Layered Double Hydroxide Materials: A Review on Their Preparation, Characterization, and Applications
Abstract
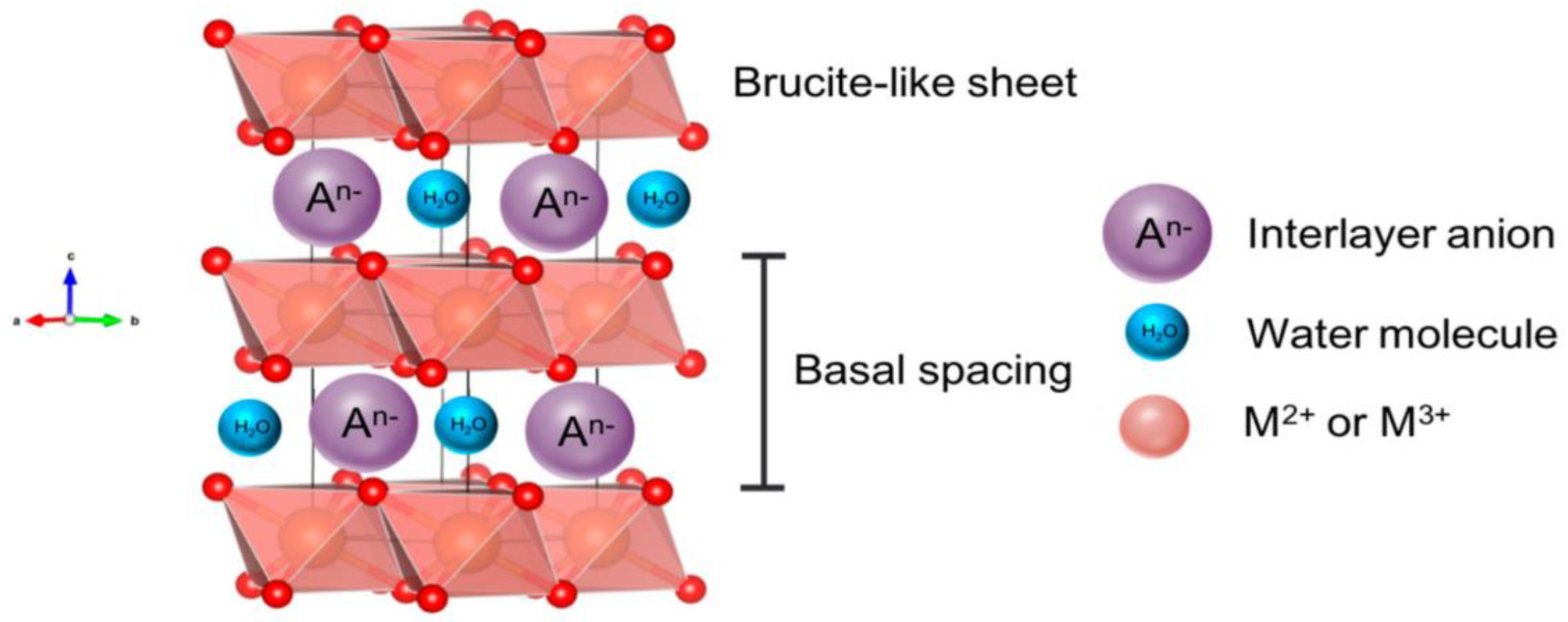
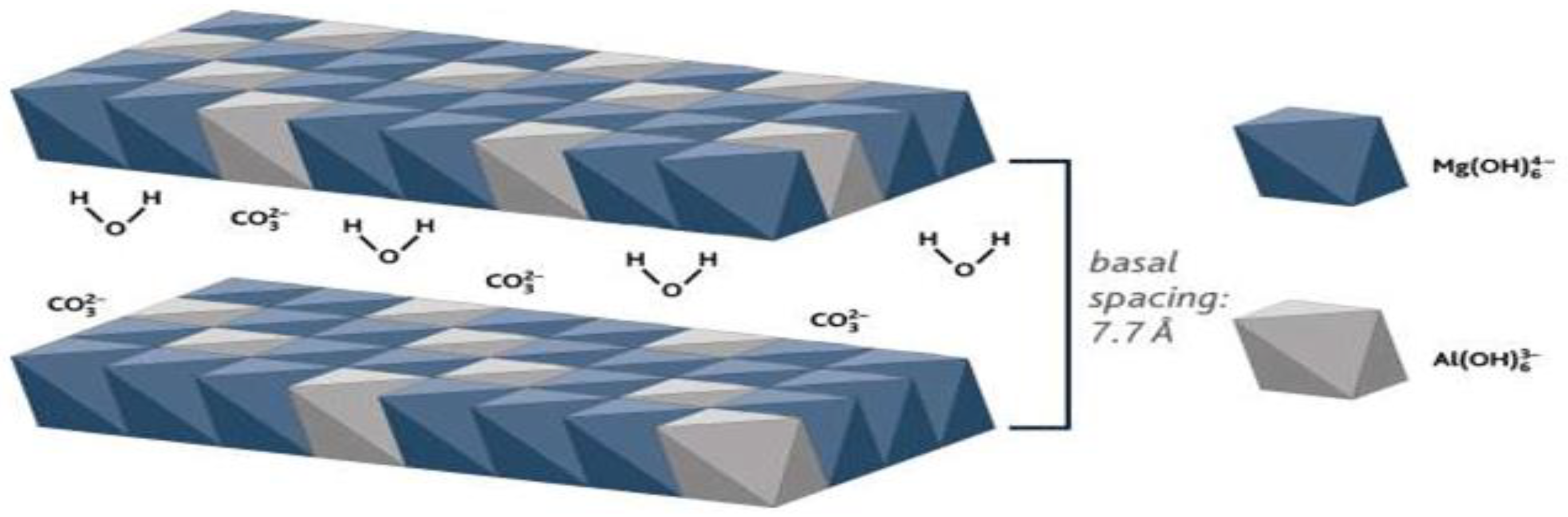
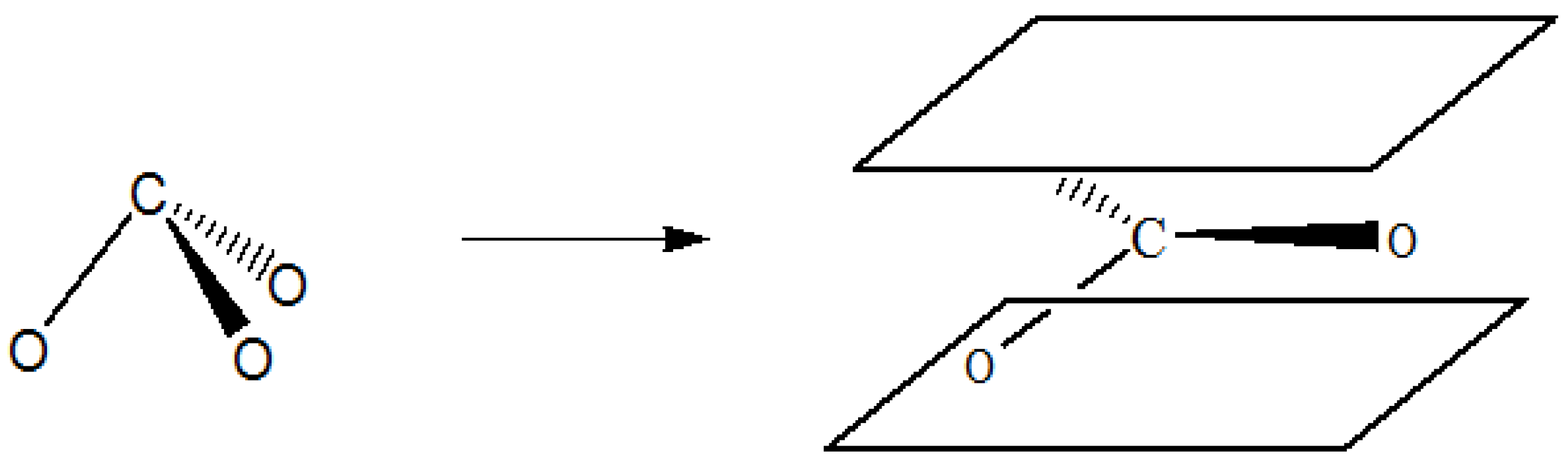
1. Introduction
2. Synthesis Techniques for LDH
2.1. Coprecipitation Method
2.2. Urea Hydrolysis
2.3. Sol–Gel Method
2.4. Hydrothermal Treatment
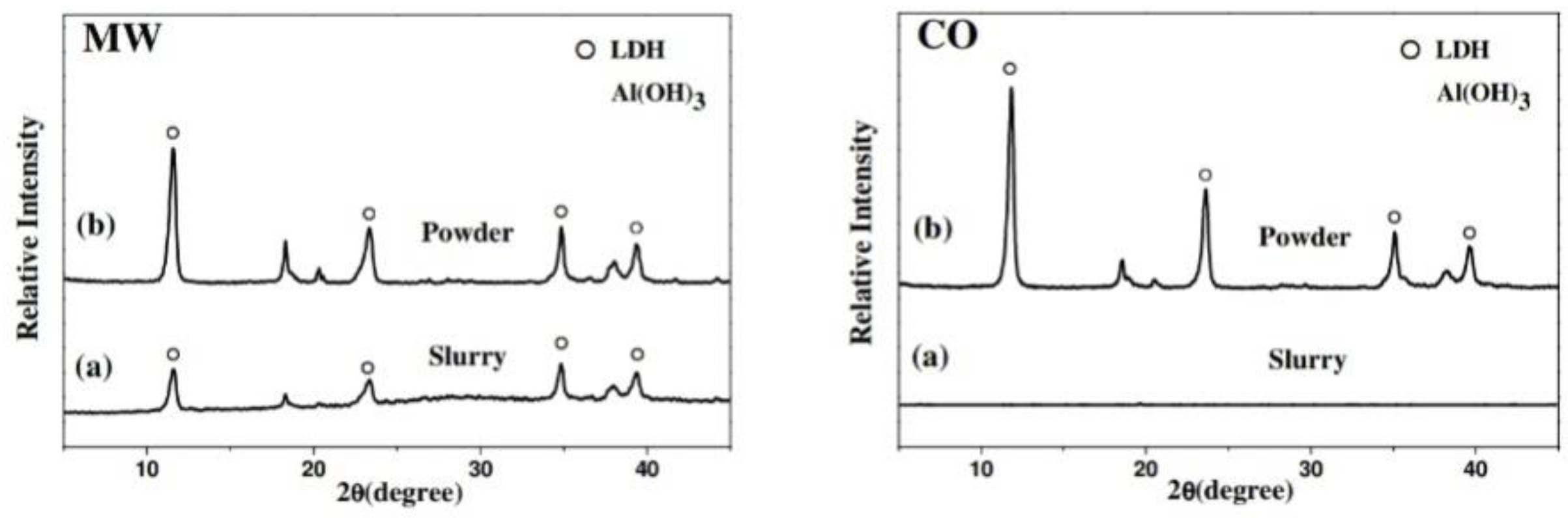
2.5. Microwave-Assisted Synthesis of LDH
2.6. Ion Exchange Process
2.7. Reconstruction
2.8. Oxide Method
2.9. Mechanochemical Methods
3. Characterization Techniques Used for LDHs
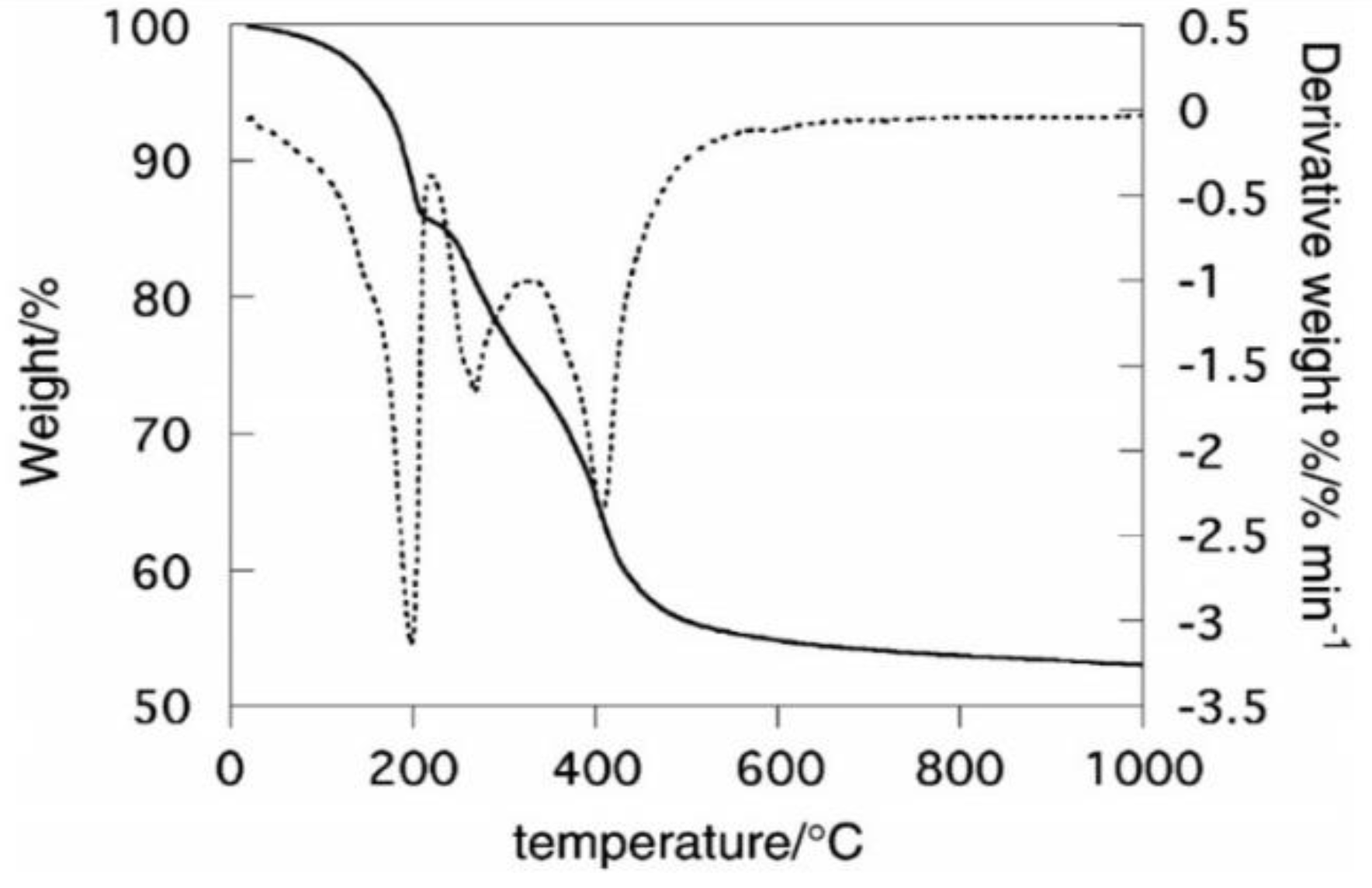
4. Thermal Properties of LDHs
- (i)
- in the first stage, physically adsorbed water molecules available on the outside surface of the crystallites are removed;
- (ii)
- the second step follows removal of the interlayer water molecules;
- (iii)
- the third step occurs as removal of the hydroxyl groups from the layers as water vapors; and
- (iv)
- the fourth and final step is responsible for the removal of the interlayer anion.
5. Applications of LDHs
5.1. LDHs as Catalysts
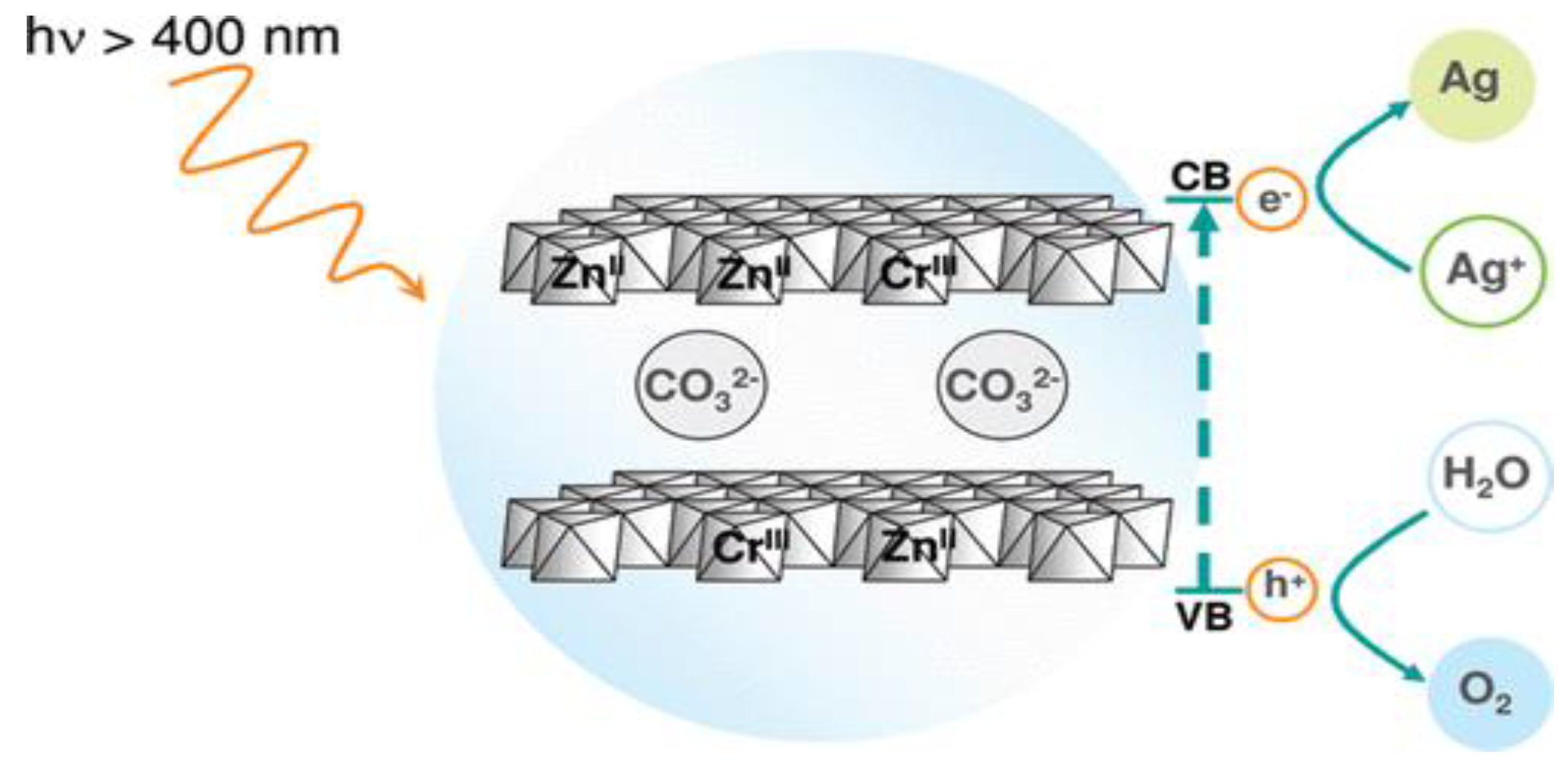
5.2. Photocatalysis
5.3. LDHs for Water Treatment and Environmental Remediation
- (1)
- The endothermic decomposition of LDH works as a heat sink.
- (2)
- Decomposition of LDH leads to formation of mixed metal oxides, which act as an insulating film on the surface.
- (3)
- Generation of bound water and carbon dioxide thereby diluting the flammable gases.
5.4. LDHs for Removal of Greenhouse Gases
5.5. LDHs for Removal of Pesticides and Related Persistent Organic Pollutants (POP)
5.6. Source of Nutrient Storage for Plants
5.7. LDHs as Adsorbents for Anionic Pollutants
5.8. LDHs for Biomedical Applications
5.9. LDH as Biosensors
5.10. LDH as Supercapacitors
5.11. Applications of LDHs in Display and Sensing
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Brito, A.; Borges, M.E.; Garín, M.; Hernández, A. Biodiesel Production from Waste Oil Using Mg−Al Layered Double Hydroxide Catalysts. Energy Fuels 2009, 23, 2952–2958. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Talib, Z.A.; bin Hussein, M.Z.; Zakaria, A. Zn–Al layered double hydroxide prepared at different molar ratios: Preparation, characterization, optical and dielectric properties. J. Solid State Chem. 2012, 191, 271–278. [Google Scholar] [CrossRef]
