Fabrication and Luminescent Properties of Er-Doped Sr5(PO4)3F Ceramics
Abstract
:1. Introduction
2. Results and Discussion
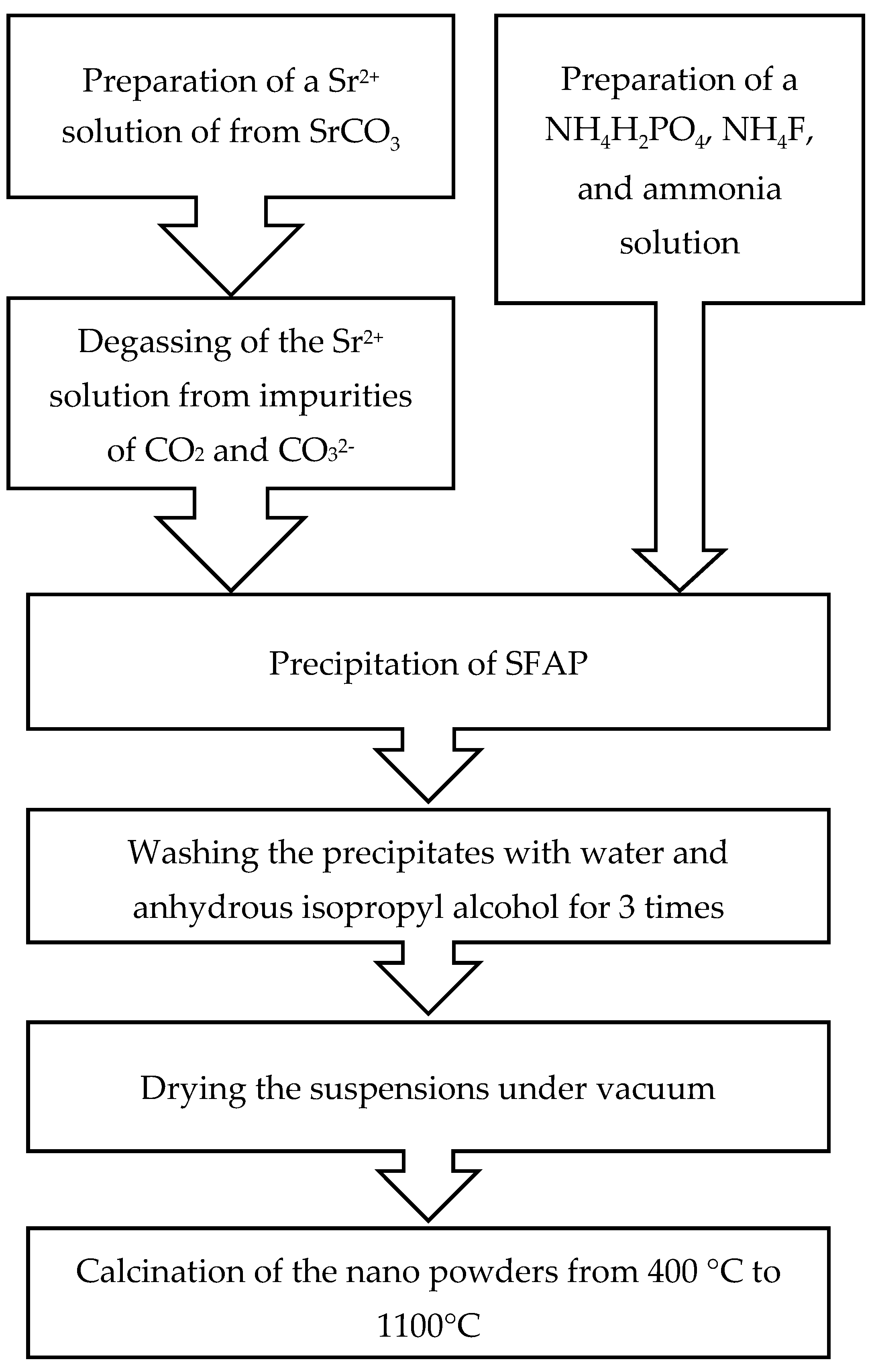
2.1. Effect of Strontium Source on the Morphology of SFAP Powders
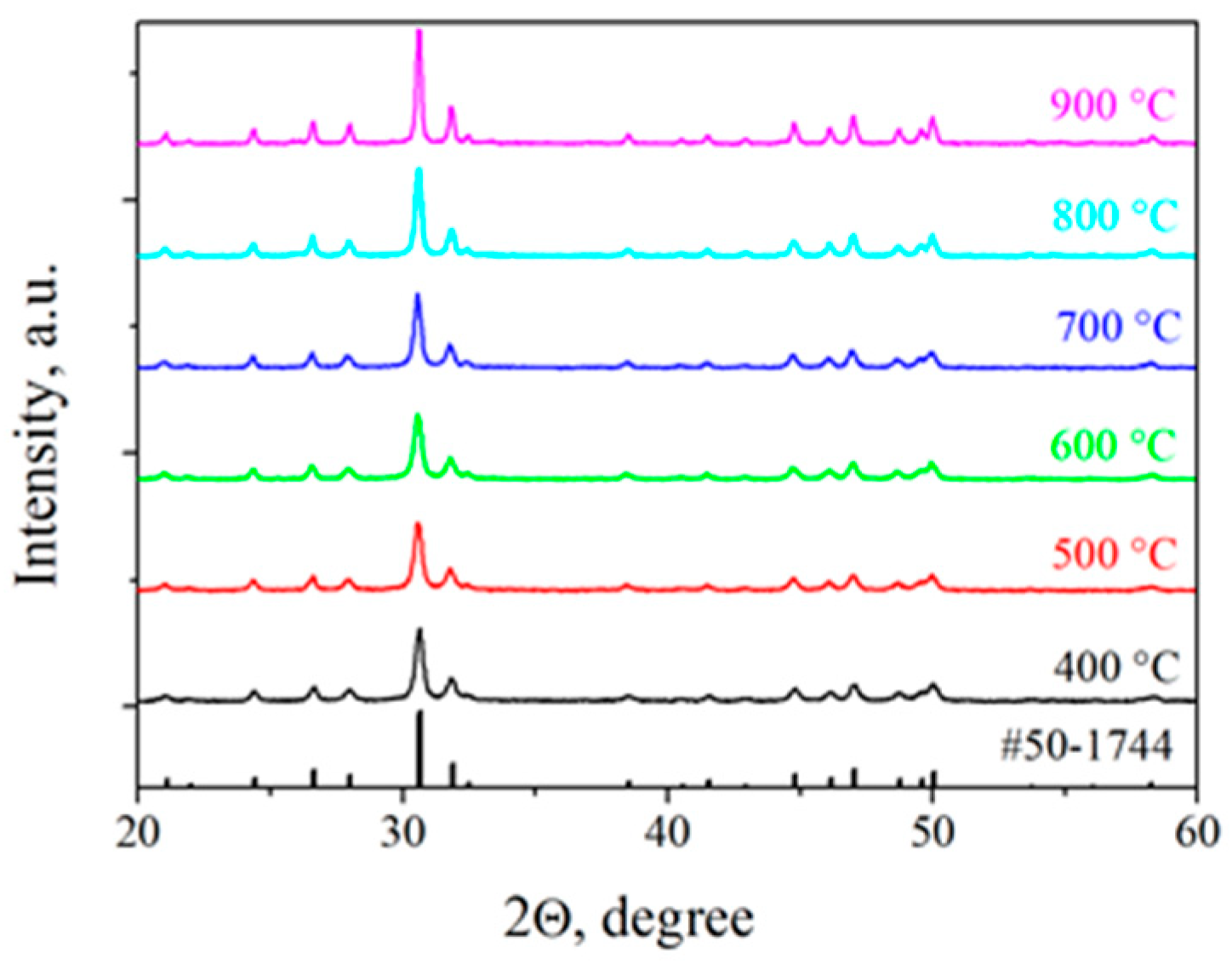
2.2. Effect of Calcination Temperature on the Phase Composition of the SFAP Powders
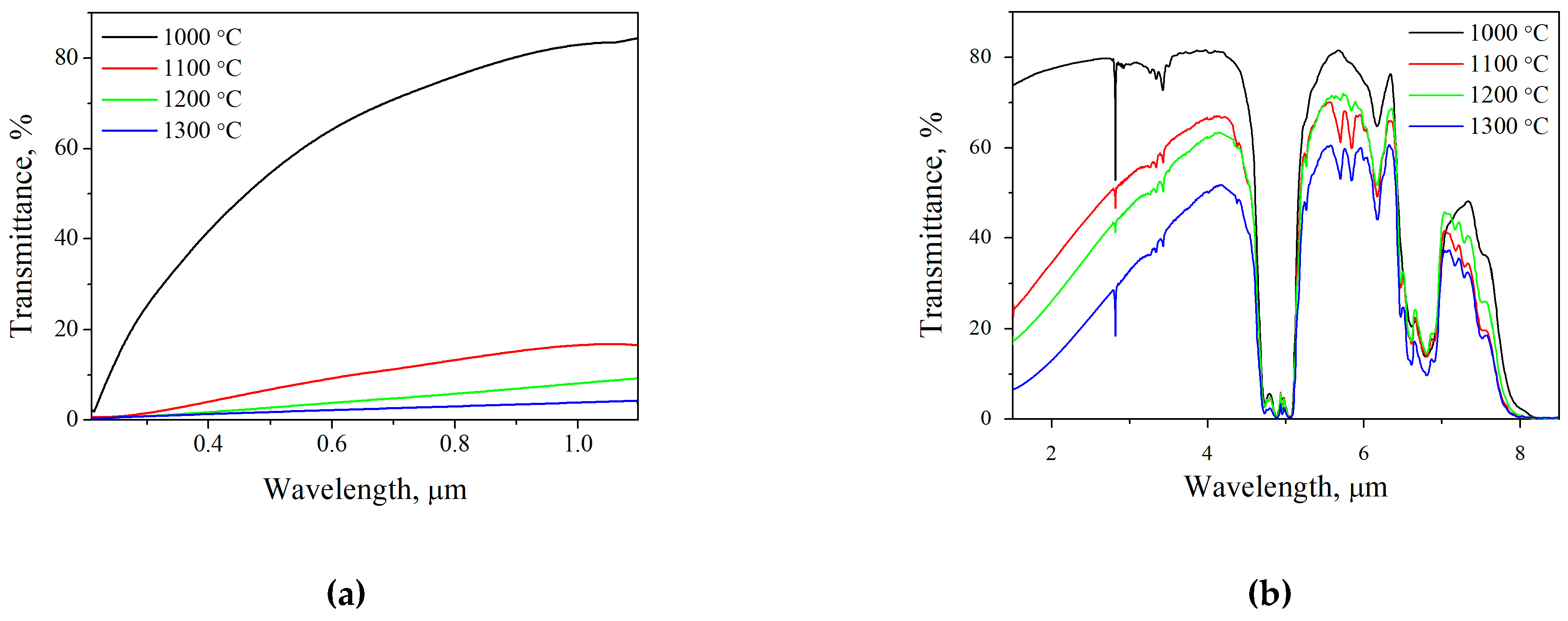
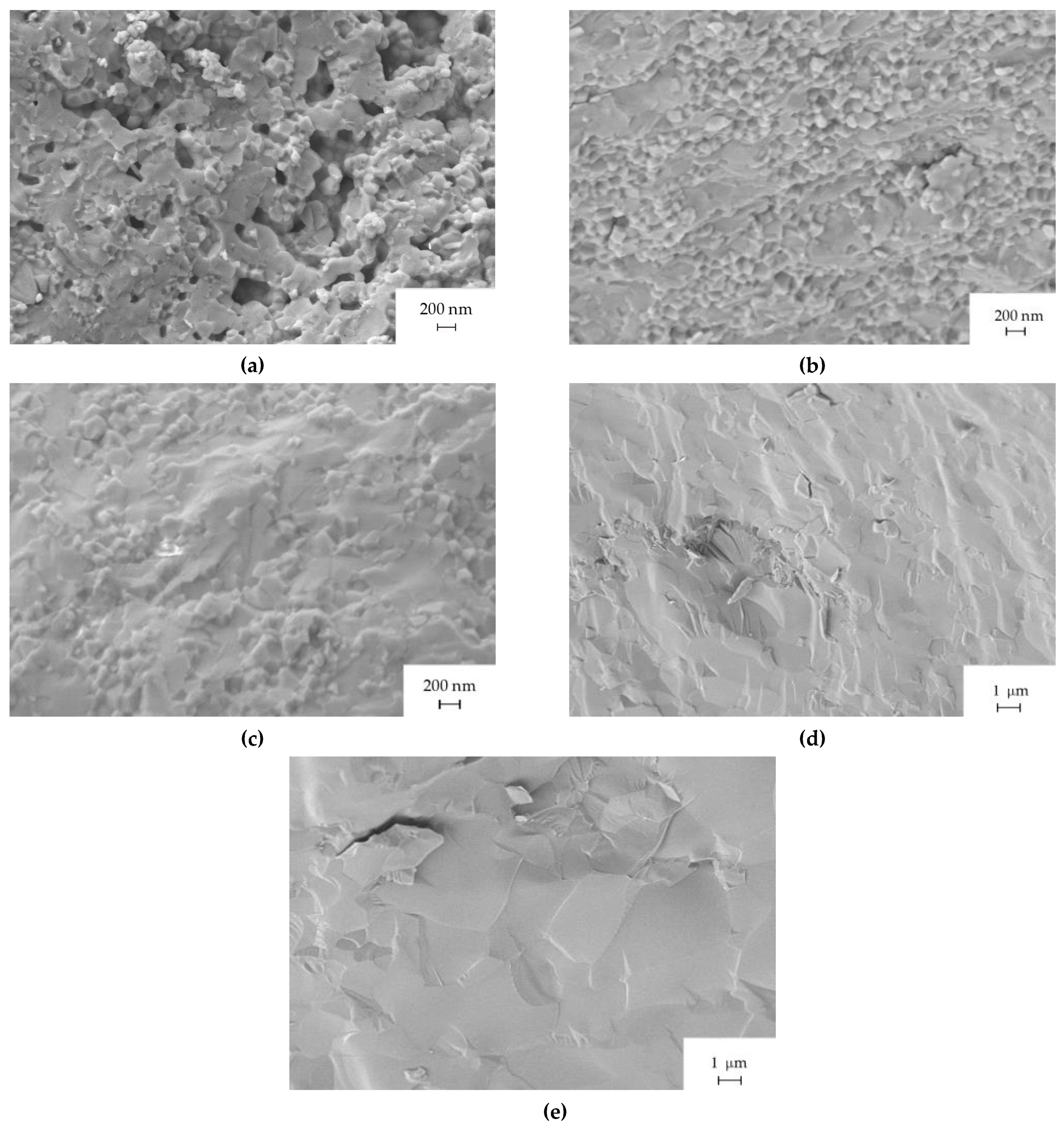
2.3. Microstructure and Optical Properties of SFAP Ceramics
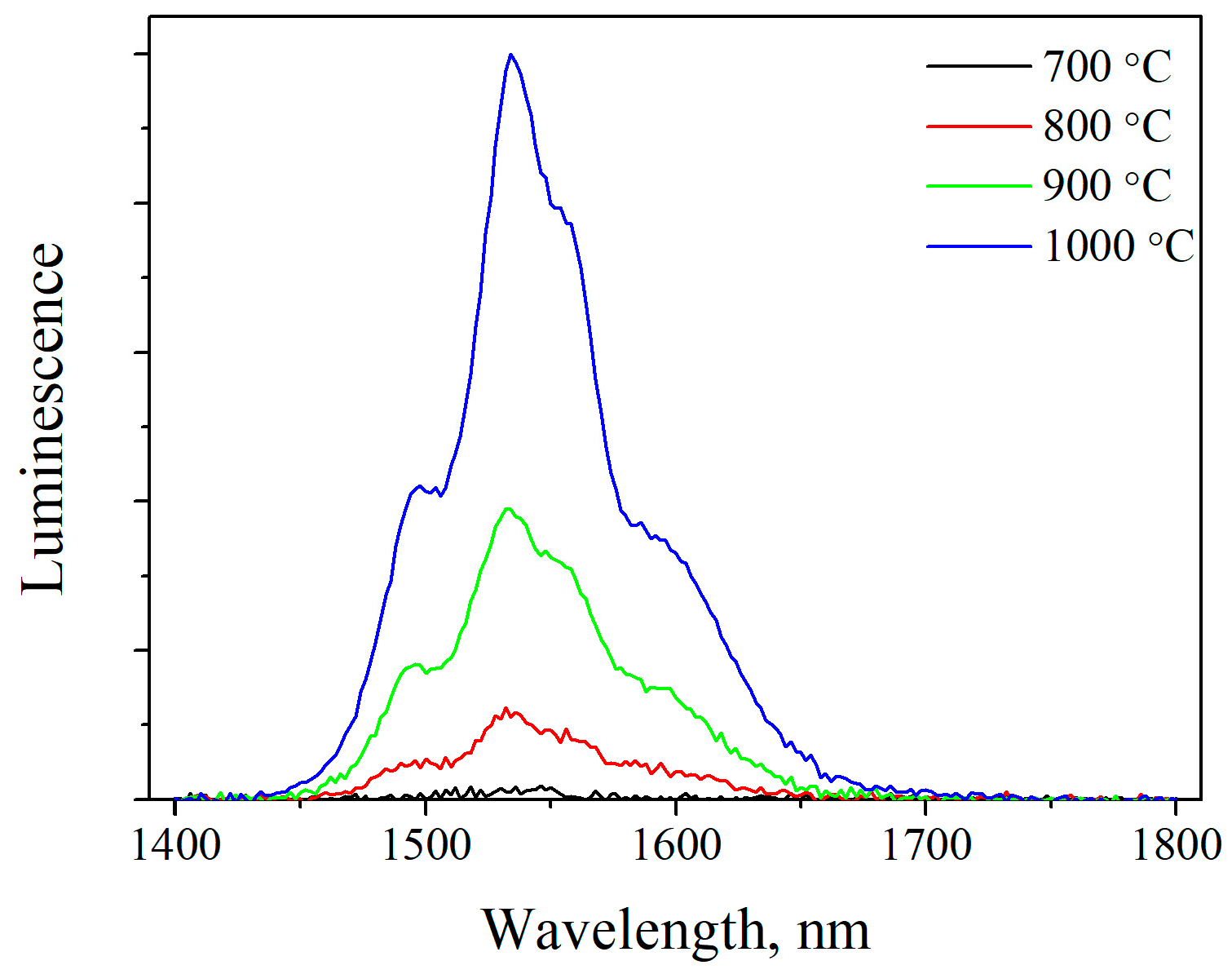
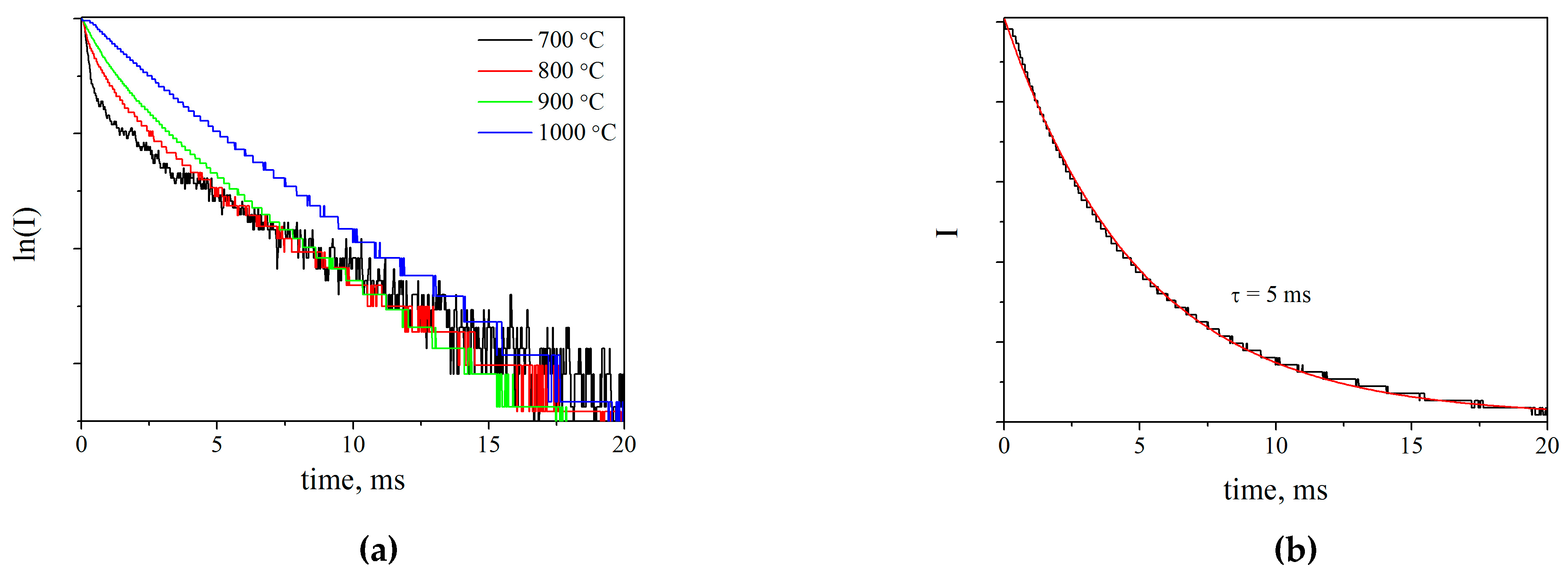
2.4. Luminescence of Erbium Ions in the SFAP Matrix
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erlandson, A.; Aceves, S.; Bayramian, A.; Bullington, A.; Beach, R.; Boley, C.; Caird, J.; Deri, R.; Dunne, A.; Flowers, D.; et al. Comparison of Nd:phosphate glass, Yb:YAG and Yb:S-FAP laser beamlines for laser inertial fusion energy (LIFE). Opt. Mater. Express. 2011, 1, 1341–1352. [Google Scholar] [CrossRef]
- Steinbruegge, K.; Henningsen, T.; Hopkins, R.; Mazelsky, R.; Melamed, N.; Riedel, E.; Roland, G. Laser Properties of Nd+3 and Ho+3 Doped Crystals with the Apatite Structure. Appl. Opt. 1972, 11, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Schaffers, K.; Tassano, J.; Bayramian, A.; Morris, R. Growth of Yb: S-FAP [Yb3+:Sr5(PO4)3F] crystals for the Mercury laser. J. Cryst. Growth. 2003, 253, 297–306. [Google Scholar] [CrossRef]
