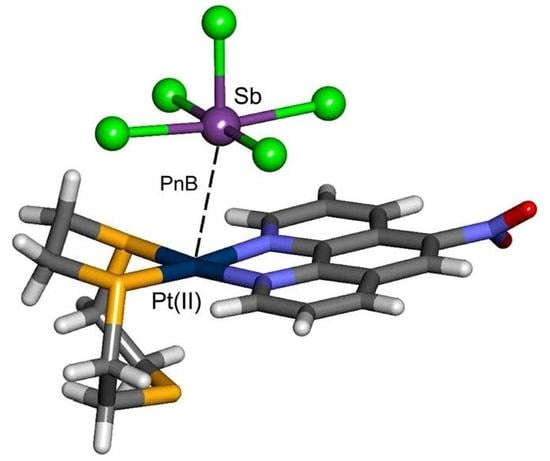
Square Planar Pt(II) Ion as Electron Donor in Pnictogen Bonding Interactions
Abstract
:1. Introduction
2. Results and Discussion
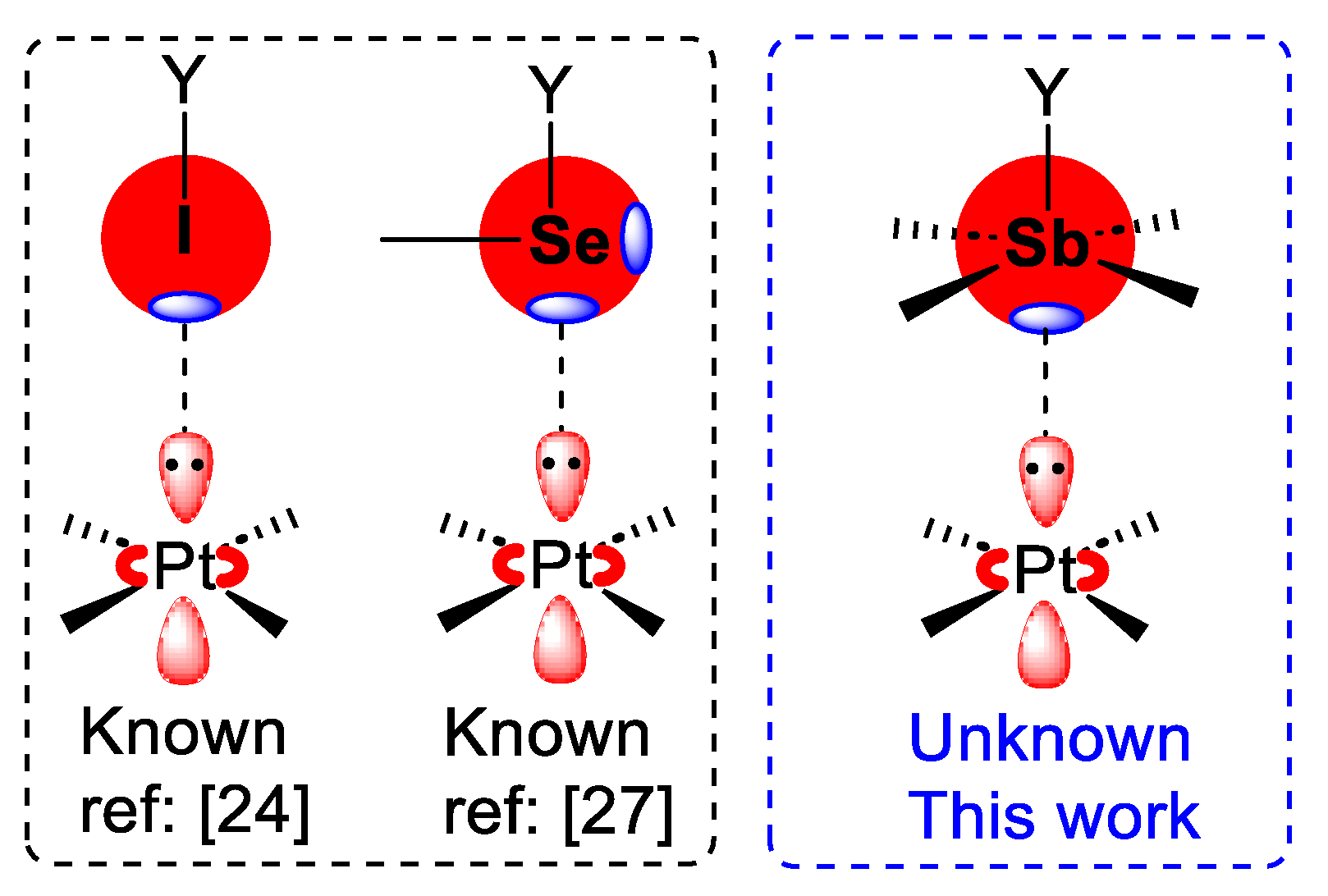
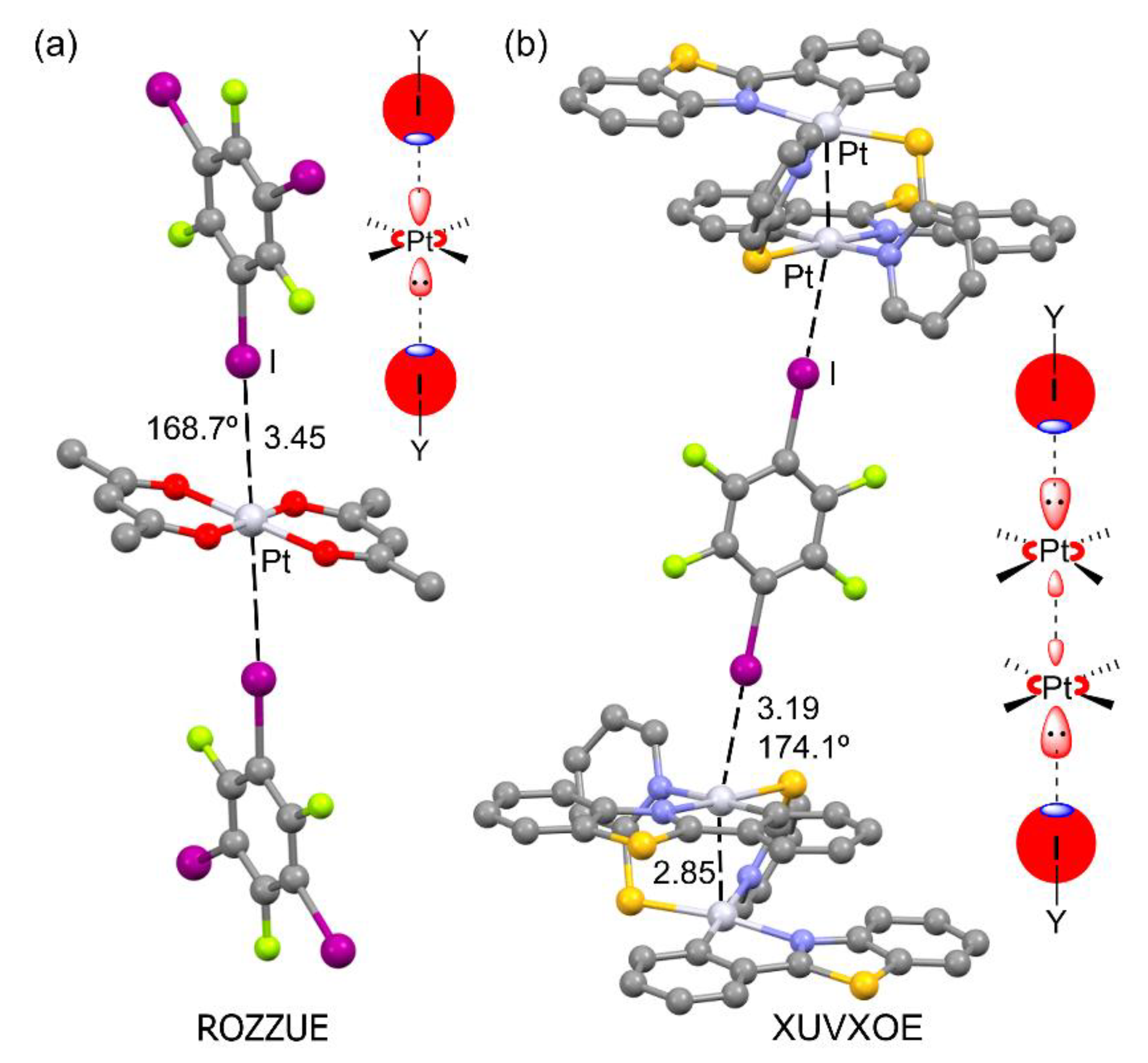
2.1. Preliminary Work on Pt···Halogen and Pt···Chalcogen Bonds
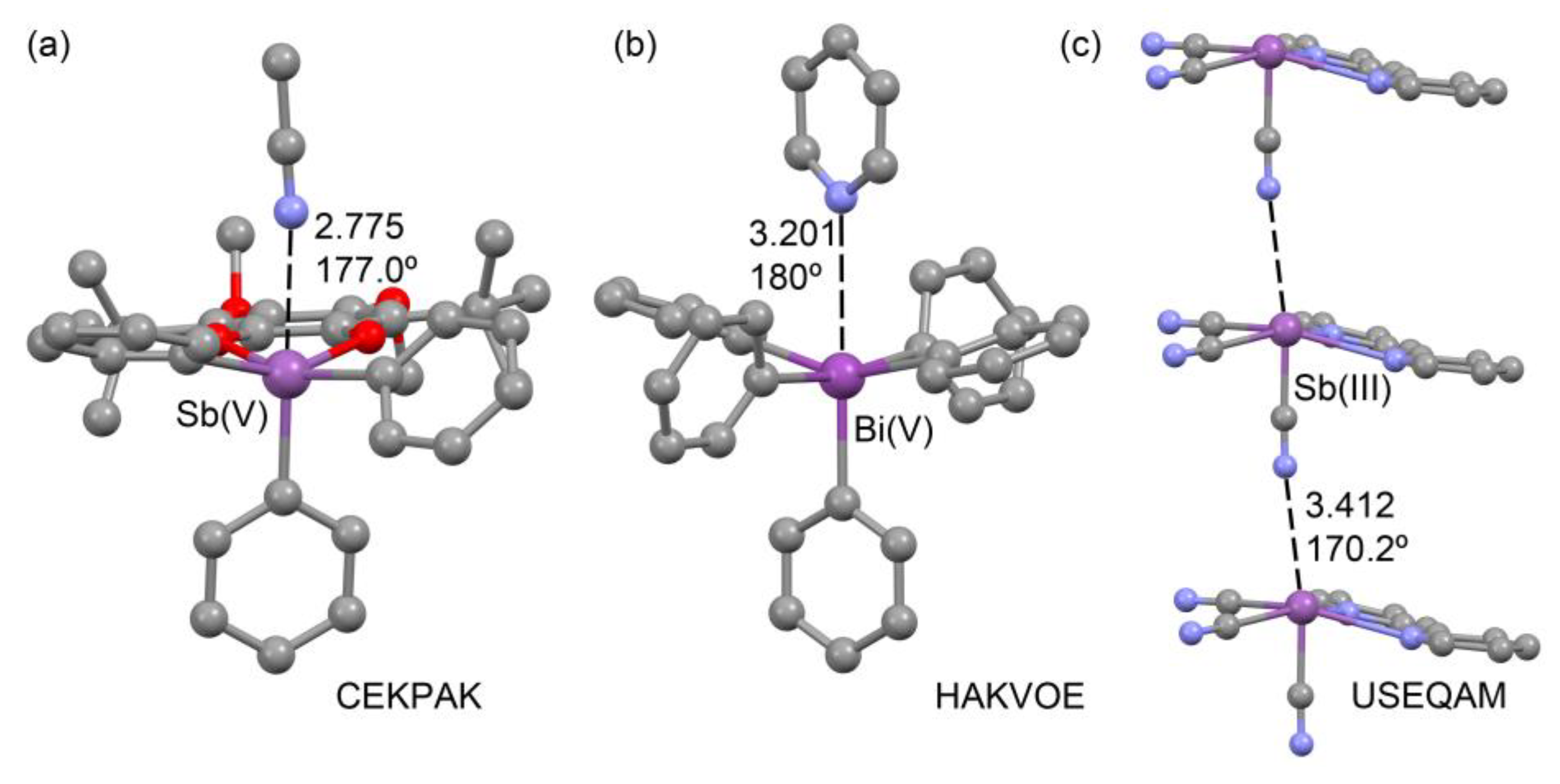
2.2. X-ray Structures Exhibiting Sb,Bi···N Pnictogen Bonds
2.3. X-ray Structures Exhibiting Sb···O Pnictogen Bonds
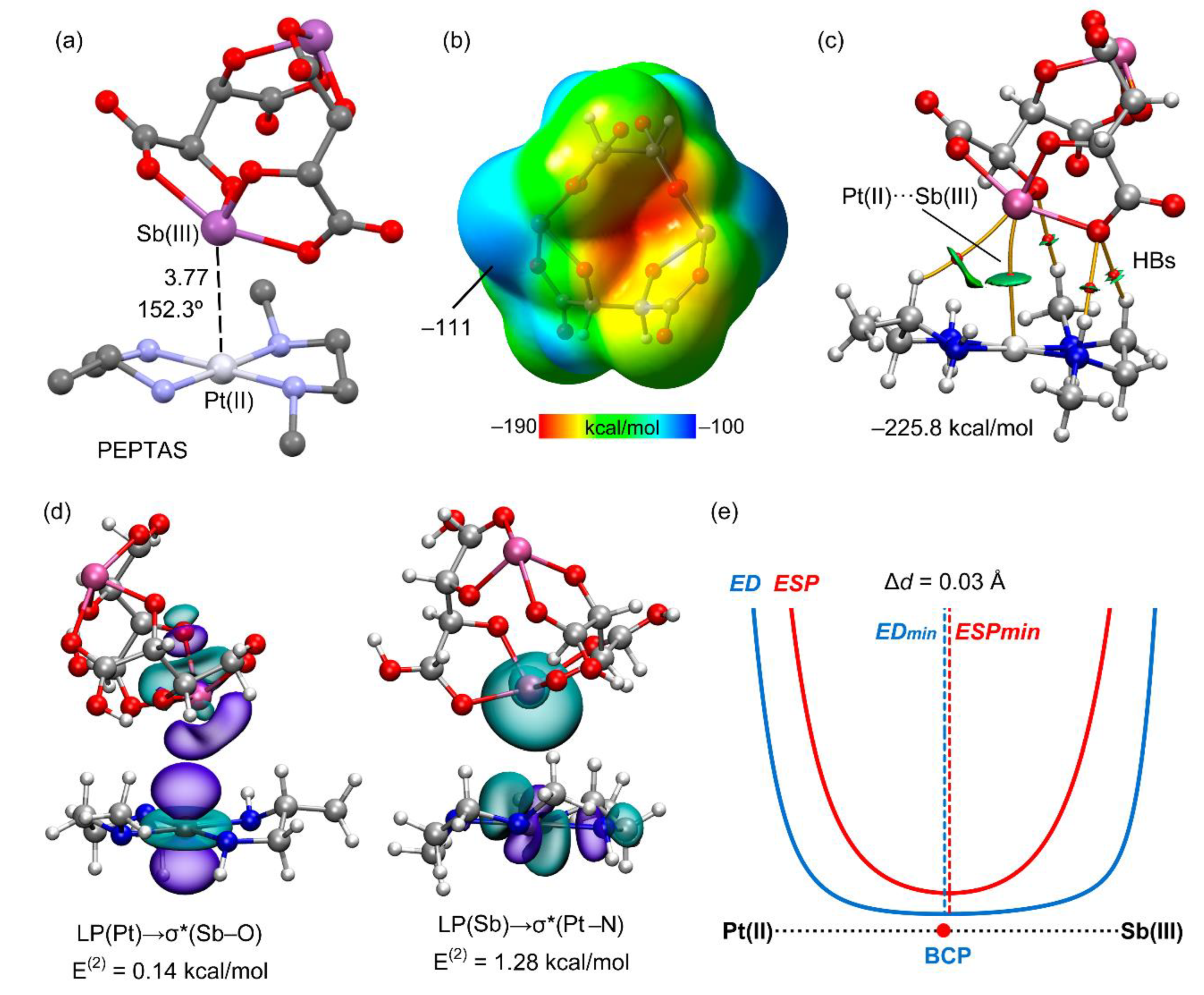
2.4. X-ray Structures Exhibiting Sb···Pt Pnictogen Bonds
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkorta, I.; Elguero, J.; Frontera, A. Not only hydrogen bonds: Other noncovalent interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Bauzá, A.; Frontera, A. On the Importance of σ–Hole Interactions in Crystal Structures. Crystals 2021, 11, 1205. [Google Scholar]
- Metrangolo, P.; Resnati, G. Halogen bonding: A paradigm in supramolecular chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Vogel, L.; Wonner, P.; Huber, S.M. Chalcogen bonding: An overview. Angew. Chem. Int. Ed. 2019, 58, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Chalcogen bonding in synthesis, catalysis and design of materials. Dalton Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Frontera, A.; Gutiérrez López, M.A.; Sakai, N.; Matile, S. Pnictogen-Bonding Catalysts, Compared to Tetrel-Bonding Catalysts: More Than Just Weak Lewis Acids. Helv. Chim. Acta 2022, 105, e202200119. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Fourmigué, M.; Dhaka, A. Chalcogen bonding in crystalline diselenides and selenocyanates: From molecules of pharmaceutical interest to conducting materials. Coord. Chem. Rev. 2020, 403, 213084. [Google Scholar] [CrossRef]
