Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound
Abstract
1. Introduction
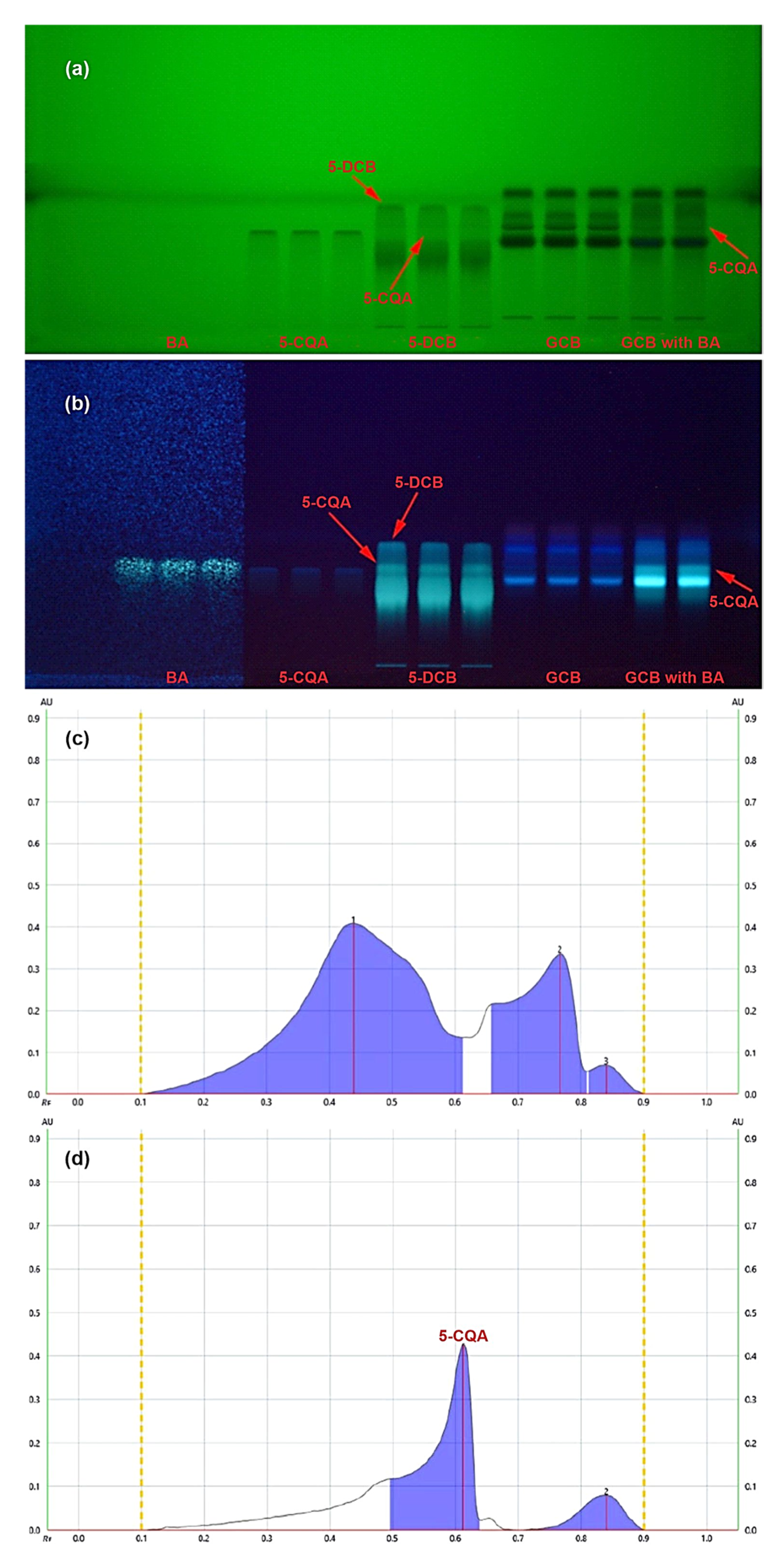
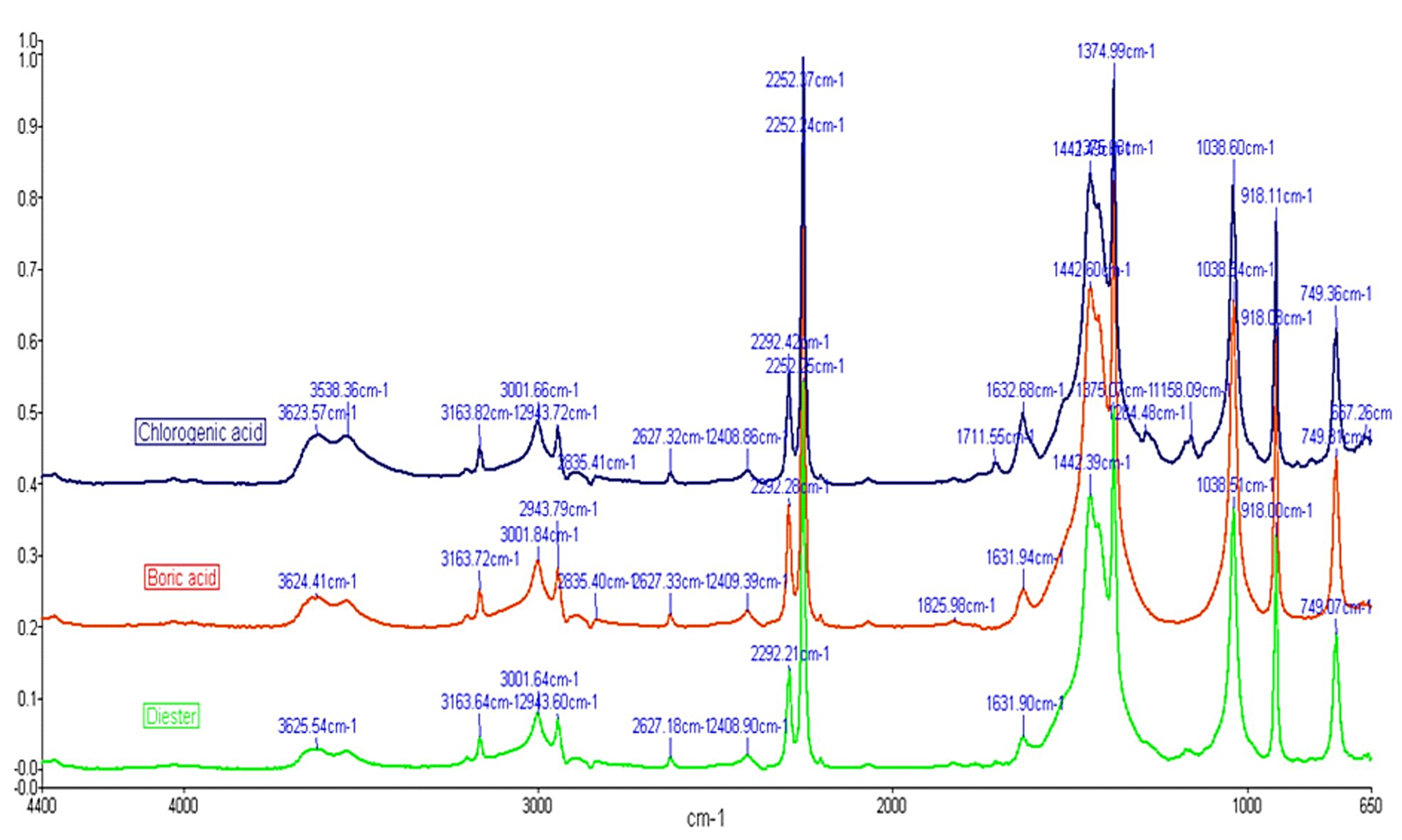
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solvents
4.2. Plant Material and GCB Extract
4.3. Semisynthesis of 5-DCB
4.4. Methods of Analysis
4.4.1. HPTLC/UV Densitometry with Confirmation by Online HPTLC/ESI–MS
4.4.2. UHPLC/MS Analysis
4.4.3. FTIR Spectroscopy
4.4.4. 1H-NMR
4.5. In Vitro Simulation of 5-DCB Digestion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donoiu, I.; Militaru, C.; Obleagă, O.; Hunter, J.M.; Neamţu, J.; Biţă, A.; Scorei, I.R.; Rogoveanu, O.C. Effects of boron-containing compounds on cardiovascular disease risk factors—A review. J. Trace Elem. Med. Biol. 2018, 50, 47–56. [Google Scholar] [CrossRef]
- Hunter, J.M.; Nemzer, B.V.; Rangavajla, N.; Biţă, A.; Rogoveanu, O.C.; Neamţu, J.; Scorei, I.R.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; et al. The fructoborates: Part of a family of naturally occurring sugar–borate complexes—Biochemistry, physiology, and impact on human health: A review. Biol. Trace Elem. Res. 2019, 188, 11–25. [Google Scholar] [CrossRef]
- Stangoulis, J.; Tate, M.; Graham, R.; Bucknall, M.; Palmer, L.; Boughton, B.; Reid, R. The mechanism of boron mobility in wheat and canola phloem. Plant Physiol. 2010, 153, 876–881. [Google Scholar] [CrossRef]
- Dinca, L.; Scorei, R. Boron in human nutrition and its regulations use. J. Nutr. Ther. 2013, 2, 22–29. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Smoum, R.; Al-Quntar, A.A.; Ali, H.A.; Pergament, I.; Srebnik, M. Natural occurrence of boron-containing compounds in plants, algae and microorganisms. Plant Sci. 2002, 163, 931–942. [Google Scholar] [CrossRef]
- Köse, D.A.; Zümreoglu-Karan, B. Mixed-ligand complexes of boric acid with organic biomolecules. Chem. Pap. 2012, 66, 54–60. [Google Scholar] [CrossRef]
