Numerical Simulation of Nitrogen-Doped Titanium Dioxide as an Inorganic Hole Transport Layer in Mixed Halide Perovskite Structures Using SCAPS 1-D
Abstract
:1. Introduction
1.1. An Overview of Perovskite Solar Cells
1.2. Semi-Transparent Perovskite Solar Cells
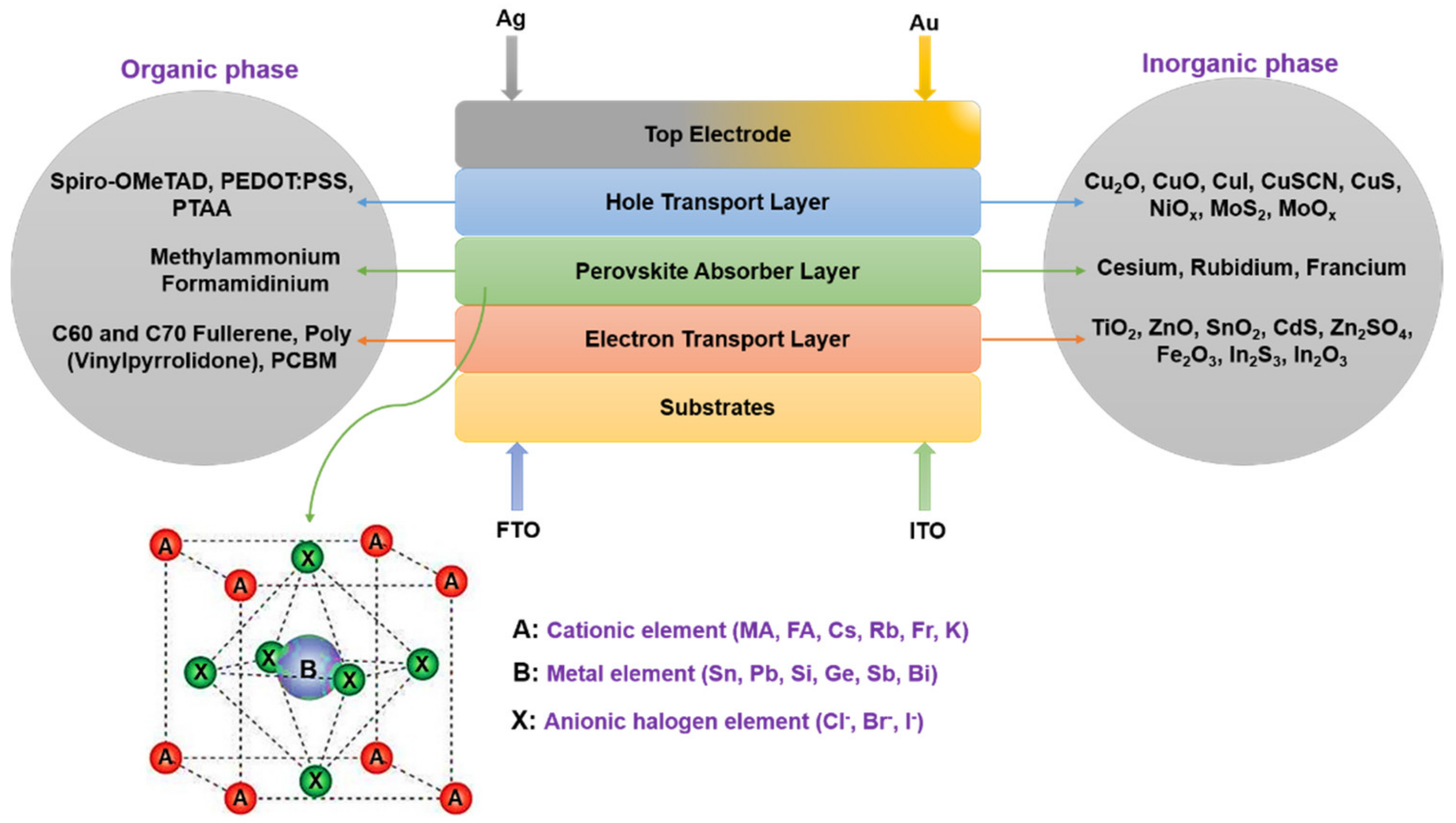
1.3. The Absorber and Charge Transport Layers in an ST PSC
1.4. Nitrogen-Doped Titanium Dioxide—A Proposed Novel HTL
1.5. Overview of This Research
2. Results and Discussion
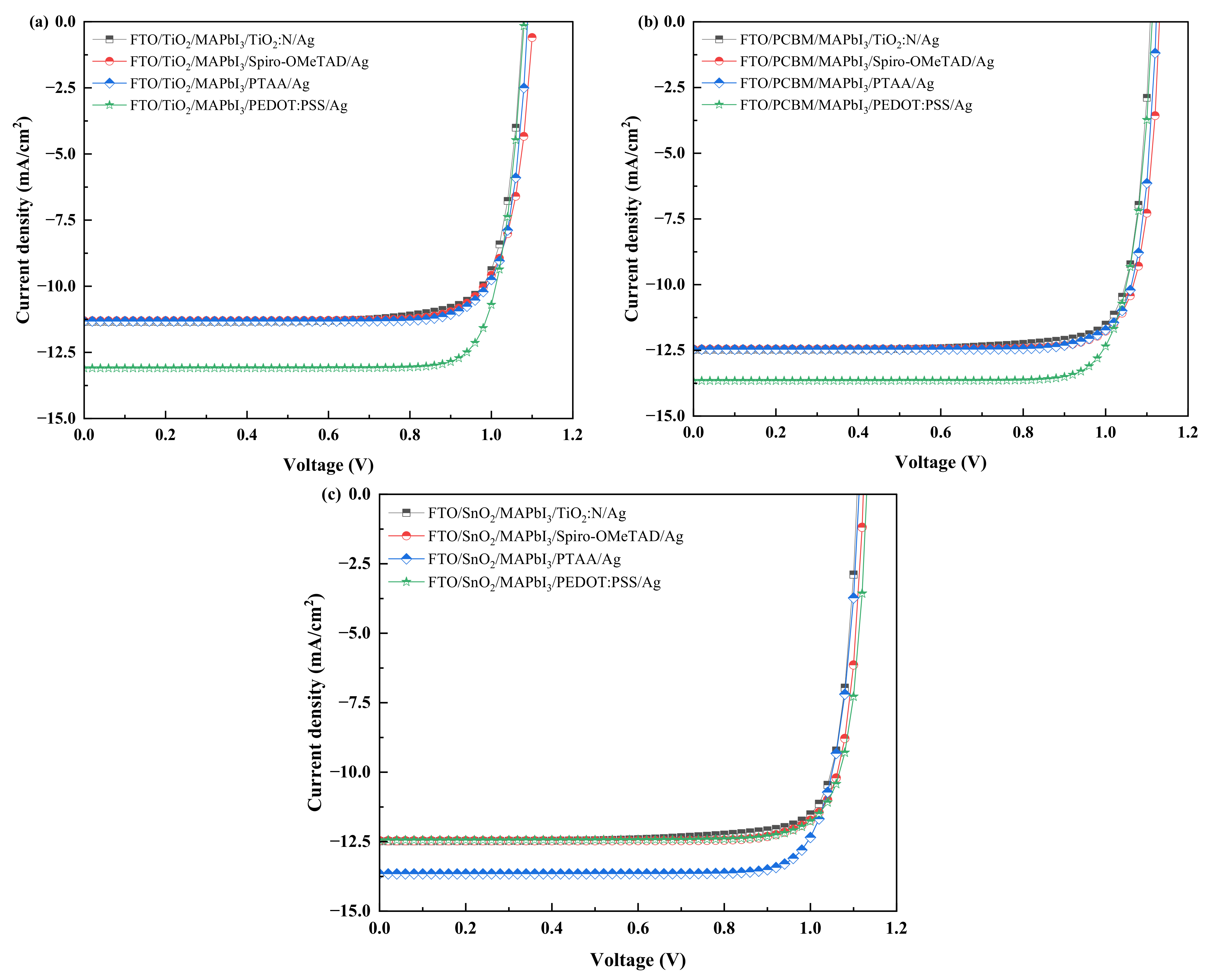
2.1. Influence of Absorber Layer Thickness on the Electrical Performance