- Biot, N.; Bonifazi, D. Chalcogen-bond driven molecular recognition at work. Coord. Chem. Rev. 2020, 413, 213243. [Google Scholar] [CrossRef]
- Navarro-García, E.; Galmés, B.; Esquivel, J.L.; Velasco, M.D.; Bastida, A.; Zapata, F.; Caballero, A.; Frontera, A. Host–guest complexes vs. supramolecular polymers in chalcogen bonding receptors: An experimental and theoretical study. Dalton Trans. 2022, 51, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Navarro-García, E.; Galmés, B.; Velasco, M.D.; Frontera, A.; Caballero, A. Anion Recognition by Neutral Chalcogen Bonding Receptors: Experimental and Theoretical Investigations. Chem. Eur. J. 2020, 26, 4706–4713. [Google Scholar] [CrossRef]
- Frontera, A.; Bauza, A. Metal Coordination Enhances Chalcogen Bonds: CSD Survey and Theoretical Calculations. Int. J. Mol. Sci. 2022, 23, 4188. [Google Scholar] [CrossRef]
- Scheiner, S. The pnicogen bond: Its relation to hydrogen, halogen, and other noncovalent bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, J.E.; Alkorta, I.; Elguero, J. The Pnicogen Bond in Review: Structures, Binding Energies, Bonding Properties, and Spin–spin Coupling Constants of Complexes Stabilized by Pnicogen Bonds. In Noncovalent Forces; Scheiner, S., Ed.; Springer: Heidelberg/Berlin, Germany, 2015; pp. 191–264. [Google Scholar]
- Brammer, L. Halogen bonding, chalcogen bonding, pnictogen bonding, tetrel bonding: Origins, current status and discussion. Faraday Discuss. 2017, 203, 485–507. [Google Scholar] [CrossRef] [Green Version]
