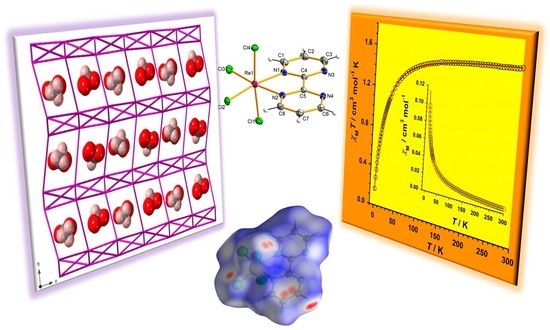
Effect of the Solvent on the Crystallographic and Magnetic Properties of Rhenium(IV) Complexes Based on 2,2′-Bipyrimidine Ligand
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of the Complexes
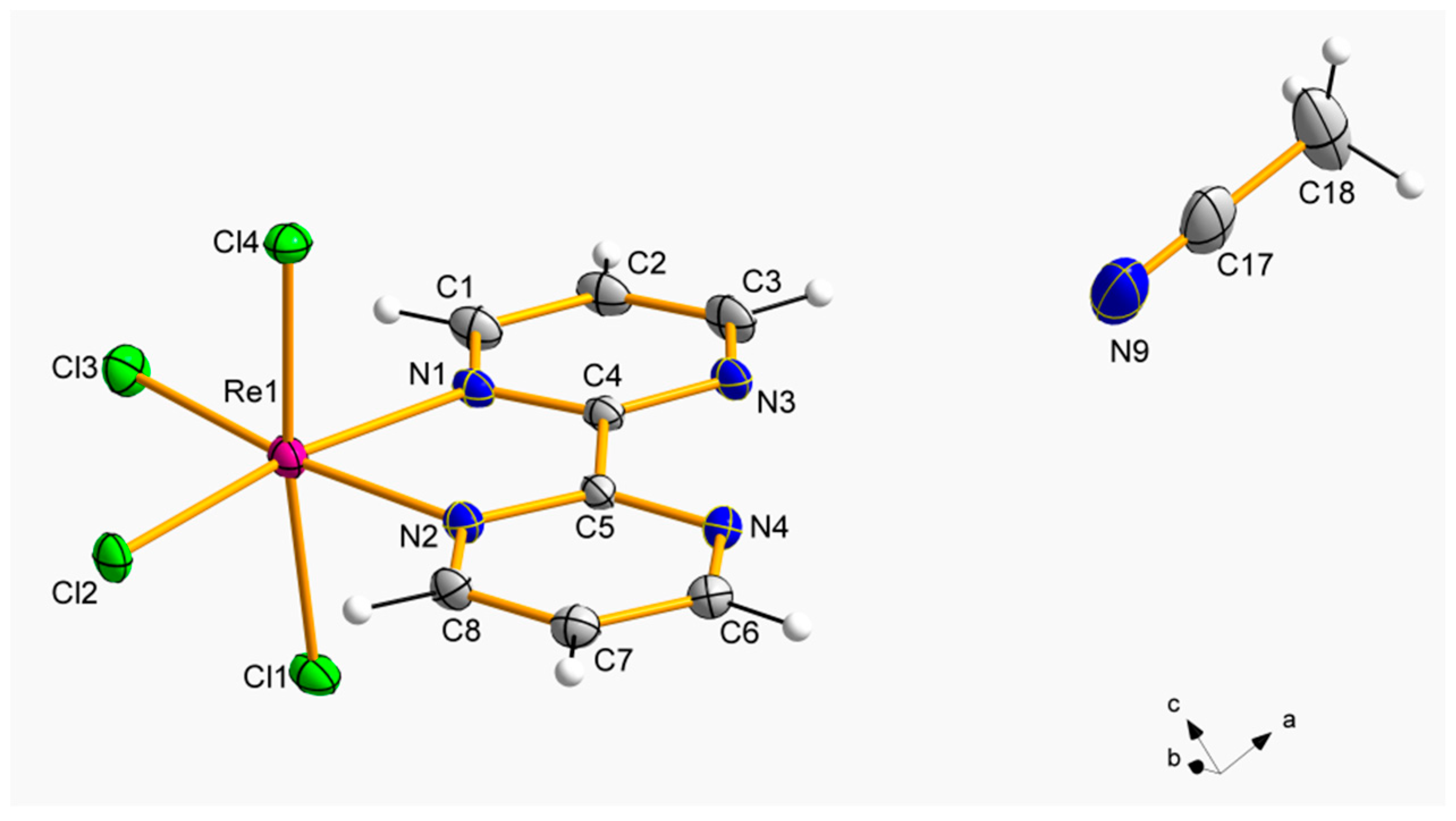
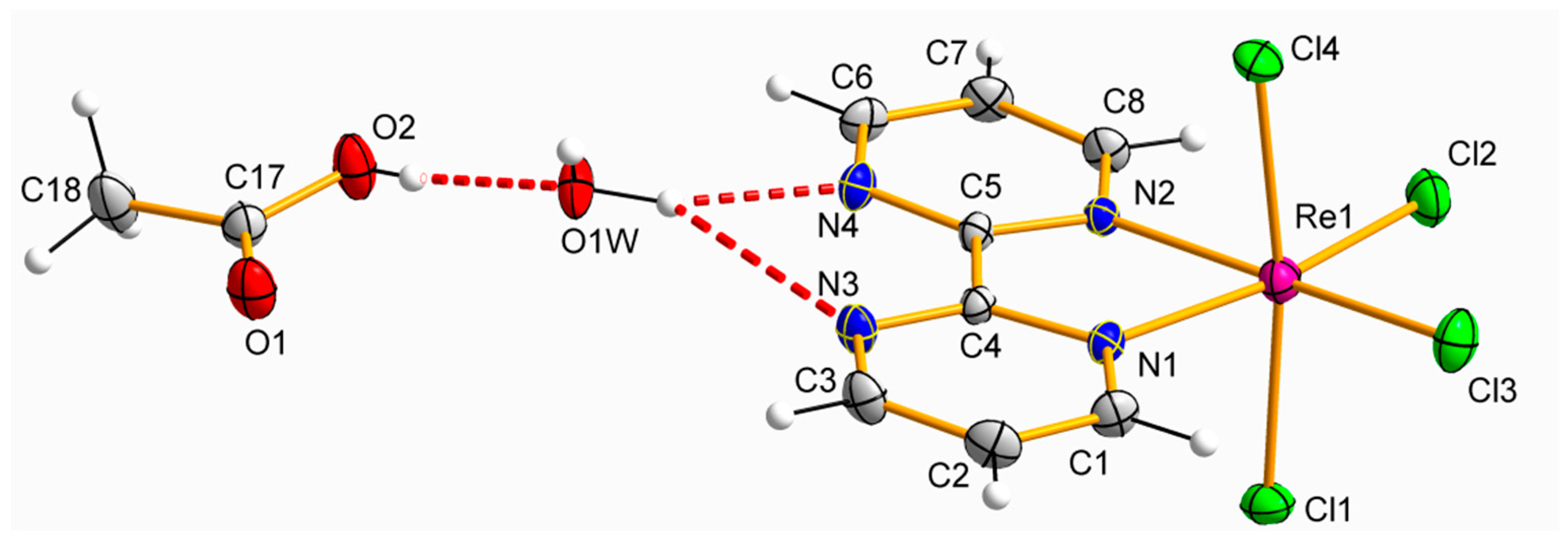
2.2. Description of the Crystal Structures
2.3. Hirshfeld Surface Analysis
2.4. Magnetic Properties
3. Experimental Section
3.1. Materials
3.2. Synthesis of the Complexes
3.2.1. Synthesis and Crystallization of [ReCl4(bpym)]·MeCN (1)
3.2.2. Synthesis and Crystallization of [ReCl4(bpym)]·CH3COOH·H2O (2)
3.3. X-ray Data Collection and Structure Refinement
3.4. Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, C.P.; Glick, G.D.; Matzger, A.J. Dissecting the Behavior of a Promiscuous Solvate Former. Angew. Chem. Int. Ed. 2006, 45, 2062–2066. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Zaworotko, M.J. The role of cocrystals in pharmaceutical science. Drug Discov. 2008, 13, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and fictions about polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef] [PubMed]
- Takieddin, K.; Khimyak, Y.Z.; Fábián, L. Prediction of Hydrate and Solvate Formation Using Statistical Models. Cryst. Growth Des. 2016, 16, 70–81. [Google Scholar] [CrossRef]
- Braga, D.; Casali, L.; Grepioni, F. The Relevance of Crystal Forms in the Pharmaceutical Field: Sword of Damocles or Innovation Tools? Int. J. Mol. Sci. 2022, 23, 9013. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; Mastropietro, T.F.; Julve, M.; Faus, J.; De Munno, G. Self-Assembled One- and Two-Dimensional Networks Based on NH2Me2[ReX5(DMF)] (X = Cl and Br) Species: Polymorphism and Supramolecular Isomerism in Re(IV) Compounds. Cryst. Growth Des. 2011, 11, 1733–1741. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Faus, J.; Lloret, F.; Julve, J. Towards multifunctional magnetic systems through molecular-programmed self assembly of Re(IV) metalloligands. Coord. Chem. Rev. 2015, 289–290, 215–237. [Google Scholar] [CrossRef]
