Polymeric Protection for Silver Nanowire-Based Transparent Conductive Electrodes: Performance and Applications
Abstract
:1. Introduction
2. Diverse Polymeric Overcoating
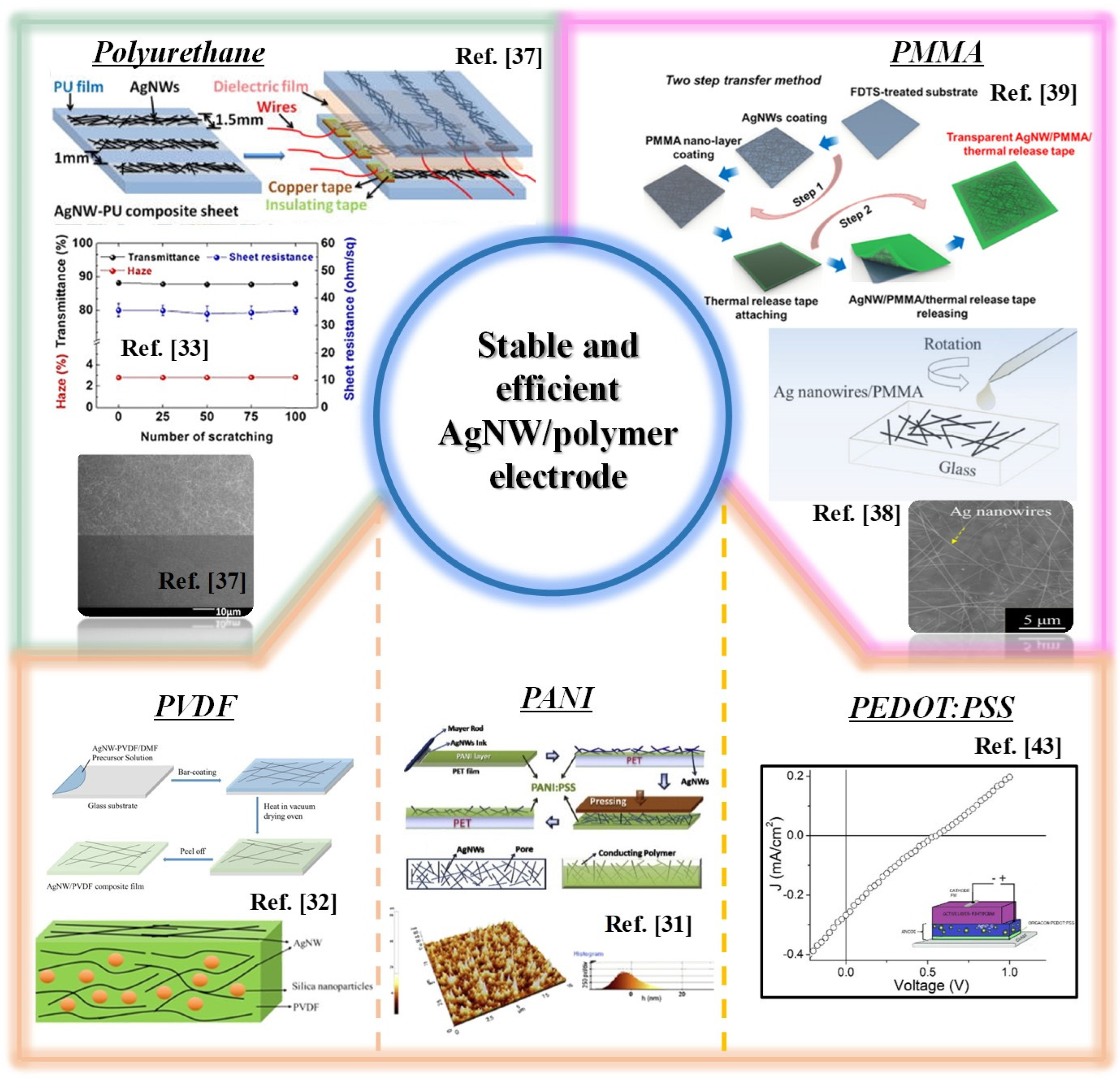
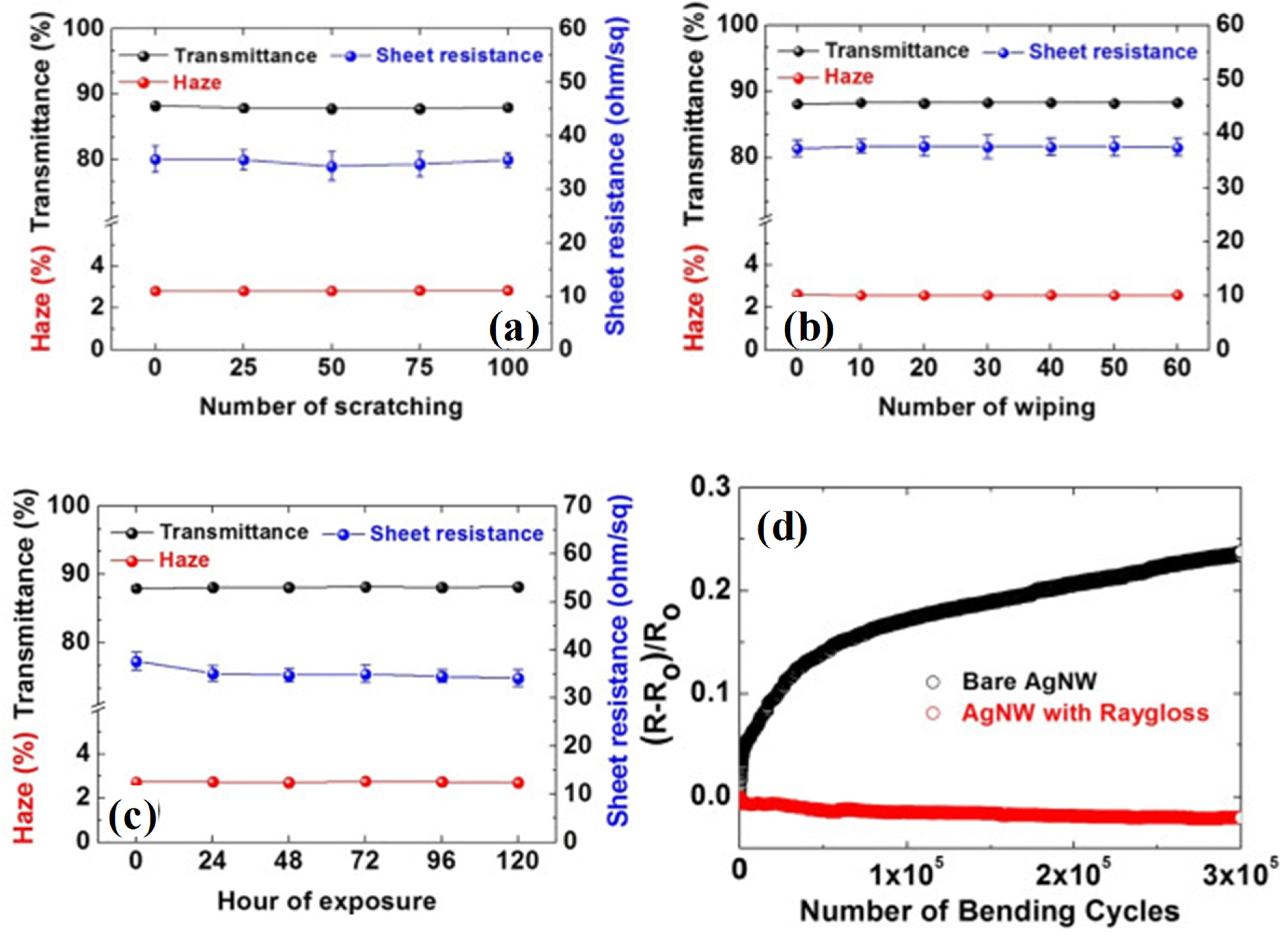
2.1. Polyurethane (PU)/AgNW
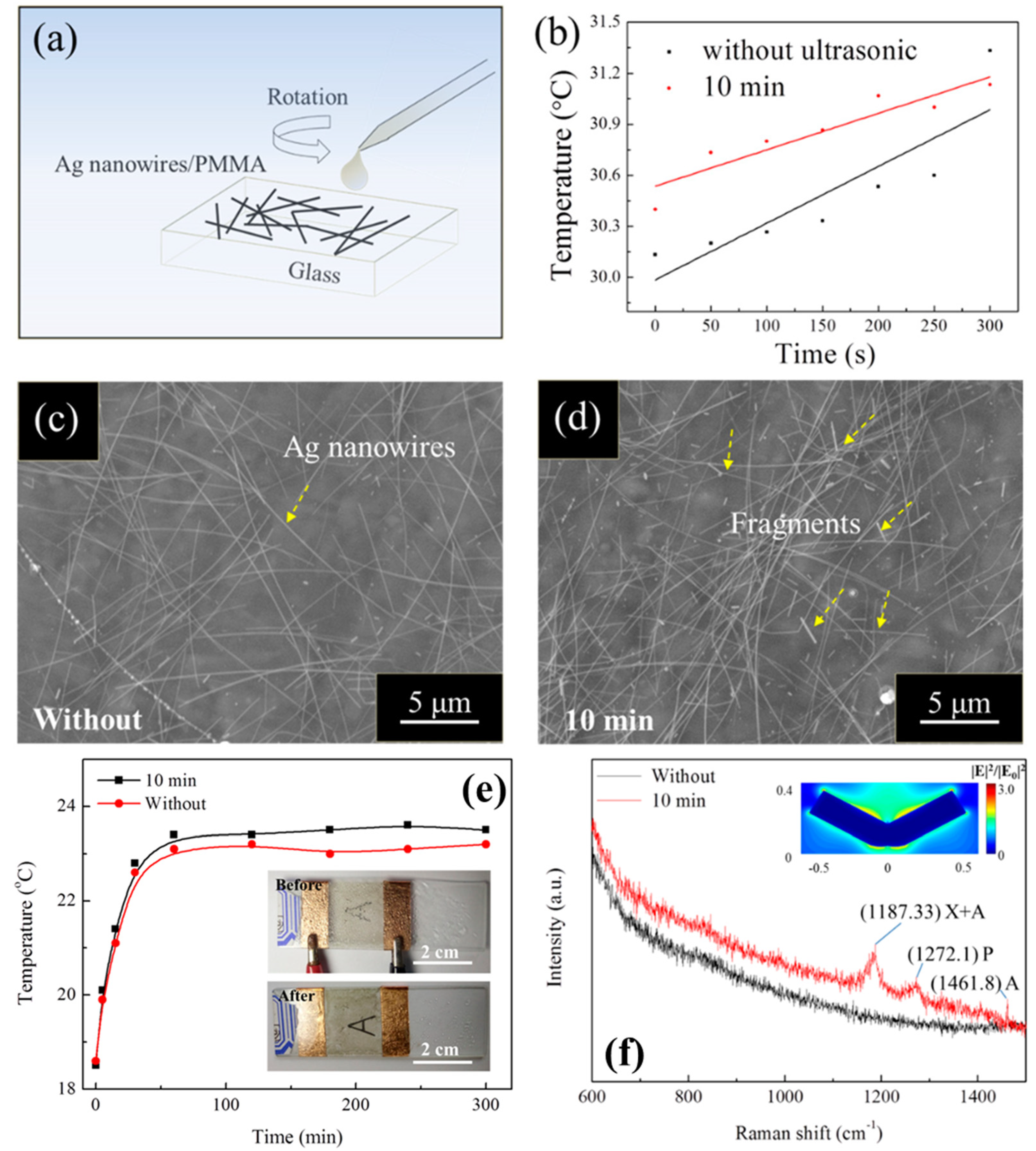
2.2. Polymethyl Methacrylate (PMMA) on AgNW
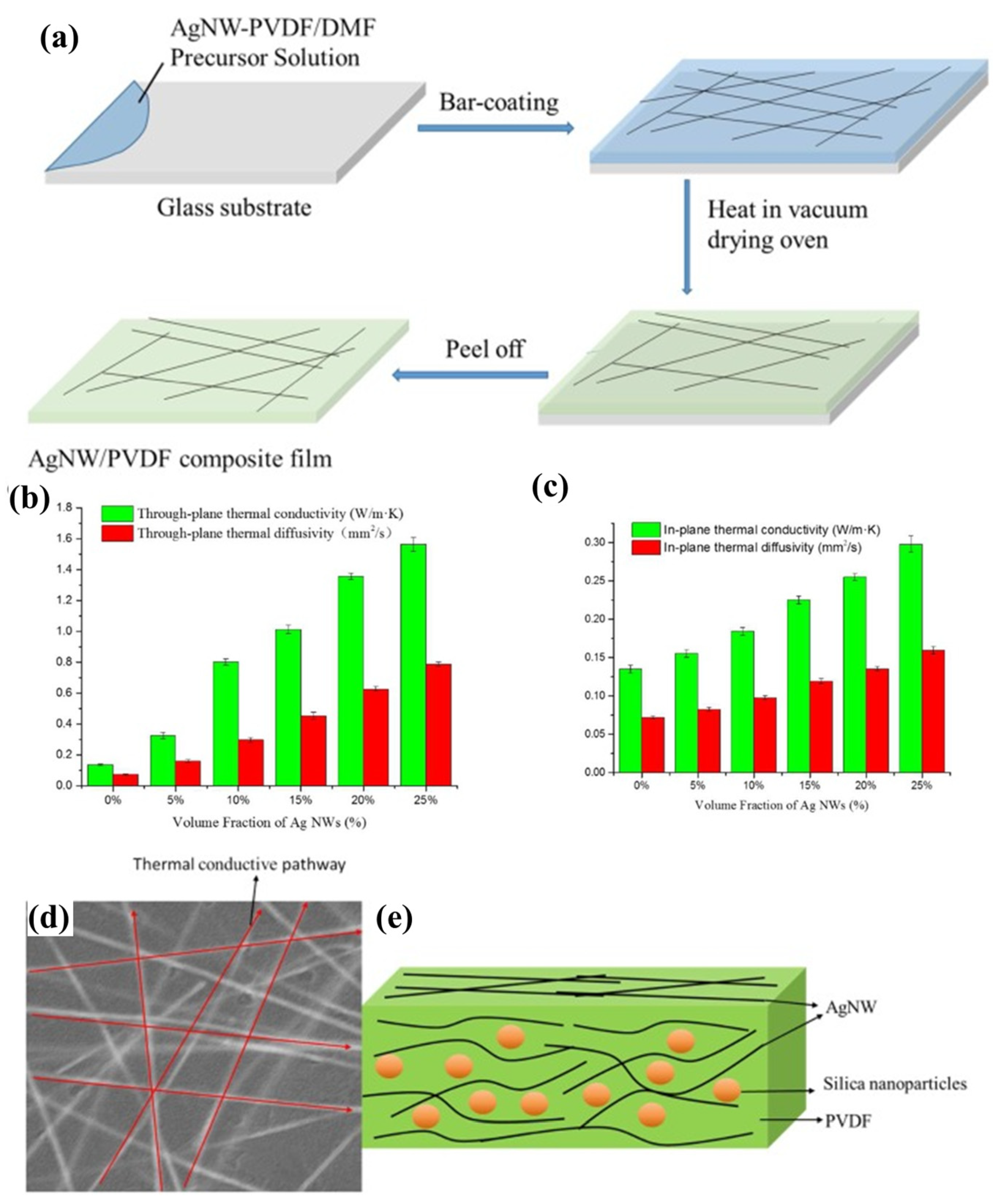
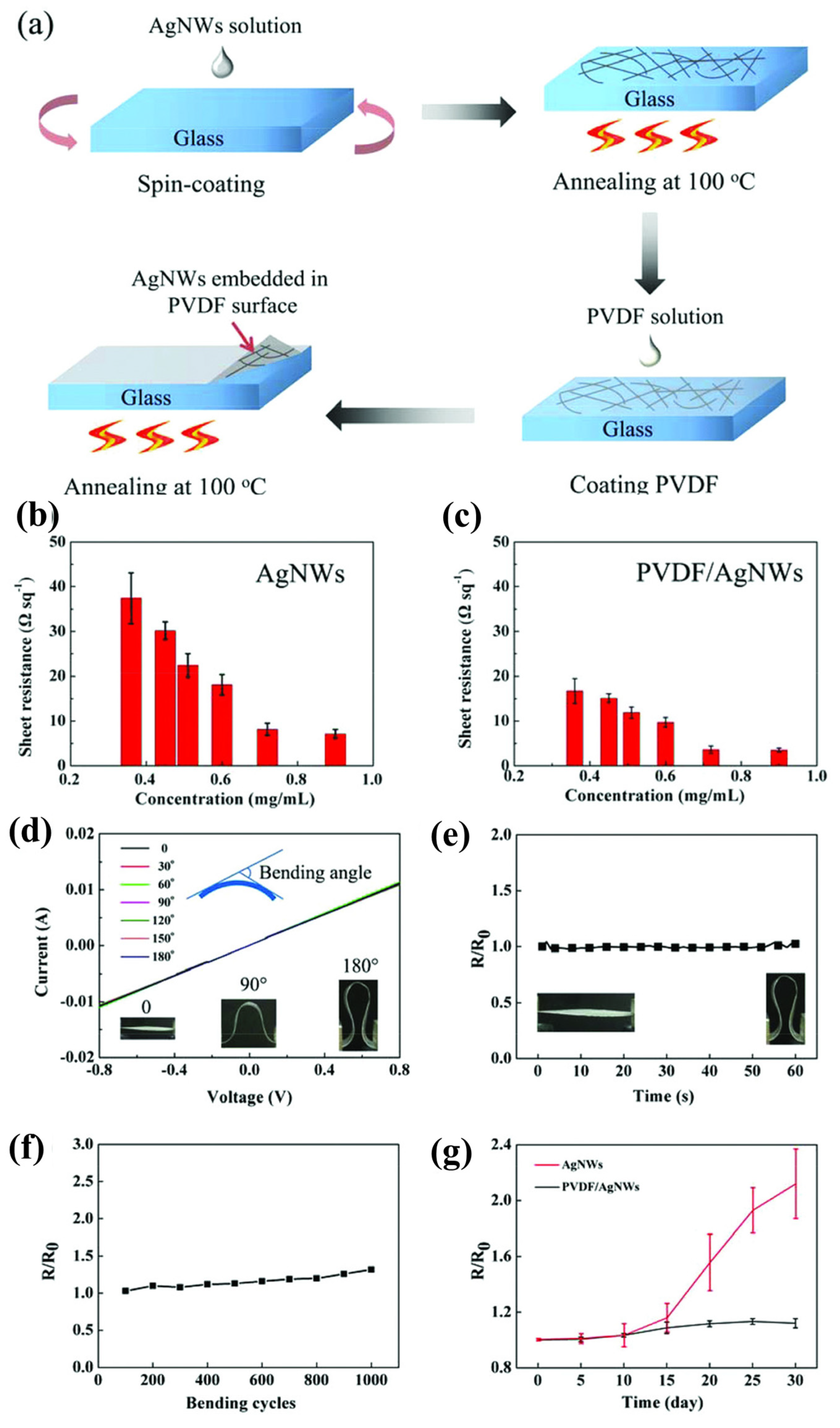
2.3. Polyvinylidene Difluoride (PVDF) on AgNW
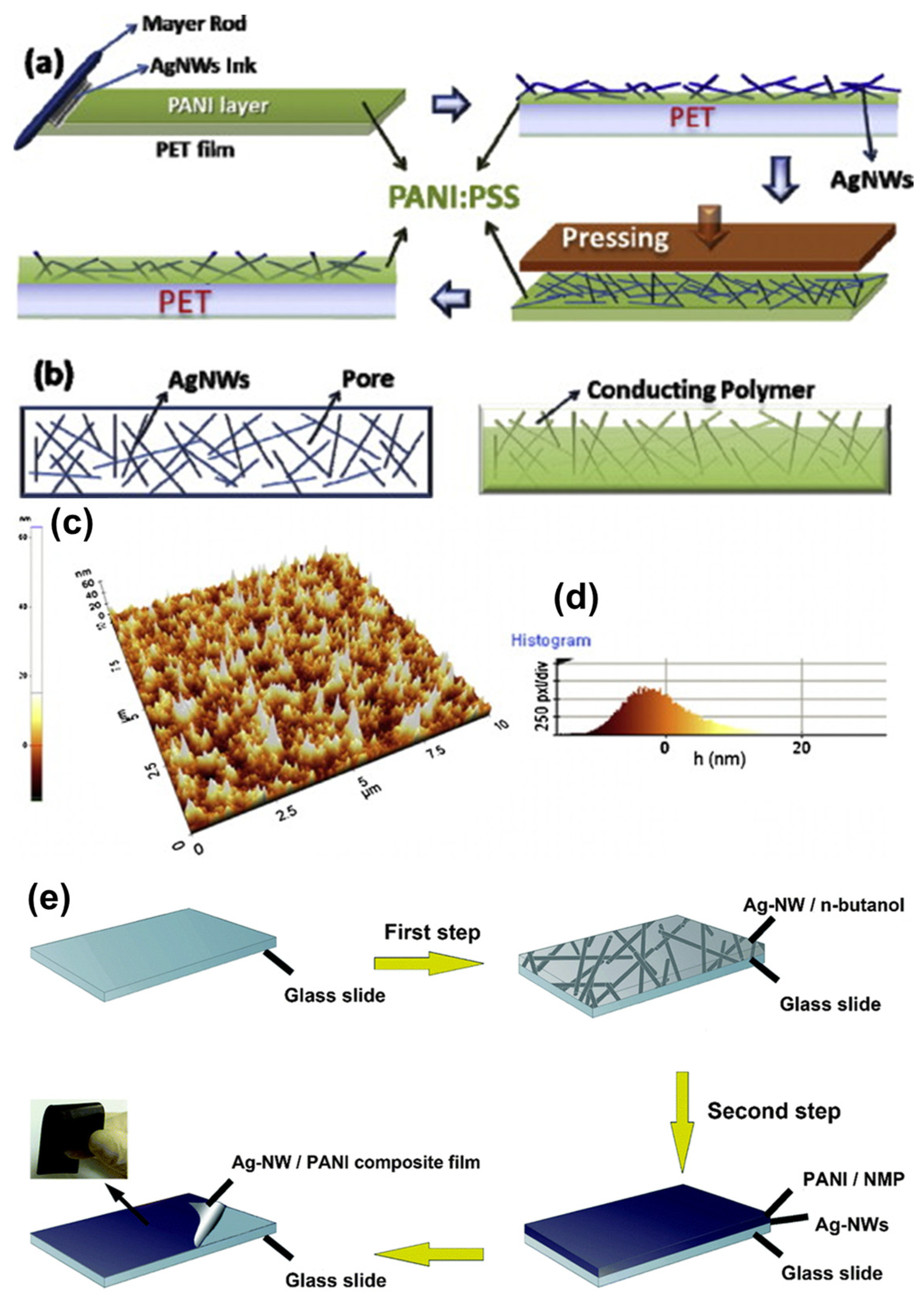
2.4. Polyaniline (PANI)/AgNW
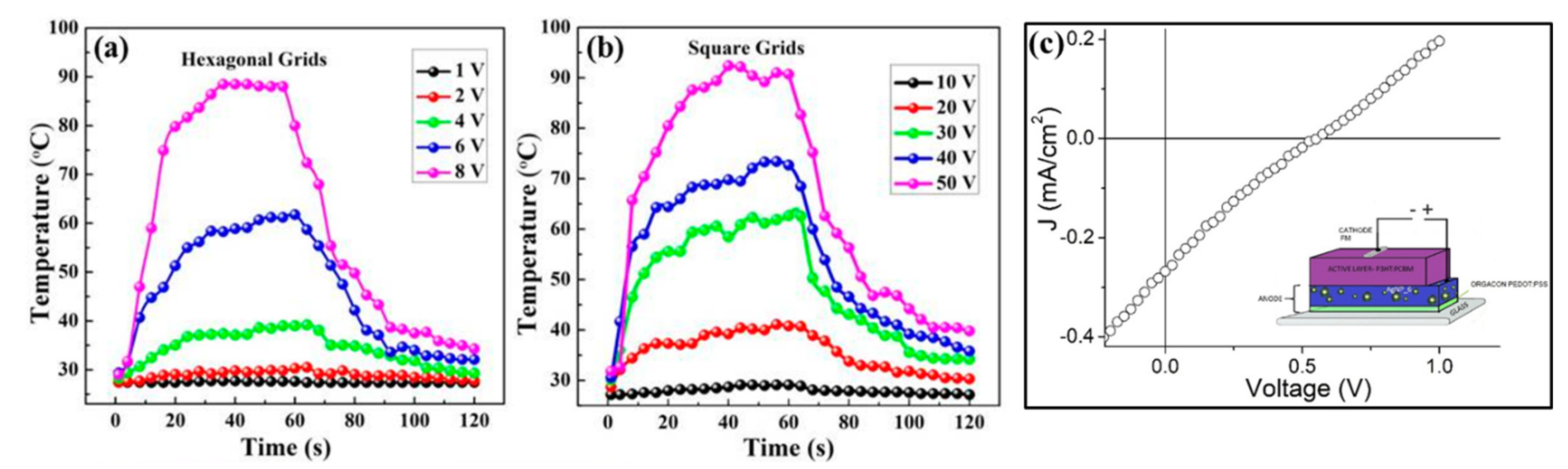
2.5. Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate (PEDOT:PSS)/AgNW
2.6. Polyimide (PI)/AgNW
3. Challenges and Future Perspectives
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Lu, K.; Xu, Z.; Lin, Z.; Ning, H.; Qiu, T.; Yang, Z.; Zheng, H.; Yao, R.; Peng, J. Recent Developments in Flexible Transparent Electrode. Crystals 2021, 11, 511. [Google Scholar] [CrossRef]
- Pang, S.; Hernandez, Y.; Feng, X.; Müllen, K. Graphene as Transparent Electrode Material for Organic Electronics. Adv. Mater. 2011, 23, 2779–2795. [Google Scholar] [CrossRef]
- Morales-Masis, M.; De Wolf, S.; Woods-Robinson, R.; Ager, J.W.; Ballif, C. Transparent Electrodes for Efficient Optoelectronics. Adv. Electron. Mater. 2017, 3, 1600529. [Google Scholar] [CrossRef]
- Kim, H.; Qaiser, N.; Hwang, B. Electro-mechanical response of stretchable pdms composites with a hybrid filler system. Facta Univ. Ser. Mech. Eng. 2023, 21, 51–61. [Google Scholar] [CrossRef]
- Jin, I.S.; Lee, H.D.; Hong, S.I.; Lee, W.; Jung, J.W. Facile Post Treatment of Ag Nanowire/Polymer Composites for Flexible Transparent Electrodes and Thin Film Heaters. Polymers 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Lee, S.; Hee Lee, D.; Hee Park, I.; Seong Bae, J.; Woo Lee, T.; Kim, J.-Y.; Hun Park, J.; Chan Cho, Y.; Ryong Cho, C.; et al. Cu Mesh for Flexible Transparent Conductive Electrodes. Sci. Rep. 2015, 5, 10715. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Liu, Y.; Huang, W.; Qiao, W.; Zhou, Y.; Ye, Y.; Chen, L.-S. Towards Flexible Transparent Electrodes Based on Carbon and Metallic Materials. Micromachines 2017, 8, 12. [Google Scholar] [CrossRef]