- Daolio, A.; Scilabra, P.; Terraneo, G.; Resnati, G. C(sp3) atoms as tetrel bond donors: A crystallographic survey. Coord. Chem. Rev. 2020, 413, 213265. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Haukka, M.; Levin, O.V.; Frontera, A.; Kukushkin, V.Y. Supramolecular Assembly of Metal Complexes by (Aryl) dz2 [PtII] Halogen Bond. Chem. Eur. J. 2020, 26, 7692–7701. [Google Scholar] [CrossRef]
- Bikbaeva, Z.M.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Electrophilic–Nucleophilic Dualism of Nickel(II) toward Ni·I Noncovalent Interactions: Semicoordination of Iodine Centers via Electron Belt and Halogen Bonding via σ-Hole. Inorg. Chem. 2017, 56, 13562–13578. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Eliseeva, A.A.; Baykov, S.V.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. One-Pot Route to X-perfluoroarenes (X = Br, I) Based on FeIII-Assisted C–F Functionalization and Utilization of These Arenes as Building Blocks for Crystal Engineering Involving Halogen Bonding. Cryst. Growth Des. 2020, 20, 5908–5921. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Rozhkov, A.V.; Ananyev, I.V.; Frontera, A.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Two Rhodium (I) Centers as an Integrated σ–Hole Acceptor. JACS Au 2021, 1, 354–361. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Bokach, N.A.; Kukushkin, V.Y.; Frontera, A. Metal Centers as Nucleophiles: Oxymoron of Halogen Bond-Involving Crystal Engineering. Chem. Eur. J. 2022, 28, e202103173. [Google Scholar] [PubMed]