- Wijitwongwan, R.; Intasa-Ard, S.; Ogawa, M. Preparation of Layered Double Hydroxides toward Precisely Designed Hierarchical Organization. Chemengineering 2019, 3, 68. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirb, F.; Vaccari, A. Hydrotalcite-Type Anlonlc Clays: Preparation, Properties And Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Butenko, E. Use of Layered Double Hydroxides to Create New Environmental Technologies. Int. J. Sci. Res. Environ. Sci. Toxicol. 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Olfs, H.-W.; Torres-Dorante, L.O.; Eckelt, R.; Kosslick, H. Comparison of different synthesis routes for Mg–Al layered double hydroxides (LDH): Characterization of the structural phases and anion exchange properties. Appl. Clay Sci. 2009, 43, 459–464. [Google Scholar] [CrossRef]
- Xu, Z.P.; Stevenson, G.; Lu, C.-Q.; Lu, G. Dispersion and Size Control of Layered Double Hydroxide Nanoparticles in Aqueous Solutions. J. Phys. Chem. B 2006, 110, 16923–16929. [Google Scholar] [CrossRef]
- Arai, Y.; Ogawa, M. Preparation of Co–Al layered double hydroxides by the hydrothermal urea method for controlled particle size. Appl. Clay Sci. 2009, 42, 601–604. [Google Scholar] [CrossRef]
- He, J.; Wei, M.; Li, B.; Kang, Y.; Evans, D.G.; Duan, X. Preparation of Layered Double Hydroxides. Layer. Double Hydroxides 2005, 119, 89–119. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yin, X.; Wang, C.; Sun, H.; Ma, A.; Zhang, G.; Wang, N. Sorption of cadmium onto Mg-Fe Layered Double Hydroxide (LDH)-Kiwi branch biochar. Environ. Pollut. Bioavailab. 2019, 31, 189–197. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Kishida, M.; Nakamura, T.; Kawasaki, N. Interaction between phosphate ions and Fe-Mg type hydrotalcite for purification of wastewater. J. Environ. Chem. Eng. 2019, 7, 102897. [Google Scholar] [CrossRef]
- Hashim, N.; Sharif, S.N.M.; Hussein, M.Z.; Isa, I.M.; Kamari, A.; Mohamed, A.; Ali, N.M.; Bakar, S.A.; Mamat, M. Layered hydroxide anion exchanger and their applications related to pesticides: A brief review. Mater. Res. Innov. 2017, 21, 129–145. [Google Scholar] [CrossRef]
- Calisto, J.S.; Pacheco, I.S.; Freitas, L.L.; Santana, L.K.; Fagundes, W.S.; Amaral, F.A.; Canobre, S.C. Adsorption kinetic and thermodynamic studies of the 2, 4–dichlorophenoxyacetate (2,4-D) by the [Co–Al–Cl] layered double hydroxide. Heliyon 2019, 5, e02553. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.; Habib, M.; Bharath, G.; Al-Harthi, M. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)–Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- Barahuie, F.; Hussein, M.Z.; Arulselvan, P.; Fakurazi, S.; Zainal, Z. Drug delivery system for an anticancer agent, chlorogenate-Zn/Al-layered double hydroxide nanohybrid synthesised using direct co-precipitation and ion exchange methods. J. Solid State Chem. 2014, 217, 31–41. [Google Scholar] [CrossRef]
