Synthesis and X-ray Structure Analysis of the Polymeric [Ag2(4-Amino-4H-1,2,4-triazole)2(NO3)]n(NO3)n Adduct: Anticancer, and Antimicrobial Applications
Abstract
:1. Introduction
2. Results and Discussion
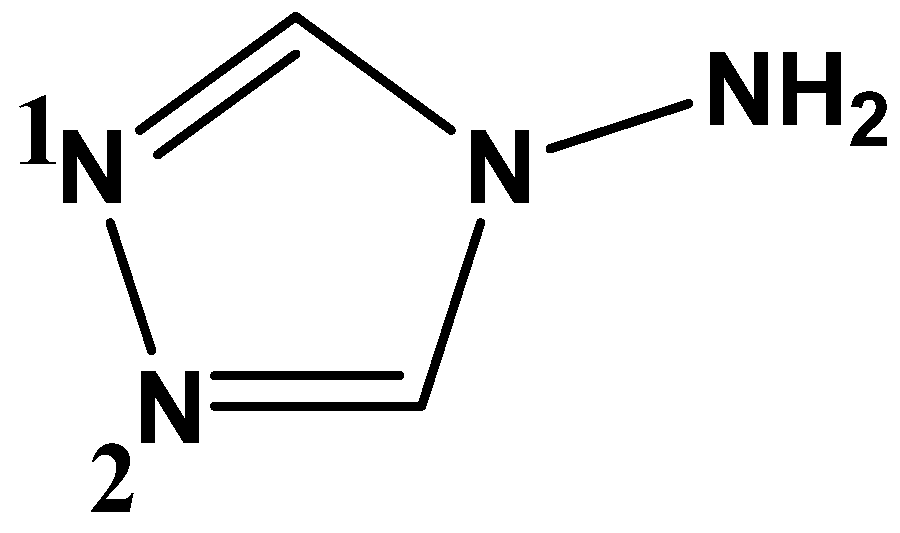
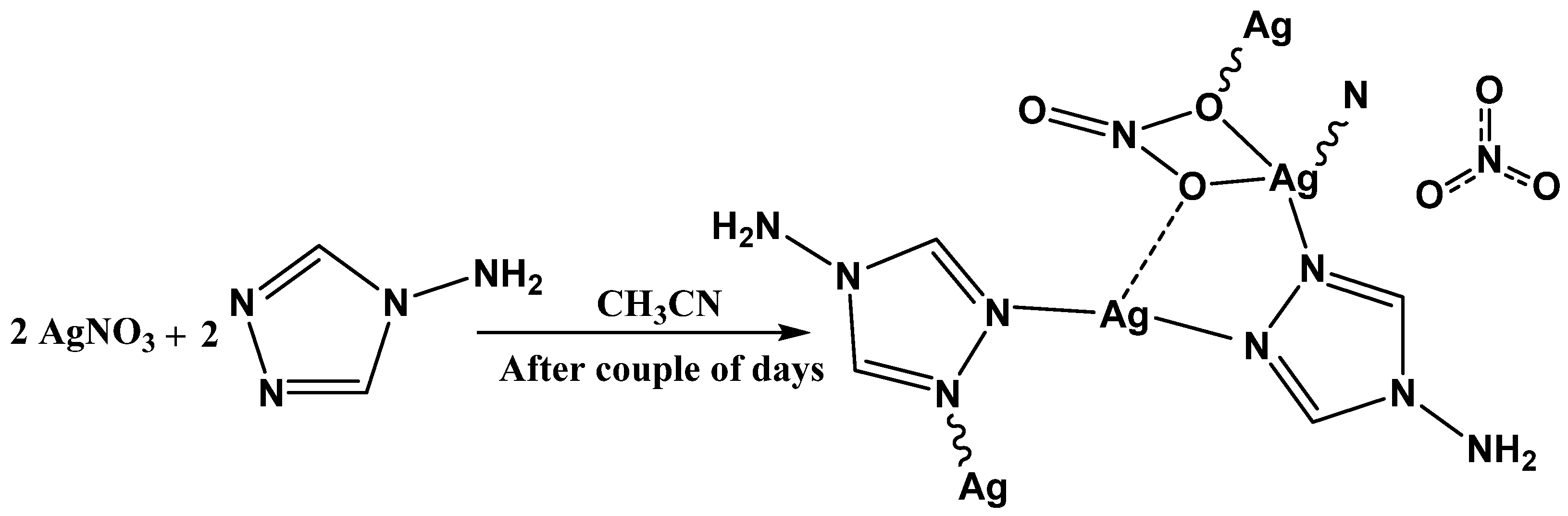
2.1. Synthesis and Characterization
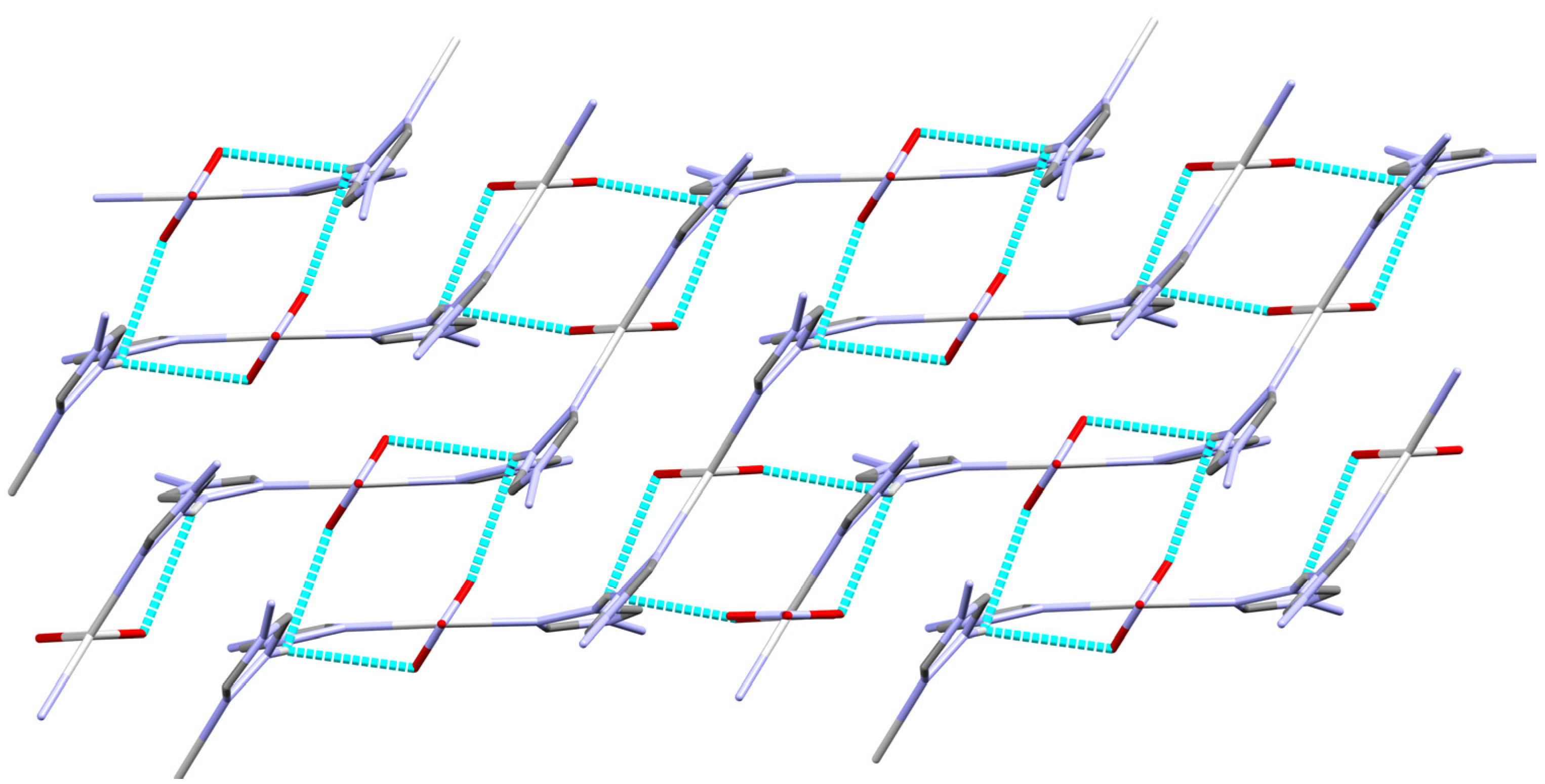
2.2. X-ray Crystal Structure of [Ag2(L)2(NO3)]n(NO3)n
2.3. FTIR Spectra
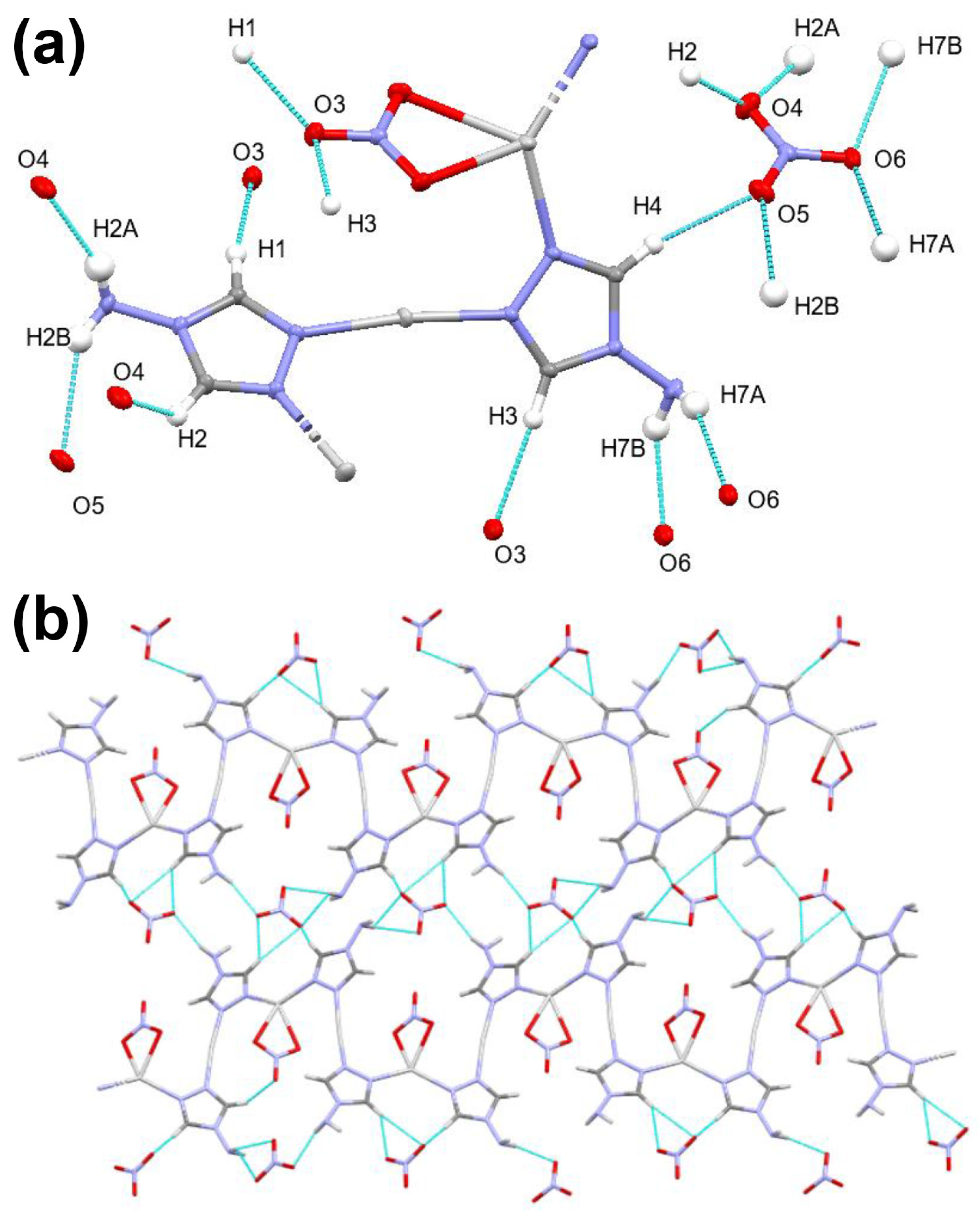
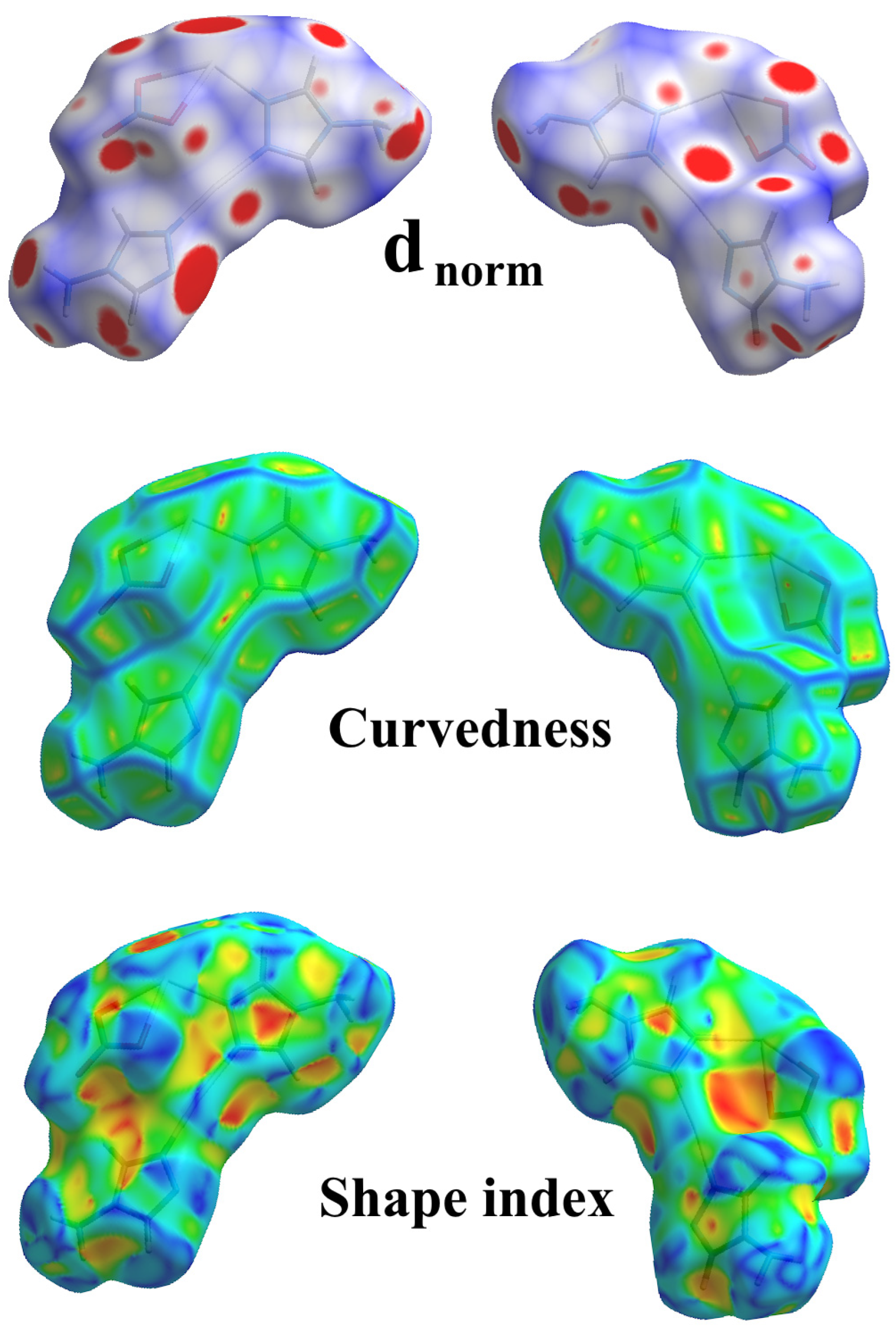
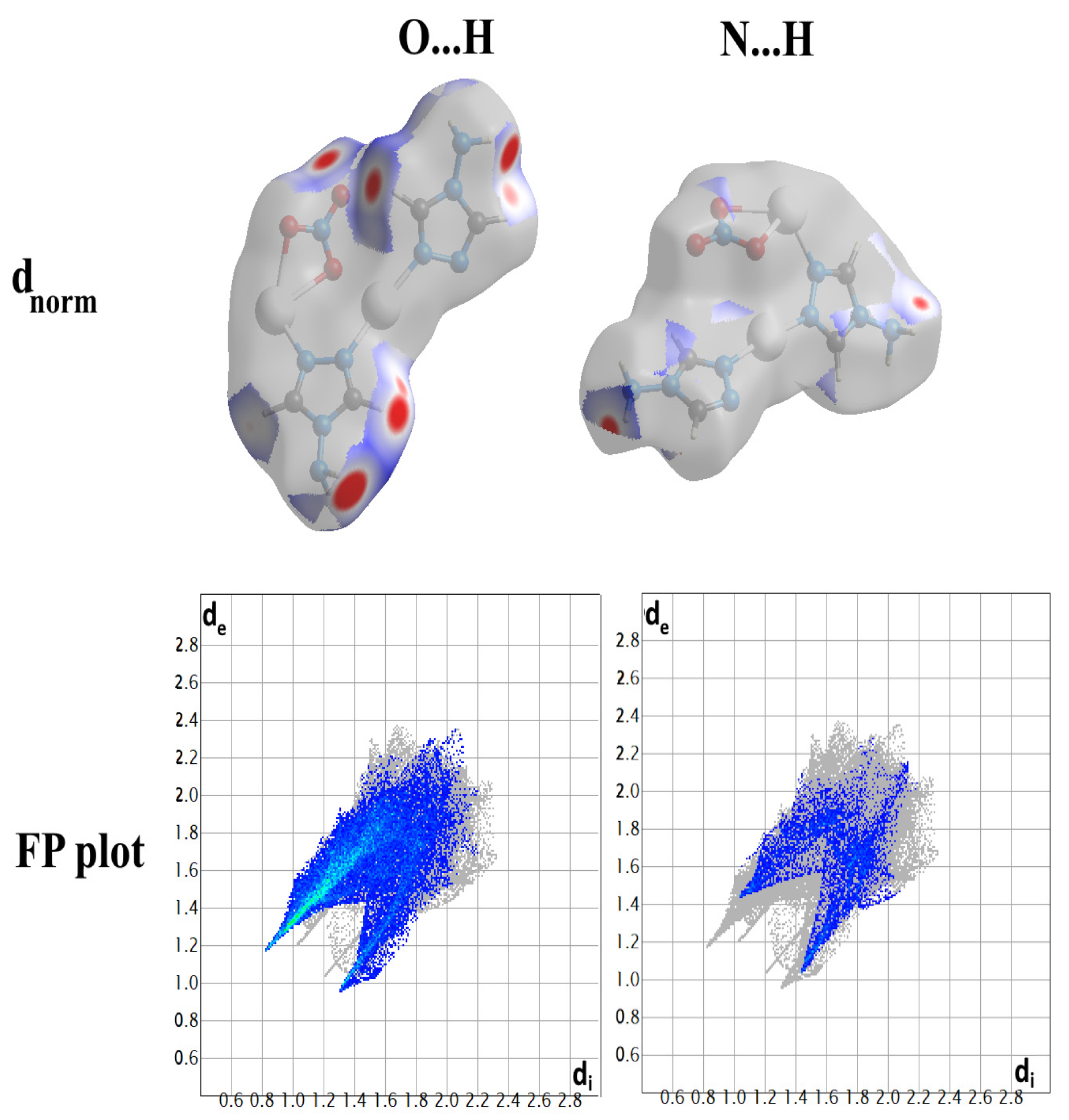
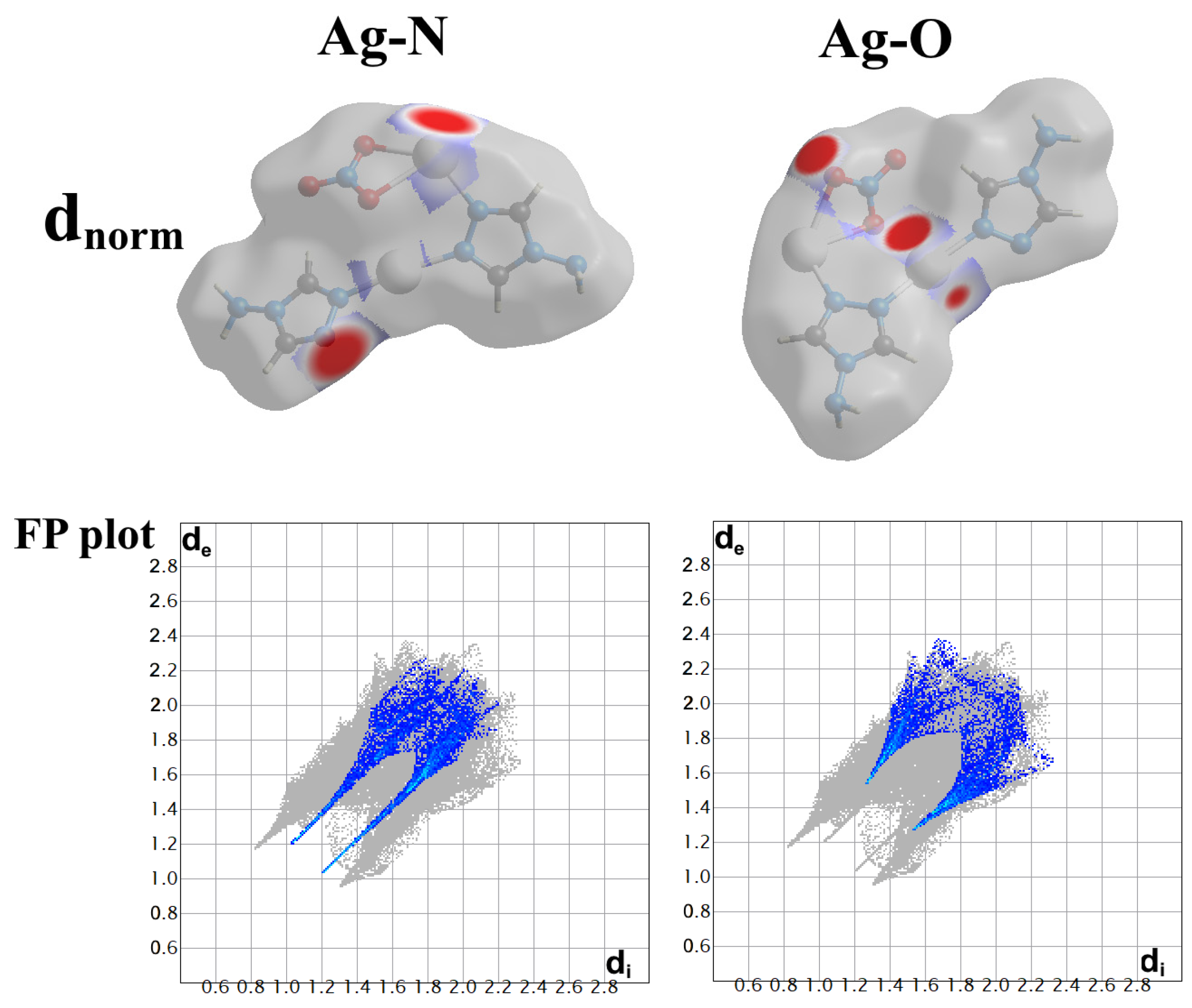
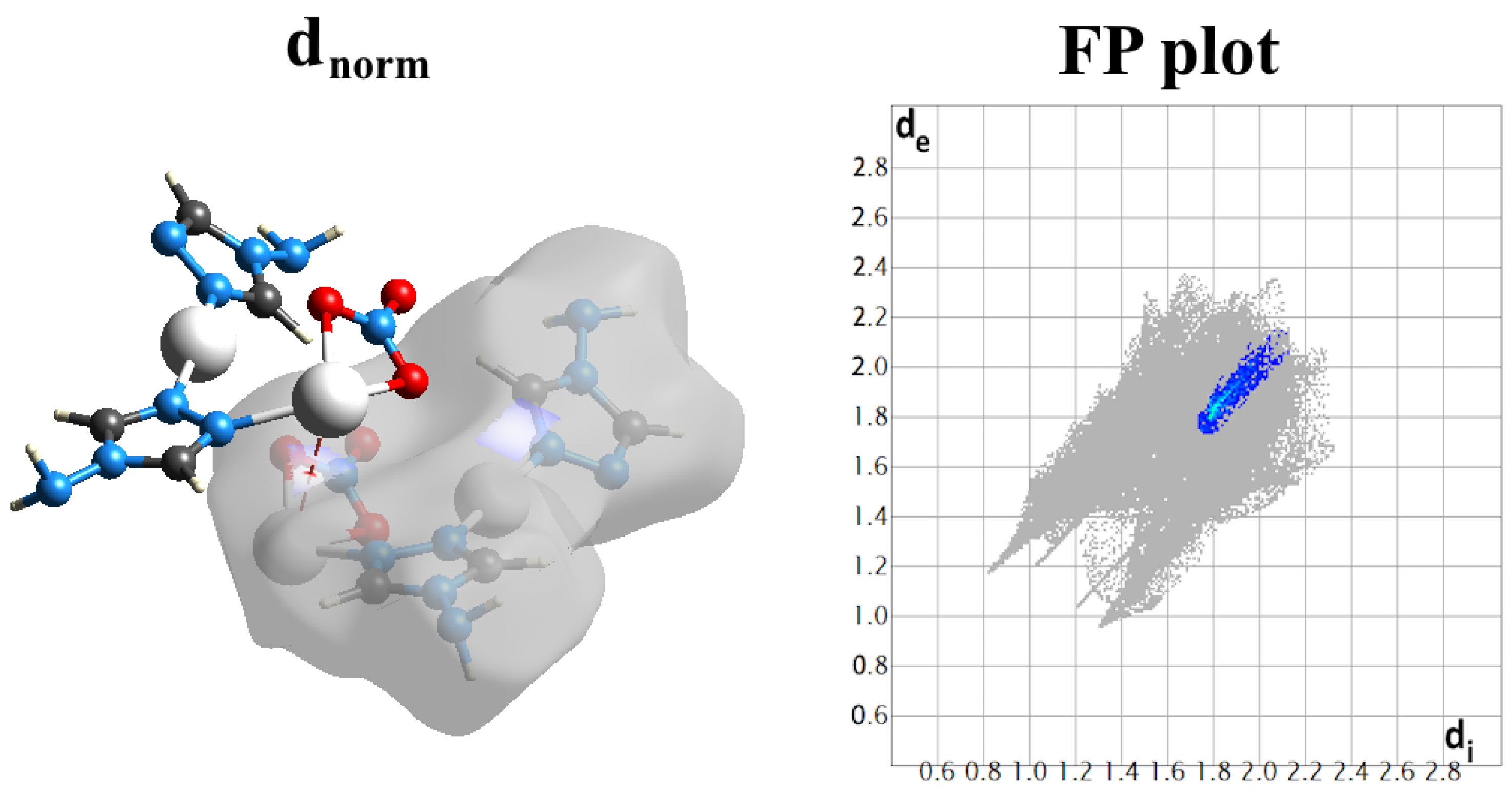
2.4. Analysis of Molecular Packing
2.5. Cytotoxic Activity
2.6. Antimicrobial Activity
3. Materials and Methods
3.1. Chemicals and Physicochemical Characterizations
3.2. Synthesis of [Ag2(L)2(NO3)]n(NO3)n
3.3. Crystal Structure Analysis
3.4. Hirshfeld Surface Analysis
3.5. Determination of Cytotoxic and Antimicrobial Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar, M.A.; Sharaf, M.M.; Albering, J.H.; Abu-Youssef, M.A.M.; Kassem, T.S.; Soliman, S.M.; Badr, A.M.A. One Pot Synthesis of Two Potent Ag(I) Complexes with Quinoxaline Ligand, X-Ray Structure, Hirshfeld Analysis, Antimicrobial, and Antitumor Investigations. Sci. Rep. 2022, 12, 20881. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.A.; Abu-Youssef, M.A.M.; Soliman, S.M.; Haukka, M.; Al-Majid, A.M.; Barakat, A.; Badr, A.M.A. Synthesis, X-Ray Structure, Hirshfeld, and Antimicrobial Studies of New Ag(I) Complexes Based on Pyridine-Type Ligands. J. Mol. Struct. 2022, 1264, 133210. [Google Scholar] [CrossRef]
- Tan, S.J.; Yan, Y.K.; Lee, P.P.F.; Lim, K.H. Copper, Gold and Silver Compounds as Potential New Anti-Tumor Metallodrugs. Future Med. Chem. 2010, 2, 1591–1608. [Google Scholar] [CrossRef]
- Journal of Pharmaceuticals, G.; Kumar Raju, S.; Karunakaran, A.; Kumar, S.; Sekar, P.; Murugesan, M.; Karthikeyan, M. Silver Complexes as Anticancer Agents: A Perspective Review. Ger. J. Pharm. Biomater. 2022, 1, 6–28. [Google Scholar]
- Altowyan, M.S.; El-Naggar, M.A.; Abu-Youssef, M.A.M.; Soliman, S.M.; Haukka, M.; Barakat, A.; Badr, A.M.A. Synthesis, X-Ray Structure, Antimicrobial and Anticancer Activity of a Novel [Ag(Ethyl-3-Quinolate)2(Citrate)] Complex. Crystals 2022, 12, 356. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.M.; Soliman, S.M.; Langer, V.; Gohar, Y.M.; Hasanen, A.A.; Makhyoun, M.A.; Zaky, A.H.; Öhrström, L.R. Synthesis, Crystal Structure, Quantum Chemical Calculations, DNA Interactions, and Antimicrobial Activity of [Ag(2-Amino-3-Methylpyridine)2]NO3 and [Ag(Pyridine-2-Carboxaldoxime)NO3]. Inorg. Chem. 2010, 49, 9788–9797. [Google Scholar] [CrossRef]