- Schaffers, K. Yb:S-FAP lasers. Opt. Mater. 2004, 26, 391–394. [Google Scholar] [CrossRef]
- Schaffers, K.; Bayramian, A.; Marshall, C.; Tassano, J.; Payne, S. Analysis of Sr5-xBax(PO4)3F:Yb3+ crystals for improved laser performance with diode-pumping. In Advanced Solid State Lasers; Pollock, B., Bosenberg, W., Eds.; Optica Publishing Group: Orlando, FL, USA, 1997; p. SC4. [Google Scholar] [CrossRef] [Green Version]
- Marshall, C.; Payne, S.; Smith, L.; Beach, R.; Emanuel, M.; Skidmore, J.; Powell, H.; Krupke, W.; Chai, B. Diode-Pumped Yb:Sr5(PO4)3F Laser Performance. In Advanced Solid State Lasers; Chai, B., Payne, S., Eds.; Optica Publishing Group: Memphis, TN, USA, 1995; p. YL2. [Google Scholar] [CrossRef]
- Yagi, H.; Takaichi, K.; Ueda, K.; Yamasaki, Y.; Yanagitani, T.; Kaminskii, A. The physical properties of composite YAG ceramics. Laser Phys. 2005, 15, 1338–1344. [Google Scholar]
- Gavrishchuk, E.; Ikonnikov, V.; Kazantsev, S.; Kononov, I.; Rodin, S.; Savin, D.; Timofeeva, N.; Firsov, K. Scaling of energy characteristics of polycrystalline laser at room temperature. Quantum Electron. 2015, 45, 823. [Google Scholar] [CrossRef]