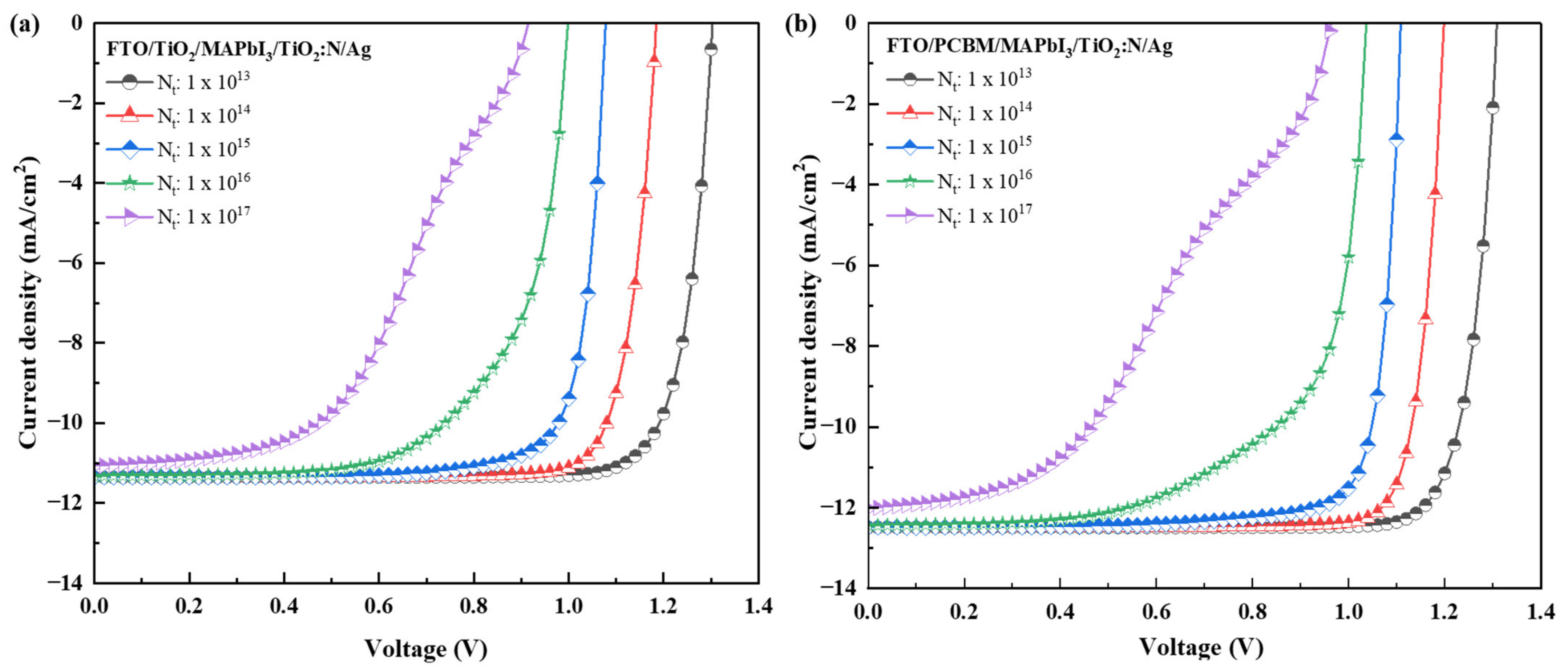
2.2. Influence of Absorber Layer Defect Density on the Electrical Performance
3. Methodology
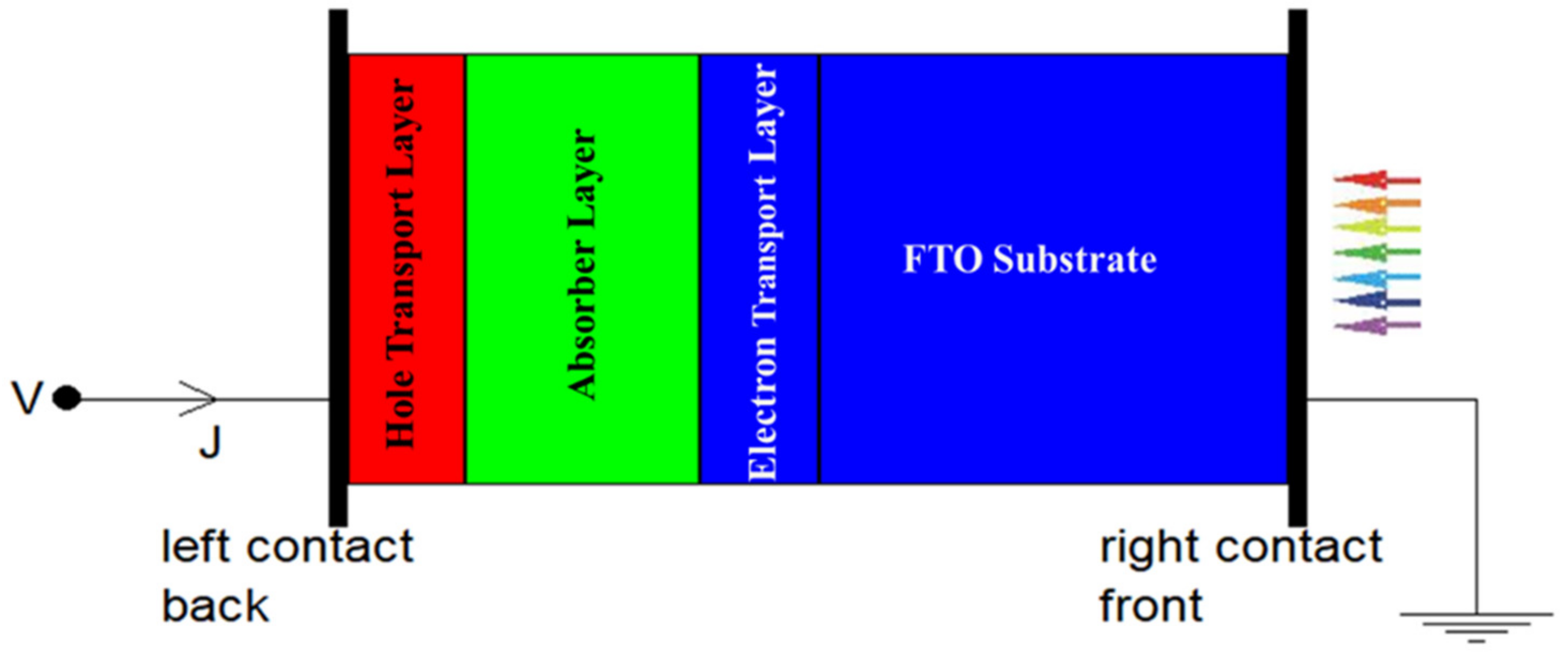
3.1. SCAPS Software Simulation
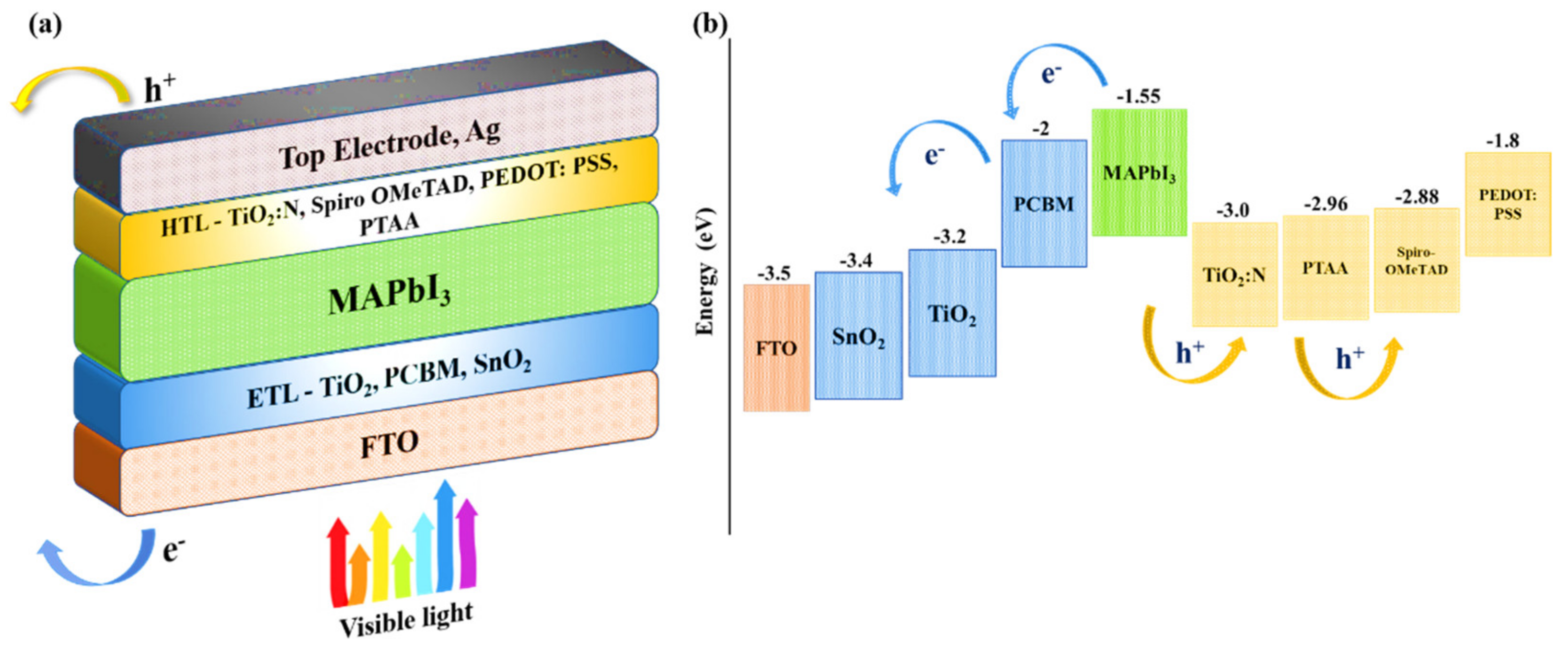
3.2. TiO2:N as a p-Type HTL
3.3. The Proposed Perovskite Solar Cell Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Nomenclature
| Ψ: | electrostatic potential | ph: | hole distribution |
| q: | electric charge | pe: | electron distribution |
| ε: | dielectric constant | Jn: | current density for electron |
| p: | hole concentration | Jp: | current density for the hole |
| n: | electron concentration | G: | Generation rates for carriers |
| ND: | doping concentration for the donor | R: | recombination rates for carriers |
| NA: | doping concentration for acceptor | ||
References
- Rao, V.T.; Sekhar, Y.R. Exergo-Economic and CO2 Emission Analysis of Bi-Symmetrical Web Flow Photovoltaic-Thermal System Under Diurnal Conditions. J. Energy Resour. Technol. 2022, 145, 032001. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart—2022. National Renewable Energy Laboratory (NREL). Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 17 October 2022).