- Bell, C.F.; Gallagher, B.C.; Lott, K.A.K.; Short, E.L.; Walton, L. Boric acid complexes of phenolic acids. Polyhedron 1991, 10, 613–618. [Google Scholar] [CrossRef]
- Wolkenstein, K.; Gross, J.H.; Falk, H. Boron-containing organic pigments from a Jurassic red alga. Proc. Natl. Acad. Sci. USA 2010, 107, 19374–19378. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Rodrigues Mota, T.; de Oliveira, D.M.; de Paiva Foletto-Felipe, M.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Liu, X.; Wang, Z.; Jiang, M.; Wang, R.; Xie, L.; Liu, Q.; Xie, X.; Shang, D.; et al. Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat. Commun. 2020, 11, 5371. [Google Scholar] [CrossRef]
- Mandal, R.K.; Jiang, T.; Al-Rubaye, A.A.; Rhoads, D.D.; Wideman, R.F.; Zhao, J.; Pevzner, I.; Kwon, Y.M. An investigation into blood microbiota and its potential association with bacterial chondronecrosis with osteomyelitis (BCO) in broilers. Sci. Rep. 2016, 6, 25882. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Shimamura, M.; Kumaki, T.; Hashimoto, S.; Saeki, K.; Ayabe, S.I.; Higashitani, A.; Akashi, T.; Sato, S.; Aoki, T. Phenolic acids induce nod factor production in Lotus japonicus–Mesorhizobium symbiosis. Microbes Environ. 2022, 37, ME21094. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic acid-induced gut microbiota improves metabolic endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef]
- Neales, T.F. The detection and chromatography on paper of boric acid, sodium tetraborate and benzene boronic acid and the use of chlorogenic and caffeic acids to detect the ions of B, W, Mo and Ge. J. Chromatogr. A. 1964, 16, 262–264. [Google Scholar] [CrossRef]
