Hydrothermally Synthesized Fluorine Added O3-NaFe1-xMgxO2 Cathodes for Sodium Ion Batteries
Abstract
1. Introduction
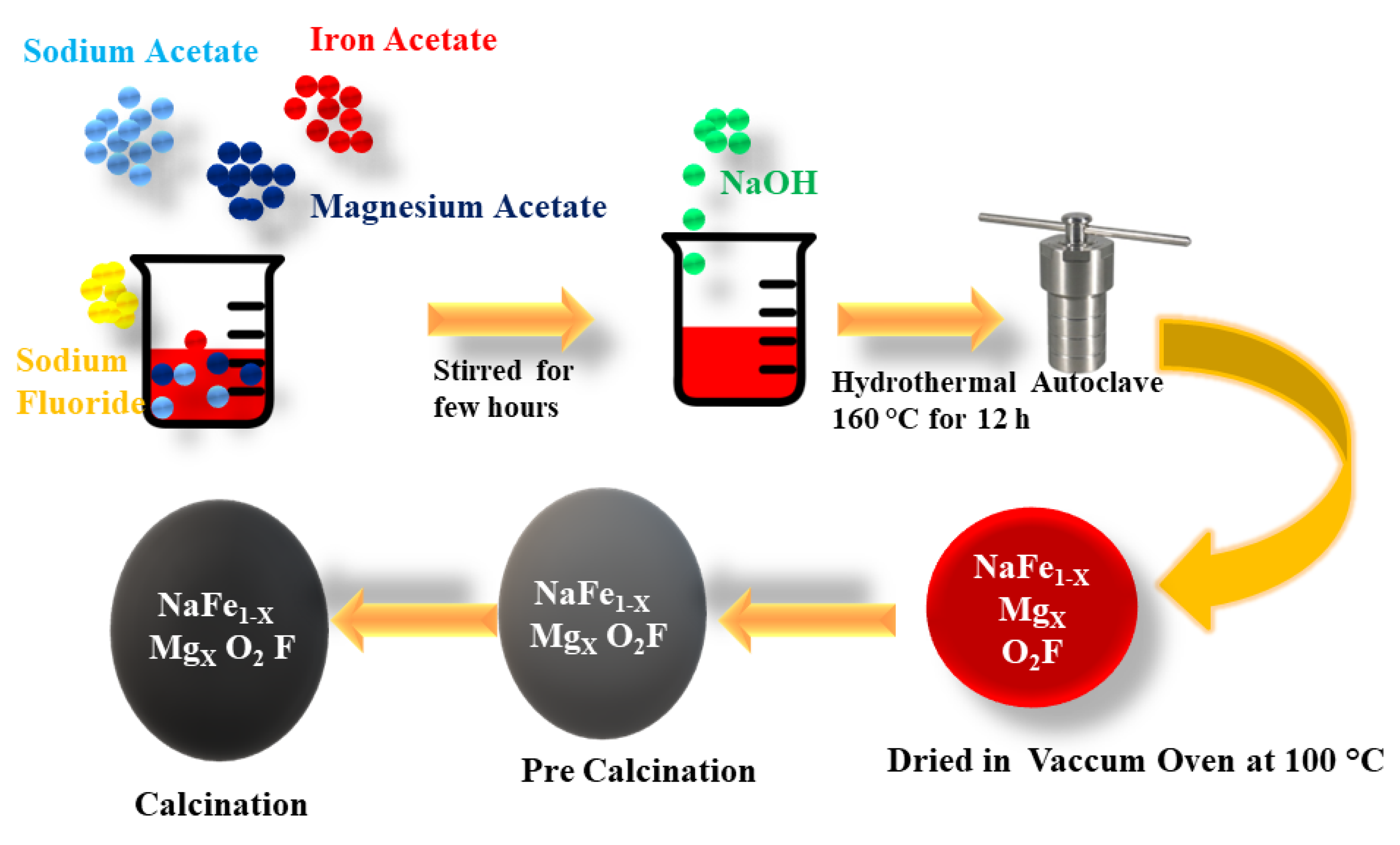
2. Experimental Procedure
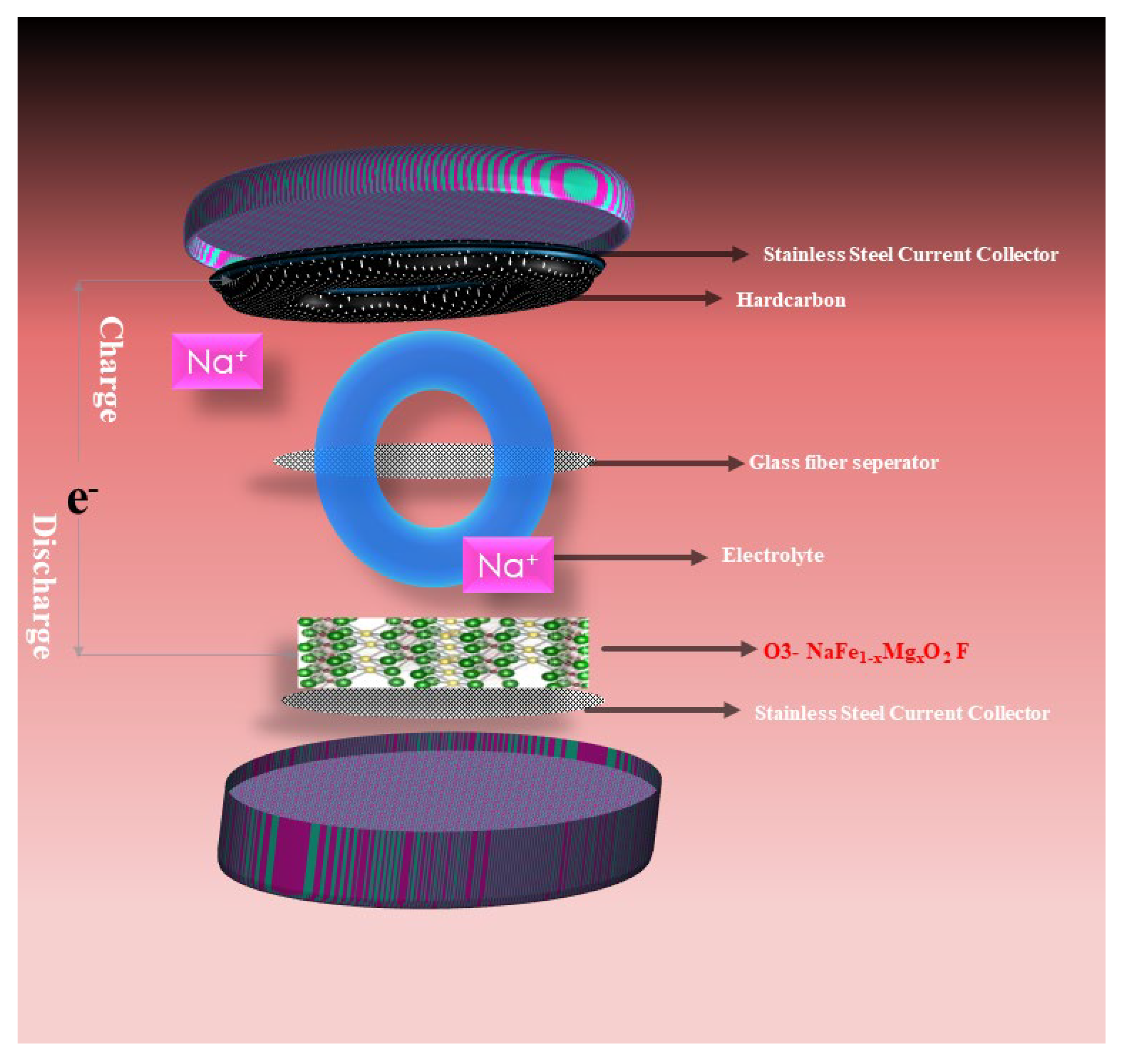
2.1. Process for Electrochemical Study
2.2. Materials Characterization
3. Results and Discussion
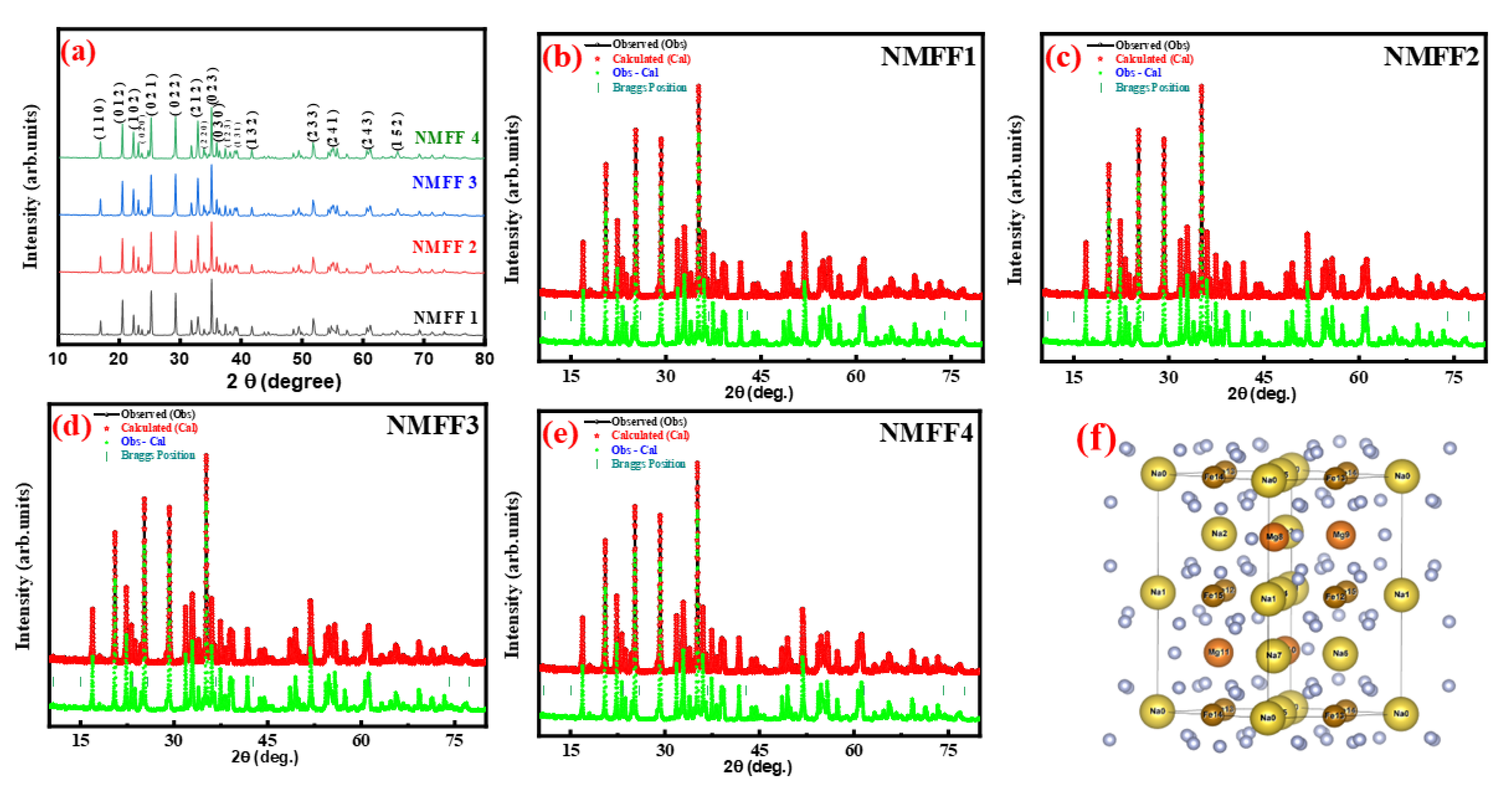
3.1. XRD and Rietveld Analysis
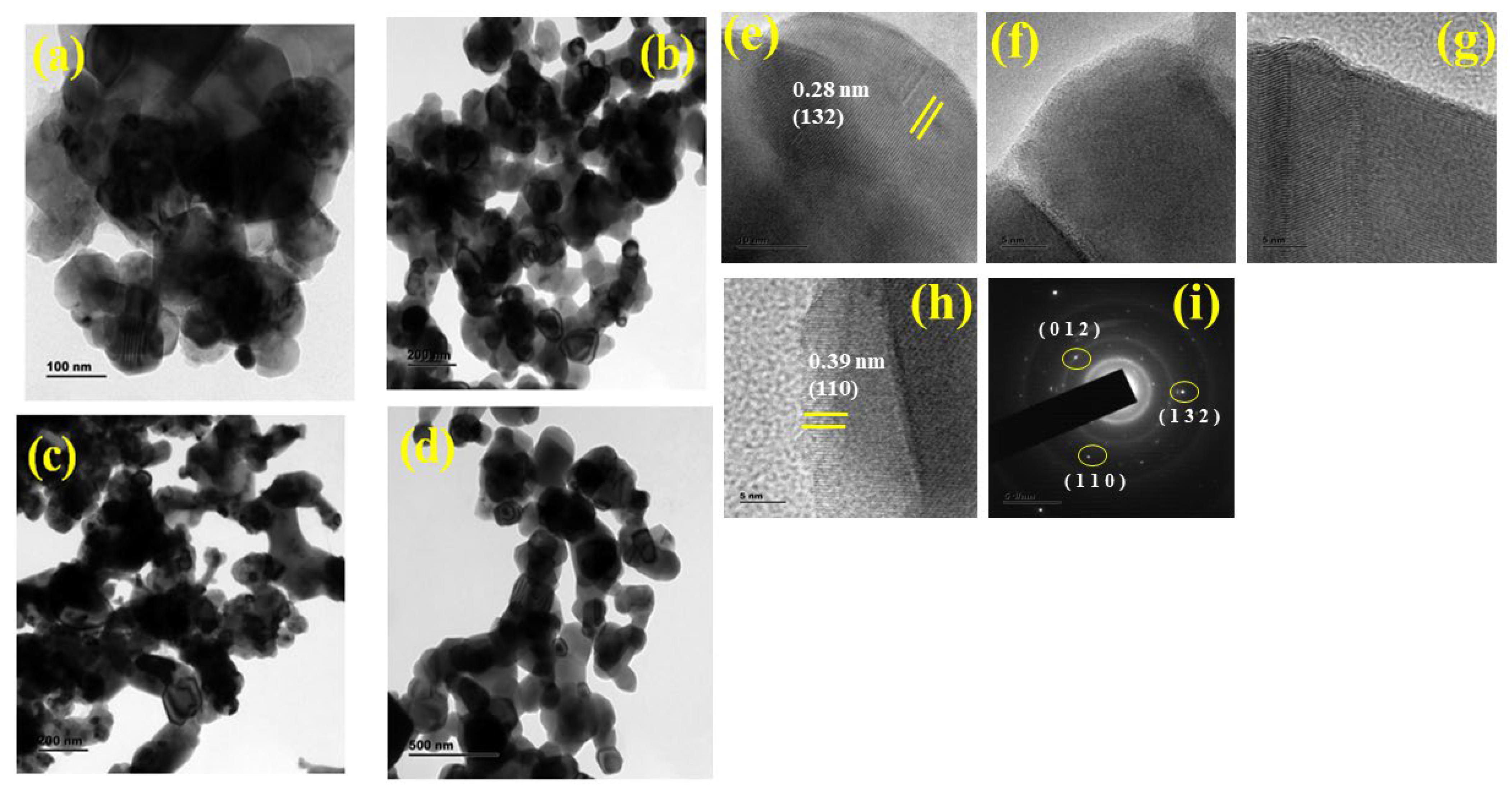
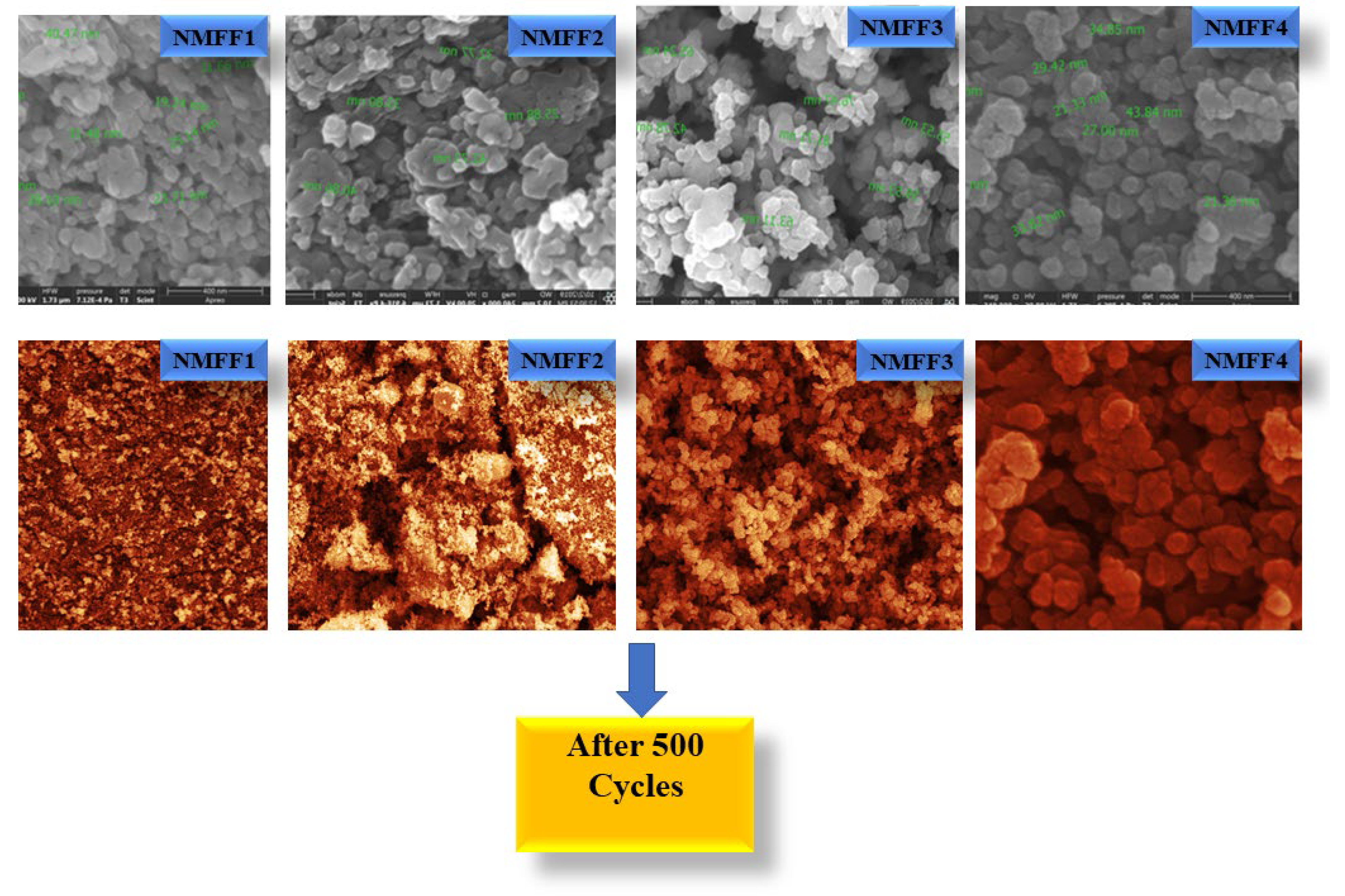
3.2. FESEM Analysis
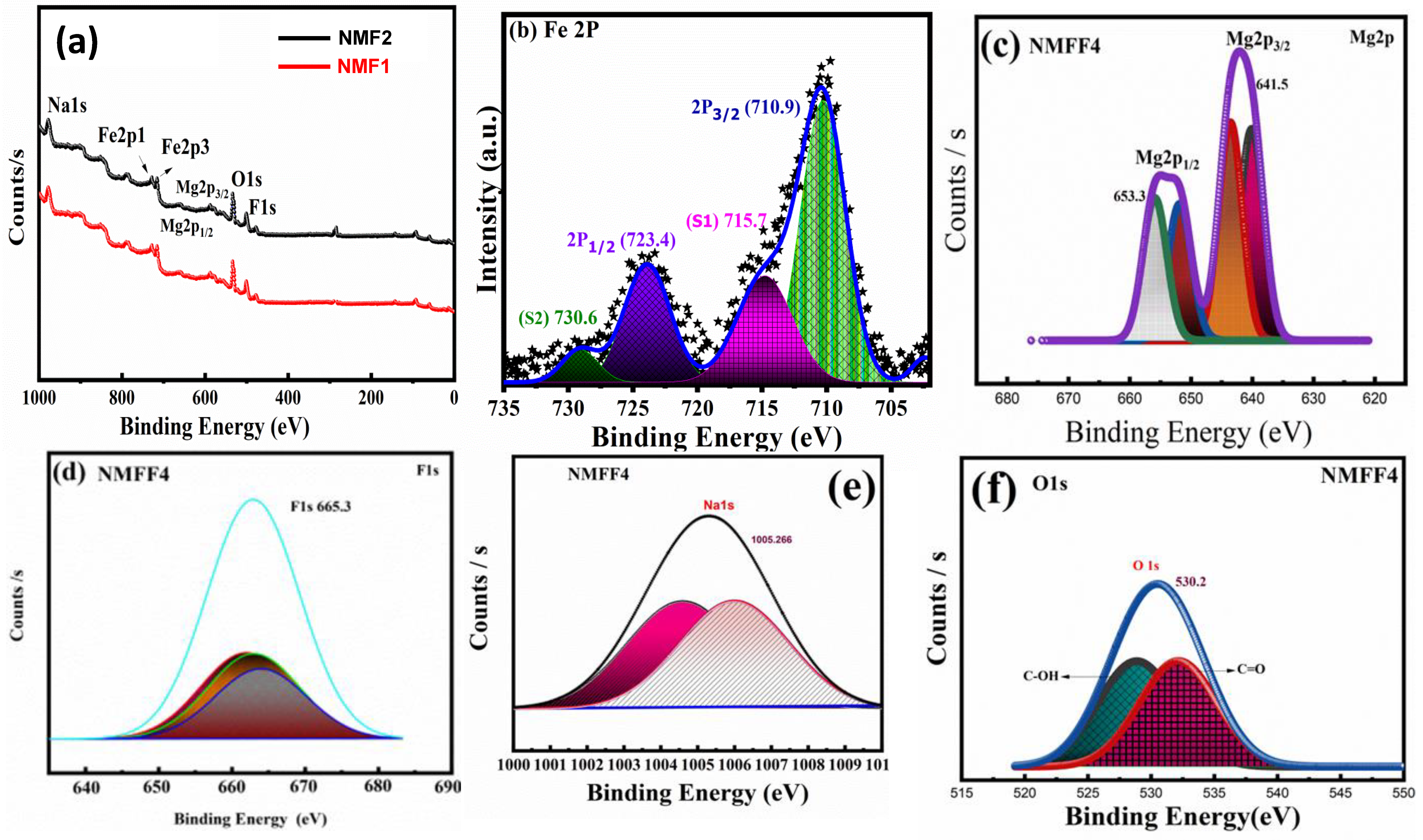
3.3. XPS Analysis
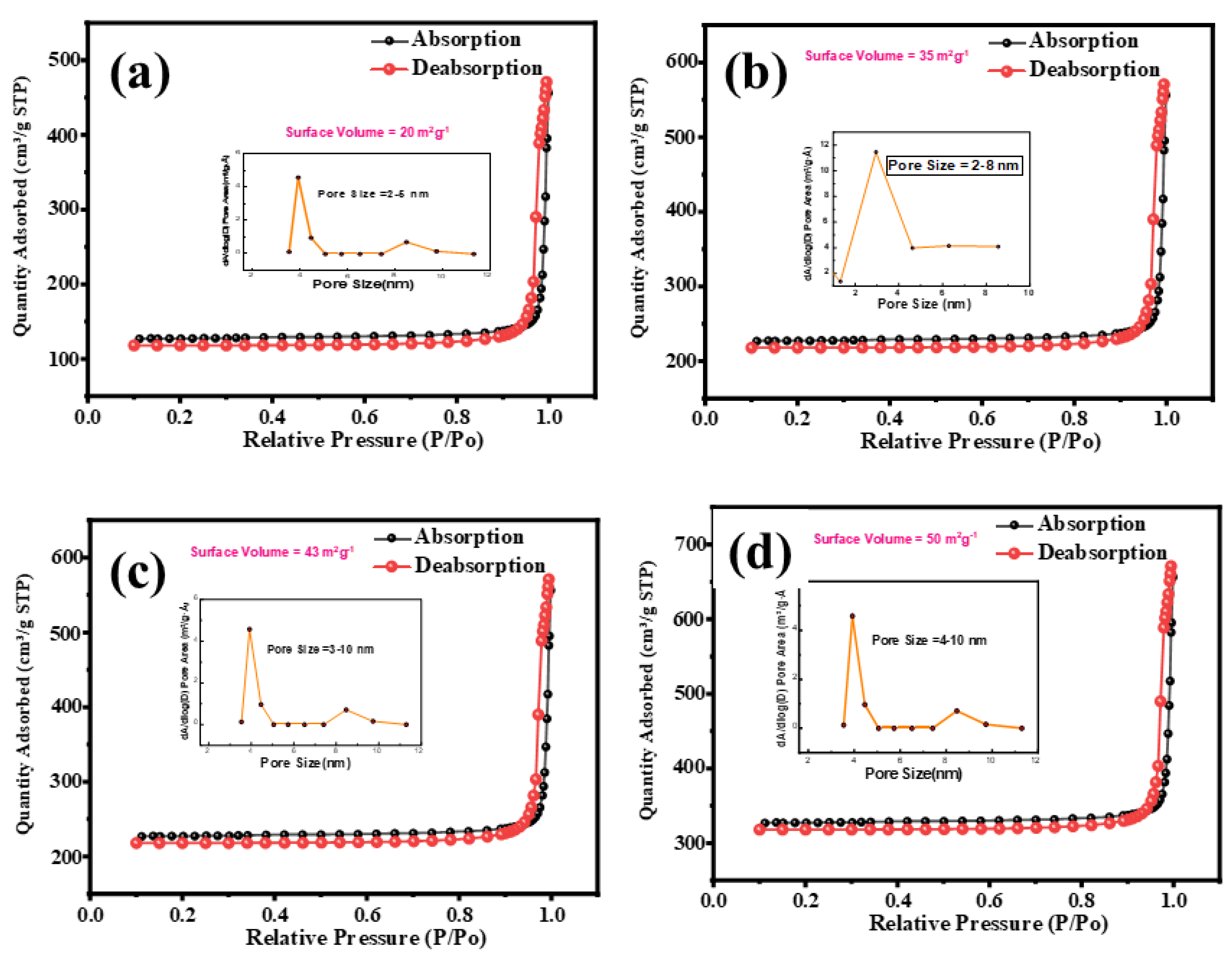
3.4. BET Analysis
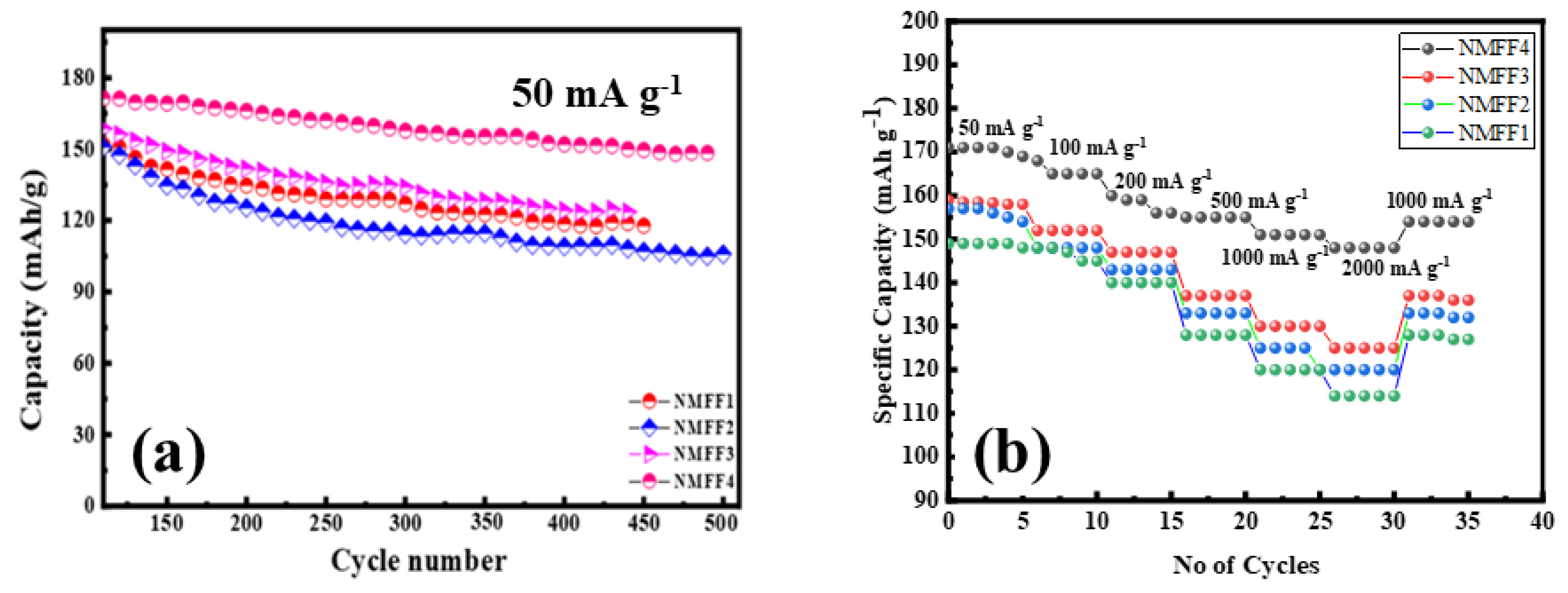
3.5. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubota, K.; Kumakura, S.; Yoda, Y.; Kuroki, K.; Komaba, S. Electrochemistry and Solid state chemistry of NaMeO2. Adv. Energy Mater. 2018, 8, 1703415. [Google Scholar] [CrossRef]
- Joshua, J.R.; Lee, Y.S.; Maiyalagan, T.; Nallamuthu, N.; Yuvraj, P.; Sivakumar, N.J. Na0.4(Mn0.33Ni0.33Co0.33)O2 surface grafter with SnO nanorods:A cathode material for rechargeable sodium ion batteries. Electroanal. Chem. 2020, 856, 113633. [Google Scholar] [CrossRef]
- Jayachitra, J.; Joshua, J.R.; Balamurugan, A.; Sivakumar, N.; Sharmila, V.; Shanavas, S.; Haija, M.A.; Alam, M.W.; BaQais, A. High electrode performance of hydrothermally developed activated C coated O3-NaFeO2 electrode for Na ion battery applications. Ceram. Int. 2023, 49, 48–56. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.Y.; Kim, H.; Kim, S.W.; Kang, K. Aqueous rechargeable Li and Na Ion batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef]