- Xie, H.; Yang, X.; Du, D.; Zhao, Y.; Wang, Y. Flexible Transparent Conductive Film Based on Random Networks of Silver Nanowires. Micromachines 2018, 9, 295. [Google Scholar] [CrossRef]
- Tokuno, T.; Nogi, M.; Karakawa, M.; Jiu, J.; Nge, T.T.; Aso, Y.; Suganuma, K. Fabrication of silver nanowire transparent electrodes at room temperature. Nano Res. 2011, 4, 1215–1222. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, Y.; Lee, Y. Improved Thermal Oxidation Stability of Solution-Processable Silver Nanowire Transparent Electrode by Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2012, 4, 6410–6414. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, Z.; Li, J.; Jiang, F.; Zhang, K.; Zhang, Y.; Zhou, Y.; Zhou, J.; Hu, B. High-Performance Hazy Silver Nanowire Transparent Electrodes through Diameter Tailoring for Semitransparent Photovoltaics. Adv. Funct. Mater. 2018, 28, 1705409. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, C.S.; Jo, S. Spray Deposition of Ag Nanowire–Graphene Oxide Hybrid Electrodes for Flexible Polymer–Dispersed Liquid Crystal Displays. Materials 2018, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, P.; Bahrami, A.; Mehr, M.Y.; van Driel, W.D.; Zhang, K. Facile Synthesis of Ag Nanowire/TiO2 and Ag Nanowire/TiO2/GO Nanocomposites for Photocatalytic Degradation of Rhodamine B. Materials 2012, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Nam, V.B.; Lee, D. Copper Nanowires and Their Applications for Flexible, Transparent Conducting Films: A Review. Nanomaterials 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.; Han, Y.; Matteini, P. Bending fatigue behavior of ag nanowire/cu thin-film hybrid interconnects for wearable electronics. Facta Univ. Ser. Mech. Eng. 2022, 20, 553–560. [Google Scholar] [CrossRef]
- Hwang, B.; Matteini, P. Research Trends on Silk-Based Conductive Fibers with the Enhanced Machine Washa-bility by Adopting PEDOT:PSS. J. Nat. Fibers 2023, 20, 2148152. [Google Scholar] [CrossRef]
- Limkatanyu, S.; Sae-Long, W.; Rungamornrat, J.; Buachart, C.; Sukontasukkul, P.; Keawsawasvong, S.; Chindaprasirt, P. Bending, buckling and free vibration analyses of nanobeam-substrate medium systems. Facta Univ. Ser. Mech. Eng. 2022, 20, 561–587. [Google Scholar] [CrossRef]