- Li, K.; Wang, G.; Li, D.; Lin, Y.; Duan, X. Intercalation Assembly Method and Intercalation Process Control of Layered Intercalated Functional Materials. Chin. J. Chem. Eng. 2013, 21, 453–462. [Google Scholar] [CrossRef]
- Miyata, S. The Syntheses of Hydrotalcite-like Compounds and Their Structures and Physico-Chemical Properties—I: The Systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO−4, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kushwaha, P.K.; Srivastava, V.K.; Bhatt, S.D.; Jasra, R.V. Effect of Hydrothermal Conditions on Structural and Textural Properties of Synthetic Hydrotalcites of Varying Mg/Al Ratio. Ind. Eng. Chem. Res. 2007, 46, 4856–4865. [Google Scholar] [CrossRef]
- Klemkaite, K.; Prosycevas, I.; Taraskevicius, R.; Khinsky, A.; Kareiva, A. Synthesis and characterization of layered double hydroxides with different cations (Mg, Co, Ni, Al), decomposition and reformation of mixed metal oxides to layered structures. Open Chem. 2011, 9, 275–282. [Google Scholar] [CrossRef]
- El Rouby, W.M.A.; El-Dek, S.I.; Goher, M.E.; Noaemy, S.G. Efficient water decontamination using layered double hydroxide beads nanocomposites. Environ. Sci. Pollut. Res. 2020, 27, 18985–19003. [Google Scholar] [CrossRef]
- Amer, A.; Sayed, G.H.; Ramadan, R.M.; Rabie, A.M.; Negm, N.A.; Farag, A.A.; Mohammed, E.A. Assessment of 3-amino-1H-1,2,4-triazole modified layered double hydroxide in effective remediation of heavy metal ions from aqueous environment. J. Mol. Liq. 2021, 341, 116935. [Google Scholar] [CrossRef]
- Yang, P.; Yu, J.; Wang, Z.; Liu, Q.; Wu, T. Urea method for the synthesis of hydrotalcites. React. Kinet. Catal. Lett. 2004, 83, 275–282. [Google Scholar] [CrossRef]
- Berber, M.R.; Hafez, I.H.; Minagawa, K.; Katoh, M.; Mori, T.; Tanaka, M. Uniform nanoparticles of hydrotalcite-like materials and their textural properties at optimized conditions of urea hydrothermal treatment. J. Mol. Struct. 2013, 1033, 104–112. [Google Scholar] [CrossRef]
- Park, S.; Kwon, D.; Kang, J.Y.; Jung, J.C. Influence of the preparation method on the catalytic activity of Mg Al hydrotalcites as solid base catalysts. Green Energy Environ. 2019, 4, 287–292. [Google Scholar] [CrossRef]
- Inayat, A.; Klumpp, M.; Schwieger, W. The urea method for the direct synthesis of ZnAl layered double hydroxides with nitrate as the interlayer anion. Appl. Clay Sci. 2011, 51, 452–459. [Google Scholar] [CrossRef]
- Bish, D.L. Anion-exchange in takovite: Applications to other hydroxide minerals. Bull. De Mineral. 1980, 103, 170–175. [Google Scholar] [CrossRef]
- Iyi, N.; Matsumoto, T.; Kaneko, A.Y.; Kitamura, K. Deintercalation of Carbonate Ions from a Hydrotalcite-Like Compound: Enhanced Decarbonation Using Acid−Salt Mixed Solution. Chem. Mater. 2004, 16, 2926–2932. [Google Scholar] [CrossRef]