- Blasi, D.; Nicolai, V.; Gomila, R.M.; Mercandelli, P.; Frontera, A.; Carlucci, L. Unprecedented {dz2-CuIIO4}⋯π-hole interactions: The case of a cocrystal of a Cu(II) bis-β-diketonate complex with 1,4-diiodotetrafluoro-benzene. Chem. Commun. 2022, 58, 9524. [Google Scholar] [CrossRef] [PubMed]
- Rogachev, A.Y.; Hoffmann, R. Iodine (I2) as a Janus-Faced Ligand in Organometallics. J. Am. Chem. Soc. 2013, 135, 3262–3275. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Katlenok, E.A.; Zhmykhova, M.V.; Ivanov, A.Y.; Kuznetsov, M.L.; Bokach, N.A.; Kukushkin, V.Y. Metal-Involving Chalcogen Bond. The Case of Platinum(II) Interaction with Se/Te-Based σ-Hole Donors. J. Am. Chem. Soc. 2021, 143, 15701–15710. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.J.; Wade, C.R.; Gabbaï, F.P. Redox and Anion Exchange Chemistry of a Stibine-Nickel Complex: Writing the L, X, Z Ligand Alphabet with a Single Element. Angew. Chem. Int. Ed. 2014, 53, 8876–8879. [Google Scholar] [CrossRef]
- Ke, I.-S.; Gabbaï, F.G. σ-Donor/Acceptor-Confused Ligands: The Case of a Clorostibine. Inorg. Chem. 2013, 52, 7145–7151. [Google Scholar] [CrossRef] [PubMed]
- Gomila, R.M.; Frontera, A. On the energetic stability of halogen bonds involving metals: Implications in crystal engineering. CrystEngComm 2022, 24, 4440–4446. [Google Scholar]
- Rozhkov, A.V.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-involving halogen bond Ar–I⋯[dz2PtII] in a platinum acetylacetonate complex. CrystEngComm 2020, 22, 554–563. [Google Scholar] [CrossRef]