- Kuznetsov, S.; Alexandrov, A.; Fedorov, P. Optical Fluoride Nanoceramics. Inorg. Mater. 2021, 57, 555–578. [Google Scholar] [CrossRef]
- Akiyama, J.; Sato, Y.; Taira, T. Laser ceramics with rare-earth-doped anisotropic materials. Opt. Lett. 2010, 35, 3598–3600. [Google Scholar] [CrossRef]
- Sato, Y.; Arzakantsyan, M.; Akiyama, J.; Taira, T. Anisotropic Yb:FAP laser ceramics by micro-domain control. Opt. Mater. Express. 2014, 4, 2006–2015. [Google Scholar] [CrossRef]
- Chai, B.; Hao, L.; Mao, X.; Xu, X.; Li, X.; Jiang, B.; Zhang, L. Precipitation and Growth Mechanism of Diverse Sr5(PO4)3F Particles. J. Am. Ceram. Soc. 2016, 99, 1498–1503. [Google Scholar] [CrossRef]
- Wu, Y.; Du, J.; Clark, R. Synthesis of Yb3+ doped Sr5(PO4)3F nanoparticles through co-precipitation. Mater. Lett. 2013, 107, 68–70. [Google Scholar] [CrossRef]
- Wu, Y. Nanostructured transparent ceramics with an anisotropic crystalline structure. Opt. Mater. Express. 2014, 4, 2026–2031. [Google Scholar] [CrossRef]
- Liu, X.; Tan, G.; Zhou, Z.; Mei, B. Fabrication, microstructure, mechanical and luminescence properties of transparent Yb3+-doped Sr5(PO4)3F nanostructured ceramics. J. Eur. Ceram. Soc. 2022, 42, 6642–6653. [Google Scholar] [CrossRef]