- Costantino, U.; Marmottini, F.; Nocchetti, M.; Vivani, R. New synthetic routes to hydrotalcite-like compounds—Characterization and properties of the obtained materials. Eur. J. Inorg. Chem. 1998, 10, 1439–1446. [Google Scholar] [CrossRef]
- Lee, G.; Kang, J.Y.; Yan, N.; Suh, Y.-W.; Jung, J.C. Simple preparation method for Mg–Al hydrotalcites as base catalysts. J. Mol. Catal. A Chem. 2016, 423, 347–355. [Google Scholar] [CrossRef]
- Prince, J.; Montoya, A.; Ferrat, G.; Valente, J.S. Proposed General Sol−Gel Method to Prepare Multimetallic Layered Double Hydroxides: Synthesis, Characterization, and Envisaged Application. Chem. Mater. 2009, 21, 5826–5835. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Velty, A. Activated hydrotalcites as catalysts for the synthesis of chalcones of pharmaceutical interest. J. Catal. 2004, 221, 474–482. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. The effects of synthesis pH and hydrothermal treatment on the formation of zinc aluminum hydrotalcites. J. Solid State Chem. 2004, 177, 4047–4057. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, C.H.; Kim, S.; Kim, S.H.; Lee, K.B. Hydrothermal Synthesis of K2CO3-Promoted Hydrotalcite from Hydroxide-Form Precursors for Novel High-Temperature CO2 Sorbent. ACS Appl. Mater. Interfaces 2014, 6, 6914–6919. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, X.; Hao, J.; Ning, P.; Qu, G.; Tang, L.; Xie, Y.; Du, C.; He, Y. Preparation of CuZnAl hydrotalcite-like catalysts for AsH3 abatement at low temperatures. Catal. Commun. 2019, 118, 51–55. [Google Scholar] [CrossRef]
- Ogawa, M.; Asai, S. Hydrothermal Synthesis of Layered Double Hydroxide−Deoxycholate Intercalation Compounds. Chem. Mater. 2000, 12, 3253–3255. [Google Scholar] [CrossRef]
- Labuschagné, F.J.W.J.; Wiid, A.; Venter, H.P.; Gevers, B.R.; Leuteritz, A. Green synthesis of hydrotalcite from untreated magnesium oxide and aluminum hydroxide. Green Chem. Lett. Rev. 2018, 11, 18–28. [Google Scholar] [CrossRef]
- Jubri, Z.; Hussein, M.Z.; Yahaya, A.; Zainal, Z. The effect of microwave-assisted synthesis on the physico-chemical properties of pamoate-intercalated layered double hydroxide. Nanosci. Methods 2012, 1, 152–163. [Google Scholar] [CrossRef]
- Benito, P.; Guinea, I.; Herrero, M.; Labajos, F.M.; Rives, V. Incidence of Microwave Hydrothermal Treatments on the Crystallinity Properties of Hydrotalcite-like Compounds. Z. Für Anorg. Und Allg. Chem. 2007, 633, 1815–1819. [Google Scholar] [CrossRef]