- Isab, A.A.; Nawaz, S.; Saleem, M.; Altaf, M.; Monim-ul-Mehboob, M.; Ahmad, S.; Evans, H.S. Synthesis, Characterization and Antimicrobial Studies of Mixed Ligand Silver(I) Complexes of Thioureas and Triphenylphosphine; Crystal Structure of {[Ag(PPh3)(Thiourea)(NO3)]2·[Ag(PPh3)(Thiourea)]2(NO3)2}. Polyhedron 2010, 29, 1251–1256. [Google Scholar] [CrossRef]
- Tan, X.J.; Liu, H.Z.; Ye, C.Z.; Lou, J.F.; Liu, Y.; Xing, D.X.; Li, S.P.; Liu, S.L.; Song, L.Z. Synthesis, Characterization and in Vitro Cytotoxic Properties of New Silver(I) Complexes of Two Novel Schiff Bases Derived from Thiazole and Pyrazine. Polyhedron 2014, 71, 119–132. [Google Scholar] [CrossRef]
- Nawaz, S.; Isab, A.A.; Merz, K.; Vasylyeva, V.; Metzler-Nolte, N.; Saleem, M.; Ahmad, S. Synthesis, Characterization and Antimicrobial Studies of Mixed Ligand Silver(I) Complexes of Triphenylphosphine and Heterocyclic Thiones: Crystal Structure of Bis[{(Μ2-Diazinane-2-Thione)(Diazinane-2-Thione)(Triphenylphosphine)Silver(I) Nitrate}]. Polyhedron 2011, 30, 1502–1506. [Google Scholar] [CrossRef]
- Nomiya, K.; Tsuda, K.; Sudoh, T.; Oda, M.; Bongoza, U.; Zamisa, S.J.; Munzeiwa, W.A.; Omondi, B.; Celik, S.; Yurdakul, S.; et al. Ag(I)-N Bond-Containing Compound Showing Wide Spectra in Effective Antimicrobial Activities: Polymeric Silver(I) Imidazolate. J. Inorg. Biochem. 1997, 68, 39–44. [Google Scholar] [CrossRef]
- Mohan, M.; Gupta, S.K.; Kalra, V.K.; Vajpayee, R.B.; Sachdev, M.S. Topical Silver Sulphadiazine--a New Drug for Ocular Keratomycosis. Br. J. Ophthalmol. 1988, 72, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Klasen, H.J. A Historical Review of the Use of Silver in the Treatment of Burns. II. Renewed Interest for Silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef] [PubMed]
- De Gracia, C.G. An Open Study Comparing Topical Silver Sulfadiazine and Topical Silver Sulfadiazine–Cerium Nitrate in the Treatment of Moderate and Severe Burns. Burns 2001, 27, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Glynn, F.; Phelan, S.; Sheahan, P.; Crotty, P.; McShane, D. Silver Nitrate Cauterisation, Does Concentration Matter? Clin. Otolaryngol. 2007, 32, 197–199. [Google Scholar]
- Spacciapoli, P.; Buxton, D.; Rothstein, D.; Friden, P. Antimicrobial Activity of Silver Nitrate against Periodontal Pathogens. J. Periodontal Res. 2001, 36, 108–113. [Google Scholar] [CrossRef]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef]