- Klauke, K.; Gruber, I.; Knedel, T.-O.; Schmolke, L.; Barthel, J.; Breitzke, H.; Buntkowsky, G.; Janiak, C. Silver, Gold, Palladium, and Platinum N-heterocyclic Carbene Complexes Containing a Selenoether-Functionalized Imidazol-2-ylidene Moiety. Organometallics 2018, 37, 298–308. [Google Scholar] [CrossRef]
- Kirsten, L.; Schwade, L.D.; Selter, L.; Hagenbach, A.; Piquini, P.C.; Lang, E.S.; Abram, U. Pt, Pd and Hg Complexes with Potentially Tridentate Telluroether Ligands. Eur. J. Inorg. Chem. 2015, 2015, 3748–3757. [Google Scholar] [CrossRef]
- Gomila, R.M.; Bauzá, A.; Frontera, A. Enhancing chalcogen bonding by metal coordination. Dalton Trans. 2022, 51, 5977–5982. [Google Scholar] [CrossRef]
- Aliyeva, V.; Gurbanov, A.V.; Mahmoud, A.G.; Gomila, R.M.; Frontera, A.; Mahmudov, K.T.; Pombeiro, A.J.L. Chalcogen bonding in copper(II)-mediated synthesis. Faraday Discuss. 2023; in press. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Jin, S.; Ye, X.; Hu, K.; Xu, W.; Wang, D. Syntheses and structural characterization of eight inorganic-organic hybrid solids based on N-Brønsted bases and halometallates of Zn, Cu, Sb and Bi. Inorg. Nano-Metal Chem. 2022, 52, 284–296. [Google Scholar] [CrossRef]
- Liu, B.; Xu, L.; Guo, G.-C.; Huang, J.-S. Three inorganic–organic hybrids of bismuth(III) iodide complexes containing substituted 1,2,4-triazole organic components with charaterizations of diffuse reflectance spectra. J. Solid State Chem. 2006, 179, 1611–1617. [Google Scholar] [CrossRef]
- Kotov, V.Y.; Ilyukhin, A.B.; Buikin, P.A.; Simonenko, N.P.; Korlyukov, A.A.; Smol’yakov, A.F.; Yorov, K.E.; Gavrikov, A.V. Unexpected hydrolytic transformation of new type hybrid bromobismuthates with methylpyrazinium dications. Dalton Trans. 2019, 48, 7602–7611. [Google Scholar] [CrossRef]