- Rahmany, S.; Etgar, L. Semitransparent Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 1519–1531. [Google Scholar] [CrossRef]
- Nalianya, M.A.; Awino, C.; Barasa, H.; Odari, V.; Gaitho, F.; Omogo, B.; Mageto, M. Numerical study of lead free CsSn0.5Ge0.5I3 perovskite solar cell by SCAPS-1D. Optik 2021, 248, 168060. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Z.; Wang, L.; Zhang, K.; Xie, X. A combined chrome oxide and titanium oxide-based electron-transport layer for high-performance perovskite solar cells. Chem. Phys. Lett. 2021, 771, 138496. [Google Scholar] [CrossRef]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Tai, Q.; Tang, K.-C.; Yan, F. Recent progress of inorganic perovskite solar cells. Energy Environ. Sci. 2019, 12, 2375–2405. [Google Scholar] [CrossRef]
- Nam, J.K.; Chai, S.U.; Cha, W.; Choi, Y.J.; Kim, W.; Jung, M.S.; Kwon, J.; Kim, D.; Park, J.H. Potassium Incorporation for Enhanced Performance and Stability of Fully Inorganic Cesium Lead Halide Perovskite Solar Cells. Nano Lett. 2017, 17, 2028–2033. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, S.; Song, H. Photon management to reduce energy loss in perovskite solar cells. Chem. Soc. Rev. 2021, 50, 7250–7329. [Google Scholar] [CrossRef]
- Zhang, Y.; Grancini, G.; Feng, Y.; Asiri, A.M.; Nazeeruddin, M.K. Optimization of Stable Quasi-Cubic FAxMA1–xPbI3 Perovskite Structure for Solar Cells with Efficiency beyond 20%. ACS Energy Lett. 2017, 2, 802–806. [Google Scholar] [CrossRef]
- Chiara, R.; Morana, M.; Malavasi, L. Germanium-Based Halide Perovskites: Materials, Properties, and Applications. ChemPlusChem 2021, 86, 879–888. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Nienhaus, L.; Kurchin, R.C.; Shin, S.S.; Wieghold, S.; Hartono, N.T.P.; Layurova, M.; Klein, N.D.; Poindexter, J.R.; Polizzotti, A.; et al. A-Site Cation in Inorganic A3Sb2I9 Perovskite Influences Structural Dimensionality, Exciton Binding Energy, and Solar Cell Performance. Chem. Mater. 2018, 30, 3734–3742. [Google Scholar] [CrossRef]
- Gkini, K.E.; Antoniadou, M.; Balis, N.; Kaltzoglou, A.; Kontos, A.G.; Falaras, P. Mixing cations and halide anions in perovskite solar cells. Mater. Today Proc. 2019, 19, 73–78. [Google Scholar] [CrossRef]
- Shao, S.; Loi, M.A. The Role of the Interfaces in Perovskite Solar Cells. Adv. Mater. Interfaces 2019, 7, 1901469. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Zhou, T.; Tian, Y.; Zhu, X.; Tu, Y. Perovskite-Based Solar Cells: Materials, Methods, and Future Perspectives. J. Nanomater. 2018, 2018, 8148072. [Google Scholar] [CrossRef]
- Nhari, L.M.; El-Shishtawy, R.M.; Asiri, A.M. Recent progress in organic hole transport materials for energy applications. Dye. Pigment. 2021, 193, 109465. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, Z.; Su, J.; Hu, Z.; Chang, J.; Hao, Y. Recent progress of inorganic hole transport materials for efficient and stable perovskite solar cells. Nano Sel. 2021, 2, 1055–1080. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.S.; Myung, C.W. Efficient electron extraction of SnO2 electron transport layer for lead halide perovskite solar cell. npj Comput. Mater. 2020, 6, 100. [Google Scholar] [CrossRef]
- Mujahid, M.; Chen, C.; Zhang, J.; Li, C.; Duan, Y. Recent advances in semitransparent perovskite solar cells. InfoMat 2021, 3, 101–124. [Google Scholar] [CrossRef]
- Pulli, E.; Rozzi, E.; Bella, F. Transparent photovoltaic technologies: Current trends towards upscaling. Energy Convers. Manag. 2020, 219, 112982. [Google Scholar] [CrossRef]
- You, P.; Liu, Z.; Tai, Q.; Liu, S.; Yan, F. Efficient Semitransparent Perovskite Solar Cells with Graphene Electrodes. Adv. Mater. 2015, 27, 3632–3638. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Z.; Duan, R.; Huang, P.; Zhang, K.; Chen, Q.; Allam, N.K.; Zhou, Y.; Song, B.; Li, Y. Semi-transparent perovskite solar cells: Unveiling the trade-off between transparency and efficiency. J. Mater. Chem. A 2018, 6, 19696–19702. [Google Scholar] [CrossRef]
- Wang, G.; Chang, J.; Tang, W.; Xie, W.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 293002. [Google Scholar] [CrossRef]
- Della Gaspera, E.; Peng, Y.; Hou, Q.; Spiccia, L.; Bach, U.; Jasieniak, J.J.; Cheng, Y.-B. Ultra-thin high efficiency semitransparent perovskite solar cells. Nano Energy 2015, 13, 249–257. [Google Scholar] [CrossRef]