- Liu, X.; Tan, G.; Li, W.; Mei, B. Conventional HP sintering of asymmetric hexagonal structure Yb3+-doped Sr5(PO4)3F transparent ceramic without additives. J. Am. Ceram. Soc. 2022, 105, 4581–4587. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, B.; Li, W.; Yang, Y.; Yi, G.; Zhou, Z.; Liu, Z. Fabrication and spectral properties of Nd:S-FAP transparent ceramics by simple route of HP method. J. Alloys Compd. 2020, 820, 153171. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Mei, B.; Yang, Y. The effect of Y3+ doping upon Nd: S-FAP transparent ceramics for effective spectral performance improvement. Ceram. Int. 2022, 49, 1362–1368. [Google Scholar] [CrossRef]
- Furuse, H.; Horiuchi, N.; Kim, B.-N. Transparent non-cubic laser ceramics with fine microstructure. Sci. Rep. 2019, 9, 10300. [Google Scholar] [CrossRef] [Green Version]
- Furuse, H.; Okabe, T.; Shirato, H.; Kato, D.; Horiuchi, N.; Morita, K.; Kim, B.-N. High-optical-quality non-cubic Yb3+-doped Ca10(PO4)6F2 (Yb:FAP) laser ceramics. Opt. Mater. Express. 2021, 11, 1756–1762. [Google Scholar] [CrossRef]
- Zhmykhov, V.; Dobretsova, E.; Tsvetkov, V.; Nikova, M.; Chikulina, I.; Vakalov, D.; Tarala, V.; Pyrkov, Y.; Kuznetsov, S.; Tsvetkov, V. Judd-Ofelt Analysis of High Erbium Content Yttrium-Aluminum and Yttrium-Scandium-Aluminum Garnet Ceramics. Inorganics 2022, 10, 170. [Google Scholar] [CrossRef]