- Biţă, A.; Mogoşanu, G.D.; Bejenaru, L.E.; Oancea, C.N.; Bejenaru, C.; Croitoru, O.; Rău, G.; Neamţu, J.; Scorei, I.D.; Scorei, I.R.; et al. Simultaneous quantitation of boric acid and calcium fructoborate in dietary supplements by HPTLC–densitometry. Anal. Sci. 2017, 33, 743–746. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Bretones, G.; Baghour, M.; Ragala, L.; Belakbir, A.; Romero, L. Relationship between boron and phenolic metabolism in tobacco leaves. Phytochemistry 1998, 48, 269–272. [Google Scholar] [CrossRef]
- Hajiboland, R.; Bahrami-Rad, S.; Bastani, S. Phenolics metabolism in boron-deficient tea [Camellia sinensis (L.) O. Kuntze] plants. Acta Biol. Hung. 2013, 64, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Yardım, Y.; Keskin, E.; Şentürk, Z. Voltammetric determination of mixtures of caffeine and chlorogenic acid in beverage samples using a boron-doped diamond electrode. Talanta 2013, 116, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Pizer, R.; Babcock, L. Mechanism of the complexation of boron acids with catechol and substituted catechols. Inorg. Chem. 1977, 16, 1677–1681. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; et al. New insights into boron essentiality in humans and animals. Int. J. Mol. Sci. 2022, 23, 9147. [Google Scholar] [CrossRef] [PubMed]
- Biţă, A.; Scorei, I.R. Novel Boron Bioactive Compounds from Plants: Identification, Synthesis and Physical-Chemical Characterization. Romanian Ministry of Education and Research, CNCS–UEFISCDI, PNCDI III, Project No. PN-III-P1-1.1-PD-2019-0214. 2021. Available online: https://www.naturalresearch.ro/projects/# (accessed on 25 May 2022).
- Özdemir Öge, T.; Keskiner, A.Ü. Experimental and DFT calculation studies on boric acid and salicylic acid–boric acid–ethanol solution. Boron 2018, 3, 118–125. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D porous zinc-organic framework platform for loading of 5-fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Rivera, W.; Velasco, X.; Rincón, C.A. Evaluación por TGA y FTIR de los cambios de composición producidos por la tostión en granos de café (TGA and FTIR evaluation of composition changes produced by roasting of coffee beans). Rev. Col. Fís. 2013, 45, 205–208. Available online: http://fisica.udea.edu.co/ojs/ojs/index.php/rcf/article/viewArticle/450334.html (accessed on 23 May 2022).