- Guchhait, A.; Dalapati, G.K.; Sonar, P.; Gopalan, S.; Bin Suhaimi, F.; Das, T.; Dutt, V.G.V.; Mishra, N.; Mahata, C.; Kumar, A.; et al. p-i-n Structured Semitransparent Perovskite Solar Cells with Solution-Processed Electron Transport Layer. J. Electron. Mater. 2021, 50, 5732–5739. [Google Scholar] [CrossRef]
- Aharon, S.; Layani, M.; Cohen, B.-E.; Shukrun, E.; Magdassi, S.; Etgar, L. Self-Assembly of Perovskite for Fabrication of Semitransparent Perovskite Solar Cells. Adv. Mater. Interfaces 2015, 2, 1500118. [Google Scholar] [CrossRef]
- Lim, S.-H.; Seok, H.-J.; Kwak, M.-J.; Choi, D.-H.; Kim, S.-K.; Kim, D.-H.; Kim, H.-K. Semi-transparent perovskite solar cells with bidirectional transparent electrodes. Nano Energy 2021, 82, 105703. [Google Scholar] [CrossRef]
- Matteocci, F.; Rossi, D.; Castriotta, L.A.; Ory, D.; Mejaouri, S.; der Maur, M.A.; Sauvage, F.; Cacovich, S.; Di Carlo, A. Wide bandgap halide perovskite absorbers for semi-transparent photovoltaics: From theoretical design to modules. Nano Energy 2022, 101, 107560. [Google Scholar] [CrossRef]
- Ponchai, J.; Srathongsian, L.; Amratisha, K.; Boonthum, C.; Sahasithiwat, S.; Ruankham, P.; Kanjanaboos, P. Modified colored semi-transparent perovskite solar cells with enhanced stability. J. Alloys Compd. 2021, 875, 159781. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, A.; Ghosh, D.S. High performance of NiO-Ag-NiO based semi-transparent perovskite solar cell. Mater. Today Proc. 2022, 66, 3224–3227. [Google Scholar] [CrossRef]
- Singh, A.; Matteocci, F.; Zhu, H.; Rossi, D.; Mejaouri, S.; Cacovich, S.; Der Maur, M.A.; Sauvage, F.; Gagliardi, A.; Grätzel, M.; et al. Methylamine Gas Treatment Affords Improving Semitransparency, Efficiency, and Stability of CH3NH3PbBr3-Based Perovskite Solar Cells. Sol. RRL 2021, 5, 2100277. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Cheng, P.-P.; Tan, W.-Y.; Min, Y. Balance the thickness, transparency and stability of semi-transparent perovskite solar cells by solvent engineering and using a bifunctional additive. Appl. Surf. Sci. 2021, 537, 147908. [Google Scholar] [CrossRef]
- Dewi, H.A.; Wang, H.; Li, J.; Thway, M.; Sridharan, R.; Stangl, R.; Lin, F.; Aberle, A.G.; Mathews, N.; Bruno, A.; et al. Highly Efficient Semitransparent Perovskite Solar Cells for Four Terminal Perovskite-Silicon Tandems. ACS Appl. Mater. Interfaces 2019, 11, 34178–34187. [Google Scholar] [CrossRef]
- Han, K.; Xie, M.; Zhang, L.; Yan, L.; Wei, J.; Ji, G.; Luo, Q.; Lin, J.; Hao, Y.; Ma, C.-Q. Fully solution processed semi-transparent perovskite solar cells with spray-coated silver nanowires/ZnO composite top electrode. Sol. Energy Mater. Sol. Cells 2018, 185, 399–405. [Google Scholar] [CrossRef]
- Chen, B.-X.; Rao, H.-S.; Chen, H.-Y.; Li, W.-G.; Kuang, D.-B.; Su, C.-Y. Ordered macroporous CH3NH3PbI3 perovskite semitransparent film for high-performance solar cells. J. Mater. Chem. A 2016, 4, 15662–15669. [Google Scholar] [CrossRef]
- Hörantner, M.T.; Nayak, P.K.; Mukhopadhyay, S.; Wojciechowski, K.; Beck, C.; McMeekin, D.; Kamino, B.; Eperon, G.E.; Snaith, H.J. Shunt-Blocking Layers for Semitransparent Perovskite Solar Cells. Adv. Mater. Interfaces 2016, 3, 1500837. [Google Scholar] [CrossRef]
- Lee, K.-T.; Guo, L.J.; Park, H.J. Neutral- and Multi-Colored Semitransparent Perovskite Solar Cells. Molecules 2016, 21, 475. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-Y.; Chang, Y.-C.; Huang, W.-K.; Lee, K.-T.; Cho, A.-C.; Hsu, C.-C. Enhanced Performance and Stability of Semitransparent Perovskite Solar Cells Using Solution-Processed Thiol-Functionalized Cationic Surfactant as Cathode Buffer Layer. Chem. Mater. 2015, 27, 7119–7127. [Google Scholar] [CrossRef]
- Heo, J.H.; Jang, M.H.; Lee, M.H.; Han, H.J.; Kang, M.G.; Lee, M.L.; Im, S.H. Efficiency enhancement of semi-transparent sandwich type CH3NH3PbI3 perovskite solar cells with island morphology perovskite film by introduction of polystyrene passivation layer. J. Mater. Chem. A 2016, 4, 16324–16329. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, H.J.; Lee, M.; Song, M.; Kim, D.H.; Im, S.H. Stable semi-transparent CH3NH3PbI3planar sandwich solar cells. Energy Environ. Sci. 2015, 8, 2922–2927. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Goriely, A.; Snaith, H.J. Neutral Color Semitransparent Microstructured Perovskite Solar Cells. ACS Nano 2014, 8, 591–598. [Google Scholar] [CrossRef]
- Lynn, N.; Mohanty, L.; Wittkopf, S. Color rendering properties of semi-transparent thin-film PV modules. Build. Environ. 2012, 54, 148–158. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Yun, J.-Y.; Kim, D.-H. Performance of inverted organic photovoltaic cells with nitrogen doped TiO2 films by atomic layer deposition. Korean J. Chem. Eng. 2018, 35, 567–573. [Google Scholar] [CrossRef]
