Sol-Gel Synthesized Nickel-Oxide-Based Fabrication of Arsenic (As3+) Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of NiO Nanoparticles
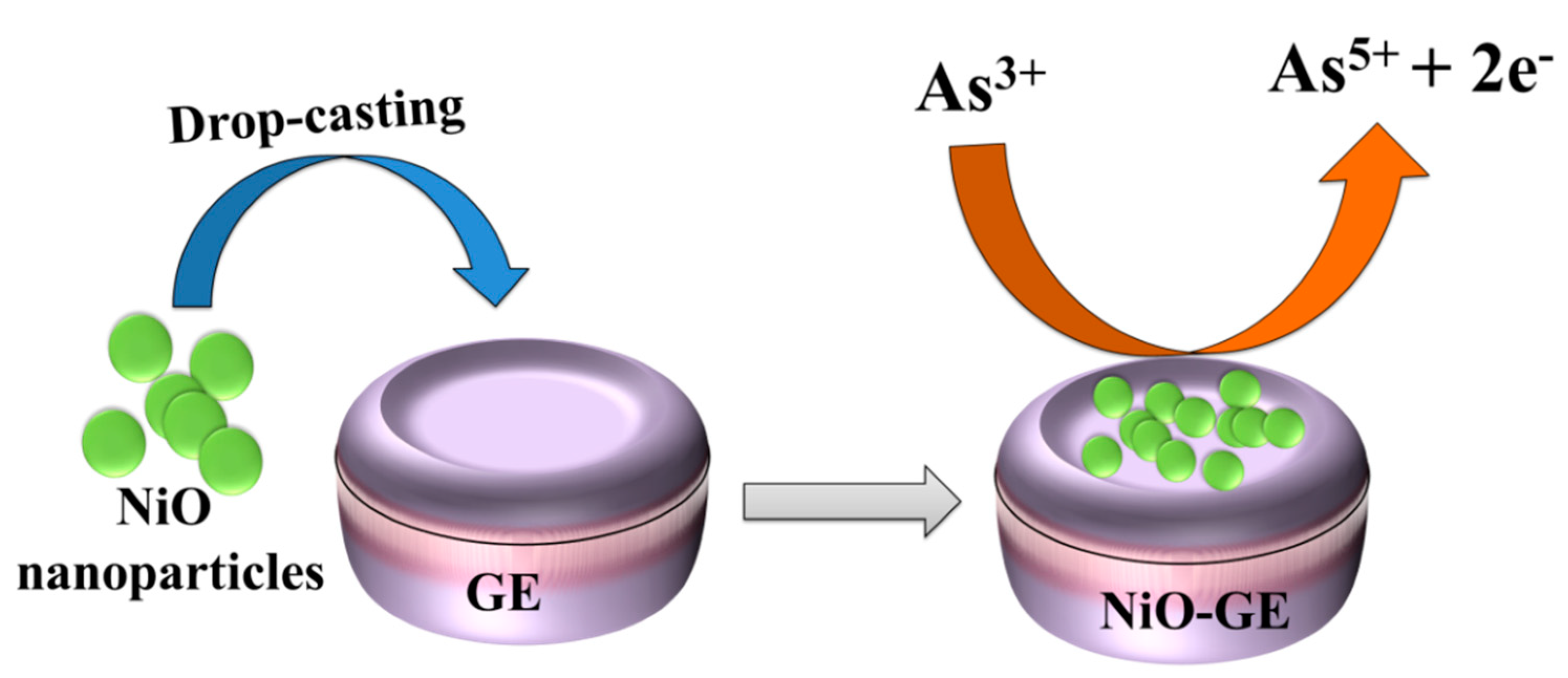
2.3. Fabrication of the Glassy Carbon Electrode
2.4. Instrumental
3. Results and Discussion
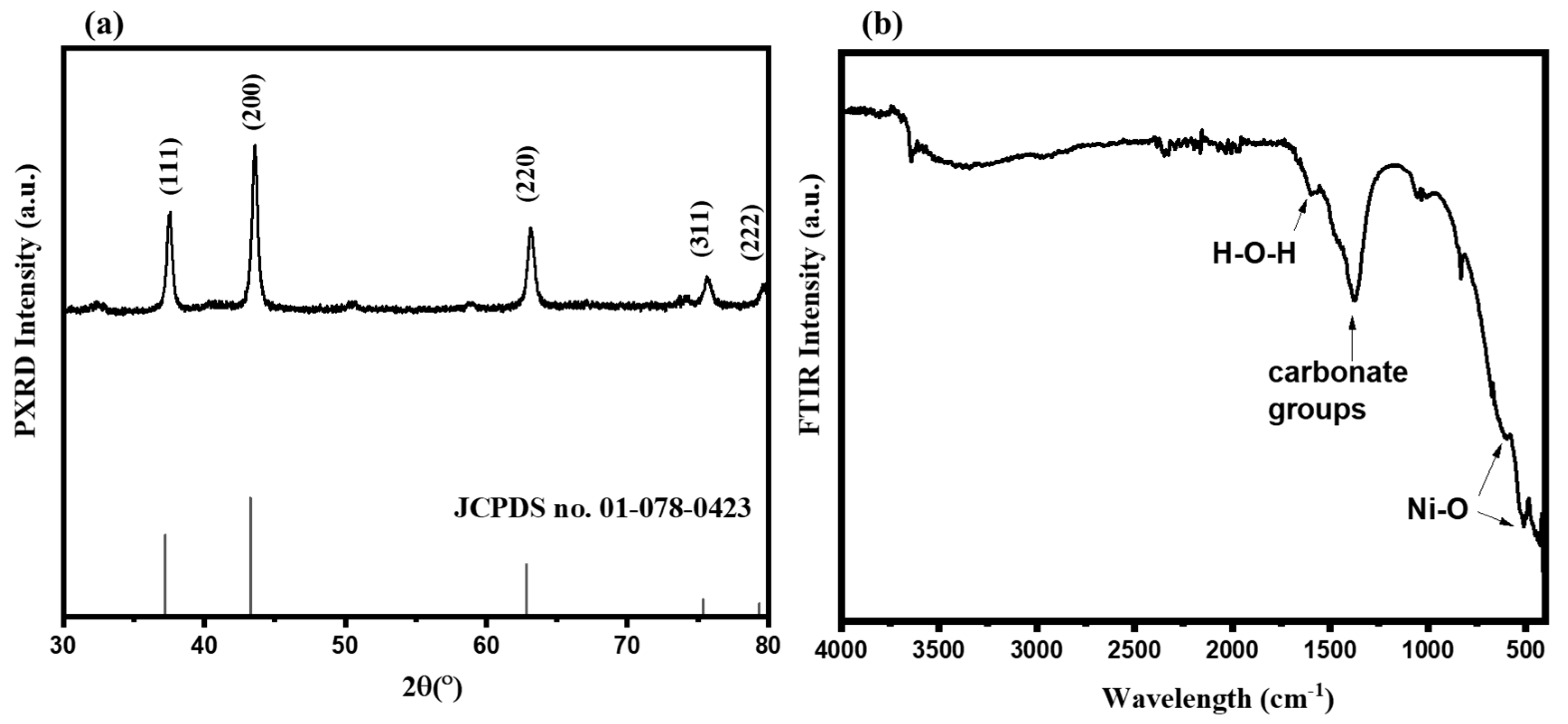
3.1. Physiochemical Characterization
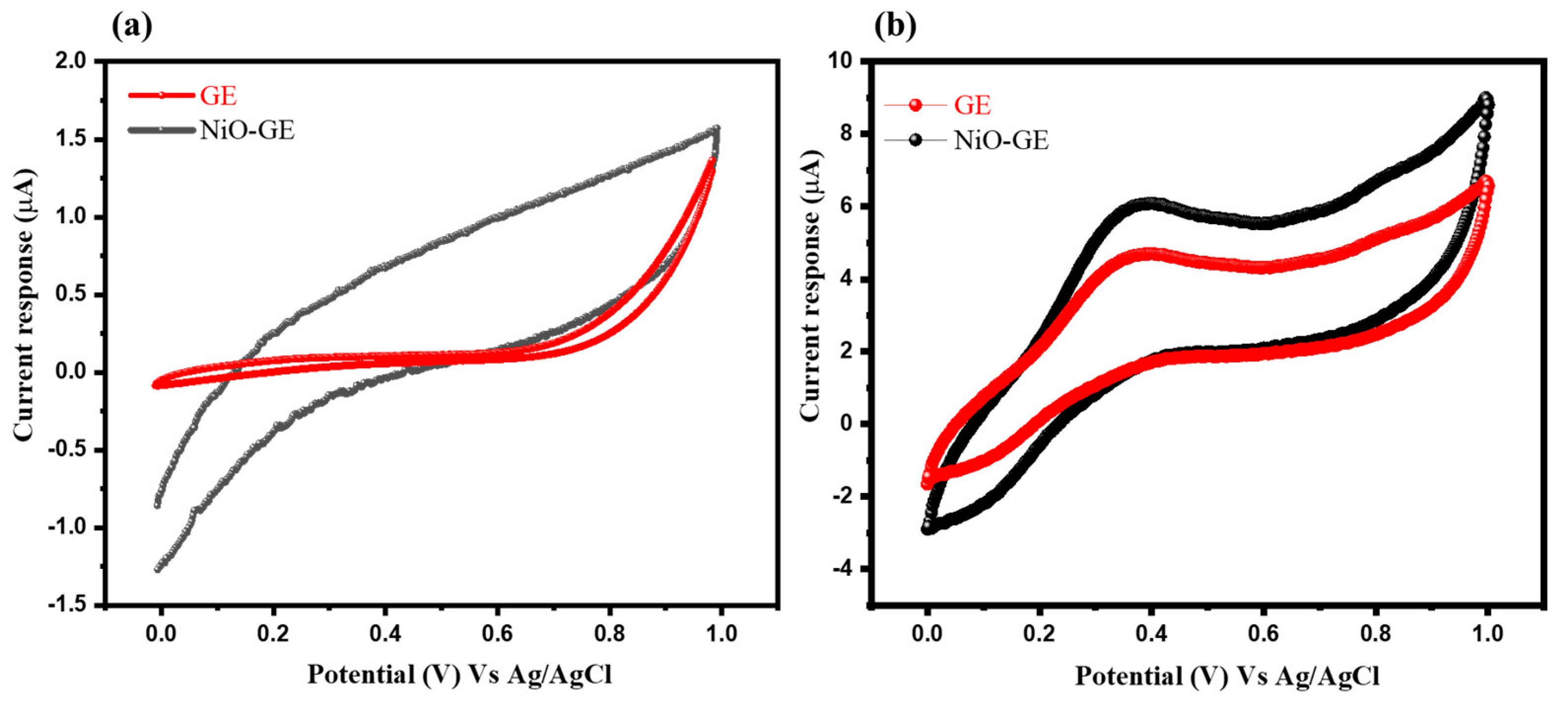
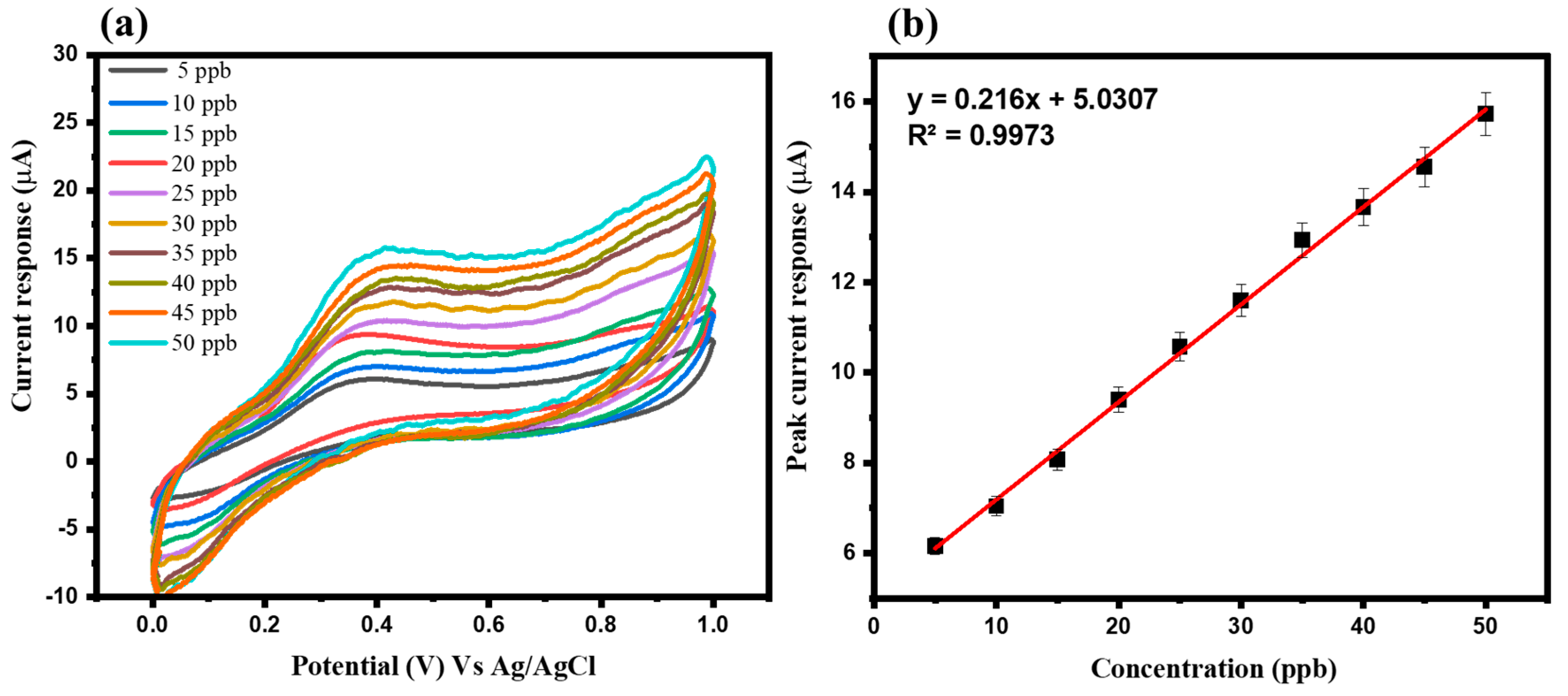
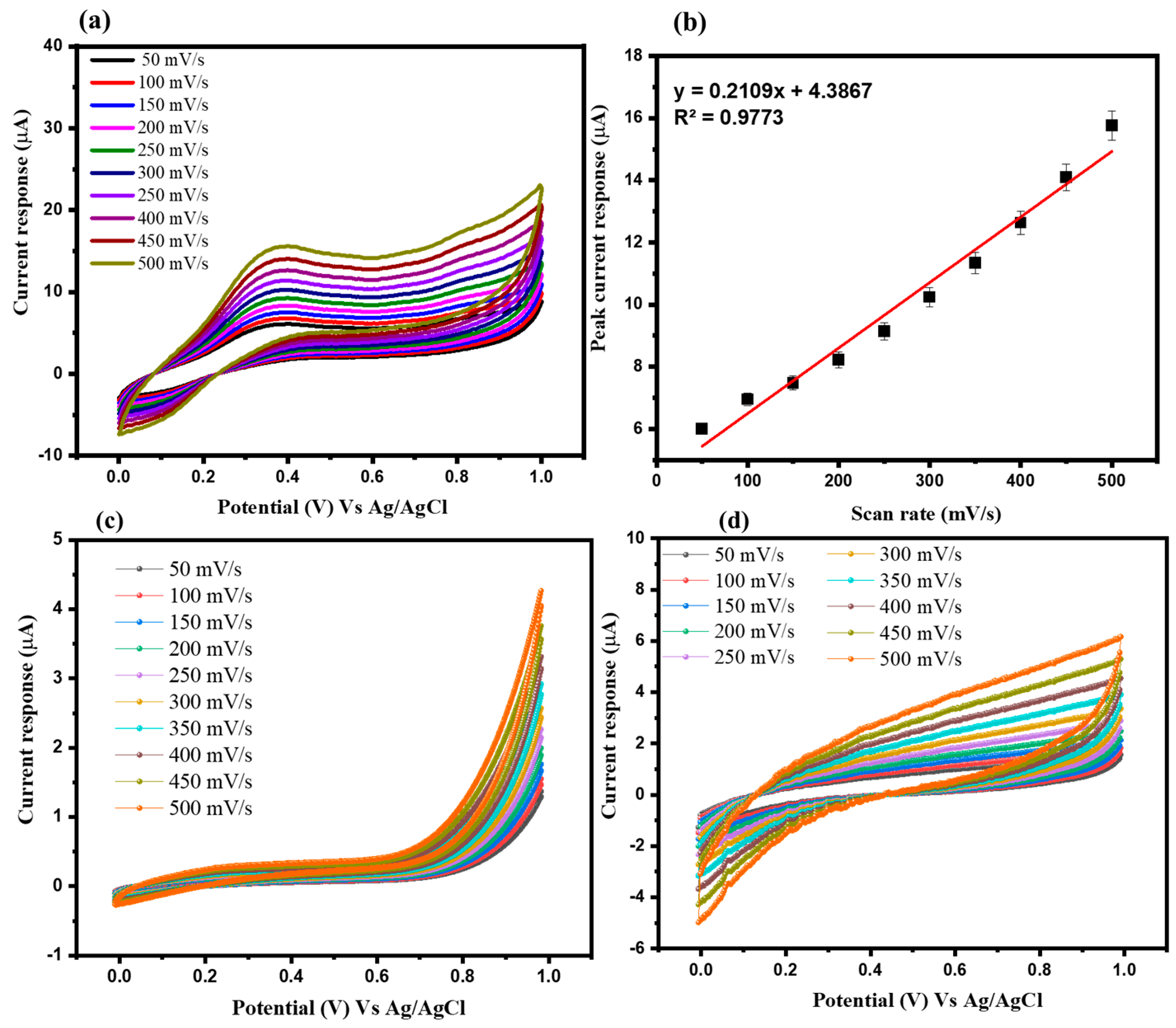
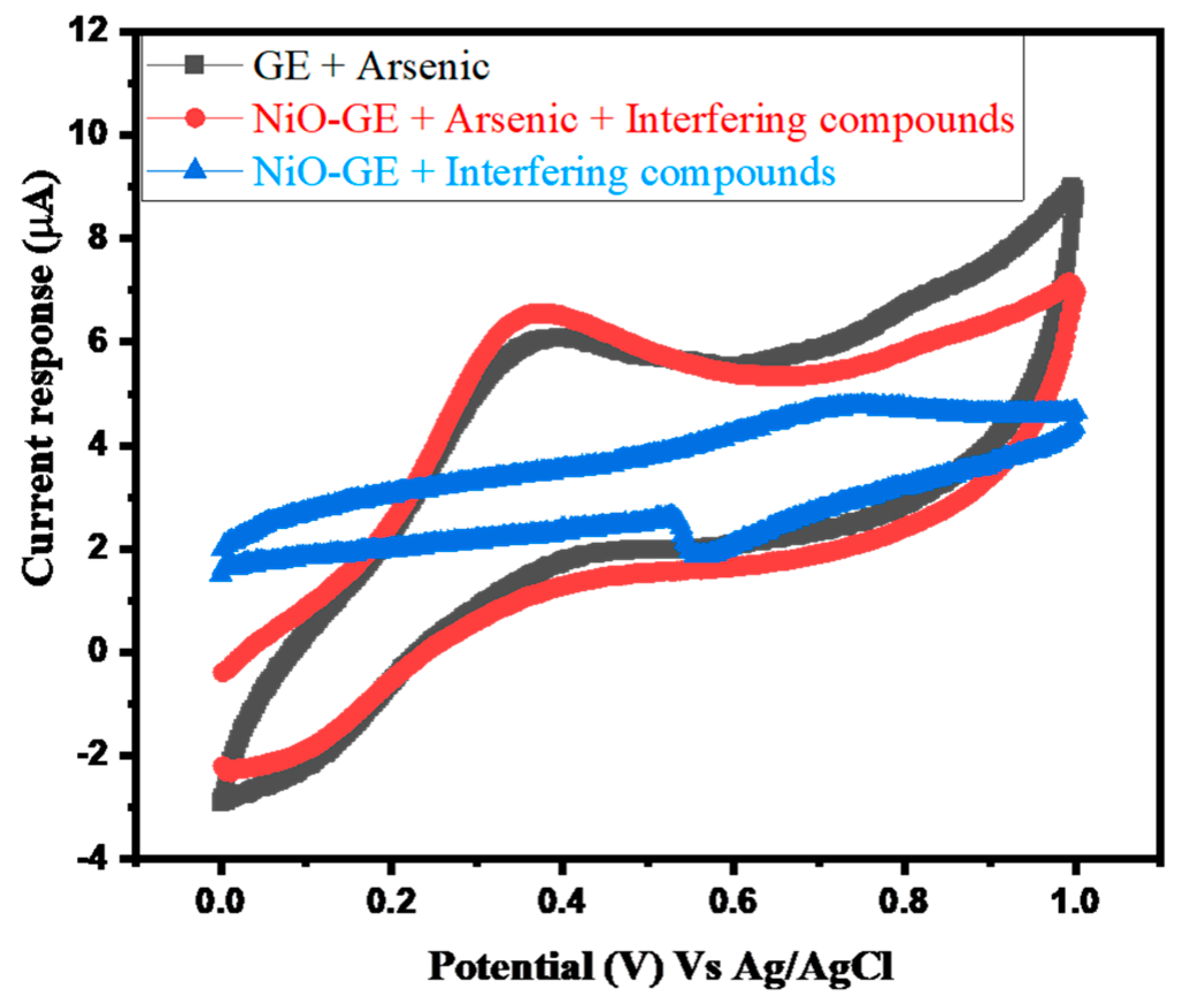
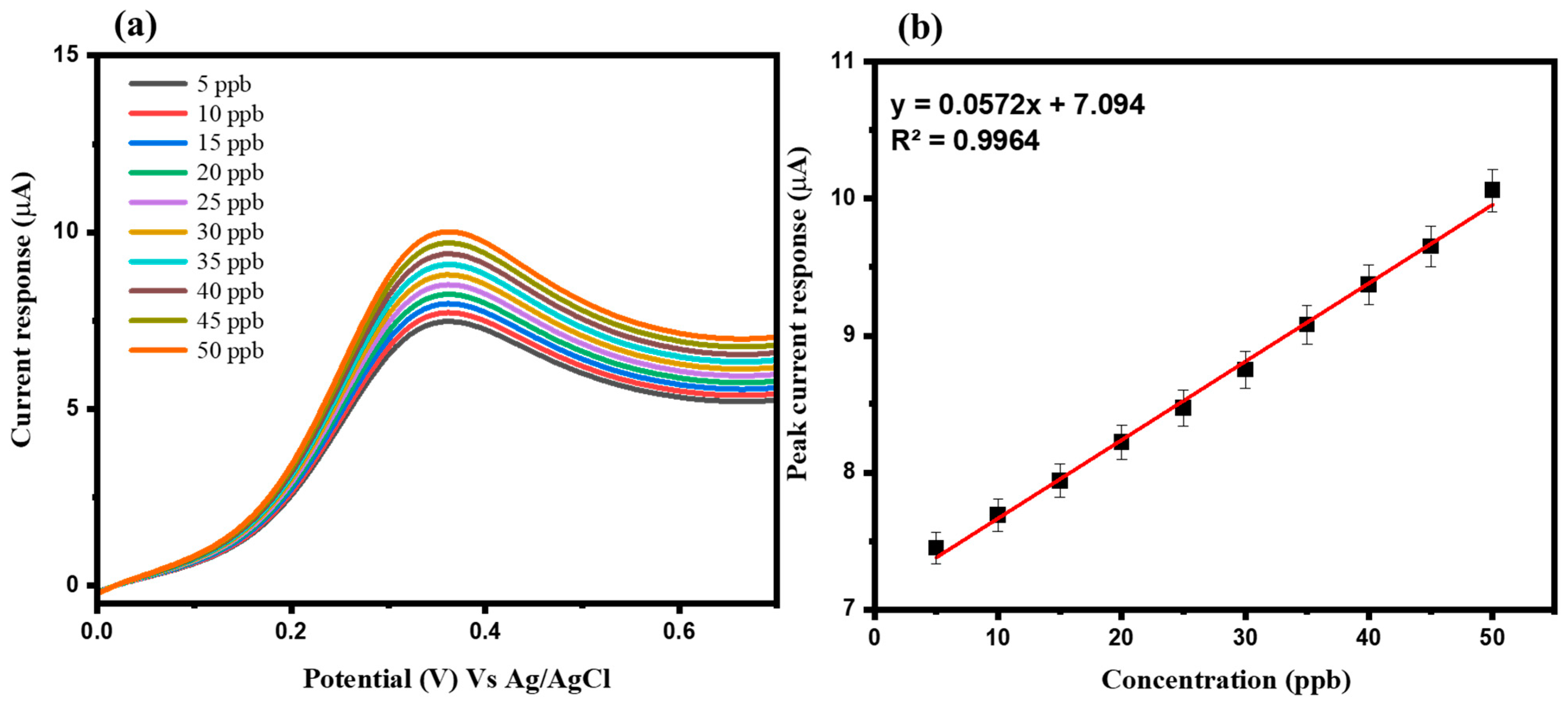
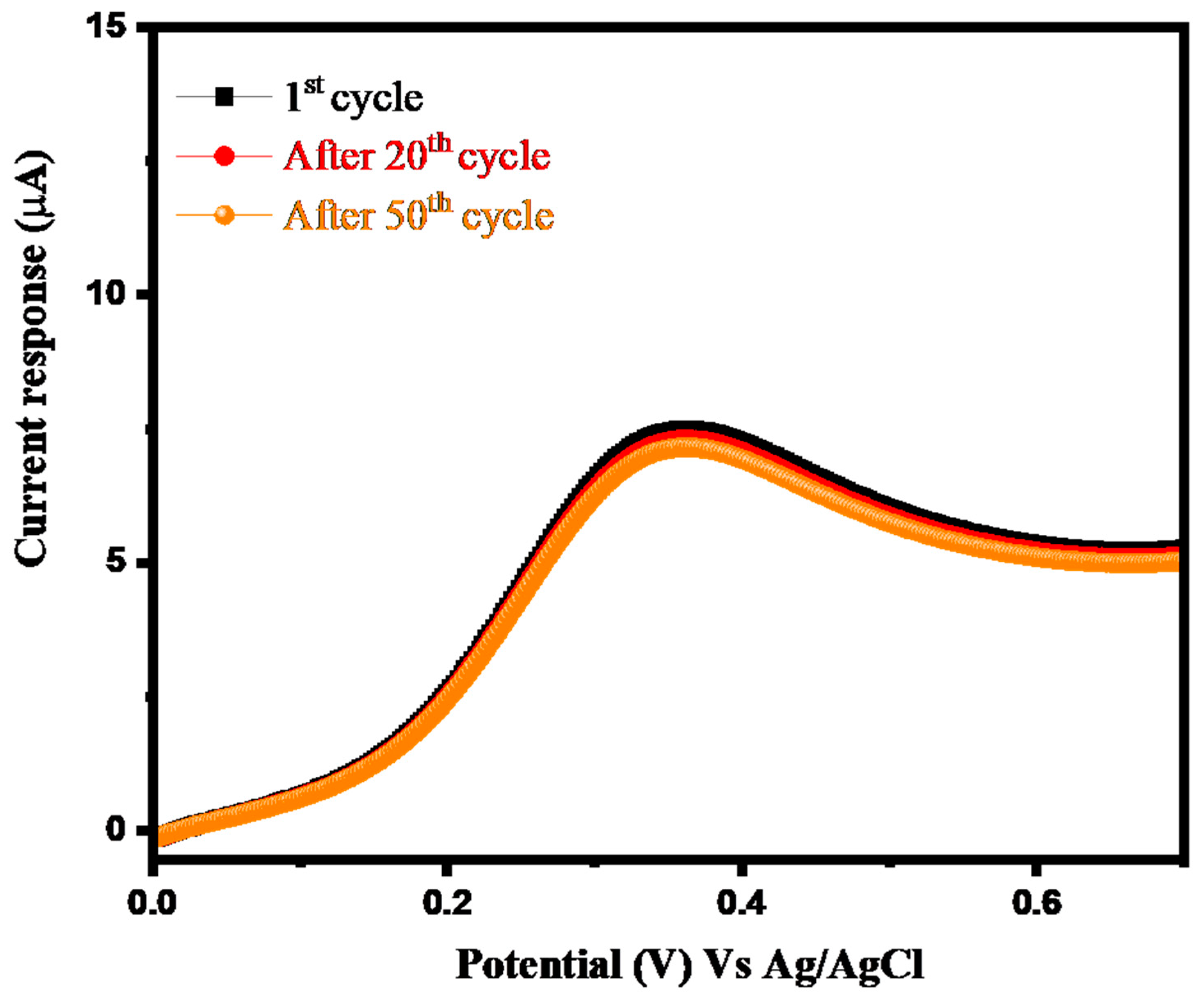
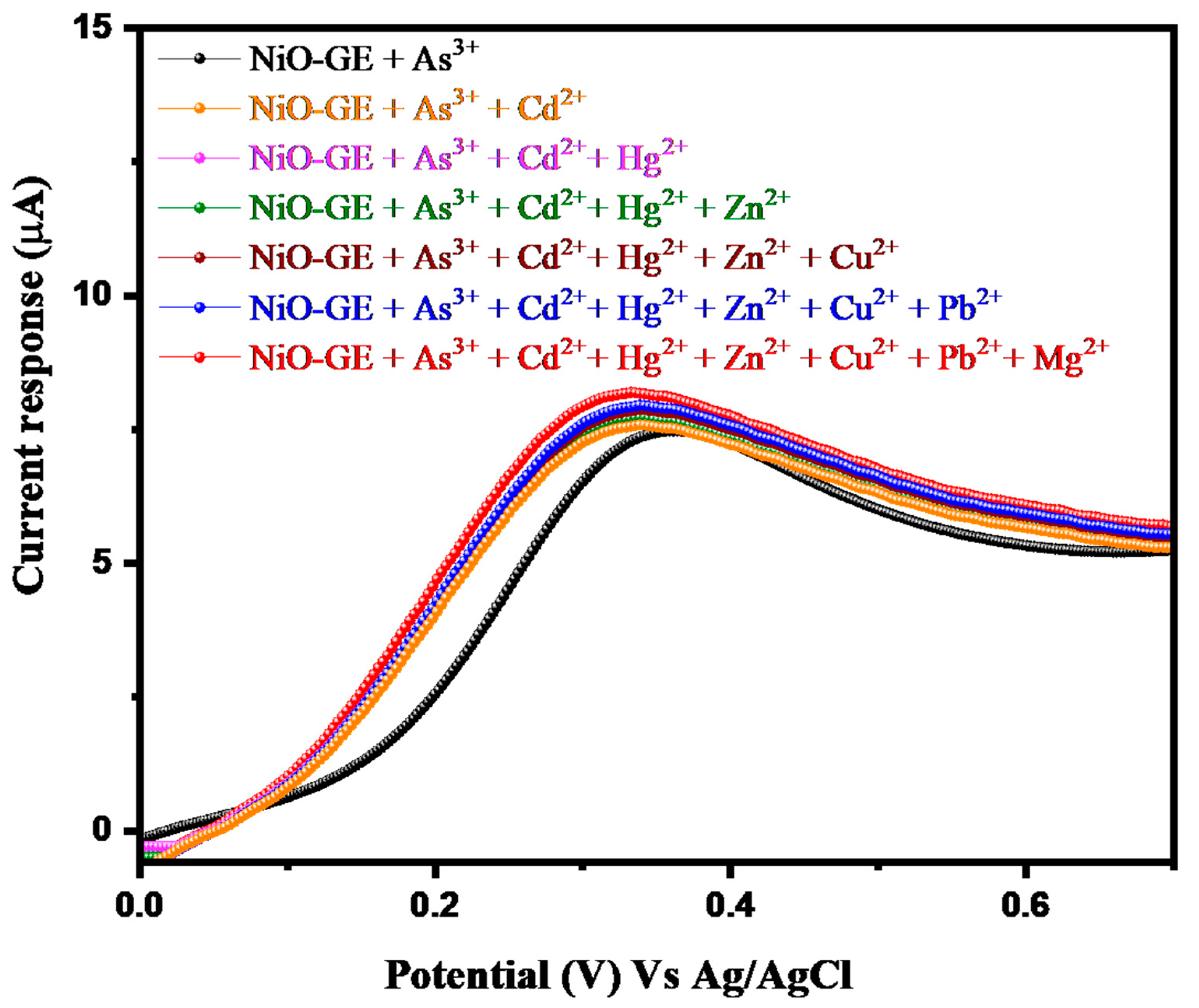
3.2. Electrochemical Study of Arsenic Sensor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, K.; Mobin, S.M. Recent Progress and Challenges in A3Sb2X9-Based Perovskite Solar Cells. ACS Omega 2020, 5, 28404–28412. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ahmad, K.; Dagar, J.; Unger, E.; Mobin, S.M. Two-Step Deposition Approach for Lead Free (NH4)3Sb2I9 Perovskite Solar Cells with Enhanced Open Circuit Voltage and Performance. ChemElectroChem 2021, 8, 3150–3154. [Google Scholar] [CrossRef]
- Ahmad, K.; Kumar, P.K.; Mobin, S.M. Hydrothermally Grown SnO2 Flowers as Efficient Electrode Modifier for Simultaneous Detection of Catechol and Hydroquinone. J. Electrochem. Soc. 2019, 166, B1577. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, E.A.; Noskova, G.N.; Antonova, S.G.; Kabakaev, A.S. Speciation of arsenic(III) and arsenic(V) by manganese-mediated stripping voltammetry at gold microelectrode ensemble in neutral and basic medium. Int. J. Environ. Anal. Chem. 2014, 94, 1478–1498. [Google Scholar] [CrossRef]
- Jiang, J.; Holm, N.; O’Brien, K. Improved Anodic Stripping Voltammetric Detection of Arsenic(III) Using Nanoporous Gold Microelectrode. ECS J. Solid State Sci. Technol. 2015, 4, S3024–S3029. [Google Scholar] [CrossRef]