- Bayston, R.; Vera, L.; Mills, A.; Ashraf, W.; Stevenson, O.; Howdle, S.M. In Vitro Antimicrobial Activity of Silver-Processed Catheters for Neurosurgery. J. Antimicrob. Chemother. 2010, 65, 258–265. [Google Scholar] [CrossRef]
- Fox, C.L.; Modak, S.M. Mechanism of Silver Sulfadiazine Action on Burn Wound Infections. Antimicrob. Agents Chemother. 1974, 5, 582–588. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Nomiya, K.; Noguchi, R.; Oda, M. Synthesis and Crystal Structure of Coinage Metal(I) Complexes with Tetrazole (Htetz) and Triphenylphosphine Ligands, and Their Antimicrobial Activities. A Helical Polymer of Silver(I) Complex [Ag(Tetz)(PPh3)2]n and a Monomeric Gold(I) Complex [Au(Tetz)(PP]. Inorg. Chim. Acta 2000, 298, 24–32. [Google Scholar] [CrossRef]
- Irisli, S.; Tiryaki, F. Silver (I) Complexes Containing Diphosphine and Bis (Phosphine) Disulphide Ligands. Asian J. Chem. 2009, 21, 355–360. [Google Scholar]
- Sharkey, M.A.; O’Gara, J.P.; Gordon, S.V.; Hackenberg, F.; Healy, C.; Paradisi, F.; Patil, S.; Schaible, B.; Tacke, M. Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3. Antibiotics 2012, 1, 25–28. [Google Scholar] [CrossRef]
- Carter, K.R.; Miller, R.D.; Hedrick, J.L. Synthesis and Properties of Imide-Aryl Ether 1,2,4-Triazole Random Copolymers. Polymer 1993, 34, 843–848. [Google Scholar] [CrossRef]
- Vatmurge, N.S.; Hazra, B.G.; Pore, V.S.; Shirazi, F.; Chavan, P.S.; Deshpande, M.V. Synthesis and Antimicrobial Activity of β-Lactam–Bile Acid Conjugates Linked via Triazole. Bioorg. Med. Chem. Lett. 2008, 18, 2043–2047. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Oikawa, S.; Goto, T.; Sawai, K.; Shirahama, H.; Matsumoto, T. Structures and Phytotoxicity of Metabolites from Valsa Ceratosperma. Agric. Biol. Chem. 2014, 50, 997–1001. [Google Scholar] [CrossRef]
- Navidpour, L.; Shafaroodi, H.; Abdi, K.; Amini, M.; Ghahremani, M.H.; Dehpour, A.R.; Shafiee, A. Design, Synthesis, and Biological Evaluation of Substituted 3-Alkylthio-4,5-Diaryl-4H-1,2,4-Triazoles as Selective COX-2 Inhibitors. Bioorg. Med. Chem. 2006, 14, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Bokor, É.; Docsa, T.; Gergely, P.; Somsák, L. C-Glucopyranosyl-1,2,4-Triazoles as New Potent Inhibitors of Glycogen Phosphorylase. ACS Med. Chem. Lett. 2013, 4, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Blank, B.; Nichols, D.M.; Vaidya, P.D. Synthesis of 1, 2, 4-Triazoles as Potential Hypoglycemic Agents. J. Med. Chem. 1972, 15, 694–696. [Google Scholar] [CrossRef]