- Sharmila, V.; Parthibavarman, M. Lithium manganese phosphate associated with MWCNT: Enhanced positive electrode for lithium hybrid batteries. J. Alloy Compd. 2021, 858, 157715. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M.; Zonouz, A.F. Effect of operational synthesis parameters on the morphology and the electrochemical properties of 3D hierarchical AlV3O9 architectures for Li-ion batteries. J. Electrochem. Soc. 2020, 1, 167. [Google Scholar] [CrossRef]
- Ma, D.; Cao, Z.; Hu, A. Si-based anode materials for Li-ion batteries: A mini review. Nano-Micro Lett. 2014, 6, 347e358. [Google Scholar] [CrossRef]
- Liu, J.; Song, K.; Zhu, C.; Chen, C.-C.; Van Aken, P.A.; Maier, J.; Yu, Y. Ge/C nanowires as high-capacity and long-life anode materials for Li-ion batteries. ACS Nano 2014, 8, 7051e7059. [Google Scholar] [CrossRef] [PubMed]
- Billaud, J.; Clément, R.J.; Armstrong, A.R.; Canales-Vázquez, J.; Rozier, P.; Grey, C.P.; Bruce, P.G. β-NaMnO2: A high-performance cathode for sodium-ion batteries. J. Am. Chem. Soc. 2014, 136, 17243–17248. [Google Scholar] [CrossRef] [PubMed]
- Clément, R.J.; Bruce, P.G.; Grey, C.P. P2-Na0.67Mn0.85Al0.15O2 and NaMn2O4 Blend as Cathode Materials for Sodium-Ion Batteries Using a Natural β-MnO2 Precursor. J. Electrochem. Soc. 2015, 162, A2589–A2604. [Google Scholar] [CrossRef]