- Chaika, M.; Balabanov, S.; Permin, D. Optical spectra and gain properties of Er3+:Lu2O3 ceramics for eye-safe 1.5-μm lasers. Opt. Mater. 2021, 112, 110785. [Google Scholar] [CrossRef]
- Liu, J.; Liu, P.; Wang, J.; Xu, X.; Li, D.; Zhang, J.; Nie, X. Fabrication and Sintering Behavior of Er:SrF2 Transparent Ceramics using Chemically Derived Powder. Materials 2018, 11, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šulc, J.; Němec, M.; Švejkar, R.; Jelínková, H.; Doroshenko, M.; Fedorov, P.; Osiko, V. Diode-pumped Er:CaF2 ceramic 2.7μm tunable laser. Opt. Lett. 2013, 38, 3406–3409. [Google Scholar] [CrossRef] [PubMed]
- Basiev, T.T.; Orlovskii, Y.V.; Polyachenkova, M.V.; Fedorov, P.P.; Kuznetsov, S.V.; Konyushkin, V.A.; Osiko, V.V.; Alimov, O.K.; Dergachev, A.Y. Continuously tunable cw lasing near 2.75 μm in diode-pumped Er3+: SrF2 and Er3+: CaF2 crystals. Quantum Electron 2006, 36, 591–594. [Google Scholar] [CrossRef]
- Malyavin, F.; Tarala, V.; Kuznetsov, S.; Kravtsov, A.; Chikulina, I.; Shama, M.; Medyanik, E.; Ziryanov, V.; Evtushenko, E.; Vakalov, D.; et al. Influence of the ceramic powder morphology and forming conditions on the optical transmittance of YAG:Yb ceramics. Ceram. Int. 2019, 45, 4418–4423. [Google Scholar] [CrossRef]