- Ha, H.; Müller, S.; Baumann, R.-P.; Hwang, B. Peakforce quantitative nanomechanical mapping for surface energy characterization on the nanoscale: A mini-review. Facta Univ. Ser. Mech. Eng. 2023. [Google Scholar] [CrossRef]
- Seo, Y.; Ha, H.; Cheong, J.Y.; Leem, M.; Darabi, S.; Matteini, P.; Müller, C.; Yun, T.G.; Hwang, B. Highly Reliable Yarn-Type Supercapacitor Using Conductive Silk Yarns with Multilayered Active Materials. J. Nat. Fibers 2021, 19, 835–846. [Google Scholar] [CrossRef]
- Shi, Y.; He, L.; Deng, Q.; Liu, Q.; Li, L.; Wang, W.; Xin, Z.; Liu, R. Synthesis and Applications of Silver Nanowires for Transparent Conductive Films. Micromachines 2019, 10, 330. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Xu, Y.; Wu, L.; Yu, A.; Lai, G.; Wei, Q.; Chi, H.; Jiang, N.; Fu, L.; et al. Intertwined Carbon Nanotubes and Ag Nanowires Constructed by Simple Solution Blending as Sensitive and Stable Chloramphenicol Sensors. Sensors 2021, 21, 1220. [Google Scholar] [CrossRef] [PubMed]
- Im, H.-G.; Jin, J.; Ko, J.-H.; Lee, J.; Lee, J.-Y.; Bae, B.-S. Flexible transparent conducting composite films using a monolithically embedded AgNW electrode with robust performance stability. Nanoscale 2013, 6, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Won, Y.; Woo, K.; Kim, C.-H.; Moon, J. Highly Transparent Low Resistance ZnO/Ag Nanowire/ZnO Composite Electrode for Thin Film Solar Cells. ACS Nano 2013, 7, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Shaikh, M.O.; Chuang, C.-H. Silver Nanowire Synthesis and Strategies for Fabricating Transparent Conducting Electrodes. Nanomaterials 2021, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Y.; Lee, E.-S.; Oh, Y.-J.; Kang, H.W. A silver nanowire mesh overcoated protection layer with graphene oxide as a transparent electrode for flexible organic solar cells. RSC Adv. 2017, 7, 52914–52922. [Google Scholar] [CrossRef]
- Jang, J.; Choi, J.-Y.; Jeon, J.; Lee, J.; Im, J.; Lee, J.; Jin, S.-W.; Park, H.-J.; Lee, S.-H.; Kim, D.-B.; et al. Flexible Transparent Electrode Characteristics of Graphene Oxide/Cysteamine/AgNP/AgNW Structure. Nanomaterials 2020, 10, 2352. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kang, S.; Pandya, A.; Shanker, R.; Khan, Z.; Lee, Y.; Park, J.; Craig, S.L.; Ko, H. Large-Area Cross-Aligned Silver Nanowire Electrodes for Flexible, Transparent, and Force-Sensitive Mechanochromic Touch Screens. ACS Nano 2017, 11, 4346–4357. [Google Scholar] [CrossRef] [PubMed]