- Ainsworth, C.; Easton, N.R.; Livezey, M.; Morrison, D.E.; Gibson, W.R. The Anticonvulsant Activity of 1,2,4-Triazoles. J. Med. Pharm. Chem. 1962, 5, 383–389. [Google Scholar] [CrossRef]
- Radhika, C.; Venkatesham, A.; Sarangapani, M. Synthesis and Antidepressant Activity of Di Substituted-5-Aryl-1,2,4- Triazoles. Med. Chem. Res. 2012, 21, 3509–3513. [Google Scholar] [CrossRef]
- Boechat, N.; Pinheiro, L.C.S.; Santos-Filho, O.A.; Silva, I.C. Design and Synthesis of New N-(5-Trifluoromethyl)-1H-1,2,4-Triazol-3-Yl Benzenesulfonamides as Possible Antimalarial Prototypes. Molecules 2011, 16, 8083–8097. [Google Scholar] [CrossRef]
- Malerich, J.P.; Lam, J.S.; Hart, B.; Fine, R.M.; Klebansky, B.; Tanga, M.J.; D’Andrea, A. Diamino-1,2,4-Triazole Derivatives Are Selective Inhibitors of TYK2 and JAK1 over JAK2 and JAK3. Bioorg. Med. Chem. Lett. 2010, 20, 7454–7457. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.J.; Hill, R.G.; Shepheard, S.L.; Hargreaves, R.J. The Anti-Migraine 5-HT1B/1D Agonist Rizatriptan Inhibits Neurogenic Dural Vasodilation in Anaesthetized Guinea-Pigs. Br. J. Pharmacol. 2001, 133, 1029–1034. [Google Scholar] [CrossRef]
- Jordão, A.K.; Afonso, P.P.; Ferreira, V.F.; de Souza, M.C.B.V.; Almeida, M.C.B.; Beltrame, C.O.; Paiva, D.P.; Wardell, S.M.S.V.; Wardell, J.L.; Tiekink, E.R.T.; et al. Antiviral Evaluation of N-Amino-1,2,3-Triazoles against Cantagalo Virus Replication in Cell Culture. Eur. J. Med. Chem. 2009, 44, 3777–3783. [Google Scholar] [CrossRef]
- Maletic, M.; Leeman, A.; Szymonifka, M.; Mundt, S.S.; Zokian, H.J.; Shah, K.; Dragovic, J.; Lyons, K.; Thieringer, R.; Vosatka, A.H.; et al. Bicyclo [2.2.2]Octyltriazole Inhibitors of 11β-Hydoxysteroid Dehydrogenase Type 1. Pharmacological Agents for the Treatment of Metabolic Syndrome. Bioorg. Med. Chem. Lett. 2011, 21, 2568–2572. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, N.; Bellasio, E. Potential Antihypertensives. Synthesis of 6-Substituted-N-(4H-1,2,4-Triazol-4-Yl)-3-Pyridazinamines and 3-Substituted-6-(3,5-Dimethyl-1H-1,2,4-Triazol-1-Yl)Pyridazines. Farmaco. Sci. 1985, 40, 517–533. [Google Scholar] [CrossRef]
- Calderone, V.; Giorgi, I.; Livi, O.; Martinotti, E.; Mantuano, E.; Martelli, A.; Nardi, A. Benzoyl and/or Benzyl Substituted 1,2,3-Triazoles as Potassium Channel Activators. VIII. Eur. J. Med. Chem. 2005, 40, 521–528. [Google Scholar] [CrossRef]