- Ribeiro, J.S.; Ferreira, M.M.C.; Salva, T.J.G. Chemometric models for the quantitative descriptive sensory analysis of Arabica coffee beverages using near infrared spectroscopy. Talanta 2011, 83, 1352–1358. [Google Scholar] [CrossRef]
- Weltner Jr, W.; Warn, J.R.W. Matrix isolation of high-temperature vapors: Boric oxide. J. Chem. Phys. 1962, 37, 292–303. [Google Scholar] [CrossRef]
- Eravuchira, P.J.; El-Abassy, R.M.; Deshpande, S.; Matei, M.F.; Mishra, S.; Tandon, P.; Kuhnert, N.; Materny, A. Raman spectroscopic characterization of different regioisomers of monoacyl and diacyl chlorogenic acid. Vib. Spectrosc. 2012, 61, 10–16. [Google Scholar] [CrossRef]
- Liang, N.; Lu, X.; Hu, Y.; Kitts, D.D. Application of attenuated total reflectance–Fourier transformed infrared (ATR–FTIR) spectroscopy to determine the chlorogenic acid isomer profile and antioxidant capacity of coffee beans. J. Agric. Food Chem. 2016, 64, 681–689. [Google Scholar] [CrossRef]
- Parsons, J.L. Vibrational spectra of orthorhombic metaboric acid. J. Chem. Phys. 1960, 33, 1860–1866. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A. Naturally occurring boron containing compounds and their biological activities. J. Nat. Prod. Resour. 2017, 3, 147–154. Available online: https://www.jacsdirectory.com/journal-of-natural-products-and-resources/articleview.php?id=40 (accessed on 10 January 2023).
- Scorei, I.R.; Bita, A.; Dinca, L.; Mogosanu, G.D.; Rangavajla, N. Borate complexes of chlorogenic acid and uses thereof. United States Patent and Trademark Office (USPTO). Patent Application No. PCT/US22/78488, 21 October 2022. Available online: https://www.uspto.gov/patents (accessed on 25 October 2022).