- Korolczuk, M.; Ochab, M.; Rutyna, I. Determination of As(III) by anodic stripping voltammetry following double deposition and stripping steps at two gold working electrodes. Talanta 2015, 144, 517–521. [Google Scholar] [CrossRef]
- Babar, N.-U.-A.; Joya, K.S.; Tayyab, M.A.; Ashiq, M.N.; Sohail, M. Highly Sensitive and Selective Detection of Arsenic Using Electrogenerated Nanotextured Gold Assemblage. ACS Omega 2019, 4, 13645–13657. [Google Scholar] [CrossRef] [Green Version]
- Majid, E.; Hrapovic, S.; Liu, Y.L.; Male, K.B.; Luong, J.H.T. Electrochemical determination of arsenite using a gold nanoparticle modified glassy carbon electrode and flow analysis. Anal. Chem. 2006, 78, 762–769. [Google Scholar] [CrossRef]
- Hassan, S.S.; Sirajuddin; Solangi, A.R.; Kazi, T.G.; Kalhoro, M.S.; Junejo, Y.; Tagar, Z.A.; Kalwar, N.H. Nafion stabilized ibuprofen-gold nanostructures modified screen printed electrode as arsenic(III) sensor. J. Electroanal. Chem. 2012, 682, 77–82. [Google Scholar] [CrossRef]
- Lu, D.; Sullivan, C.; Brack, E.M.; Drew, C.P.; Kurup, P. Simultaneous voltammetric detection of cadmium(II), arsenic(III), and selenium(IV) using gold nanostar-modified screen-printed carbon electrodes and modified Britton-Robinson buffer. Anal. Bioanal. Chem. 2020, 412, 4113–4125. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, M.; Xiao, J.; Fu, X.; Jin, J.; Li, L.; Chang, W.; Xie, C. Gold Nanoparticle Dropped Titania Microsphere Hybrids as an Enhanced Sensitive Material for Stripping Voltammetry Determination of As(III). J. Electrochem. Soc. 2013, 160, B225–B230. [Google Scholar] [CrossRef]
- Kato, D.; Kamata, T.; Kato, D.; Yanagisawa, H.; Niwa, O. Au Nanoparticle-Embedded Carbon Films for Electrochemical As3+ Detection with High Sensitivity and Stability. Anal. Chem. 2016, 88, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, X.; Liu, J.-H.; Huang, X.-J. Enhanced anti-interference on electrochemical detection of arsenite with nanoporous gold in mild condition. Sens. Actuators B Chem. 2016, 234, 404–411. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, P.K.; Satpati, A.K. Gold Nano Particle and Reduced Graphene Oxide Composite Modified Carbon Paste Electrode for the Ultra Trace Detection of Arsenic (III). Electroanalysis 2017, 29, 1400–1409. [Google Scholar] [CrossRef]
- Rani, S.; Das, R.K.; Jaiswal, A.; Singh, G.P.; Palwe, A.; Saxena, S.; Shukla, S. 4D nanoprinted sensor for facile organo-arsenic detection: A two-photon lithography-based approach. Chem. Eng. J. 2023, 454, 140130. [Google Scholar] [CrossRef]
- Wang, W.; Bao, N.; Yuan, W.; Si, N.; Bai, H.; Li, H.; Zhang, Q. Simultaneous determination of lead, arsenic, and mercury in cosmetics using a plastic based disposable electrochemical sensor. Microchem. J. 2019, 148, 240–247. [Google Scholar] [CrossRef]
- Sullivan, C.; Lu, D.; Brack, E.; Drew, C.; Kurup, P. Voltammetric codetection of arsenic(III) and copper(II) in alkaline buffering system with gold nanostar modified electrodes. Anal. Chim. Acta 2020, 1107, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Liebana, S.; Drago, G.A. Bioconjugation and stabilisation of biomolecules in biosensors. Essays Biochem. 2016, 60, 59–68. [Google Scholar]
- Dar, R.A.; Khare, N.G.; Cole, D.P.; Karna, S.P.; Srivastava, A.K. Green synthesis of a silver nanoparticle-graphene oxide composite and its application for As(III) detection. RSC Adv. 2014, 4, 14432–14440. [Google Scholar] [CrossRef]
- Salimi, A.; Hyde, M.E.; Banks, C.E.; Compton, R.G. Boron doped diamond electrode modified with iridium oxide for amperometic detection of ultra trace amounts of arsenic(III). Analyst 2004, 129, 9–14. [Google Scholar] [CrossRef]