- Rodriguez, L.V.; Dedet, J.P.; Paredes, V.; Mendoza, C.; Cardenas, F. A Randomized Trial of Amphotericin B Alone or in Combination with Itraconazole in the Treatment of Mucocutaneous Leishmaniasis. Mem. Inst. Oswaldo Cruz 1995, 90, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Ilango, K.; Valentina, P. Synthesis and Biological Activities of Novel 1,2,4-Triazolo-[3,4-b]-1,3,4-Thiadiazole. Der Pharma Chem. 2010, 2, 16–22. [Google Scholar]
- Jordão, A.K.; Ferreira, V.F.; Lima, E.S.; de Souza, M.C.B.V.; Carlos, E.C.L.; Castro, H.C.; Geraldo, R.B.; Rodrigues, C.R.; Almeida, M.C.B.; Cunha, A.C. Synthesis, Antiplatelet and in Silico Evaluations of Novel N-Substituted-Phenylamino-5-Methyl-1H-1,2,3-Triazole-4-Carbohydrazides. Bioorg. Med. Chem. 2009, 17, 3713–3719. [Google Scholar] [CrossRef]
- Gabryszewski, M.; Wieczorek, B. Silver (I) Complexes with 1, 2, 4-Triazole, 1-Ethyl-1, 2, 4-Triazole, 3-Amino-1, 2, 4-Triazole, 4-Amino-1, 2, 4-Triazole and 3, 5-Diamino-1, 2, 4-Triazole. Pol. J. Chem. 1998, 72, 2352–2355. [Google Scholar]
- Wang, Q.L.; Xu, H.; Hou, H.W.; Yang, G. Synthesis and Structures of Silver(I) Adducts with 4-Amino-4H-1,2,4- Triazole. Z. Naturforsch. B J. Chem. Sci. 2009, 64, 1143–1146. [Google Scholar] [CrossRef]
- Haasnoot, J.G. Mononuclear, Oligonuclear and Polynuclear Metal Coordination Compounds with 1,2,4-Triazole Derivatives as Ligands. Coord. Chem. Rev. 2000, 200–202, 131–185. [Google Scholar] [CrossRef]
- Yi, L.; Ding, B.; Zhao, B.; Cheng, P.; Liao, D.Z.; Yan, S.P.; Jiang, Z.H. Novel Triazole-Bridged Cadmium Coordination Polymers Varying from Zero- to Three-Dimensionality. Inorg. Chem. 2004, 43, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Housecroft, C.E.; Kocik, M.K.; Zampese, J.A. Photoactive Building Blocks for Coordination Complexes: Gilding 2,2′:6′,2″-Terpyridine. Polyhedron 2011, 30, 2704–2710. [Google Scholar] [CrossRef]
- Kang, Y.; Seward, C.; Song, D.; Wang, S. Blue Luminescent Rigid Molecular Rods Bearing N-7-Azaindolyl and 2,2′-Dipyridylamino and Their Zn(II) and Ag(I) Complexes. Inorg. Chem. 2003, 42, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.E.; Crespo, O.; Gimeno, M.C.; Jones, P.G.; Laguna, A.; Villacampa, M.D. Coordination Properties of the 1,1′-Bis[((6-Methyl)-2-Pyridyl)Amido]Ferrocene Ligand towards Group 11 Complexes. Dalton Trans. 2010, 39, 4321–4330. [Google Scholar] [CrossRef]
- Al-Mandhary, M.R.A.; Fitchett, C.M.; Steel, P.J.; Al-Mandhary, M.R.A.; Fitchett, C.M.; Steel, P.J. Discrete Metal Complexes of Two Multiply Armed Ligands. Aust. J. Chem. 2006, 59, 307–314. [Google Scholar] [CrossRef]