- Bari, B.; Lee, J.; Jang, T.; Won, P.; Ko, S.H.; Alamgir, K.; Arshad, M.; Guo, L.J. Simple hydrothermal synthesis of very-long and thin silver nanowires and their application in high quality transparent electrodes. J. Mater. Chem. A 2016, 4, 11365–11371. [Google Scholar] [CrossRef]
- Lian, L.; Dong, D.; Feng, D.; He, G. Low roughness silver nanowire flexible transparent electrode by low temper-ature solution-processing for organic light emitting diodes. Org. Electron. 2017, 49, 9–18. [Google Scholar] [CrossRef]
- Xiong, W.; Liu, H.; Chen, Y.; Zheng, M.; Zhao, Y.; Kong, X.; Wang, Y.; Zhang, X.; Kong, X.; Wang, P.; et al. Highly Conductive, Air-Stable Silver Nanowire@Iongel Composite Films toward Flexible Transparent Electrodes. Adv. Mater. 2016, 28, 7167–7172. [Google Scholar] [CrossRef]
- Fang, F.; Li, Y.-Q.; Xiao, H.-M.; Hu, N.; Fu, S.-Y. Layer-structured silver nanowire/polyaniline composite film as a high performance X-band EMI shielding material. J. Mater. Chem. C 2016, 4, 4193–4203. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Qi, R.; Xie, F.; Qi, S. Improvement of the thermal transport performance of a poly(vinylidene fluoride) composite film including silver nanowire. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Hwang, B.; An, C.-H.; Becker, S. Highly robust Ag nanowire flexible transparent electrode with UV-curable polyurethane-based overcoating layer. Mater. Des. 2017, 129, 180–185. [Google Scholar] [CrossRef]
- Hwang, B.; Qaiser, N.; Lee, C.; Matteini, P.; Yoo, S.J.; Kim, H. Effect of Al2O3/Alucone nanolayered composite overcoating on reliability of Ag nanowire electrodes under bending fatigue. J. Alloys Compd. 2020, 846, 156420. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.; Ahn, Y.; Jeong, Y.; Lee, D.-Y.; Lee, Y. Highly stable and flexible silver nanowire–graphene hybrid transparent conducting electrodes for emerging optoelectronic devices. Nanoscale 2013, 5, 7750–7755. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jian, M.; Zhang, C.; Wu, M.; Ling, X.; Zhang, J.; Wei, B.; Yang, L. Highly Stable Graphene-Based Flexible Hybrid Transparent Conductive Electrodes for Organic Solar Cells. Adv. Mater. Interfaces 2021, 9, 2101442. [Google Scholar] [CrossRef]