- Bergadà, O.; Vicente, I.; Salagre, P.; Cesteros, Y.; Medina, F.; Sueiras, J.E. Microwave effect during aging on the porosity and basic properties of hydrotalcites. Microporous Mesoporous Mater. 2007, 101, 363–373. [Google Scholar] [CrossRef]
- Lim, M.H.; Kang, R.M.; Lee, S.C.; Lee, S.H.; Kim, K.J. Effect of Microwave Heating on the Synthesis of Layered Double Hydroxide. Mat. Sci. Forum Vols. 2005, 492–493, 743–748. [Google Scholar] [CrossRef]
- Dwiasi, D.W.; Mudasir, M.; Roto, R. Ion Exchange of Benzoate in Ni-Al-Benzoate Layered Double Hydroxide by Amoxicillin. Open Chem. 2019, 17, 1043–1049. [Google Scholar] [CrossRef]
- Mascolo, G.; Mascolo, M. On the synthesis of layered double hydroxides (LDHs) by reconstruction method based on the “memory effect”. Microporous Mesoporous Mater. 2015, 214, 246–248. [Google Scholar] [CrossRef]
- Jia, Q.-Y.; Liu, Z.-L.; Li, X.-D.; Li, S.-P.; Jiang, J.-L.; Li, D.-X. Synthesis of spherical MTX/LDHs nanohybrids by the calcination-reconstruction method. J. Dispers. Sci. Technol. 2021, 42, 1673–1680. [Google Scholar] [CrossRef]
- Boehm, H.-P.; Steinle, J.; Vieweger, C. [Zn2Cr(Oh)6]X·2H2O, New Layer Compounds Capable of Anion Exchange and Intracrystalline Swelling; Institute for an Organics Chemise der Universidad Meiserstrasse: Munich, Germany, 1977; pp. 265–266. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, F.; Li, S.; Wei, S.; Zhou, J. A mechanochemical approach to get stunningly uniform particles of magnesium–aluminium-layered double hydroxides. Appl. Surf. Sci. 2012, 259, 245–251. [Google Scholar] [CrossRef]
- Madhusha, C.; Rajapaksha, K.; Munaweera, I.; de Silva, M.; Perera, C.; Wijesinghe, G.; Weerasekera, M.; Attygalle, D.; Sandaruwan, C.; Kottegoda, N. A Novel Green Approach to Synthesize Curcuminoid-Layered Double Hydroxide Nanohybrids: Adroit Biomaterials for Future Antimicrobial Applications. ACS Omega 2021, 6, 9600–9608. [Google Scholar] [CrossRef]
- Zhang, F.; Du, N.; Zhang, R.; Hou, W. Mechanochemical synthesis of Fe3O4@(Mg-Al-OH LDH) magnetic composite. Powder Technol. 2012, 228, 250–253. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Su, Q.; Chen, L.; Wu, J.; Meng, J. Preparation of Magnesium-Aluminum Hydrotalcite by Mechanochemical Method and Its Application as Heat Stabilizer in poly(vinyl chloride). Materials 2020, 13, 5223. [Google Scholar] [CrossRef]
- Zeng, M.-G.; Huo, X.-L.; Liu, S.-Q.; Li, S.-P.; Li, X.-D. Mechanochemical approach to get layered double hydroxides: Mechanism explore on crystallite growth. Appl. Surf. Sci. 2014, 292, 1059–1066. [Google Scholar] [CrossRef]
- Bhojaraj; Arulraj, J.; Kolinjavadi, M.R.; Rajamathi, M. Solvent-Mediated and Mechanochemical Methods for Anion Exchange of Carbonate from Layered Double Hydroxides Using Ammonium Salts. ACS Omega 2019, 4, 20072–20079. [Google Scholar] [CrossRef]
