RbEr2AsS7: A Rubidium-Containing Erbium Sulfide Thioarsenate(III) with (S2)2− Ligands According to RbEr2S(S2)[AsS2(S2)]
Abstract
:1. Introduction
2. Results and Discussion
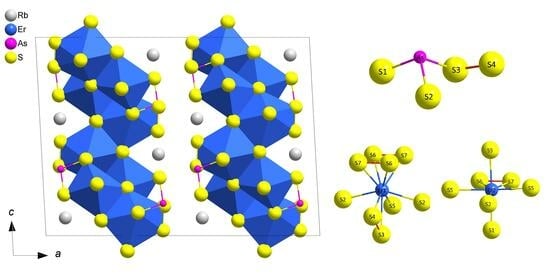
2.1. Structure Description for RbEr2AsS7 (≡ RbEr2S(S2)[AsS2(S2)])
2.2. Diffuse Reflectance Spectroscopy (DRS)
3. Experimental Section
3.1. Solid-State Synthesis
3.2. Single-Crystal X-ray Diffraction (SCXRD)
3.3. Wavelength-Dispersive X-ray Spectroscopy (WDXS)
3.4. Powder-Crystal X-ray Diffraction (PXRD)
3.5. Diffuse Reflectance Spectroscopy (DRS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, C.; Jörgens, S.; Mewis, A. Neue Thiophosphate: Die Verbindungen Li6Ln3(PS4)5 (Ln: Y, Gd, Dy, Yb, Lu) und Ag3Y(PS4)2. Z. Anorg. Allg. Chem. 2007, 633, 1633. [Google Scholar] [CrossRef]
- Gauthier, G.; Jobic, S.; Brec, R.; Rouxel, J. K3CeP2S8: A New Cerium Thiophosphate with One-Dimensional Anionic Chains. Inorg. Chem. 1998, 37, 2332. [Google Scholar] [CrossRef]
- Evenson, C.R.; Dorhout, P.K. Thiophosphate Phase Diagrams Developed in Conjunction with the Synthesis of the New Compounds KLaP2S6, K2La(P2S6)1/2(PS4), K3La(PS4)2, K4La0.67(PS4)2, K9−xLa1+x/3(PS4)4 (x = 0.5), K4Eu(PS4)2, and KEuPS4. Inorg. Chem. 2001, 40, 2884–2891. [Google Scholar] [CrossRef]
- Wu, Y.; Bensch, W. Syntheses, structures, and spectroscopic properties of K9Nd[PS4]4, K3Nd[PS4]2, Cs3Nd[PS4]2, and K3Nd3[PS4]4. Inorg. Chem. 2008, 47, 7523–7534. [Google Scholar] [CrossRef] [PubMed]
- Milot, S.; Wu, Y.; Näther, C.; Bensch, W.; Klepp, K.O. Two New Quaternary Thiophosphates with Pseudo One-dimensional Structures: Syntheses and Crystal Structures of Cs3Sm[PS4]2 and Rb3Sm[PS4]2. Z. Anorg. Allg. Chem. 2008, 634, 1575–1580. [Google Scholar] [CrossRef]
- Klepov, V.V.; Pace, K.A.; Breton, L.S.; Kocevski, V.; Besmann, T.M.; zur Loye, H.-C. Nearly Identical but Not Isotypic: Influence of Lanthanide Contraction on Cs2NaLn(PS4)2 (Ln = La–Nd, Sm, and Gd–Ho). Inorg. Chem. 2020, 59, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Schoop, L.M.; Eger, R.; Kremer, R.K.; Kuhn, A.; Nuss, J.; Lotsch, B.V. Structural Stability Diagram of ALnP2S6 Compounds (A = Na, K, Rb, Cs; Ln = Lanthanide). Inorg. Chem. 2017, 56, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Klepov, V.V.; Breton, L.S.; Pace, K.A.; Kocevski, V.; Besmann, T.M.; zur Loye, H.-C. Size-Driven Stability of Lanthanide Thiophosphates Grown from an Iodide Flux. Inorg. Chem. 2019, 58, 6565–6573. [Google Scholar] [CrossRef]
- Goh, E.-Y.; Kim, E.-J.; Kim, S.-J. Structure Modification on Quaternary Rare Earth Thiophosphates: NaYbP2S6, NaSmP2S6, and KSmP2S7. J. Solid State Chem. 2001, 160, 195–204. [Google Scholar] [CrossRef]