- Hu, W.; Niu, X.; Zhao, R.; Pei, Q. Elastomeric transparent capacitive sensors based on an interpenetrating composite of silver nanowires and polyurethane. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Li, Z.; Zhao, J.; Zhu, P.; Dong, X.; Yu, Z.; Zhao, Z.; Shi, D.; Wang, J.; et al. Ultrasonic Modification of Ag Nanowires and Their Applications in Flexible Transparent Film Heaters and SERS Detectors. Materials 2019, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Kim, D.; Kim, B.; Chae, H.; Yi, H.; Hwang, B. High-Performance Transparent Quantum Dot Light-Emitting Diode with Patchable Transparent Electrodes. ACS Appl. Mater. Interfaces 2019, 11, 26333–26338. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Gui, J.; Wang, X.; Li, R.; Liu, W.; Sun, C.; Zhao, X.; Guo, S. Efficient Welding of Silver Nanowires embedded in a Poly(vinylidene fluoride) Film for Robust Wearable Electronics. Adv. Mater. Technol. 2018, 4, 1800438. [Google Scholar] [CrossRef]
- Kumar, A.B.V.K.; Jiang, J.; Bae, C.W.; Seo, D.M.; Piao, L.; Kim, S.-H. Silver nanowire/polyaniline composite trans-parent electrode with improved surface properties. Mater. Res. Bull. 2014, 57, 52–57. [Google Scholar] [CrossRef]
- He, X.; Shen, G.; Xu, R.; Yang, W.; Zhang, C.; Liu, Z.; Chen, B.; Liu, J.; Song, M. Hexagonal and Square Patterned Silver Nanowires/PEDOT:PSS Composite Grids by Screen Printing for Uniformly Transparent Heaters. Polymers 2019, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Zappia, S.; Alloisio, M.; Valdivia, J.C.; Arias, E.; Moggio, I.; Scavia, G.; Destri, S. Silver Nanoparticle–PEDOT:PSS Composites as Water-Processable Anodes: Correlation between the Synthetic Parameters and the Optical/Morphological Properties. Polymers 2023, 15, 3675. [Google Scholar] [CrossRef]
- Li, X.; Park, H.; Lee, M.H.; Hwang, B.; Kim, S.H.; Lim, S. High resolution patterning of Ag nanowire flexible trans-parent electrode via electrohydrodynamic jet printing of acrylic polymer-silicate nanoparticle composite overcoating layer. Org. Electron. 2018, 62, 400–406. [Google Scholar] [CrossRef]
