybenzoic acid).7H2O. J. Chem. Crystallogr. 2007, 27, 219–231. [Google Scholar] [CrossRef]
- Hammud, H.H.; Holman, K.T.; Masoud, M.S.; El-Faham, A.; Beidas, H. 1-Hydroxybenzotriazole (HOBt) acidity, formation constant with different metals and thermodynamic parameters. Synthesis and characterization of some HOBt metal complexes. Crystal structures of two polymers: [Cu2(H2O)5(OBt)2(μ-OBt)2].2H2O.EtOH (1A) and [Cu(μ-OBt)(HOBt)(OBt)(EtOH)] (1B). Inorg. Chim. Acta 2009, 362, 3526–3540. [Google Scholar]
- Hammud, H.H.; Kortz, U.; Bhattacharya, S.; Demirdjian, S.; Hariri, E.; Isber, S.; Sang Choi, E.; Mirtamizdoust, B.; Mroueh, M.; Daher, C.F. Structure, DFT studies, Magnetism and Biological activity of Bis[(µ2-azido)-chloro-(1,10-phenanthroline)-copper(II)] complex. Inorg. Chim. Acta 2020, 506, 119533. [Google Scholar] [CrossRef]
- Hammud, H.H.; Zaworotko, M.J.; McManus, G.J.; Tabesh, R.N.; Islam, H.; Ibrahim, M.; Ayub, K.; Ludwig, R. The co-crystal of copper(II) phenanthroline chloride complex hydrate with p-aminobenzoic acid: Structure, cytotoxicity, thermal analysis and DFT calculation. Chem. Mon. 2021, 152, 323–336. [Google Scholar] [CrossRef]
- Zaworotko, M.; Hammud, H.; Abbas, I.; Kravtsov, V.; Masoud, M. Ampicillin acidity and formation constants with some metals and their thermodynamic parameters in different media. Crystal structures of two polymorphs isolated from the reaction of ampicillin with copper(II). J. Coord. Chem. 2006, 59, 65–84. [Google Scholar] [CrossRef]
- Hammud, H.H.; Nemer, G.; Sawma, W.; Touma, J.; Barnabe, P.; Bou-Mouglabey, Y.; Ghannoum, A.; El-Hajjar, J.; Usta, J. Copper–adenine complex, a compound, with multi-biochemical targets and potential anti-cancer effect. Chem. Biol. Interact. 2008, 173, 84–96. [Google Scholar] [CrossRef]
- Mroueh, M.; Daher, C.; Hariri, E.; Demirdjian, S.; Isber, S.; Choi, E.S.; Mirtamizdoust, B.; Hammud, H.H. Magnetic property, DFT calculation, and biological activity of bis[(μ2-chloro)chloro(1,10-phenanthroline)copper(II)] complex. Chem. Biol. Interact. 2015, 231, 53–60. [Google Scholar] [CrossRef]
- Hammud, H.H.; Holman, K.T.; Al-Noaimi, M.; Sheikh, N.S.; Ghannoum, A.M.; Bouhadir, K.H.; Masoud, M.S.; Karnati, R.K. Structures of selected transition metal complexes with 9-(2-hydroxyethyl)adenine: Potentiometric complexation and DFT Studies. J. Mol. Struct. 2020, 1205, 127548. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Denningtom, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V.J. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. Chem. Phys. 1998, 108, 664. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- How, F.N.-F.; Crouse, K.A.; Tahir, M.I.M.; Tarafder, M.; Cowley, A.R. Synthesis, characterization and biological studies of S-benzyl-β-N-(benzoyl) dithiocarbazate and its metal complexes. Polyhedron 2008, 27, 3325–3329. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, X.-Q.; Liu, H.-M.; Zhou, X.-Z.; Shao, Z.-H. Synthesis and evaluation of antitumor activities of novel chiral 1,2,4-triazole Schiff bases bearing γ-butenolide moiety. Org. Med. Chem. Lett. 2012, 2, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Gu, Z.; Yin, K.; Zhang, R.; Deng, Q.; Xiang, J. Synthesis of substituted-phenyl-1,2,4-triazol-3-thione analogues with modified D-glucopyranosyl residues and their antiproliferative activities. Eur. J. Med. Chem. 2009, 44, 4716–4720. [Google Scholar] [CrossRef]