- Yang, M.; Guo, Z.; Li, L.-N.; Huang, Y.-Y.; Liu, J.-H.; Zhou, Q.; Chen, X.; Huang, X.-J. Electrochemical determination of arsenic(III) with ultra-high anti-interference performance using Au-Cu bimetallic nanoparticles. Sens. Actuators B Chem. 2016, 231, 70–78. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Bhanjana, G.; Dilbaghi, N.; Chaudhary, S.; Kim, K.-H.; Kumar, S. Robust and Direct Electrochemical Sensing of Arsenic Using Zirconia Nanocubes. Analyst 2016, 141, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xie, B.; Lu, Y.; Zhu, J. Advances in Electrochemical Detection Electrodes for As(III). Nanomaterials 2022, 12, 781. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Mohammad, A.; Ansari, S.N.; Mobin, S.M. Construction of Graphene Oxide Sheets Based Modified Glassy Carbon Electrode (GO/GCE) for the Highly Sensitive Detection of Nitrobenzene. Mater. Res. Express 2018, 5, 075601. [Google Scholar] [CrossRef]
- Ahmad, K.; Mobin, S.M. Shape Controlled Synthesis of High Surface Area MgO Microstructures for Highly Efficient Congo Red Dye Removal and Peroxide Sensor. J. Environ. Chem. Eng. 2019, 7, 103347. [Google Scholar] [CrossRef]
- Ahmad, K.; Mobin, S.M. Design and Fabrication of Cost-Effective and Sensitive Non-Enzymatic Hydrogen Peroxide Sensor Using Co-Doped δ-MnO2 Flowers as Electrode Modifier. Anal. Bioanal. Chem. 2021, 413, 789–798. [Google Scholar] [CrossRef]
- Ahmad, K.; Kumar, P.; Mobin, S.M. Hydrothermally Grown Novel Pyramids of the CaTiO3 Perovskite as an Efficient Electrode Modifier for Sensing Applications. Mater. Adv. 2020, 1, 2003–2009. [Google Scholar] [CrossRef]
- Ahmad, K.; Mobin, S.M. Construction of Polyanilne/ITO Electrode for Electrochemical Sensor Applications. Mater. Res. Express 2019, 6, 085508. [Google Scholar] [CrossRef]
- Mobili, R.; Preda, G.; Cognata, S.L.; Toma, L.; Pasini, D.; Amendola, V. Chiroptical sensing of perrhenate in aqueous media by a chiral organic cage. Chem. Commun. 2022, 58, 3897–3900. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zha, D.; Anslyn, E.V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef] [PubMed]