- Yang, H.; Bai, S.; Guo, X.; Wang, H. Robust and smooth UV-curable layer overcoated AgNW flexible transparent conductor for EMI shielding and film heater. Appl. Surf. Sci. 2019, 483, 888–894. [Google Scholar] [CrossRef]
- Chen, S.; Song, L.; Tao, Z.; Shao, X.; Huang, Y.; Cui, Q.; Guo, X. Neutral-pH PEDOT:PSS as over-coating layer for stable silver nanowire flexible transparent conductive films. Org. Electron. 2014, 15, 3654–3659. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, K.Y.; Kim, S.W. Ultra-bendable and durable Graphene–Urethane composite/silver nanowire film for flexible transparent electrodes and electromagnetic-interference shielding. Compos. Part B Eng. 2019, 177, 107406. [Google Scholar] [CrossRef]
- Tiwari, N.; Ankit, A.; Rajput, M.; Kulkarni, M.R.; John, R.A.; Mathews, N. Healable and flexible transparent heaters. Nanoscale 2017, 9, 14990–14997. [Google Scholar] [CrossRef]
- Yu, S.; Ma, X.; Li, X.; Li, J.; Gong, B.; Wang, X. Enhanced adhesion of Ag nanowire based transparent conducting electrodes for application in flexible electrochromic devices. Opt. Mater. 2021, 120, 111414. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, Y.; Yi, P.; Peng, L.; Lai, X.; Lin, Z. Flexible Transparent Electrodes Based on Silver Nanowires: Mate-rial Synthesis, Fabrication, Performance, and Applications. Adv. Mater. Technol. 2019, 4, 1900413. [Google Scholar] [CrossRef]
- Khadtare, S.; Ko, E.J.; Kim, Y.H.; Lee, H.S.; Moon, D.K. A flexible piezoelectric nanogenerator using conducting polymer and silver nanowire hybrid electrodes for its application in real-time muscular monitoring system. Sens. Actuators A Phys. 2019, 299, 111575. [Google Scholar] [CrossRef]
- Bobinger, M.; Keddis, S.; Hinterleuthner, S.; Becherer, M.; Kluge, F.; Schwesinger, N.; Salmeron, J.F.; Lugli, P.; Rivadeneyra, A. Light and Pressure Sensors Based on PVDF With Sprayed and Transparent Electrodes for Self-Powered Wireless Sensor Nodes. IEEE Sens. J. 2018, 19, 1114–1126. [Google Scholar] [CrossRef]
- Basarir, F.; Madani, Z.; Vapaavuori, J. Recent Advances in Silver Nanowire Based Flexible Capacitive Pressure Sensors: From Structure, Fabrication to Emerging Applications. Adv. Mater. Interfaces 2022, 9, 2200866. [Google Scholar] [CrossRef]