- Barnard, B.A.; Labuschagné, F.J.W.J. Exploring the Wet Mechanochemical Synthesis of Mg-Al, Ca-Al, Zn-Al and Cu-Al Layered Double Hydroxides from Oxides, Hydroxides and Basic Carbonates. Crystals 2020, 10, 954. [Google Scholar] [CrossRef]
- Ibrahimova, K.A.; Azizov, A.; Balayeva, O.; Alosmanov, R.M.; Mammadyarova, S.C. Mechanochemical synthesis of PbS/Ni–Cr layered double hydroxide nanocomposite. Mendeleev Commun. 2021, 31, 100–103. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Zăvoianu, R.; Bucur, I.C.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V.I. Mechano-chemical versus co-precipitation for the preparation of Y-modified LDHs for cyclohexene oxidation and Claisen-Schmidt condensations. Appl. Catal. A Gen. 2020, 605, 117797. [Google Scholar] [CrossRef]
- Santosa, S.J.; Sudiono, S.; Wibawani, R.S. Solvent-free mechanochemically synthesized Zn layered hydroxide salts for the adsorption of naphtholate AS dye. Appl. Surf. Sci. 2019, 506, 144930. [Google Scholar] [CrossRef]
- Szabados, M.; Gácsi, A.; Gulyás, Y.; Kónya, Z.; Kukovecz, A.; Csányi, E.; Pálinkó, I.; Sipos, P. Conventional or mechanochemically-aided intercalation of diclofenac and naproxen anions into the interlamellar space of CaFe-layered double hydroxides and their application as dermal drug delivery systems. Appl. Clay Sci. 2021, 212, 106233. [Google Scholar] [CrossRef]
- Frederic, L. Synthesis and Characterisation of Layered Double Hydroxides and Their Application for Water Purification. Ph.D. Thesis, University Queensland University of technology, Brisbane, Australia, 2012. [Google Scholar]
- Wang, M.; Tan, G.; Ren, H.; Xia, A.; Liu, Y. Direct double Z-scheme O-g-C3N4/Zn2SnO4N/ZnO ternary heterojunction photocatalyst with enhanced visible photocatalytic activity. Appl. Surf. Sci. 2019, 492, 690–702. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Lu, S. Preparation and application of layered double hydroxide nanosheets. RSC Adv. 2021, 11, 24254–24281. [Google Scholar] [CrossRef] [PubMed]
- Sideris, P.J.; Blanc, F.; Gan, Z.; Grey, C. Identification of Cation Clustering in Mg–Al Layered Double Hydroxides Using Multinuclear Solid State Nuclear Magnetic Resonance Spectroscopy. Chem. Mater. 2012, 24, 2449–2461. [Google Scholar] [CrossRef]
- Sanchez-Valente, J.; Millet, J.M.M.; Figueras, F.; Fournes, L. Mössbauer Spectroscopic Study of Iron Containing Hydrotalcite Catalysts for the Reduction of Aromatic Nitro Compounds. Hyperfine Interact. 2000, 131, 43–50. [Google Scholar] [CrossRef]