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Go, G.-M.; Cho, H.-B.; Song, Y.; Lee, C.-G.; Choa, Y.-H. A Novel Synthetic Method for N Doped TiO2 Nanoparticles Through Plasma-Assisted Electrolysis and Photocatalytic Activity in the Visible Region. Front. Chem. 2018, 6, 458. [Google Scholar] [CrossRef] [Green Version]
- Panepinto, A.; Dervaux, J.; Cormier, P.; Boujtita, M.; Odobel, F.; Snyders, R. Synthesis of p-type N-doped TiO 2 thin films by co-reactive magnetron sputtering. Plasma Process. Polym. 2020, 17, 1900203. [Google Scholar] [CrossRef]
- Bag, A.; Radhakrishnan, R.; Nekovei, R.; Jeyakumar, R. Effect of absorber layer, hole transport layer thicknesses, and its doping density on the performance of perovskite solar cells by device simulation. Sol. Energy 2020, 196, 177–182. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, S.; Xi, T.; Li, H.; Yi, J.; Zhong, J. Gradient doping simulation of perovskite solar cells with CH3NH3Sn1− xPbxI3 as the absorber layer. Curr. Appl. Phys. 2022, 44, 55–62. [Google Scholar] [CrossRef]
- Hussain, S.S.; Riaz, S.; Nowsherwan, G.A.; Jahangir, K.; Raza, A.; Iqbal, M.J.; Sadiq, I.; Naseem, S. Numerical Modeling and Optimization of Lead-Free Hybrid Double Perovskite Solar Cell by Using SCAPS-1D. J. Renew. Energy 2021, 2021, 6668687. [Google Scholar] [CrossRef]
- Madan, J.; Singh, K.; Pandey, R. Comprehensive device simulation of 23.36% efficient two-terminal perovskite-PbS CQD tandem solar cell for low-cost applications. Sci. Rep. 2021, 11, 19829. [Google Scholar] [CrossRef]
- Santos, I.M.D.L.; Cortina-Marrero, H.J.; Ruíz-Sánchez, M.; Hechavarría-Difur, L.; Sánchez-Rodríguez, F.; Courel, M.; Hu, H. Optimization of CH3NH3PbI3 perovskite solar cells: A theoretical and experimental study. Sol. Energy 2020, 199, 198–205. [Google Scholar] [CrossRef]
- Bhavsar, K.; Lapsiwala, P. Numerical simulation of perovskite solar cell with different material as electron transport layer using SCAPS-1D Software. Semicond. Phys. Quantum Electron. Optoelectron. 2021, 24, 341–347. [Google Scholar] [CrossRef]
- Mamta; Maurya, K.; Singh, V. Sb2Se3 versus Sb2S3 solar cell: A numerical simulation. Sol. Energy 2021, 228, 540–549. [Google Scholar] [CrossRef]
- Deepthi Jayan, K.; Sebastian, V.; Kurian, J. Simulation and optimization studies on CsPbI3 based inorganic perovskite solar cells. Sol. Energy 2021, 221, 99–108. [Google Scholar] [CrossRef]
- Raza, E.; Ahmad, Z.; Aziz, F.; Asif, M.; Ahmed, A.; Riaz, K.; Bhadra, J.; Al-Thani, N.J. Numerical simulation analysis towards the effect of charge transport layers electrical properties on cesium based ternary cation perovskite solar cells performance. Sol. Energy 2021, 225, 842–850. [Google Scholar] [CrossRef]
- Lee, M.D.; Lee, G.J.; Nam, I.; Abbas, M.A.; Bang, J.H. Exploring the Effect of Cation Vacancies in TiO2: Lithiation Behavior of n-Type and p-Type TiO2. ACS Appl. Mater. Interfaces 2022, 14, 6560–6569. [Google Scholar] [CrossRef]
- Vasu, K.; Sreedhara, M.B.; Ghatak, J.; Rao, C.N.R. Atomic Layer Deposition of p-Type Epitaxial Thin Films of Undoped and N-Doped Anatase TiO2. ACS Appl. Mater. Interfaces 2016, 8, 7897–7901. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Kelaidis, N.; Polydorou, E.; Soultati, A.; Davazoglou, D.; Argitis, P.; Papadimitropoulos, G.; Tsikritzis, D.; Kennou, S.; Auras, F.; et al. Hydrogen and nitrogen codoping of anatase TiO2 for efficiency enhancement in organic solar cells. Sci. Rep. Sci. Rep. 2017, 7, 17839. [Google Scholar] [CrossRef] [Green Version]
- Anitha, V.C.; Banerjee, A.N.; Joo, S.W. Recent developments in TiO2 as n- and p-type transparent semiconductors: Synthesis, modification, properties, and energy-related applications. J. Mater. Sci. 2015, 50, 7495–7536. [Google Scholar] [CrossRef]
- Mishra, A.; Bhatt, N.; Bajpai, A. Nanostructured superhydrophobic coatings for solar panel applications. In Nanomaterials-Based Coatings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–424. [Google Scholar] [CrossRef]
- Lakhdar, N.; Hima, A. Electron transport material effect on performance of perovskite solar cells based on CH3NH3GeI3. Opt. Mater. 2020, 99, 109517. [Google Scholar] [CrossRef]
- Jayan, K.D.; Sebastian, V. Comprehensive device modelling and performance analysis of MASnI3 based perovskite solar cells with diverse ETM, HTM and back metal contacts. Sol. Energy 2021, 217, 40–48. [Google Scholar] [CrossRef]