- Cherkasov, V.K.; Abakumov, G.A.; Grunova, E.V.; Poddel’sky, A.I.; Fukin, G.K.; Baranov, E.V.; Kurskii, Y.V.; Abakumova, L.G. Triphenylantimony(v) Catecholates and o-Amidophenolates: Reversible Binding of Molecular Oxygen. Chem. Eur. J. 2006, 12, 3916–3927. [Google Scholar] [CrossRef]
- Wallenhauer, S.; Leopold, D.; Seppelt, K. Hexacoordinate organobismuth compounds. Inorg. Chem. 1993, 32, 3948–3951. [Google Scholar] [CrossRef]
- Deokar, P.; Leitz, D.; Stein, T.H.; Vasiliu, M.; Dixon, D.A.; Christe, K.O.; Haiges, R. Preparation and Characterization of Antimony and Arsenic Tricyanide and Their 2,2′-Bipyridine Adducts. Chem. Eur. J. 2016, 22, 13251–13257. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional σ/π-Hole Interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Chang, T.; Zeng, R.; Cao, S.; Zhao, J.; Han, X.; Wang, L.; Zou, B. Self-Trapped Exciton Emission in a Zero-Dimensional (TMA)2SbCl5·DMF Single Crystal and Molecular Dynamics Simulation of Structural Stability. J. Phys. Chem. Lett. 2021, 12, 7091–7099. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Senchurin, V.S.; Sharutina, O.K.; Kunkurdonova, B.B.; Burlakova, A.G. Synthesis and structure of antimony complexes [Ph3AmP]+2 [Sb2I8·2DMSO]2−, [Ph3PrP]+2[Sb2I8 · C4H8O2]2−, and [(HOCH2CH2)3NH]+4[Sb4I16]4−. Russ. J. Inorg. Chem. 2011, 56, 197–204. [Google Scholar] [CrossRef]