- Alam, M.W.; Azam, H.; Khalid, N.R.; Naeem, S.; Hussain, M.K.; BaQais, A.; Farhan, M.; Souayeh, B.; Zaidi, N.; Khan, K. Enhanced Photocatalytic Performance of Ag3PO4/Mn-ZnO Nanocomposite for the Degradation of Tetracycline Hydrochloride. Crystals 2022, 12, 1156. [Google Scholar] [CrossRef]
- Ensling, D.; Cherkashinin, G.; Schmid, S.; Bhuvaneswari, S.; Thissen, A.; Jaegermann, W. Nonrigid Band Behavior of the Electronic Structure of LiCoO2 Thin Film during Electrochemical Li Deintercalation. Chem. Mater. 2014, 26, 3948–3956. [Google Scholar] [CrossRef]
- Kang, K.; Meng, Y.S.; Bréger, J.; Grey, C.P.; Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Manthiram, A. Role of Chemical and Structural Stabilities on the Electrochemical Properties of Layered LiNi1 ∕ 3Mn1 ∕ 3Co1 ∕ 3O2 Cathodes. J. Electrochem. Soc. 2005, 152, A1714–A1718. [Google Scholar] [CrossRef]
- Mu, L.; Xu, S.; Li, Y.; Hu, Y.S.; Li, H.; Chen, L.; Huang, X. Prototype Sodium-Ion Batteries Using an Air-Stable and Co/Ni-Free O3-Layered Metal Oxide Cathode. Adv. Mater. 2015, 27, 6928–6933. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Park, J.S.; Jung, H.G.; Chung, K.Y.; Aurbach, D.; Sun, Y.K.; Myung, S.T. NaCrO2 cathode for high-rate sodium-ion batteries. Energy Environ. Sci. 2015, 8, 2019–2026. [Google Scholar] [CrossRef]