- Grisafe, D.A.; Hummel, F.A. Pentavalent Ion Substitutions in the Apatite Structure Part A. Crystal Chemistry. J. Solid State Chem. 1970, 2, 160–166. [Google Scholar] [CrossRef]
- Marincea, Ş.; Dumitraş, D.-G.; Sava Ghineţ, C.; Dal Bo, F. Carbonate-Bearing, F-Overcompensated Fluorapatite in Magnesian Exoskarns from Valea Rea, Budureasa, Romania. Minerals 2022, 12, 1083. [Google Scholar] [CrossRef]
- Baddiel, C.; Berry, E. Spectra structure correlations in hydroxy and fluorapatite. Spectrochim. Acta. 1966, 22, 1407–1416. [Google Scholar] [CrossRef]
- Weidner, V.; Carney, M.; Schermerhorn, D.; Pasteris, J.; Yoder, C. A-type substitution in carbonated strontium fluor-, chlor- and hydroxylapatites. Mineral. Mag. 2015, 79, 399–412. [Google Scholar] [CrossRef]
- Grigorjeva, L.; Smits, K.; Millers, D.; Jankoviča, D. Luminescence of Er/Yb and Tm/Yb doped FAp nanoparticles and ceramics, IOP Conf. Ser. Mater. Sci. Eng. 2015, 77, 12036. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Man, Z.; Ao, Y.; Chen, H. Investigation on the structure and upconversion fluorescence of Yb3+/Ho3+ co-doped fluorapatite crystals for potential biomedical applications. Sci. Rep. 2014, 4, 4446. [Google Scholar] [CrossRef] [Green Version]