- Benedini, S.; Zheng, Y.; Nitti, A.; Mazza, M.M.A.; Dondi, D.; Raymob, F.M.; Pasini, D. Large polarization of push–pull ‘‘Cruciforms’’ via coordination with lanthanide ions. New J. Chem. 2022, 46, 221–227. [Google Scholar] [CrossRef]
- Dan-dan, Y.; Yong, Z.; Hong-quan, Y.; Hong, Z. Low temperature synthesis of NiO/CoO nanostructures to enhance their low temperature oxygen reduction catalysis. Micron 2022, 161, 103326. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzade, M.R.; Naji, L.; Hasannezhad, F. Electrochemical deposition of NiO bunsenite nanostructures with different morphologies as the hole transport layer in polymer solar cells. J. Electroanal. Chem. 2022, 926, 116955. [Google Scholar] [CrossRef]
- Kumar, V.M.; Polaki, S.R.; Krishnan, R.; Sarguna, R.M.; Mathews, T. Binder-free vertical graphene nanosheets templated NiO petals for high-performance supercapacitor applications. J. Alloys Compd. 2023, 931, 167420. [Google Scholar] [CrossRef]
- Mishra, S.; Yogi, P.; Sagdeo, P.R.; Kumar, R. Mesoporous Nickel Oxide (NiO) Nanopetals for Ultrasensitive Glucose Sensing. Nanoscale Res. Lett. 2018, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, M.; Tomar, M.; Gupta, V. NiO nanoparticle-based urea biosensor. Biosens. Bioelectron. 2013, 41, 110–115. [Google Scholar] [CrossRef]
- Jahani, P.M.; Javar, H.A.; Mahmoudi-Moghaddam, H. A new electrochemical sensor based on Europium-doped NiO nanocomposite for detection of venlafaxine. Measurement 2021, 173, 108616. [Google Scholar] [CrossRef]
- Amin, S.; Tahira, A.; Solangi, A.; Mazzaro, R.; Ibupoto, Z.H.; Vomiero, A. A sensitive enzyme-free lactic acid sensor based on NiO nanoparticles for practical applications. Anal. Methods 2019, 11, 3578–3583. [Google Scholar] [CrossRef]
- Bakhsh, H.; Buledi, J.A.; Khand, N.H.; Junejo, B.; Solangi, A.R.; Mallah, A.; Sherazi, S.T.H. NiO nanostructures based functional none-enzymatic electrochemical sensor for ultrasensitive determination of endosulfan in vegetables. Food Meas. 2021, 15, 2695–2704. [Google Scholar] [CrossRef]