- Chatterjee, S.; Krause, J.A.; Connick, W.B.; Genre, C.; Rodrigue-Witchel, A.; Reber, C. Interaction of SbCl52− and Thioether Groups at the Open Coordination Sites of Platinum(II) Diimine Complexes. Inorg. Chem. 2010, 49, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Mata, I.; Molins, E.; Alkorta, I.; Espinosa, E. Topological Properties of the Electrostatic Potential in Weak and Moderate N···H Hydrogen Bonds. J. Phys. Chem. A 2007, 111, 6425–6433. [Google Scholar] [CrossRef]
- Bartashevich, E.V.; Matveychuk, Y.V.; Troitskaya, E.A.; Tsirelson, V.G. Characterizing the multiple non-covalent interactions in N, S-heterocycles–diiodine complexes with focus on halogen bonding. Comput. Theor. Chem. 2014, 1037, 53–62. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ooi, S.; Sakuma, M.; Kuroya, H. The Crystal Structure of (+)350-(R)-Propylenediamine-N,N′-dimethylethylenediamineplatinum(II) Di-μ-(+)-tartrato(4–)bis(antimonate(III)) Dihydrate. Bull. Chem. Soc. Jpn. 1976, 49, 2129–2132. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mat. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Mertsalov, D.F.; Gomila, R.M.; Zaytsev, V.P.; Grigoriev, M.S.; Nikitina, E.V.; Zubkov, F.I.; Frontera, A. On the Importance of Halogen Bonding Interactions in Two X-ray Structures Containing All Four (F, Cl, Br, I) Halogen Atoms. Crystals 2021, 11, 1406. [Google Scholar] [CrossRef]
- Gomila, R.M.; Frontera, A. Matere Bonds vs. Multivalent Halogen and Chalcogen Bonds: Three Case Studies. Molecules 2022, 27, 6597. [Google Scholar] [CrossRef] [PubMed]
- Roselló, Y.; Benito, M.; Molins, E.; Barceló-Oliver, M.; Frontera, A. Adenine as a Halogen Bond Acceptor: A Combined Experimental and DFT Study. Crystals 2019, 9, 224. [Google Scholar]