- Woodall, C.H.; Craig, G.A.; Prescimone, A.; Misek, M.; Cano, J.; Faus, J.; Probert, M.R.; Parsons, S.; Moggach, S.; Martínez-Lillo, J.; et al. Pressure induced enhancement of the magnetic ordering temperature in rhenium(IV) monomers. Nat. Commun. 2016, 7, 13870. [Google Scholar] [CrossRef]
- González, R.; Chiozzone, R.; Kremer, C.; Guerra, F.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. Magnetic Studies on Hexahalorhenate(IV) Salts of Ferrocenium Cations [Fe(C5R5)2]2[ReX6] (R = H, CH3; X = Cl, Br, I). Inorg. Chem. 2004, 43, 3013–3019. [Google Scholar] [CrossRef]
- Nelson, C.M.; Boyd, G.E.; Smith, W.T. Magnetochemistry of Technetium and Rhenium. J. Am. Chem. Soc. 1954, 76, 348–352. [Google Scholar] [CrossRef]
- Figgis, B.N.; Lewis, J.; Mabbs, F.E. The Magnetic Properties of Some d3-Complexes. J. Chem. Soc. 1961, 3138–3145. [Google Scholar] [CrossRef]
- Busey, R.; Sonder, E. Magnetic Susceptibility of Potassium Hexachlororhenate (IV) and Potassium Hexabromorhenate (IV) from 5° to 300 °K. J. Chem. Phys. 1962, 36, 93–97. [Google Scholar] [CrossRef]
- Rouschias, G.; Wilkinson, G. The chemistry of rhenium–nitrile complexes. J. Chem. Soc. A 1968, 489–496. [Google Scholar] [CrossRef]
- Mroziński, J. Magnetic properties of methylammonium hexachlororhenate (4) salts in the range 1.5–300 K. Bull. Pol. Acad. Sci. Chem. 1978, 26, 789–798. [Google Scholar]
- Mroziński, J. Low temperature magnetic properties of some methylammonium hexabromorhenate (4) salts. Bull. Pol. Acad. Sci. Chem. 1980, 28, 559–567. [Google Scholar]
- Reynolds, P.A.; Moubaraki, B.; Murray, K.S.; Cable, J.W.; Engelhardt, L.M.; Figgis, B.N. Metamagnetism in tetrachlorobis(N-phenylacetamidine)rhenium(IV). J. Chem. Soc. Dalton Trans. 1997, 263–268. [Google Scholar] [CrossRef]
- Małecka, J.; Jäger, L.; Wagner, C.; Mroziński, J. Preparation, crystal structure and properties of (Ph4P)2ReCl6·2CH3CN. Pol. J. Chem. 1998, 72, 1879–1885. [Google Scholar]
- Reynolds, P.A.; Figgis, B.N.; Martín y Marero, D.J. Magnetic structure and covalence in tetrachlorobis(N-phenylacetamidinato)rhenium(IV) by neutron diffraction. J. Chem. Soc. Dalton Trans. 1999, 945–950. [Google Scholar] [CrossRef]