- Jahromi, S.P.; Pandikumar, A.; Goh, B.T.; Lim, Y.S.; Basirun, W.J.; Lim, H.N.; Huang, N.M. Influence of particle size on performance of a nickel oxide nanoparticle-based supercapacitor. RSC Adv. 2015, 5, 14010–14019. [Google Scholar] [CrossRef]
- Mateos, D.; Valdez, B.; Castillo, J.R.; Nedev, N.; Curiel, M.; Perez, O.; Arias, A.; Tiznado, H. Synthesis of high purity nickel oxide by a modified sol-gel method. Ceram. Int. 2019, 45, 11403–11407. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Prabu, P.; Nagarajan, S.; Kim, C.H.; Park, J.H.; Kim, H.Y. Synthesis of nickel oxide nanoparticles using nickel acetate and poly(vinyl acetate) precursor. Mater. Sci. Eng. B 2006, 128, 111–114. [Google Scholar] [CrossRef]
- Jeremić, D.; Andjelković, L.; Milenković, M.R.; Šuljagić, M.; Ristović, M.S.; Ostojić, S.; Nikolić, A.S.; Vulić, P.; Brčeski, I.; Pavlović, V. One-Pot Combustion Synthesis of Nickel Oxide and Hematite: From Simple Coordination Compounds to High Purity Metal Oxide Nanoparticles. Sci. Sinter. 2020, 52, 481–490. [Google Scholar] [CrossRef]
- Yang, M.; Chen, X.; Jiang, T.-J.; Guo, Z.; Liu, J.-H.; Huang, X.-J. Electrochemical Detection of Trace Arsenic(III) by Nanocomposite of Nanorod-Like α-MnO2 Decorated with ∼5 nm Au Nanoparticles: Considering the Change of Arsenic Speciation. Anal. Chem. 2016, 88, 9720–9728. [Google Scholar] [CrossRef]
- Huang, J.-F.; Chen, H.-H. Gold-nanoparticle-embedded Nafion Composite Modified on Glassy Carbon Electrode for Highly Selective Detection of Arsenic (III). Talanta 2013, 116, 852–859. [Google Scholar] [CrossRef]
- Salimi, A.; Mamkhezri, H.; Hallaj, R.; Soltanian, S. Electrochemical Detection of Trace amount of Arsenic(III) at Glassy Carbon Electrode Modified with Cobalt Oxide Nanoparticles. Sens. Actuators B Chem. 2008, 129, 246. [Google Scholar] [CrossRef]