- Permin, D.; Kurashkin, S.; Novikova, A.; Savikin, A.; Gavrishchuk, E.; Balabanov, S.; Khamaletdinova, N. Synthesis and luminescence properties of Yb-doped Y2O3, Sc2O3 and Lu2O3 solid solutions nanopowders. Opt. Mater. 2018, 77, 240–245. [Google Scholar] [CrossRef]
- Gruber, J.B.; Wright, A.O.; Seltzer, M.D.; Zandi, B.; Merkle, L.D.; Hutchinson, J.A.; Morrison, C.A.; Allik, T.H.; Chai, B.H.T. Site-Selective Excitation and Polarized Absorption and Emission Spectra of Trivalent Thulium and Erbium in Strontium Fluorapatite. J. Appl. Phys. 1997, 81, 6585–6598. [Google Scholar] [CrossRef] [Green Version]
- Gheorghe, C.; Georgescu, S.; Lupei, V.; Lupei, A.; Ikesue, A. Absorption intensities and emission cross section of Er3+ in Sc2O3 transparent ceramics. J. Appl. Phys. 2008, 103, 83116. [Google Scholar] [CrossRef]

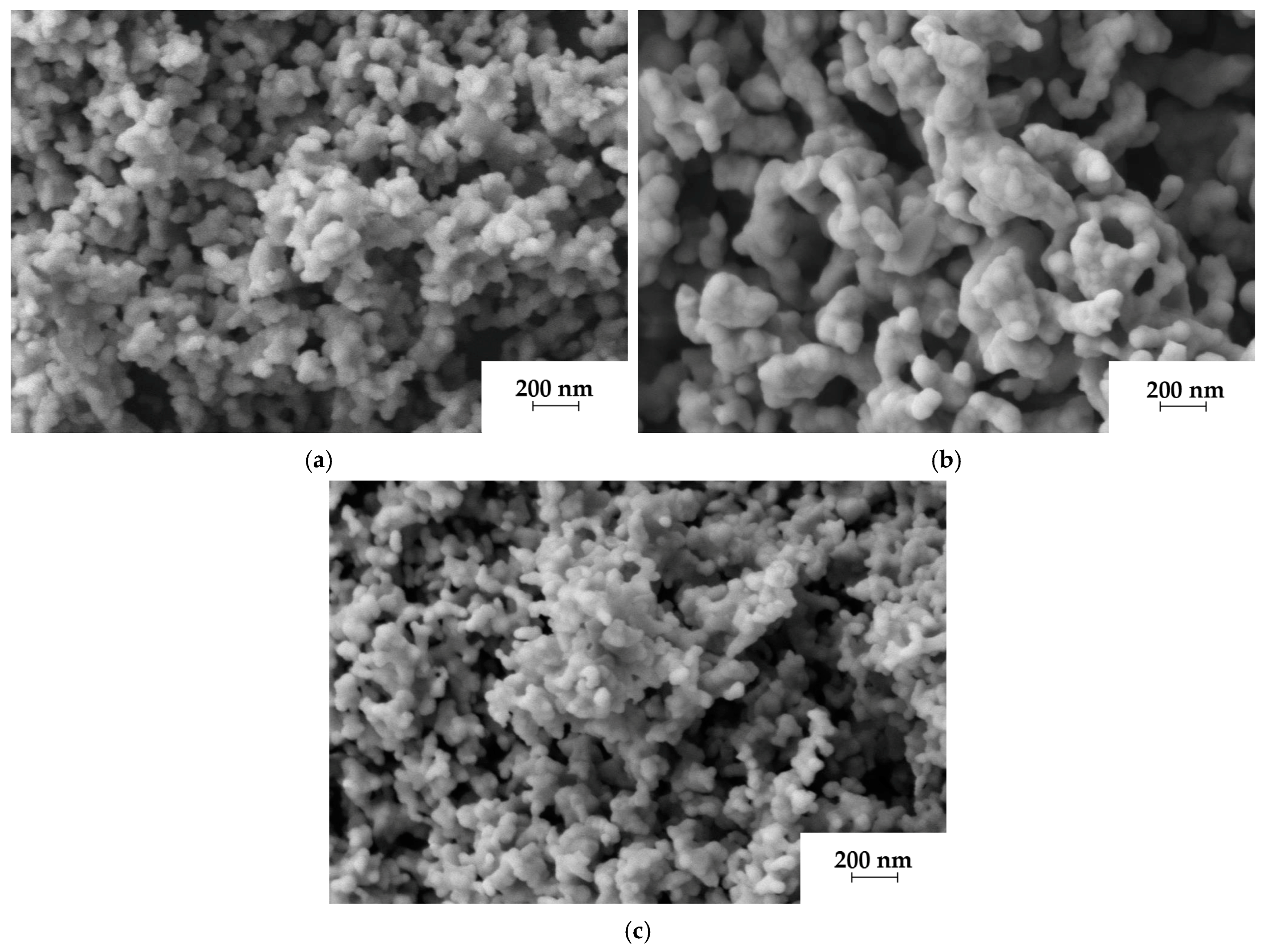

| Precursor | SBET | DBET | DXRD | n |
|---|---|---|---|---|
| Strontium nitrate | 15.1 | 96 | 30 | 3.2 |
| Strontium acetate | 10.9 | 132 | 35 | 3.8 |
| Strontium chloride | 8.5 | 170 | 33 | 5.1 |
| Temperature, °C | a, Å | c, Å | V, Å | ρ, g/cm3 |
|---|---|---|---|---|
| 400 | 9.71(2) | 7.27(2) | 593(4) | 4.15(3) |
| 500 | 9.71(3) | 7.28(2) | 595(4) | 4.14(3) |