- Yao, H.R.; Wang, P.F.; Gong, Y.; Zhang, J.; Yu, X.; Gu, L.; Ouyang, C.; Yin, Y.X.; Hu, E.; Yang, X.Q.; et al. Designing Air-Stable O3-Type Cathode Materials by Combined Structure Modulation for Na-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 8440–8443. [Google Scholar] [CrossRef]
- Kubota, K.; Yabuuchi, N.; Yoshida, H.; Dahbi, M.; Komaba, S. Layered oxides as positive electrode materials for Na-ion batteries. Mrs Bull. 2014, 39, 416–422. [Google Scholar] [CrossRef]
- Xiong, P.; Bai, P.; Tu, S.; Cheng, M.; Zhang, J.; Sun, J.; Xu, Y. Red phosphorus nanoparticle@ 3D interconnected carbon nanosheet framework composite for potassium-ion battery anodes. Small 2018, 14, 1802140. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent Progress in Electrode Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef]
- Alam, M.W.; Wang, Z.; Naka, S.; Okada, H. Performance Enhancement of Top-Contact Pentacene-Based Organic Thin-Film Transistors with Bilayer WO3/Au Electrodes. Jpn. J. Appl. Phys. 2013, 52, 03BB08. [Google Scholar] [CrossRef]
- Richards, J.J.; Maiyalagan, T.; Sivakumar, N.; Lee, Y.S. Advanced High Voltage Cathode Materials for Rechargeable Lithium Ion Batteries; CRC Press: Boca Raton, FL, USA, 2020; Volume 1, p. 33. [Google Scholar]
- Yuan, T.; Ruan, J.; Peng, C.; Sun, H.; Pang, Y.; Yang, J.; Ma, Z.-F.; Zheng, S. 3D red phosphorus/sheared CNT sponge for high performance lithium-ion battery anodes. Energy Storage Mater. 2018, 13, 267–273. [Google Scholar] [CrossRef]