- Thornton, P. 13 Manganese, technetium and rhenium. Annu. Rep. Prog. Chem. Sect. A Inorg. Chem. 2000, 96, 215–228. [Google Scholar] [CrossRef]
- Coronado, E.; Day, P. Magnetic Molecular Conductors. Chem. Rev. 2004, 104, 5419–5448. [Google Scholar] [CrossRef]
- Feng, X.; Liu, J.-L.; Pedersen, K.S.; Nehrkorn, J.; Schnegg, A.; Holldack, K.; Bendix, J.; Sigrist, M.; Mutka, H.; Samohvalov, D.; et al. Multifaceted magnetization dynamics in the mononuclear complex [ReIVCl4(CN)2]2−. Chem. Commun. 2016, 52, 12905–12908. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Sigrist, M.; Sørensen, M.A.; Barra, A.-L.; Weyhermüller, T.; Piligkos, S.; Thuesen, C.A.; Vinum, M.G.; Mutka, H.; Weihe, H.; et al. [ReF6]2−: A Robust Module for the Design of Molecule-Based Magnetic Materials. Angew. Chem. Int. Ed. 2014, 53, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Chiozzone, R.; Kremer, C.; De Munno, G.; Nicolò, F.; Lloret, F.; Julve, M.; Faus, J. Magnetic Studies on Hexaiodorhenate(IV) Salts of Univalent Cations. Spin Canting and Magnetic Ordering in K2[ReI6] with Tc = 24 K. Inorg. Chem. 2003, 42, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Kong, J.; Barros, W.P.; Faus, J.; Julve, M.; Brechin, E.K. Metamagnetic behaviour in a new Cu(II)Re(IV) chain based on the hexachlororhenate(IV) anion. Chem. Commun. 2014, 50, 5840–5842. [Google Scholar] [CrossRef]
- Louis-Jean, J.; Balasekaran, S.M.; Lawler, K.V.; Sanchis-Perucho, A.; Martínez-Lillo, J.; Smith, D.; Forster, P.M.; Salamat, A.; Poineau, F. Coexistence of metamagnetism and slow relaxation of magnetization in ammonium hexafluoridorhenate. RSC Adv. 2021, 11, 6353–6360. [Google Scholar] [CrossRef]
- Chiozzone, R.; González, R.; Kremer, C.; Cerdá, M.F.; Armentano, D.; De Munno, G.; Martínez-Lillo, J.; Faus, J. A novel series of rhenium-bipyrimidine complexes: Synthesis, crystal structure and electrochemical properties. Dalton Trans. 2007, 6, 653–660. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Mastropietro, T.F.; Lappano, R.; Madeo, A.; Alberto, M.E.; Russo, N.; Maggiolini, M.; De Munno, G. Rhenium(IV) compounds inducing apoptosis in cancer cells. Chem. Commun. 2011, 47, 5283–5285. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Lloret, F.; Julve, M.; Faus, J. Spin canting in Re(IV) complexes: Magnetic properties of [ReX4(bpym)] (X = Cl and Br; bpym = 2,2′-bipyrimidine). J. Coord. Chem. 2008, 62, 92–99. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M.; Faus, J. First Magnetostructural Study on a Heterodinuclear 2,2′-Bipyrimidine-Bridged Complex. Inorg. Chem. 2011, 50, 12405–12407. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; Fortea-Pérez, F.R.; Stiriba, S.E.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. A Chiral, Photoluminescent, and Spin-Canted {CuIReIV2}n Branched Chain. Inorg. Chem. 2015, 54, 4594–4596. [Google Scholar] [CrossRef]
- Armentano, D.; Sanchis-Perucho, A.; Rojas-Dotti, C.; Martínez-Lillo, J. Halogen⋯halogen interactions in the self-assembly of one-dimensional 2,2′-bipyrimidine-based CuIIReIV systems. CrystEngComm 2018, 20, 4575–4581. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Faus, J. Magneto-structural study on a series of rhenium(IV) complexes containing biimH2, pyim and bipy ligands. Polyhedron 2008, 27, 1447–1454. [Google Scholar] [CrossRef]
- Roy, N.; Sen, U.; Moharana, P.; Babu, L.T.; Kar, B.; Vardhan, S.; Sahoo, S.K.; Bose, B.; Paira, P. 2,2′-Bipyrimidine-based luminescent Ru(II)/Ir(III)–arene monometallic and homo- and hetero-bimetallic complexes for therapy against MDA-MB-468 and caco-2 cells. Dalton Trans. 2021, 50, 11725–11729. [Google Scholar] [CrossRef]
- Yao, S.-Y.; Ou, Y.-L.; Ye, B.-H. Asymmetric Synthesis of Enantiomerically Pure Mono- and Binuclear Bis(cyclometalated) Iridium(III) Complexes. Inorg. Chem. 2016, 55, 6018–6026. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Li, S.; Bu, R.; Gou, R.-J.; Zhang, C. Hirshfeld Surface Method and Its Application in Energetic Crystals. Cryst. Growth Des. 2021, 21, 6619–6634. [Google Scholar] [CrossRef]