- Wang, B.-L.; Zhang, L.-Y.; Liu, X.-H.; Ma, Y.; Zhang, Y.; Li, Z.-M.; Zhang, X. Synthesis, biological activities and SAR studies of new 3-substitutedphenyl-4-substitutedbenzylideneamino-1,2,4-triazole Mannich bases and bis-Mannich bases as ketol-acid reductoisomerase inhibitors. Bioorganic Med. Chem. Lett. 2017, 27, 5457–5462. [Google Scholar] [CrossRef]








| Atoms * | Experimental | CAM-B3LYP | ωB97XD | B3LYP | MPW1PW91 | B3PW91 | PBEPBE | |
|---|---|---|---|---|---|---|---|---|
| 1 | Cu1–Cl65 | 2.2552 | 2.32245 | 2.32583 | 2.35151 | 2.32697 | 2.33641 | 2.36238 |
| 2 | Cu1–N6 | 1.9603 | 1.98980 | 1.99478 | 2.01025 | 1.99702 | 1.99994 | 1.99434 |
| 3 | N6–N12 | 1.3780 | 1.36072 | 1.35936 | 1.36874 | 1.35584 | 1.35925 | 1.37069 |
| 4 | N6–C7 | 1.3010 | 1.31391 | 1.31499 | 1.32261 | 1.31716 | 1.32039 | 1.33439 |
| 5 | N12–C8 | 1.2890 | 1.29647 | 1.29993 | 1.30322 | 1.29924 | 1.30190 | 1.31503 |
| 6 | N13–C8 | 1.3610 | 1.37361 | 1.37337 | 1.38006 | 1.37290 | 1.37556 | 1.38594 |
| 7 | N13–C7 | 1.3600 | 1.36775 | 1.36506 | 1.37806 | 1.36936 | 1.37323 | 1.38636 |
| 8 | C7–C25 | 1.4540 | 1.46381 | 1.46333 | 1.46439 | 1.45853 | 1.46011 | 1.46150 |
| 9 | N13–N37 | 1.3840 | 1.37593 | 1.37701 | 1.37927 | 1.36791 | 1.37086 | 1.37838 |
| 10 | N37–C39 | 1.2670 | 1.27635 | 1.27852 | 1.28583 | 1.28112 | 1.28432 | 1.29893 |
| 11 | C39–C52 | 1.4490 | 1.46268 | 1.46373 | 1.46148 | 1.45706 | 1.45818 | 1.45929 |
| 12 | Cl63–C59 | 1.7300 | 1.77717 | 1.77651 | 1.79155 | 1.77405 | 1.77941 | 1.79058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammud, H.H.; Wehbie, M.; Abdul-Ghani, M.M.; Gal, Z.A.; Sheikh Abdul Hamid, M.H.; Sheikh, N.S. Synthesis, DFT and X-ray Studies of Trans CuCl2L2 with L Is (E)-(4-Chlorophenyl)-N-(3-phenyl-4H-1,2,4-triazol-4-yl)methanimine. Inorganics 2023, 11, 18. https://doi.org/10.3390/inorganics11010018
Hammud HH, Wehbie M, Abdul-Ghani MM, Gal ZA, Sheikh Abdul Hamid MH, Sheikh NS. Synthesis, DFT and X-ray Studies of Trans CuCl2L2 with L Is (E)-(4-Chlorophenyl)-N-(3-phenyl-4H-1,2,4-triazol-4-yl)methanimine. Inorganics. 2023; 11(1):18. https://doi.org/10.3390/inorganics11010018
Chicago/Turabian StyleHammud, Hassan H., Moheddine Wehbie, Mohamed M. Abdul-Ghani, Zoltan A. Gal, Malai Haniti Sheikh Abdul Hamid, and Nadeem S. Sheikh. 2023. "Synthesis, DFT and X-ray Studies of Trans CuCl2L2 with L Is (E)-(4-Chlorophenyl)-N-(3-phenyl-4H-1,2,4-triazol-4-yl)methanimine" Inorganics 11, no. 1: 18. https://doi.org/10.3390/inorganics11010018
APA StyleHammud, H. H., Wehbie, M., Abdul-Ghani, M. M., Gal, Z. A., Sheikh Abdul Hamid, M. H., & Sheikh, N. S. (2023). Synthesis, DFT and X-ray Studies of Trans CuCl2L2 with L Is (E)-(4-Chlorophenyl)-N-(3-phenyl-4H-1,2,4-triazol-4-yl)methanimine. Inorganics, 11(1), 18. https://doi.org/10.3390/inorganics11010018