- Tan, Q.; Yuan, L.; Liang, G.; Gu, A. Flexible, transparent, strong and high dielectric constant composite film based on polyionic liquid coated silver nanowire hybrid. Appl. Surf. Sci. 2021, 576, 151827. [Google Scholar] [CrossRef]
- Bobinger, M.; Hinterleuthner, S.; Becherer, M.; Keddis, S.; Schwesinger, N.; Lugli, P. Energy harvesting from ambient light using PVDF with highly conductive and transparent silver nanowire/PEDOT:PSS hybride electrodes. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 426–429. [Google Scholar]
- Gao, D.; Zhao, P.; Liu, J.; Zhou, Y.; Lyu, B.; Ma, J.; Shao, L. Polyaniline/silver nanowire cotton fiber: A flexible electrode material for supercapacitor. Adv. Powder Technol. 2021, 32, 3954–3963. [Google Scholar] [CrossRef]
- Fang, F.; Huang, G.-W.; Xiao, H.-M.; Li, Y.-Q.; Hu, N.; Fu, S.-Y. Largely enhanced electrical conductivity of layer-structured silver nanowire/polyimide composite films by polyaniline. Compos. Sci. Technol. 2018, 156, 144–150. [Google Scholar] [CrossRef]
- Che, B.; Zhou, D.; Li, H.; He, C.; Liu, E.; Lu, X. A highly bendable transparent electrode for organic electrochromic devices. Org. Electron. 2018, 66, 86–93. [Google Scholar] [CrossRef]
- Bai, S.; Guo, X.; Chen, T.; Zhang, Y.; Zhang, X.; Yang, H.; Zhao, X. Solution processed fabrication of silver nanowire-MXene@PEDOT: PSS flexible transparent electrodes for flexible organic light-emitting diodes. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106088. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Feng, J.; Ou, X.-L.; Cui, H.-F.; Xu, M.; Sun, H.-B. Ultrasmooth, highly conductive and transparent PE-DOT:PSS/silver nanowire composite electrode for flexible organic light-emitting devices. Org. Electron. 2016, 31, 247–252. [Google Scholar] [CrossRef]
- Nair, N.M.; Pakkathillam, J.K.; Kumar, K.; Arunachalam, K.; Ray, D.; Swaminathan, P. Printable Silver Nanowire and PEDOT:PSS Nanocomposite Ink for Flexible Transparent Conducting Applications. ACS Appl. Electron. Mater. 2020, 2, 1000–1010. [Google Scholar] [CrossRef]
- Ghosh, D.S.; Chen, T.L.; Mkhitaryan, V.; Pruneri, V. Ultrathin Transparent Conductive Polyimide Foil Embedding Silver Nanowires. ACS Appl. Mater. Interfaces 2014, 6, 20943–20948. [Google Scholar] [CrossRef]