- Liu, C.S.; Chen, P.Q.; Chang, Z.; Wang, J.J.; Yan, L.F.; Sun, H.W.; Bu, X.H.; Lin, Z.; Li, Z.M.; Batten, S.R. A Photoluminescent Hexanuclear Silver(I) Complex Exhibiting C–H⋯Ag Close Interactions. Inorg. Chem. Commun. 2008, 11, 159–163. [Google Scholar] [CrossRef]
- Zhao, J.; Li, D.S.; Ke, X.J.; Liu, B.; Zou, K.; Hu, H.M. Auxiliary Ligand-Directed Structural Variation from 2D→3D Polythreaded Net to 3-Fold Interpenetrating 3D Pillar-Layered Framework: Syntheses, Crystal Structures and Magnetic Properties. Dalt. Trans. 2012, 41, 2560–2563. [Google Scholar] [CrossRef]
- Hanton, L.R.; Young, A.G. Square-Planar Silver(I)-Containing Polymers Formed from π-Stacked Entities. Cryst. Growth Des. 2006, 6, 833–835. [Google Scholar] [CrossRef]
- Liu, T.F.; Lü, J.; Cao, R. Coordination Polymers Based on Flexible Ditopic Carboxylate or Nitrogen-Donor Ligands. CrystEngComm 2010, 12, 660–670. [Google Scholar] [CrossRef]
- Yoon, I.; Lee, Y.H.; Jung, J.H.; Park, K.M.; Kim, J.; Lee, S.S. Assembly of a Tennis Ball-like Supramolecule Coordinatively Encapsulating Disilver. Inorg. Chem. Commun. 2002, 5, 820–823. [Google Scholar] [CrossRef]
- Njogu, E.M.; Omondi, B.; Nyamori, V.O. Review: Multimetallic Silver(I)–Pyridinyl Complexes: Coordination of Silver(I) and Luminescence. J. Coord. Chem. 2015, 68, 3389–3431. [Google Scholar] [CrossRef]
- Rogovoy, M.I.; Frolova, T.S.; Samsonenko, D.G.; Berezin, A.S.; Bagryanskaya, I.Y.; Nedolya, N.A.; Tarasova, O.A.; Fedin, V.P.; Artem’ev, A.V. 0D to 3D Coordination Assemblies Engineered on Silver(I) Salts and 2-(Alkylsulfanyl)Azine Ligands: Crystal Structures, Dual Luminescence, and Cytotoxic Activity. Eur. J. Inorg. Chem. 2020, 2020, 1635–1644. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic Interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef]
- Lippert, B. Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug; Helvetica Chimica Acta: Zürich, Switzerland, 2006; pp. 1–563. [Google Scholar] [CrossRef]
- Adams, M.; Kerby, I.J.; Rocker, I.; Evans, A.; Johansen, K.; Franks, C.R. A Comparison of the Toxicity and Efficacy of Cisplatin and Carboplatin in Advanced Ovarian Cancer. The Swons Gynaecological Cancer Group. Acta Oncol. 1989, 28, 57–60. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Piccart, M.J.; Lamb, H.; Vermorken, J.B. Current and Future Potential Roles of the Platinum Drugs in the Treatment of Ovarian Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001, 12, 1195–1203. [Google Scholar] [CrossRef]