- Jayachitra, J.; Balamurugan, A.; Joshua, J.R.; Sharmila, V.; Sivakumar, N.; Alshahrani, T.; Shkir, M. Enhancing the electrochemical performance by structural evolution in O3-NaFe1-xMgxO2 cathodes for sodium ion batteries. J. Inorg. Chem. Commun. 2021, 129, 108528. [Google Scholar] [CrossRef]
- Komaba, S.; Yabuuchi, N.; Nakayama, T.; Ogata, A.; Ishikawa, T.; Nakai, I. Study on the reversible electrode reaction of Na(1-x)Ni(0.5)Mn(0.5)O2 for a rechargeable sodium-ion battery. Inorg. Chem. 2012, 51, 6211–6220. [Google Scholar] [CrossRef]
- Palomares, V.; Casas-Cabanas, M.; Castillo-Martínez, E.; Han, M.H.; Rojo, T. Update on Na-based battery materials. A growing research path. Energy Environ. Sci. 2013, 6, 2312. [Google Scholar] [CrossRef]
- Vassilaras, P.; Ma, X.; Li, X.; Ceder, G. Electrochemical Properties of Monoclinic NaNiO2. J. Electrochem. Soc. 2013, 160, A207–A211. [Google Scholar] [CrossRef]
- Mendiboure, A.; Delmas, C.; Hagenmuller, P. Electrochemical intercalation and deintercalation of NaxMnO2 bronzes. J. Solid State Chem. 1985, 57, 323–331. [Google Scholar] [CrossRef]
- Saadoune, I.; Maazaz, A.; Menetrier, M.; Delmas, C. On the NaxNi0.6Co0.4O2System: Physical and Electrochemical Studies. J. Solid State Chem. 1996, 122, 111–117. [Google Scholar] [CrossRef]
- Lee, D.H.; Xu, J.; Meng, Y.S. An advanced cathode for Na-ion batteries with high rate and excellent structural stability. Phys. Chem. Chem. Phys. 2013, 15, 3304–3312. [Google Scholar] [CrossRef]