- del Arco, M.; Rives, V.; Trujillano, R.; Malet, P. Thermal behaviour of Zn–Cr layered double hydroxides with hydrotalcite-like structures containing carbonate or decavanadate. J. Mater. Chem. 1996, 6, 1419–1428. [Google Scholar] [CrossRef]
- Kooli, F.; Crespo, I.; Barriga, C.; Ulibarri, M.A.; Rives, V. Precursor dependence of the nature and structure of non-stoichiometric magnesium aluminium vanadates. J. Mater. Chem. 1996, 6, 1199–1206. [Google Scholar] [CrossRef]
- Labajos, F.M.; Rives, V.; Ulibarri, M.A. Effect of hydrothermal and thermal treatments on the physicochemical properties of Mg-Al hydrotalcite-like materials. J. Mater. Sci. 1992, 27, 1546–1552. [Google Scholar] [CrossRef]
- Benício, L.; Silv, R.; Lopes, L.; Eulálio, D.; Santos, R.; De Aquino, L.; Vergütz, L.; Novais, R.; Da Costa, L.; Pinto, F.; et al. Layered double hydroxides: Nanomaterials for applications in agriculture. Hidróxidos duplos lamelares: Nanomateriais Para Aplicaçoes na agricultura. Rev. De Cienc. Do Solo 2015, 39, 1–13. [Google Scholar] [CrossRef]
- Rives, V. Comment on “Direct Observation of a Metastable Solid Phase of Mg/Al/CO3-Layered Double Hydroxide by Means of High-Temperature in Situ Powder XRD and DTA/TG”. Inorg. Chem. 1999, 38, 406–407. [Google Scholar] [CrossRef]
- Bera, P.; Rajamathi, M.; Hegde, M.S.; Kamath, P.V. Thermal behaviour of hydroxides, hydroxysalts and hydrotalcites. Bull. Mater. Sci. 2000, 23, 141–145. [Google Scholar] [CrossRef]
- Trujillano, R.; González-García, I.; Morato, A.; Rives, V. Controlling the Synthesis Conditions for Tuning the Properties of Hydrotalcite-Like Materials at the Nano Scale. Chemengineering 2018, 2, 31. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zhang, J.; Adebajo, M.; Zhang, H.; Zhou, C. Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 2011, 53, 139–150. [Google Scholar] [CrossRef]
- Dejoz, A.; Nieto, J.M.L.; Melo, F.; Vázquez, I. Kinetic Study of the Oxidation of n-Butane on Vanadium Oxide Supported on Al/Mg Mixed Oxide. Ind. Eng. Chem. Res. 1997, 36, 2588–2596. [Google Scholar] [CrossRef]
- Dinka, P.; Prandová, K.; Hronec, M. Reaction of methanol and n-propanol over hydrotalcite-like catalysts containing vanadium oxide. Appl. Clay Sci. 1998, 13, 467–477. [Google Scholar] [CrossRef]
- Kakiuchi, N.; Nishimura, T.; Inoue, M.; Uemura, S. Palladium (II) Supported by Hydrotalcite [Pd (II)-Hydrotalcite] -Catalyzed Selective Oxidation of Alcohols Using Molecular Oxygen. In Proceedings of the ECSOC-4, the Fourth International Electronic Conference on Synthetic Organic Chemistry, Basel, Switzerland, 1–30 September 2000; Wirth, T., Kappe, C.O., Felder, E., Diederichsen, U., Lin, S., Eds.; Available online: http://www.mdpi.org/ecsoc-4.htm (accessed on 21 January 2022).