- Alipour, H.; Ghadimi, A. Optimization of lead-free perovskite solar cells in normal-structure with WO3 and water-free PEDOT: PSS composite for hole transport layer by SCAPS-1D simulation. Opt. Mater. 2021, 120, 111432. [Google Scholar] [CrossRef]
- Kumar, M.H.; Dharani, S.; Leong, W.L.; Boix, P.P.; Prabhakar, R.R.; Baikie, T.; Shi, C.; Ding, H.; Ramesh, R.; Asta, M.; et al. Lead-Free Halide Perovskite Solar Cells with High Photocurrents Realized Through Vacancy Modulation. Adv. Mater. 2014, 26, 7122–7127. [Google Scholar] [CrossRef]
- Jeyakumar, R.; Bag, A. Methylammonium lead bromide based planar perovskite solar cells using various electron transport layers. Sol. Energy 2021, 221, 456–467. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, S.; Mahapatra, A.; Baral, J.K.; Pradhan, B. Performance optimization of lead free-MASnI3 based solar cell with 27% efficiency by numerical simulation. Opt. Mater. 2021, 117, 111193. [Google Scholar] [CrossRef]
- Abdelaziz, S.; Zekry, A.; Shaker, A.; Abouelatta, M. Investigation of lead-free MASnI3-MASnIBr2 tandem solar cell: Numerical simulation. Opt. Mater. 2022, 123, 111893. [Google Scholar] [CrossRef]
- Fatema, K.; Arefin, S. Enhancing the efficiency of Pb-based and Sn-based perovskite solar cell by applying different ETL and HTL using SCAPS-ID. Opt. Mater. 2022, 125, 112036. [Google Scholar] [CrossRef]
- Karthick, S.; Bouclé, J.; Velumani, S. Effect of bismuth iodide (BiI3) interfacial layer with different HTL’s in FAPI based per-ovskite solar cell–SCAPS–1D study. Sol. Energy 2021, 218, 157–168. [Google Scholar] [CrossRef]
- Lin, C.; Liu, G.; Xi, X.; Wang, L.; Wang, Q.; Sun, Q.; Li, M.; Zhu, B.; de Lara, D.P.; Zai, H. The Investigation of the Influence of a Cu2O Buffer Layer on Hole Transport Layers in MAPbI3-Based Perovskite Solar Cells. Materials 2022, 15, 8142. [Google Scholar] [CrossRef] [PubMed]



| S. No. | Author | ST PSC Device Structure (Top Electrode/HTL/Absorber Layer/ETL/Substrate) | PCE (%) | AVT (%) | Reference | Year |
|---|---|---|---|---|---|---|
| 1 | Matteocci et al. | ITO/PTAA/MAPbBr1−xClx/c-TiO2/m-TiO2/FTO | 64.9 | 6.3 | [28] | 2022 |
| 2 | Ponchai et al. | Au/Spiro-OMeTAD/(PEA)2MAn-1PbnBr3n+1/TiO2/FTO | 4.86 | 26.12 | [29] | 2021 |
| 3 | Rani et al. | Ag/NiOx/MAPbI3/TiO2/ZnO/ITO | - | 31.49 | [30] | 2021 |
| 4 | Singh et al. | Au/PTAA/MAPbBr3/c-TiO2/mp-TiO2/FTO | 7.6 | 52 | [31] | 2021 |
| 5 | Zhang et al. | Ag/Zr/PCBM/MAPbI3/NiOx/ITO | 11.74 | 23 | [32] | 2021 |
| 6 | Dew et al. | ITO/Ag/PEDOT: PSS/MAPbI3/PC61BM/ITO | 7.4 | 8 | [33] | 2019 |
| 7 | Yuan et al. | Ag/ZnO/PC61M/MAPbI3/PEDOT: PSS/ITO | 8.5 | 28.4 | [22] | 2018 |
| 8 | Han et al. | Ag/PCBM/MAPbI3-xClx/PEDOT: PSS/ITO | 13.27 | 16.3 | [34] | 2018 |
| 9 | Chen et al. | Au/Spiro-OMeTAD/MAPbI3/TiO2/FTO | 11.7 | 36 | [35] | 2016 |
| 10 | Hörantner et al. | Au/Spiro-OMeTAD/MAPbI3/c-TiO2/FTO | 6.1 | 38 | [36] | 2016 |
| 11 | Lee et al. | Ag/PEDOT: PSS/MAPbI3/PCBM/C60/ITO | 8.2 | 34 | [37] | 2016 |
| 12 | Chang et al. | Ag/PEDOT: PSS/MAPbI3/PC61BM/ITO | 11.8 | 20.8 | [38] | 2016 |
| 13 | Heo et al. | Ag/ZnO/PC61BM/MAPbI3-xClx/PEDOT: PSS/ITO | 7.8 | 37 | [39] | 2016 |
| 14 | Heo et al. | Au/Spiro-OMeTAD/MAPbI3/c-TiO2/mp-TiO2/FTO | 4.9 | 19 | [40] | 2015 |
| 15 | Della Gaspera et al. | SnOx/Ag/PEDOT: PSS/MAPbI3/PCBM/ITO | 11.8 | 29 | [24] | 2015 |
| 16 | Eperon et al. | ITO/PEDOT: PSS/PTAA/MAPbI3/TiO2/FTO | 10.6 | 20.9 | [41] | 2014 |
| 17 | Lynn et al. | Au/Spiro-OMeTAD/MAPbI3/c-TiO2/FTO | 3.5 | 30 | [42] | 2012 |
| Device Structure | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
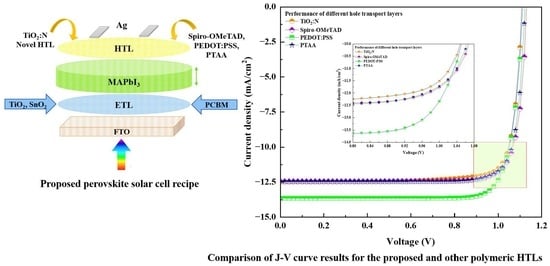
| FTO/TiO2/MAPbI3/TiO2:N/Ag | 1.07 | 11.35 | 81.09 | 9.92 |
| FTO/TiO2/MAPbI3/Spiro-OMeTAD/Ag | 1.10 | 11.30 | 80.30 | 10.00 |
| FTO/TiO2/MAPbI3/PTAA/Ag | 1.08 | 11.32 | 82.00 | 10.12 |
| FTO/TiO2/MAPbI3/PEDOT: PSS/Ag | 1.08 | 13.09 | 83.00 | 11.75 |
| FTO/PCBM/MAPbI3/TiO2:N/Ag | 1.10 | 12.48 | 83.32 | 11.54 |
| FTO/PCBM/MAPbI3/PTAA/Ag | 1.12 | 12.46 | 83.75 | 11.73 |
| FTO/PCBM/MAPbI3/Spiro-OMeTAD/Ag | 1.13 | 12.44 | 83.55 | 11.77 |
| FTO/PCBM/MAPbI3/PEDOT: PSS/Ag | 1.11 | 13.66 | 82.71 | 12.58 |
| FTO/SnO2/MAPbI3/TiO2:N/Ag | 1.08 | 11.33 | 82.00 | 10.11 |
| FTO/SnO2/MAPbI3/Spiro-OMeTAD/Ag | 1.11 | 11.28 | 81.62 | 10.23 |
| FTO/SnO2/MAPbI3/PEDOT: PSS/Ag | 1.10 | 11.31 | 86.84 | 10.89 |
| FTO/SnO2/MAPbI3/PTAA/Ag | 1.09 | 13.08 | 82.94 | 11.84 |