- Hamani, D.; Ati, M.; Tarascon, J.M.; Rozier, P. NaxVO2 as possible electrode for Na-ion batteries. Electrochem. Commun. 2011, 13, 938–941. [Google Scholar] [CrossRef]
- Guignard, M.; Didier, C.; Darriet, J.; Bordet, P.; Elkaïm, E.; Delmas, C. P2-Na(x)VO2 system as electrodes for batteries and electron-correlated materials. Nat. Mater. 2013, 12, 74–80. [Google Scholar] [CrossRef]
- Didier, C.; Guignard, M.; Darriet, J.; Delmas, C. O′3–NaxVO2 System: A Superstructure for Na1/2VO2. Inorg. Chem. 2012, 51, 11007–11016. [Google Scholar] [CrossRef] [PubMed]
- Didier, C.; Guignard, M.; Denage, C.; Szajwaj, O.; Ito, S.; Saadoune, I.; Darriet, J.; Delmas, C. Electrochemical Na-Deintercalation from NaVO2, Electrochem. Solid State Lett. 2011, 14, A75–A78. [Google Scholar] [CrossRef]
- Komaba, S.; Takei, C.; Nakayama, T.; Ogata, A.; Yabuuchi, N. Electrochemical intercalation activity of layered NaCrO2 vs. LiCrO2. Electrochem. Commun. 2010, 12, 355–358. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Gao, R.; Sun, L.; Hu, Z.; Liu, X. Zr-doped P2-Na0.75Mn0.55Ni0.25Co0.05Fe0.10Zr0.05O2 as high-rate performance cathode material for sodium ion batteries. Electrochim. Acta 2017, 223, 92–99. [Google Scholar] [CrossRef]
- Ding, J.J.; Zhou, Y.N.; Sun, Q.; Fu, Z.W. Cycle performance improvement of NaCrO2 cathode by carbon coating for sodium ion batteries. Electrochem. Commun. 2012, 22, 85–88. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Matsumoto, K.; Nohira, T.; Hagiwara, R.; Fukunaga, A.; Sakai, S.; Jitta, K.; Inazawa, S. Electrochemical and structural investigation of NaCrO2 as a positive electrode for sodium secondary battery using inorganic ionic liquid NaFSA–KFSA. J. Power Sources 2013, 237, 52–57. [Google Scholar] [CrossRef]
- Doeff, M.M.; Richardson, T.J.; Hollingsworth, J.; Yuan, C.W.; Gonzales, M. Synthesis and characterization of a copper-substituted manganese oxide with the Na0.44MnO2 structure. J. Power Sources 2002, 112, 294–297. [Google Scholar] [CrossRef]
- Qi, X.; Liu, L.; Song, N.; Gao, F.; Yang, K.; Lu, Y.; Yang, H.; Hu, Y.S.; Cheng, Z.H.; Chen, L. Design and Comparative Study of O3/P2 Hybrid Structures for Room Temperature Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 40215–40223. [Google Scholar] [CrossRef]
- Allagui, A.; Baboukani, A.R.; Elwakil, A.S.; Wang, C. Electrochemical stability analysis of red phosphorus-based anode for lithium-ion batteries. J. Electr. Acta 2021, 395, 139149. [Google Scholar] [CrossRef]


| Na | Fe | Mg | F | O | |
|---|---|---|---|---|---|
| Calculated Ratio | |||||
| NMFF1 | 0.400 | 0.900 | 0.100 | 0.150 | 0.200 |
| NMFF2 | 0.400 | 0.800 | 0.200 | 0.150 | 0.200 |
| NMFF3 | 0.400 | 0.600 | 0.400 | 0.150 | 0.200 |
| NMFF4 | 0.400 | 0.500 | 0.500 | 0.150 | 0.200 |
| Ratios by ICP analysis | |||||
| NMFF1 | 0.401 | 0.899 | 0.101 | 0.150 | 0.200 |
| NMFF2 | 0.401 | 0.788 | 0.212 | 0.150 | 0.200 |