| 600 | 9.71(2) | 7.28(2) | 595(4) | 4.14(3) |
| 700 | 9.72(2) | 7.28(2) | 596(3) | 4.13(3) |
| 800 | 9.71(2) | 7.283(14) | 595(2) | 4.14(3) |
| 900 | 9.715(10) | 7.283(7) | 595.3(13) | 4.14(3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permin, D.; Nazmutdinov, M.; Kurashkin, S.; Balabanov, S.; Belyaev, A.; Novikova, A.; Koshkin, V. Fabrication and Luminescent Properties of Er-Doped Sr5(PO4)3F Ceramics. Inorganics 2023, 11, 57. https://doi.org/10.3390/inorganics11020057
Permin D, Nazmutdinov M, Kurashkin S, Balabanov S, Belyaev A, Novikova A, Koshkin V. Fabrication and Luminescent Properties of Er-Doped Sr5(PO4)3F Ceramics. Inorganics. 2023; 11(2):57. https://doi.org/10.3390/inorganics11020057
Chicago/Turabian StylePermin, Dmitry, Marsel Nazmutdinov, Sergey Kurashkin, Stanislav Balabanov, Alexander Belyaev, Anastasia Novikova, and Vitaliy Koshkin. 2023. "Fabrication and Luminescent Properties of Er-Doped Sr5(PO4)3F Ceramics" Inorganics 11, no. 2: 57. https://doi.org/10.3390/inorganics11020057
APA StylePermin, D., Nazmutdinov, M., Kurashkin, S., Balabanov, S., Belyaev, A., Novikova, A., & Koshkin, V. (2023). Fabrication and Luminescent Properties of Er-Doped Sr5(PO4)3F Ceramics. Inorganics, 11(2), 57. https://doi.org/10.3390/inorganics11020057