- Manríquez, V.; Galdámez, A.; Cerda-Monje, A.; Peña, O.; Ávila, R.E. Electrical and magnetic properties of quaternary rare earth thiophosphate: K4Sm2[PS4]2[P2S6]. J. Braz. Chem. Soc. 2009, 20, 1499–1503. [Google Scholar] [CrossRef]
- Aslani, C.K.; Breton, L.S.; Klepov, V.V.; zur Loye, H.-C. A series of Rb4Ln2(P2S6)(PS4)2 (Ln = La, Ce, Pr, Nd, Sm, Gd) rare earth thiophosphates with two distinct thiophosphate units [PVS4]3– and [PIV2S6]4–. Dalton Trans. 2021, 50, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Komm, T.; Gudat, D.; Schleid, T. Die Lanthanid(III)-ortho-Thiophosphate(V) vomTyp M[PS4] (M = La–Nd, Sm, Gd–Er): Synthese, Kristallstruktur und 31P-NMR-Untersuchungen. Z. Naturforsch. B 2006, 61, 766–774. [Google Scholar] [CrossRef]
- Cleary, D.; Twamley, B. Synthesis and structure of a new layered phase in the lanthanide thiophosphates: LuPS4. Inorg. Chim. Acta 2003, 353, 183–186. [Google Scholar] [CrossRef]
- Scholz, T.; Pielnhofer, F.; Eger, R.; Lotsch, B.V. Lanthanide ortho-thiophosphates revisited: Single-crystal X-ray, Raman, and DFT studies of TmPS4 and YbPS4. Z. Naturforsch. B 2020, 75, 225–231. [Google Scholar] [CrossRef]
- Wu, Y.; Näther, C.; Bensch, W. K3Ln(AsS4)2 (Ln = Nd, Sm, Gd): The First Rare Earth Thioarsenate Compounds with Infinite Straight 1∞[Ln(AsS4)2]3– chains. Inorg. Chem. 2006, 45, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bensch, W. Syntheses, crystal structures and spectroscopic properties of KEu[AsS4], K3Dy[AsS4]2 and Rb4Nd0.67[AsS4]2. Solid State Sci. 2009, 11, 1542–1548. [Google Scholar] [CrossRef]
- Engel, K.; Schleid, T. Die Serie caesiumhaltiger Thioarsenate(V) der Lanthanoide vom Formeltyp Cs3Ln[AsS4]2 mit Ln = La − Nd und Sm. Z. Naturforsch. B 2023, in press. [Google Scholar]
- Kang, D.-H. Oxidoarsenate(III/V) und Thioarsenate(III) der Selten-Erd-Metalle. Doctoral Dissertation, Universität Stuttgart, Stuttgart, Germany, 2015. [Google Scholar]
- Kang, D.-H.; Schleid, T. Cs2CeCl2[AsS3]: Ein neues chlorid-derivatisiertes Caesium-Cer(III)-Thioarsenat(III). Z. Anorg. Allg. Chem. 2008, 634, 2050. [Google Scholar] [CrossRef]
- Ledderboge, F.; Schleid, T. Cs4Pr2As4S11: A New Quaternary Thioarsenate(III) According to Cs4Pr2[AsS3]2[As2S5]. Z. Krist. S 2016, 36, 70. [Google Scholar]
- Bera, T.K.; Kanatzidis, M.G. AEuAsS3 (A = Li, K, Rb, and Cs): New As3+ Species from an Arsenic-Rich Polysulfide Flux. Inorg. Chem. 2008, 47, 7068–7070. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX: Program for Crystal-Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinemement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Böttcher, P. Darstellung und Kristallstruktur der Dialkalimetalltrichalkogenide Rb2S3, Rb2Se3, Cs2S3 und Cs2Se3. Z. Anorg. Allg. Chem. 1980, 461, 13–21. [Google Scholar] [CrossRef]