- Mitruţ, I.; Scorei, I.R.; Manolea, H.O.; Biţă, A.; Mogoantă, L.; Neamţu, J.; Bejenaru, L.E.; Ciocîlteu, M.V.; Bejenaru, C.; Rău, G.; et al. Boron-containing compounds in Dentistry: A narrative review. Rom. J. Morphol. Embryol. 2022, 63, 477–483. [Google Scholar] [CrossRef]
- Neamţu, A.S.; Biţă, A.; Scorei, I.R.; Rău, G.; Bejenaru, L.E.; Bejenaru, C.; Rogoveanu, O.; Oancea, C.N.; Radu, A.; Pisoschi, C.G.; et al. Simultaneous quantitation of nicotinamide riboside and nicotinamide in dietary supplements via HPTLC–UV with confirmation by online HPTLC–ESI–MS. Acta Chromatogr. 2020, 32, 128–133. [Google Scholar] [CrossRef]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The role of microbiome in brain development and neurodegenerative diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef]
- Thompson, J.A.; Oliveira, R.A.; Xavier, K.B. Chemical conversations in the gut microbiota. Gut Microbes 2016, 7, 163–170. [Google Scholar] [CrossRef]
- Kremer, D.; Pieters, T.T.; Verhaar, M.C.; Berger, S.P.; Bakker, S.J.L.; van Zuilen, A.D.; Joles, J.A.; Vernooij, R.W.M.; van Balkom, B.W.M. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: Lessons to be learned. Am. J. Transplant. 2021, 21, 3936–3945. [Google Scholar] [CrossRef]
- Weber, K.S.; Ratjen, I.; Enderle, J.; Seidel, U.; Rimbach, G.; Lieb, W. Plasma boron concentrations in the general population: A cross-sectional analysis of cardio-metabolic and dietary correlates. Eur. J. Nutr. 2022, 61, 1363–1375. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]

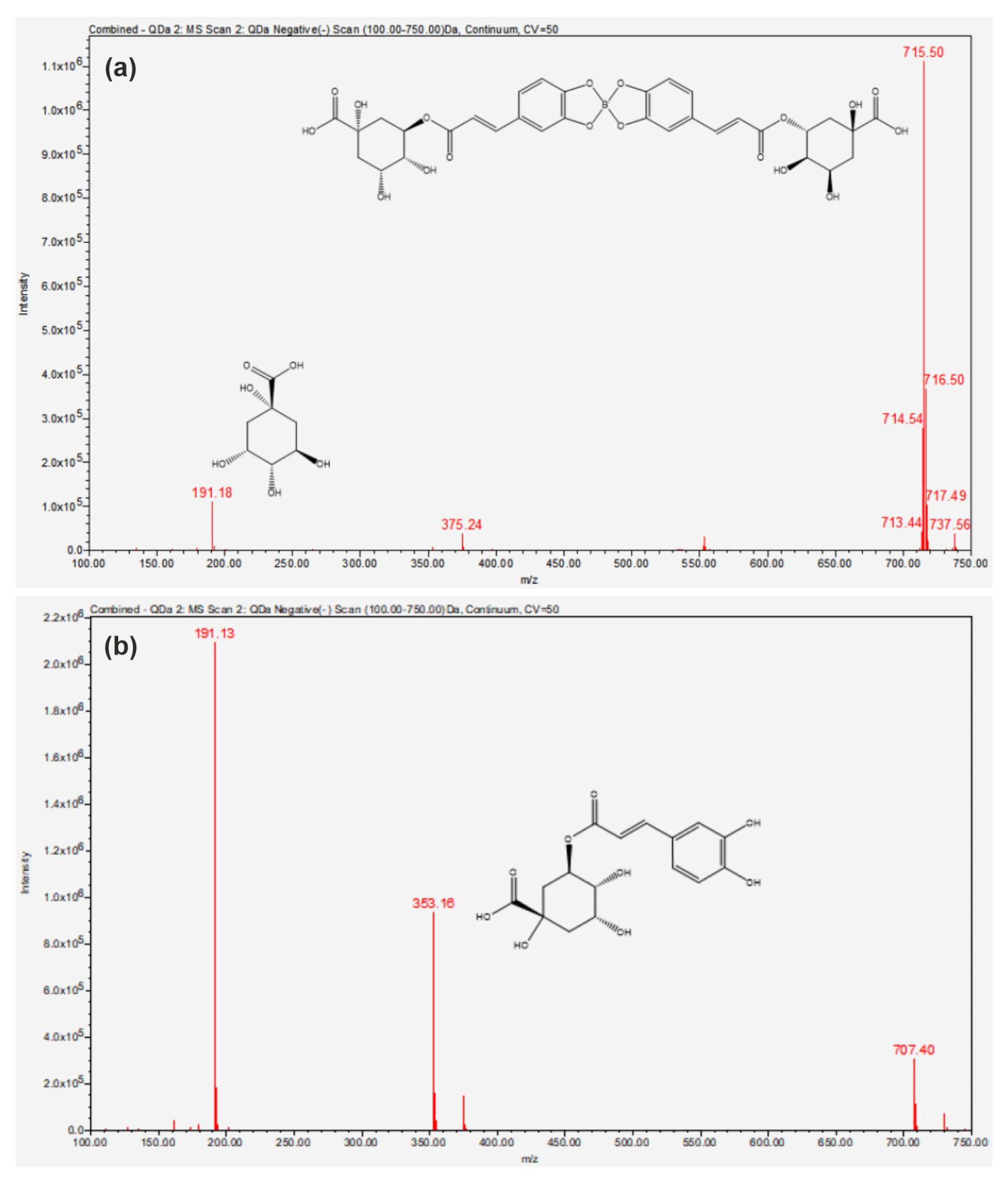



| Compound | Fragment Ions (m/z) | Molecular Structure |
|---|---|---|