- Xiao, L.; Wildgoose, G.G.; Compton, R.G. Sensitive Electrochemical Detection of Arsenic (III) using Gold Nanoparticle Modified Carbon Nanotubes via Anodic Stripping Voltammetry. Anal. Chim. Acta 2008, 620, 44–49. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Jia, X.; Han, Y.; Wang, E. Electrochemical Determination of Arsenic(III) on Mercaptoethylamine Modified Au Electrode in Neutral Media. Anal. Chim. Acta 2012, 733, 23–27. [Google Scholar] [CrossRef]
- Bhanjana, G.; Mehta, N.; Chaudhary, G.R.; Dilbaghi, N.; Kim, K.-H.; Kumar, S. Novel electrochemical sensing of arsenic ions using a simple graphite pencil electrode modified with tin oxide nanoneedles. J. Mol. Liq. 2018, 264, 198–204. [Google Scholar] [CrossRef]
- Agustiany, T.; Khalil, M.; Einaga, Y.; Jiwanti, P.K.; Ivandini, T.A. Stable iridium-modified boron-doped diamond electrode for the application in electrochemical detection of arsenic (III). Mater. Chem. Phys. 2020, 244, 122723. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Q.; Zhou, L.; Zhang, Z. Stripping Analysis of Trace Arsenic Based on the MnOx/AuNPs Composite Film Modified Electrode in Alkaline Media. Electroanalysis 2014, 26, 1840–1849. [Google Scholar] [CrossRef]
- Zhou, S.; Han, X.; Fan, H.; Liu, Y. Electrochemical Sensing toward Trace As(III) Based on Mesoporous MnFe2O4/Au Hybrid Nanospheres Modified Glass Carbon Electrode. Sensors 2016, 16, 935. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Li, P.-H.; Xu, W.-H.; Wei, Y.; Li, L.-N.; Huang, Y.-Y.; Sun, Y.-F.; Chen, X.; Liu, J.-H.; Huang, X.-J. Reliable electrochemical sensing arsenic (III) in nearly groundwater pH based on efficient adsorption and excellent electrocatalytic ability of AuNPs/CeO2-ZrO2 nanocomposite. Sens. Actuators B 2018, 255, 226–234. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Sampath, S. In situ formation of graphene–lead oxide composite and its use in trace arsenic detection. Sens. Actuators B 2011, 160, 306–311. [Google Scholar] [CrossRef]