- El-Naggar, M.A.; Albering, J.H.; Barakat, A.; Abu-Youssef, M.A.M.; Soliman, S.M.; Badr, A.M.A. New Bioactive 1D Ag(I) Coordination Polymers with Pyrazole and Triazine Ligands; Synthesis, X-Ray Structure, Hirshfeld Analysis and DFT Studies. Inorg. Chim. Acta 2022, 537, 120948. [Google Scholar] [CrossRef]
- Ali, K.A.; Abd-Elzaher, M.M.; Mahmoud, K. Synthesis and Anticancer Properties of Silver(I) Complexes Containing 2,6-Bis(Substituted)Pyridine Derivatives. Int. J. Med. Chem. 2013, 2013, 256836. [Google Scholar] [CrossRef] [PubMed]
- Banti, C.N.; Hadjikakou, S.K. Anti-Proliferative and Anti-Tumor Activity of Silver(i) Compounds. Metallomics 2013, 5, 569–596. [Google Scholar]
- Zaki, M.; Arjmand, F.; Tabassum, S. Current and Future Potential of Metallo Drugs: Revisiting DNA-Binding of Metal Containing Molecules and Their Diverse Mechanism of Action. Inorg. Chim. Acta 2016, 444, 1–22. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble Metals in Medicine: Latest Advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Yousri, A.; Haukka, M.; Abu-Youssef, M.A.M.; Ayoup, M.S.; Ismail, M.M.F.; El Menofy, N.G.; Soliman, S.M.; Barakat, A.; Noa, F.M.A.; Öhrström, L. Synthesis, Structure Diversity, and Antimicrobial Studies of Ag(I) Complexes with Quinoline-Type Ligands. CrystEngComm 2023, 25, 3922–3930. [Google Scholar] [CrossRef]
- El-Naggar, M.A.; Abu-Youssef, M.A.M.; Haukka, M.; Barakat, A.; Sharaf, M.M.; Soliman, S.M. Synthesis, X-ray Structure, and Hirshfeld Analysis of [Ag(3-amino-5,6-dimethyl-1,2,4-triazine)(NO3)]n: A Potent Anticancer and Antimicrobial Agent. Inorganics 2023, 11, 350. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Oxfordshire, UK, 2020. [Google Scholar]
- Sheldrick, G.M. Shelxt–integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]


| Bond | Distance | Bond | Distance |
|---|---|---|---|
| Ag(1)–N(4) | 2.1375(9) | Ag(2)–N(8) | 2.2184(9) |
| Ag(1)–N(5) | 2.1407(9) | Ag(2)–O(1) | 2.5044(9) |
| Ag(2)–N(3)#1 | 2.2087(9) | Ag(2)–O(2) | 2.5202(9) |
| Bonds | Angle | Bonds | Angle |
| N(4)–Ag(1)–N(5) | 171.52(4) | N(3)#1–Ag(2)–O(2) | 127.71(3) |
| N(3)#1–Ag(2)–N(8) | 132.98(3) | N(8)–Ag(2)–O(2) | 95.06(3) |
| N(3)#1–Ag(2)–O(1) | 100.41(3) | O(1)–Ag(2)–O(2) | 51.31(3) |
| N(8)–Ag(2)–O(1) | 123.76(3) |
| D–H···A | d(D–H) | d(H···A) | d(D···A) | <(DHA) |
|---|---|---|---|---|
| C(2)–H(2)···O(4)#1 | 0.95 | 2.34 | 3.0520(15) | 131.2 |
| N(2)–H(2A)···O(4)#2 | 0.85(2) | 2.12(2) | 2.9308(15) | 160.5(19) |
| N(2)–H(2B)···O(5)#3 | 0.876(19) | 2.281(19) | 3.0142(14) | 141.2(17) |