- Mullen, D.J.E.; Nowacki, W. Refinement of the crystal structures of realgar, AsS, and orpiment, As2S3. Z. Krist. 1972, 136, 48–65. [Google Scholar] [CrossRef]
- Schleid, T.; Lissner, F. Einkristalle von A-Nd2S3, U-Ho2S3, D-Er2S3 und E-Lu2S3 durch Oxidation reduzierter Chloride der Lanthanide mit Schwefel. Z. Anorg. Allg. Chem. 1992, 615, 19–26. [Google Scholar] [CrossRef]
- Fang, C.M.; Meetsma, A.; Wiegers, G.A.; Boom, G. Synthesis and crystal structure of F-type erbium sesquisulfide, F-Er2S3. J. Alloys Compd. 1993, 201, 255–259. [Google Scholar] [CrossRef]
- Hamani, D.; Masson, O.; Thomas, P. Localization and steric effect of the lone electron pair of the tellurium Te4+ cation and other cations of the p-block elements. A systematic study. J. Appl. Cryst. 2020, 53, 1243–1251. [Google Scholar] [CrossRef]
- Iyer, R.G.; Kanatzidis, M.G. Controlling Lewis Basicity in Polythioarsenate Fluxes: Stabilization of KSnAsS5 and K2SnAs2S6. Extended Chains and Slabs Based on Pyramidal ß-[AsS4]3– and [AsS3]3– Units. Inorg. Chem. 2002, 41, 3605–3607. [Google Scholar] [CrossRef]
- Iyer, R.G.; Do, J.; Kanatzidis, M.G. Flux Synthesis of the Noncentrosymmetric Cluster Compounds Cs2SnAs2Q9 (Q = S, Se) Containing Two Different Polychalcoarsenite ß-[AsQ4]3– and [AsQ5]3– Ligands. Inorg. Chem. 2003, 42, 1475–1482. [Google Scholar] [CrossRef]
- Chou, J.-H.; Kanatzidis, M.G. Pt2+ vs. Pt4+ in AsS33– Solutions and Isolation of the Clusters [Pt(As3S5)2]2– and [Pt3(AsS4)3]3–. Observation of Unique Thioarsenate ligands and Pt–As Bonds. Inorg. Chem. 1994, 33, 5372–5373. [Google Scholar] [CrossRef]
- Iyer, R.G.; Kanatzidis, M.G. [Mn2(AsS4)4]8– and [Cd2(AsS4)2(AsS5)2]8–: Discrete Clusters with High Negative Charge from Alkali Metal Polythioarsenate Fluxes. Inorg. Chem. 2004, 43, 3656–3662. [Google Scholar] [CrossRef]
- Wang, J.; Lee, K.; Kovnir, K. Synthesis, Crystal, and Electronic Structure of Ba3Sb2Q7 (Q = S, Se). Z. Anorg. Allg. Chem. 2015, 641, 1087–1092. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Liu, P.-F. Ba3[LiSbS2(S2)2Cl2]: The first zero-dimensional (0D) lithium metal thioantimonate featuring molecular anions of [LiSbS2(S2)2Cl2]6–. J. Solid State Chem. 2021, 294, 121873. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An article on optics of paint layers. Z. Tech. Phys. 1931, 12, 259–274. [Google Scholar]
- Hüttig, G.F. Apparat zur gleichzeitigen Druck- und Raummessung von Gasen (Tensi-Eudiometer). Z. Anorg. Allg. Chem. 1920, 114, 161–173. [Google Scholar] [CrossRef]
- Engel, K. Einblicke in die Welt der Lanthanoid-Thioarsenate: In preparation. Doctoral Dissertation, Universität Stuttgart, Stuttgart, Germany.