- Martin, A.D.; Britton, J.; Easun, T.L.; Blake, A.J.; Lewis, W.; Schröder, M. Hirshfeld Surface Investigation of Structure-Directing Interactions within Dipicolinic Acid Derivatives. Cryst. Growth Des. 2015, 15, 1697–1706. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Castro, I.; Lloret, F.; Martínez-Lillo, J. Molecular Self-Assembly in a Family of Oxo-Bridged Dinuclear Ruthenium(IV) Systems. Cryst. Growth Des. 2020, 20, 2044–2056. [Google Scholar] [CrossRef]
- Sanchis-Perucho, A.; Orts-Arroyo, M.; Camús-Hernández, J.; Rojas-Dotti, C.; Escrivà, E.; Lloret, F.; Martínez-Lillo, J. Hexahalorhenate(IV) salts of protonated ciprofloxacin: Antibiotic-based single-ion magnets. CrystEngComm 2021, 23, 8579–8587. [Google Scholar] [CrossRef]
- Sanchis-Perucho, A.; Orts-Arroyo, M.; Castro, I.; Lloret, F.; Martínez-Lillo, J. Crystal polymorphism in 2,2’-bipyrimidine-based iridium(III) complexes. J. Coord. Chem. 2022, 75, 2495–2507. [Google Scholar] [CrossRef]
- McAdams, S.G.; Ariciu, A.M.; Kostopoulos, A.K.; Walsh, J.P.S.; Tuna, F. Molecular single-ion magnets based on lanthanides and actinides: Design considerations and new advances in the context of quantum technologies. Coord. Chem. Rev. 2017, 346, 216–239. [Google Scholar] [CrossRef] [Green Version]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-ac to dysprosium metallocenes, a travel in time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Uysal, Ş. The Synthesis and Characterization of Star Shaped Metal Complexes of Triazine Cored Schiff Bases: Their Thermal Decompositions and Magnetic Moment Values. J. Inorg. Organomet. Pol. Mat. 2011, 21, 291–296. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Álvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Chiozzone, R.; González, R.; Kremer, C.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M.; Faus, J. Synthesis, Crystal Structure, and Magnetic Properties of Tetraphenylarsonium Tetrachloro(oxalato)rhenate(IV) and Bis(2,2‘-bipyridine)tetrachloro(μ-oxalato)copper(II)rhenium(IV). Inorg. Chem. 1999, 38, 4745–4752. [Google Scholar] [CrossRef]
- SHELXTL-2013/4; Bruker Analytical X-ray Instruments. Bruker: Madison, WI, USA, 2013.