- Rolfes, J.D.; Neese, F.; Pantazis, D.A. All-electron scalar relativistic basis sets for the elements Rb–Xe. J. Comput. Chem. 2020, 41, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Canal Neto, A.; Ferreira, I.B.; Jorge, F.E.; de Oliveira, A.Z. All-electron triple zeta basis sets for ZORA calculations: Application in studies of atoms and molecules. Chem. Phys. Lett. 2021, 771, 138548. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting non-covalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural bond orbital methods. WIREs Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Karafiloglou, P.; Landis, C.R.; Weinhold, F. NBO 7.0: New Vistas in Localized and Delocalized Chemical Bonding Theory; Theoretical Chemistry Institute, University of Wisconsin-Madison: Madison, WI, USA, 2018. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burguera, S.; Gomila, R.M.; Bauzá, A.; Frontera, A. Square Planar Pt(II) Ion as Electron Donor in Pnictogen Bonding Interactions. Inorganics 2023, 11, 80. https://doi.org/10.3390/inorganics11020080
Burguera S, Gomila RM, Bauzá A, Frontera A. Square Planar Pt(II) Ion as Electron Donor in Pnictogen Bonding Interactions. Inorganics. 2023; 11(2):80. https://doi.org/10.3390/inorganics11020080
Chicago/Turabian StyleBurguera, Sergi, Rosa M. Gomila, Antonio Bauzá, and Antonio Frontera. 2023. "Square Planar Pt(II) Ion as Electron Donor in Pnictogen Bonding Interactions" Inorganics 11, no. 2: 80. https://doi.org/10.3390/inorganics11020080
APA StyleBurguera, S., Gomila, R. M., Bauzá, A., & Frontera, A. (2023). Square Planar Pt(II) Ion as Electron Donor in Pnictogen Bonding Interactions. Inorganics, 11(2), 80. https://doi.org/10.3390/inorganics11020080