| NMFF3 | 0.401 | 0.600 | 0.400 | 0.150 | 0.200 |
| NMFF4 | 0.401 | 0.500 | 0.500 | 0.150 | 0.200 |
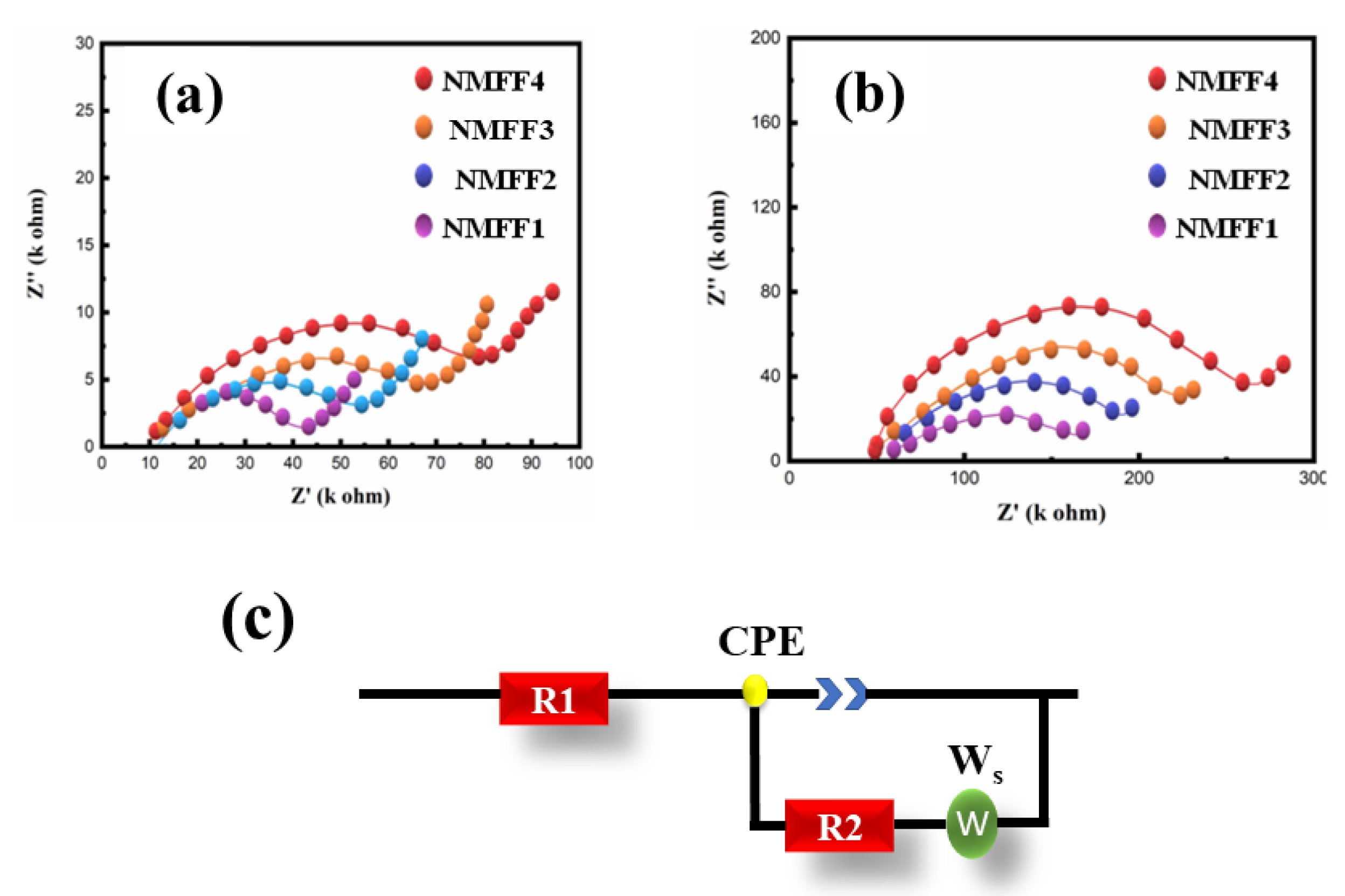
| Before Cycling | R1/kΩ | R2/kΩ |
| NMFF1 | 10.11 | 16.06 |
| NMFF2 | 8.66 | 14.21 |
| NMFF3 | 7.23 | 13.12 |
| NMFF4 | 6.52 | 11.47 |
| After Cycling | R1/kΩ | R2/kΩ |
| NMFF1 | 11.25 | 82.32 |
| NMFF2 | 10.78 | 80.54 |
| NMFF3 | 8.56 | 75.56 |
| NMFF4 | 7.98 | 71.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.W.; BaQais, A.; Nahvi, I.; Yasin, A.; Mir, T.A.; Shajahan, S. Hydrothermally Synthesized Fluorine Added O3-NaFe1-xMgxO2 Cathodes for Sodium Ion Batteries. Inorganics 2023, 11, 37. https://doi.org/10.3390/inorganics11010037
Alam MW, BaQais A, Nahvi I, Yasin A, Mir TA, Shajahan S. Hydrothermally Synthesized Fluorine Added O3-NaFe1-xMgxO2 Cathodes for Sodium Ion Batteries. Inorganics. 2023; 11(1):37. https://doi.org/10.3390/inorganics11010037
Chicago/Turabian StyleAlam, Mir Waqas, Amal BaQais, Insha Nahvi, Amina Yasin, Tanveer Ahmad Mir, and Shanavas Shajahan. 2023. "Hydrothermally Synthesized Fluorine Added O3-NaFe1-xMgxO2 Cathodes for Sodium Ion Batteries" Inorganics 11, no. 1: 37. https://doi.org/10.3390/inorganics11010037
APA StyleAlam, M. W., BaQais, A., Nahvi, I., Yasin, A., Mir, T. A., & Shajahan, S. (2023). Hydrothermally Synthesized Fluorine Added O3-NaFe1-xMgxO2 Cathodes for Sodium Ion Batteries. Inorganics, 11(1), 37. https://doi.org/10.3390/inorganics11010037