- Diamond, version 4.5.0; Crystal Impact GbR: Bonn, Germany, 2018.
- CrystalMaker, version 8.5.1; CrystalMaker Software Ltd.: Oxford, UK.
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]







| Compound | 1 | 2 |
|---|---|---|
| CIF | 2,233,243 | 2,233,244 |
| Formula | C18H15Cl8N9Re2 | C18H18Cl8N8O3Re2 |
| Fw/g mol−1 | 1013.39 | 1050.40 |
| Temperature/K | 120(2) | 120(2) |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/n | P21/c |
| a/Å | 13.239(1) | 11.960(1) |
| b/Å | 11.852(1) | 18.089(1) |
| c/Å | 17.666(1) | 13.433(1) |
| α/° | 90 | 90 |
| β/° | 90.02(1) | 93.02(1) |
| γ/° | 90 | 90 |
| V/Å3 | 2771.97(13) | 2902.30(10) |
| Z | 4 | 4 |
| Dc/g cm−3 | 2.428 | 2.404 |
| μ(Mo-Kα)/mm−1 | 9.526 | 9.110 |
| F(000) | 1888 | 1968 |
| Goodness-of-fit on F2 | 1.307 | 1.061 |
| R1 [I > 2σ(I)]/all data | 0.0221/0.0252 | 0.0377/0.0424 |
| wR2 [I > 2σ(I)] /all data | 0.0649/0.0773 | 0.0793/0.0816 |
| D-H⋯A | D-H/Å | H⋯A/Å | D⋯A/Å | (DHA)/° |
|---|---|---|---|---|
| O(1w)-H(1wB)⋯N(4) | 0.927 | 1.91(1) | 2.804(1) | 162.7(1) |
| O(1w)-H(1wA)⋯N(7a) | 0.935 | 1.97(1) | 2.866(1) | 160.8(1) |
| O(1w)-H(1wA)⋯N(8a) | 0.935 | 2.67(1) | 3.301(1) | 125.4(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchis-Perucho, A.; Orts-Arroyo, M.; Moliner, N.; Martínez-Lillo, J. Effect of the Solvent on the Crystallographic and Magnetic Properties of Rhenium(IV) Complexes Based on 2,2′-Bipyrimidine Ligand. Inorganics 2023, 11, 78. https://doi.org/10.3390/inorganics11020078
Sanchis-Perucho A, Orts-Arroyo M, Moliner N, Martínez-Lillo J. Effect of the Solvent on the Crystallographic and Magnetic Properties of Rhenium(IV) Complexes Based on 2,2′-Bipyrimidine Ligand. Inorganics. 2023; 11(2):78. https://doi.org/10.3390/inorganics11020078
Chicago/Turabian StyleSanchis-Perucho, Adrián, Marta Orts-Arroyo, Nicolas Moliner, and José Martínez-Lillo. 2023. "Effect of the Solvent on the Crystallographic and Magnetic Properties of Rhenium(IV) Complexes Based on 2,2′-Bipyrimidine Ligand" Inorganics 11, no. 2: 78. https://doi.org/10.3390/inorganics11020078
APA StyleSanchis-Perucho, A., Orts-Arroyo, M., Moliner, N., & Martínez-Lillo, J. (2023). Effect of the Solvent on the Crystallographic and Magnetic Properties of Rhenium(IV) Complexes Based on 2,2′-Bipyrimidine Ligand. Inorganics, 11(2), 78. https://doi.org/10.3390/inorganics11020078