| Empirical Formula | RbEr2AsS7 |
|---|---|
| Structured formula | RbEr2S(S2)[AsS2(S2)] |
| Crystal system | monoclinic |
| Space group | C2/c (no. 15) |
| Lattice constants | |
| a/pm | 2339.86(12) |
| b/pm | 541.78(3) |
| c/pm | 1686.71(9) |
| β/° | 93.109(3) |
| Unit cell volume, Vuc/nm3 | 2.135 |
| Number of formula units | Z = 8 |
| Diffractometer | κ-CCD (Bruker-Nonius) |
| Radiation | Mo-Kα (λ = 71.07 pm) |
| Structure solution and refinement | SHELX-97 [22,23,24] |
| Index range, ±hmax/±kmax/±lmax | 30/7/21 |
| Number of e− per unit cell, F(000) | 2544 |
| Absorption coefficient, µ/mm−1 | 24.52 |
| Number of collected/unique reflections | 28015/2449 |
| Rint/Rσ | 0.068/0.028 |
| R1/wR2 for all reflections | 0.026/0.068 |
| Goodness of fit (GooF) | 1.030 |
| Residual electron density, ρmax/min/10−6 pm3 | 1.64/−1.51 |
| CSD number | 2219896 |
| Atom | x/a | y/b | z/c | Ueq/pm2 |
|---|---|---|---|---|
| Rb | 0.42638(3) | 0.26898(9) | 0.41040(4) | 299(2) |
| Er1 | 0.31925(1) | 0.24529(4) | 0.08041(2) | 150(1) |
| Er2 | 0.19026(1) | 0.25347(4) | 0.22655(2) | 149(1) |
| As | 0.06627(3) | 0.28248(9) | 0.33685(4) | 183(2) |
| S1 | 0.07440(7) | 0.2712(3) | 0.20711(9) | 213(3) |
| S2 | 0.16020(7) | 0.2460(3) | 0.38410(9) | 156(3) |
| S3 | 0.43645(7) | 0.2077(3) | 0.14952(9) | 224(3) |
| S4 | 0.42774(7) | 0.2307(3) | 0.02700(9) | 227(4) |
| S5 | 0.30438(7) | 0.2523(3) | 0.24007(9) | 144(3) |
| S6 | 0.20702(7) | 0.4488(3) | 0.07613(9) | 195(4) |
| S7 | 0.20447(7) | 0.0591(3) | 0.07745(9) | 201(4) |
| LP | 0.0282 | 0.2077 | 0.4047 | – |
| Atom Pair | d/pm | Atom Pair | d/pm |
|---|---|---|---|
| Er1–S5 | 273.4(1) | Rb–S4 | 334.5(2) |
| Er1–S4 | 274.0(2) | Rb–S1 | 334.7(2) |
| Er1–S2 | 280.6(1) | Rb–S4 | 334.8(2) |
| Er1–S2 | 281.4(1) | Rb–S1 | 336.6(2) |
| Er1–S6 | 284.5(2) | Rb–S3 | 343.3(2) |
| Er1–S7 | 286.7(2) | Rb–S7 | 345.8(2) |
| Er1–S6 | 287.7(2) | Rb–S4 | 352.4(2) |
| Er1–S7 | 289.2(2) | Rb–S6 | 358.9(2) |
| Er1–S3 | 292.8(2) | Rb–S5 | 394.0(2) |
| Er2–S5 | 266.8(2) | As–S1 | 220.7(2) |
| Er2–S1 | 271.5(2) | As–S2 | 230.5(2) |
| Er2–S5 | 276.2(1) | As–S3 | 231.6(2) |
| Er2–S7 | 276.3(2) | As–LP | 154 |
| Er2–S5 | 277.5(1) | ||
| Er2–S2 | 278.6(2) | S3–S4 | 206.9(2) |
| Er2–S6 | 279.6(2) | S6–S7 | 211.2(2) |
| Atom Triple | ∢/° |
|---|---|
| S1–As–S2 | 102.04(6) |
| S1–As–S3 | 97.49(6) |
| S2–As–S3 | 94.69(6) |
| As–S3–S4 | 99.06(7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engel, K.; Schleid, T. RbEr2AsS7: A Rubidium-Containing Erbium Sulfide Thioarsenate(III) with (S2)2− Ligands According to RbEr2S(S2)[AsS2(S2)]. Inorganics 2023, 11, 465. https://doi.org/10.3390/inorganics11120465
Engel K, Schleid T. RbEr2AsS7: A Rubidium-Containing Erbium Sulfide Thioarsenate(III) with (S2)2− Ligands According to RbEr2S(S2)[AsS2(S2)]. Inorganics. 2023; 11(12):465. https://doi.org/10.3390/inorganics11120465
Chicago/Turabian StyleEngel, Katja, and Thomas Schleid. 2023. "RbEr2AsS7: A Rubidium-Containing Erbium Sulfide Thioarsenate(III) with (S2)2− Ligands According to RbEr2S(S2)[AsS2(S2)]" Inorganics 11, no. 12: 465. https://doi.org/10.3390/inorganics11120465
APA StyleEngel, K., & Schleid, T. (2023). RbEr2AsS7: A Rubidium-Containing Erbium Sulfide Thioarsenate(III) with (S2)2− Ligands According to RbEr2S(S2)[AsS2(S2)]. Inorganics, 11(12), 465. https://doi.org/10.3390/inorganics11120465