- Huang, Q.; Shen, W.; Fang, X.; Chen, G.; Guo, J.; Xu, W.; Tan, R.; Song, W. Highly flexible and transparent film heaters based on polyimide films embedded with silver nanowires. RSC Adv. 2015, 5, 45836–45842. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Zhang, G.; Xiao, C.; Wei, Y.; Li, W. Ultrathin Flexible Transparent Composite Electrode via Semi-embedding Silver Nanowires in a Colorless Polyimide for High-Performance Ultraflexible Organic Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 5699–5708. [Google Scholar] [CrossRef]


| Polymeric Materials | Conductivity | Optical Transmittance | Mechanical Stability | Chemical Stability | Applications | Refs. |
|---|---|---|---|---|---|---|
| PU | ~50 Ω/sq | ~88% | Excellent | Excellent | Transparent electrode | [33] |
| ~8 Ω/sq | ~74.6% | Excellent | N/A | Touch panel | [37] | |
| PMMA | ~8 Ω/sq | ~85% | Excellent | N/A | Thin film heater/SERS detector | [38] |
| ~16.1 Ω/sq | ~87% | Excellent | Excellent | QLED | [39] | |
| PVDF | ~8 Ω/sq | N/A | N/A | Excellent | Thermal conduction | [32] |
| ~5 Ω/sq | ~73% | Excellent | Excellent | Touch sensor | [40] | |
| PANI | ~25 Ω/sq | ~83.5% | N/A | Excellent | Solar cells | [41] |
| ~5300 S/cm | N/A | Excellent | N/A | EMI shielding | [31] | |
| PEDOT:PSS | ~2.3 Ω/sq | ~70.5% | N/A | Excellent | Thin film heater | [42] |
| ~12 Ω/sq | ~80% | N/A | N/A | Solar cells | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, H.; Cheong, J.Y.; Yun, T.G.; Hwang, B. Polymeric Protection for Silver Nanowire-Based Transparent Conductive Electrodes: Performance and Applications. Inorganics 2023, 11, 409. https://doi.org/10.3390/inorganics11100409
Ha H, Cheong JY, Yun TG, Hwang B. Polymeric Protection for Silver Nanowire-Based Transparent Conductive Electrodes: Performance and Applications. Inorganics. 2023; 11(10):409. https://doi.org/10.3390/inorganics11100409
Chicago/Turabian StyleHa, Heebo, Jun Young Cheong, Tae Gwang Yun, and Byungil Hwang. 2023. "Polymeric Protection for Silver Nanowire-Based Transparent Conductive Electrodes: Performance and Applications" Inorganics 11, no. 10: 409. https://doi.org/10.3390/inorganics11100409
APA StyleHa, H., Cheong, J. Y., Yun, T. G., & Hwang, B. (2023). Polymeric Protection for Silver Nanowire-Based Transparent Conductive Electrodes: Performance and Applications. Inorganics, 11(10), 409. https://doi.org/10.3390/inorganics11100409