| C(3)–H(3)···O(3)#3 | 0.95 | 2.36 | 3.2420(13) | 154.7 |
| N(7)–H(7A)···O(6)#4 | 0.828(19) | 2.301(19) | 3.0714(15) | 154.9(17) |
| N(7)–H(7B)···O(6)#5 | 0.878(19) | 2.119(19) | 2.9295(14) | 153.1(16) |
| C(1)–H(1)···O(3)#6 | 0.95 | 2.4 | 3.3135(14) | 160.3 |
| C(4)–H(4)···O(5) | 0.95 | 2.29 | 3.1399(14) | 147.9 |
| Compound | A-549 | MCF-7 |
|---|---|---|
| [Ag2(L)2(NO3)]n(NO3)n | 3.50 ± 0.37 | 2.98 ± 0.26 |
| L | 366.99 ± 13.94 | 270.39 ± 11.86 |
| AgNO3 | 14.70 ± 0.53 | 2.81 ± 0.97 |
| cis-Platin | 7.5 ± 0.69 | 4.59 ± 0.53 |
| Compound | Gram-Positive Bacteria | Gram-Negatvie Bacteria | Fungi | |||
|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. vulgaris | A. fumigatus | C. albicans | |
| [Ag2(L)2(NO3)]n(NO3)n. | 30.5 | 64 | 6.1 | 32 | ND | 156 |
| AgNO3 | 64 | 312.5 | 32 | 156 | ND | 128 |
| L | ND | ND | ND | ND | ND | ND |
| Control | 9.7 a | 4.8 a | 4.8 a | 4.8 a | 156.25 b | 312.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Naggar, M.A.; Al-Rasheed, H.H.; AL-khamis, S.A.; El-Faham, A.; Abu-Youssef, M.A.M.; Haukka, M.; Barakat, A.; Sharaf, M.M.; Soliman, S.M. Synthesis and X-ray Structure Analysis of the Polymeric [Ag2(4-Amino-4H-1,2,4-triazole)2(NO3)]n(NO3)n Adduct: Anticancer, and Antimicrobial Applications. Inorganics 2023, 11, 395. https://doi.org/10.3390/inorganics11100395
El-Naggar MA, Al-Rasheed HH, AL-khamis SA, El-Faham A, Abu-Youssef MAM, Haukka M, Barakat A, Sharaf MM, Soliman SM. Synthesis and X-ray Structure Analysis of the Polymeric [Ag2(4-Amino-4H-1,2,4-triazole)2(NO3)]n(NO3)n Adduct: Anticancer, and Antimicrobial Applications. Inorganics. 2023; 11(10):395. https://doi.org/10.3390/inorganics11100395
Chicago/Turabian StyleEl-Naggar, Mostafa A., Hessa H. Al-Rasheed, Sarah A. AL-khamis, Ayman El-Faham, Morsy A. M. Abu-Youssef, Matti Haukka, Assem Barakat, Mona M. Sharaf, and Saied M. Soliman. 2023. "Synthesis and X-ray Structure Analysis of the Polymeric [Ag2(4-Amino-4H-1,2,4-triazole)2(NO3)]n(NO3)n Adduct: Anticancer, and Antimicrobial Applications" Inorganics 11, no. 10: 395. https://doi.org/10.3390/inorganics11100395
APA StyleEl-Naggar, M. A., Al-Rasheed, H. H., AL-khamis, S. A., El-Faham, A., Abu-Youssef, M. A. M., Haukka, M., Barakat, A., Sharaf, M. M., & Soliman, S. M. (2023). Synthesis and X-ray Structure Analysis of the Polymeric [Ag2(4-Amino-4H-1,2,4-triazole)2(NO3)]n(NO3)n Adduct: Anticancer, and Antimicrobial Applications. Inorganics, 11(10), 395. https://doi.org/10.3390/inorganics11100395