- Chowdhury, A.-N.; Ferdousi, S.; Islam, M.M.; Okajima, T.; Ohsaka, T. Arsenic detection by nanogold/conductingpolymer-modified glassy carbon electrodes. J. Appl. Polym. Sci. 2007, 104, 1306–1311. [Google Scholar] [CrossRef]





| Materials | LOD (ppb) | Sensitivity (μAppb−1) | Dynamic Linear Range (ppb) | References |
|---|---|---|---|---|
| Au/α-MnO2 | 0.019 | 0.828 | 1–10 | [46] |
| Au NPs/nafion | 0.047 | - | 0.1–12 | [47] |
| CoOx/GCE | 0.83 | 0.11 | 15–300 | [48] |
| Au/CNTs/GCE | 0.1 | 0.00075 | 0.75–7.5 | [49] |
| ZrO2 | 5 | - | 5–60 | [24] |
| Mercaptoethylamine/Au | 0.02 | - | 0.2–300 | [50] |
| SnO2 | 10 | - | - | [51] |
| Fe3O4/rGO | 0.38 | - | - | [52] |
| MnOx/Au NP | 0.057 | - | - | [53] |
| MnFe2O4 | 3.37 | - | - | [54] |
| AuNPs/CeO2-ZrO2 | 0.137 | - | - | [55] |
| Graphene/PbO2 | 0.01 | - | - | [56] |
| Au NP/polyaniline | 0.4 | - | - | [57] |
| NiO-GE | 1.94 | 3.10 | 5–50 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsalme, A.; Alsaeedi, H.; Altowairqi, M.F.; Khan, R.A.; Alharbi, G.M.; Alhamed, A.A. Sol-Gel Synthesized Nickel-Oxide-Based Fabrication of Arsenic (As3+) Sensor. Inorganics 2023, 11, 83. https://doi.org/10.3390/inorganics11020083
Alsalme A, Alsaeedi H, Altowairqi MF, Khan RA, Alharbi GM, Alhamed AA. Sol-Gel Synthesized Nickel-Oxide-Based Fabrication of Arsenic (As3+) Sensor. Inorganics. 2023; 11(2):83. https://doi.org/10.3390/inorganics11020083
Chicago/Turabian StyleAlsalme, Ali, Huda Alsaeedi, Malak Faisal Altowairqi, Rais Ahmad Khan, Ghadah M. Alharbi, and Afnan A. Alhamed. 2023. "Sol-Gel Synthesized Nickel-Oxide-Based Fabrication of Arsenic (As3+) Sensor" Inorganics 11, no. 2: 83. https://doi.org/10.3390/inorganics11020083
APA StyleAlsalme, A., Alsaeedi, H., Altowairqi, M. F., Khan, R. A., Alharbi, G. M., & Alhamed, A. A. (2023). Sol-Gel Synthesized Nickel-Oxide-Based Fabrication of Arsenic (As3+) Sensor. Inorganics, 11(2), 83. https://doi.org/10.3390/inorganics11020083