| PSC Structure | Thickness (nm) | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| FTO/TiO2/MAPbI3/TiO2:N/Ag | 100 | 1.07 | 11.35 | 81.09 | 9.92 |
| 200 | 1.05 | 17.20 | 78.48 | 14.30 | |
| 300 | 1.04 | 20.48 | 77.26 | 16.54 | |
| 400 | 1.03 | 22.40 | 76.39 | 17.69 | |
| 500 | 1.02 | 23.58 | 75.50 | 18.24 | |
| 600 | 1.01 | 24.33 | 74.57 | 18.43 | |
| 700 | 1.00 | 24.82 | 73.54 | 18.41 | |
| FTO/PCBM/MAPbI3/TiO2:N/Ag | 100 | 1.10 | 12.48 | 83.32 | 11.54 |
| 200 | 1.07 | 17.66 | 79.58 | 15.12 | |
| 300 | 1.05 | 20.64 | 77.64 | 16.93 | |
| 400 | 1.04 | 22.42 | 76.59 | 17.90 | |
| 500 | 1.03 | 23.53 | 75.74 | 18.37 | |
| 600 | 1.02 | 24.25 | 74.81 | 18.53 | |
| 700 | 1.01 | 24.72 | 73.83 | 18.49 | |
| FTO/SnO2/MAPbI3/TiO2:N/Ag | 100 | 1.08 | 11.33 | 82.00 | 10.11 |
| 200 | 1.06 | 17.19 | 78.95 | 14.46 | |
| 300 | 1.04 | 20.47 | 77.42 | 16.64 | |
| 400 | 1.03 | 22.40 | 76.49 | 17.77 | |
| 500 | 1.02 | 23.58 | 75.61 | 18.31 | |
| 600 | 1.01 | 24.32 | 74.59 | 18.53 | |
| 700 | 1.01 | 24.81 | 73.68 | 18.48 |
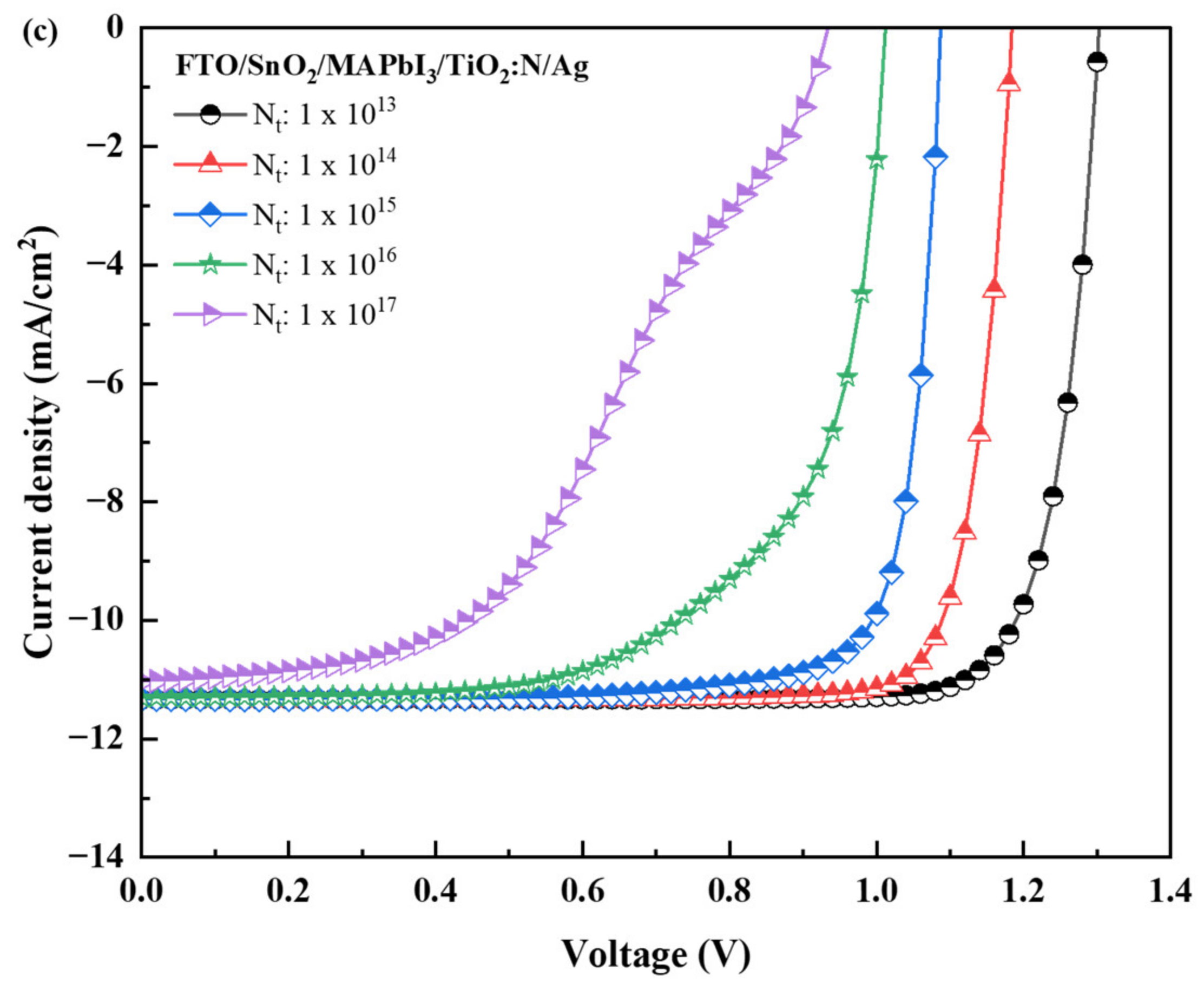
| Recipe | Absorber Layer Defect Density (Nt, cm−3) | Voc (V) | Jsc (mA/cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| FTO/TiO2/MAPbI3/TiO2:N/Ag | 1 1013 | 1.30 | 11.35 | 83.42 | 12.34 |
| 1 1014 | 1.18 | 11.35 | 83.74 | 11.26 | |
| 1 1015 | 1.07 | 11.35 | 81.09 | 9.92 | |
| 1 1016 | 0.99 | 11.32 | 65.63 | 7.42 | |
| 1 1017 | 0.91 | 11.07 | 49.18 | 4.98 | |
| FTO/PCBM/MAPbI3/TiO2:N/Ag | 1 1013 | 1.30 | 12.48 | 84.79 | 13.86 |
| 1 1014 | 1.19 | 12.48 | 85.87 | 12.85 | |
| 1 1015 | 1.10 | 12.48 | 83.32 | 11.54 | |
| 1 1016 | 1.03 | 12.43 | 66.05 | 8.52 | |
| 1 1017 | 0.96 | 12.02 | 40.62 | 4.70 | |
| FTO/SnO2/MAPbI3/TiO2:N/Ag | 1 1013 | 1.30 | 11.33 | 83.69 | 12.36 |
| 1 1014 | 1.18 | 11.33 | 84.80 | 11.39 | |
| 1 1015 | 1.08 | 11.33 | 82.00 | 10.11 | |
| 1 1016 | 1.02 | 11.30 | 65.08 | 7.45 | |
| 1 1017 | 0.93 | 11.03 | 45.99 | 4.74 |
| Parameters | Substrate | ETL | Perovskite Absorber Layer | Novel HTL | Polymeric HTLs | ||||
|---|---|---|---|---|---|---|---|---|---|
| FTO | TiO2 | PCBM | SnO2 | MAPbI3 | TiO2:N | Spiro-OMeTAD | PEDOT: PSS | PTAA | |
| Thickness ‘t’ (nm) | 200 | 50 | 50 | 50 | 100 * | 50 | 50 | 50 | 50 |
| Bandgap ‘Eg’ (eV) | 3.5 | 3.2 | 2 | 3.4 | 1.55 | 3 | 2.88 | 1.8 | 2.96 |
| Electron affinity ‘χ’ (eV) | 4 | 4 | 3.9 | 4 | 3.9 | 2.2 | 2.05 | 3.4 | 2.3 |
| Dielectric Permittivity ‘εr’ | 9 | 9 | 4 | 9 | 6.5 | 3 | 3 | 3 | 9 |
| CB EDOS ‘Nc’ (cm−3) | 2.2 × 1018 | 2.2 × 1018 | 1 × 1021 | 2.2 × 1018 | 2.2 × 1019 | 1.3 × 1014 | 2.2 × 1018 | 2.2 × 1018 | 1 × 1021 |
| VB EDOS ‘Nv’ (cm−3) | 2.2 × 1018 | 1.8 × 1019 | 2 × 1020 | 1.8 × 1019 | 1.3 × 1019 | 1.3 × 1015 | 1.8 × 1019 | 1.8 × 1019 | 1 × 1021 |
| e- thermal velocity (cm.s−1) | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 |
| h+ thermal velocity (cm.s−1) | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 |
| Electron mobility ‘μn’ (cm2/V.s) | 20 | 20 | 1 × 10−2 | 20 | 2.7 | 2 | 2 × 10−4 | 100 | 1 |
| Hole mobility ‘μh’ (cm2/V.s) | 10 | 10 | 1 × 10−2 | 10 | 1.8 | 2 | 2 × 10−4 | 4 | 40 |
| Shallow donor density ‘ND’ (cm−3) | 2 × 1019 | 1 × 1016 | 1 × 1020 | 1 × 1017 | 1.3 × 1016 | 0 | 0 | 0 | 0 |
| Shallow Acceptor density ‘NA’ (cm−3) | 0 | 0 | 0 | 0 | 1.3 × 1016 | 1.3 × 1014 | 2 × 1019 | 2 × 1019 | 2 × 1019 |
| Defect density ‘Nt’ (cm−3) | 1 × 1015 | 1 × 1015 | 1 × 1014 | 1 × 1015 | 1 × 1015 * | 1 × 1015 | 1 × 1015 | 1 × 1014 | 1 × 1015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pochont, N.R.; Sekhar, Y.R. Numerical Simulation of Nitrogen-Doped Titanium Dioxide as an Inorganic Hole Transport Layer in Mixed Halide Perovskite Structures Using SCAPS 1-D. Inorganics 2023, 11, 3. https://doi.org/10.3390/inorganics11010003
Pochont NR, Sekhar YR. Numerical Simulation of Nitrogen-Doped Titanium Dioxide as an Inorganic Hole Transport Layer in Mixed Halide Perovskite Structures Using SCAPS 1-D. Inorganics. 2023; 11(1):3. https://doi.org/10.3390/inorganics11010003
Chicago/Turabian StylePochont, Nitin Ralph, and Yendaluru Raja Sekhar. 2023. "Numerical Simulation of Nitrogen-Doped Titanium Dioxide as an Inorganic Hole Transport Layer in Mixed Halide Perovskite Structures Using SCAPS 1-D" Inorganics 11, no. 1: 3. https://doi.org/10.3390/inorganics11010003
APA StylePochont, N. R., & Sekhar, Y. R. (2023). Numerical Simulation of Nitrogen-Doped Titanium Dioxide as an Inorganic Hole Transport Layer in Mixed Halide Perovskite Structures Using SCAPS 1-D. Inorganics, 11(1), 3. https://doi.org/10.3390/inorganics11010003