| 5-CQA | 191 | 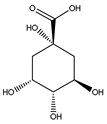 Quinic acid |
| 353 |  5-CQA | |
| 707 | 5-CQA dimer | |
| 5-DCB complex | 191 | Quinic acid |
| 715 | 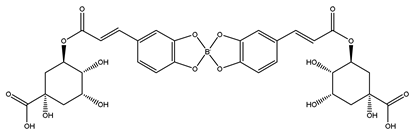 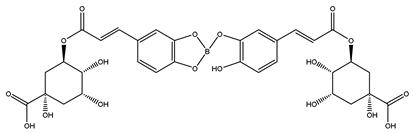 Newly formed 5-DCB complex |
| Compound | RT [min] | Relative Area |
|---|---|---|
| 5-CQA | 2.478 | 20 921 574 |
| 5-DCB 10B | 5.742 | 173 776 |
| 5-DCB 11B | 5.742 | 818 884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biţă, A.; Scorei, I.R.; Rangavajla, N.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; Ciocîlteu, M.V.; Dincă, L.; Neamţu, J.; Bunaciu, A.; et al. Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound. Inorganics 2023, 11, 112. https://doi.org/10.3390/inorganics11030112
Biţă A, Scorei IR, Rangavajla N, Bejenaru LE, Rău G, Bejenaru C, Ciocîlteu MV, Dincă L, Neamţu J, Bunaciu A, et al. Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound. Inorganics. 2023; 11(3):112. https://doi.org/10.3390/inorganics11030112
Chicago/Turabian StyleBiţă, Andrei, Ion Romulus Scorei, Nagendra Rangavajla, Ludovic Everard Bejenaru, Gabriela Rău, Cornelia Bejenaru, Maria Viorica Ciocîlteu, Laura Dincă, Johny Neamţu, Andrei Bunaciu, and et al. 2023. "Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound" Inorganics 11, no. 3: 112. https://doi.org/10.3390/inorganics11030112
APA StyleBiţă, A., Scorei, I. R., Rangavajla, N., Bejenaru, L. E., Rău, G., Bejenaru, C., Ciocîlteu, M. V., Dincă, L., Neamţu, J., Bunaciu, A., Rogoveanu, O. C., Pop, M. I., & Mogoşanu, G. D. (2023). Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound. Inorganics, 11(3), 112. https://doi.org/10.3390/inorganics11030112