- Choudary, B.M.; Bharathi, B.; Reddy, C.V.; Kantam, M.L.; Raghavan, K.V. The first example of catalytic N-oxidation of tertiary amines by tungstate-exchanged Mg–Al layered double hydroxide in water: A green protocol. Chem. Commun. 2001, 1736–1737. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.M.; Medina, F.; Tichit, D.; Pérez-Ramírez, J.; Rodríguez, X.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Study of alkaline-doping agents on the performance of reconstructed Mg–Al hydrotalcites in aldol condensations. Appl. Catal. A Gen. 2005, 281, 191–198. [Google Scholar] [CrossRef]
- Kantam, M.L.; Ravindra, A.; Reddy, C.V.; Sreedhar, B.; Choudary, B.M. Layered Double Hydroxides-Supported Diisopropylamide: Synthesis, Characterization and Application in Organic Reactions. Adv. Synth. Catal. 2006, 348, 569–578. [Google Scholar] [CrossRef]
- Zümreoğlu-Karan, B.; Ay, A.N. Layered double hydroxides—multifunctional nanomaterials. Chem. Pap. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Likhar, P.R.; Arundhathi, R.; Kantam, M.L. A recyclable Cu/Al-HT catalyst for amination of aryl chlorides. Tetrahedron Lett. 2007, 48, 3911–3914. [Google Scholar] [CrossRef]
- Choudary, B.; Reddy, C.; Prakash, B.; Bharathi, B.; Kantam, M. Oxidation of secondary and tertiary amines by a solid base catalyst. J. Mol. Catal. A Chem. 2004, 217, 81–85. [Google Scholar] [CrossRef]
- Arrabito, G.; Bonasera, A.; Prestopino, G.; Orsini, A.; Mattoccia, A.; Martinelli, E.; Pignataro, B.; Medaglia, P. Layered Double Hydroxides: A Toolbox for Chemistry and Biology. Crystals 2019, 9, 361. [Google Scholar] [CrossRef]
- Fu, Y.; Ning, F.; Xu, S.; An, H.; Shao, M.; Wei, M. Terbium doped ZnCr-layered double hydroxides with largely enhanced visible light photocatalytic performance. J. Mater. Chem. A 2016, 4, 3907–3913. [Google Scholar] [CrossRef]
- Silva, C.G.; Bouizi, Y.; Fornés, V.; García, H. Layered Double Hydroxides as Highly Efficient Photocatalysts for Visible Light Oxygen Generation from Water. J. Am. Chem. Soc. 2009, 131, 13833–13839. [Google Scholar] [CrossRef]
- Wein, L.A.; Zhang, H.; Urushidate, K.; Miyano, M.; Izumi, Y. Optimized photoreduction of CO2 exclusively into methanol utilizing liberated reaction space in layered double hydroxides comprising zinc, copper, and gallium. Appl. Surf. Sci. 2018, 447, 687–696. [Google Scholar] [CrossRef]
- Gama, B.; Selvasembian, R.; Giannakoudakis, D.; Triantafyllidis, K.; McKay, G.; Meili, L. Layered Double Hydroxides as Ris-ing-Star Adsorbents for Water Purification: A Brief Discussion. Molecules 2022, 27, 4900. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, K.; Xu, Z.P.; Lu, G. Layered double hydroxide nanoparticles in gene and drug delivery. Expert Opin. Drug Deliv. 2009, 6, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide-based Nanomaterials as Highly Efficient Catalysts and Adsorbents. Small 2014, 10, 4469–4486. [Google Scholar] [CrossRef] [PubMed]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Del Hoyo, C. Layered double hydroxides and human health: An overview. Appl. Clay Sci. 2015, 36, 103–121. [Google Scholar] [CrossRef]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Zhang, Q.; Huang, J.-Q.; Wei, F. Hierarchical Nanocomposites Derived from Nanocarbons and Layered Double Hydroxides-Properties, Synthesis, and Applications. Adv. Funct. Mater. 2012, 22, 675–694. [Google Scholar] [CrossRef]
- Wu, F.; Liang, J.; Peng, Z.; Liu, B. Electrochemical deposition and characterization of Zn-Al layered double hydroxides (LDHs) films on magnesium alloy. Appl. Surf. Sci. 2014, 313, 834–840. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Webber, M.R.; Gorman, W.R.; Rogers, R.E. Removal of Copper Ions from Aqueous Solutions via Adsorption on Carbon Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 15674–15680. [Google Scholar] [CrossRef]
- Thirunavukkarasu, A.; Nithya, R.; Sivashankar, R. A review on the role of nanomaterials in the removal of organic pollutants from wastewater. Rev. Environ. Sci. Bio/Technol. 2020, 19, 1–28. [Google Scholar] [CrossRef]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Chapter 1. Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. In Heavy Metals in Water; RSC Publishing: Cambridge, UK, 2014; pp. 1–24. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Yang, L.; Han, M.; Zhao, J.; Cheng, X. Nanomaterials as Sorbents to Remove Heavy Metal Ions in Wastewater Treatment. J. Environ. Anal. Toxicol. 2012, 2, 154–158. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Faur-Brasquet, C.; Le Cloirec, P. Removal of Cu(II), Pb(II), and Ni(II) by Adsorption onto Activated Carbon Cloths. Langmuir 2000, 16, 8404–8409. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Shamsayei, M. Highly selective and efficient removal of arsenic(V), chromium(VI) and selenium(VI) oxyanions by layered double hydroxide intercalated with zwitterionic glycine. J. Hazard. Mater. 2017, 339, 239–247. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.; Tadé, M. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Lazaridis, N.; Matis, K.; Webb, M. Flotation of metal-loaded clay anion exchangers. Part I: The case of chromates. Chemosphere 2001, 42, 373–378. [Google Scholar] [CrossRef]
- Lazaridis, N.; Hourzemanoglou, A.; Matis, K. Flotation of metal-loaded clay anion exchangers. Part II: The case of arsenates. Chemosphere 2002, 47, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gillman, G. A simple technology for arsenic removal from drinking water using hydrotalcite. Sci. Total. Environ. 2006, 366, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Kuzawa, K.; Jung, Y.-J.; Kiso, Y.; Yamada, T.; Nagai, M.; Lee, T.-G. Phosphate removal and recovery with a synthetic hydrotalcite as an adsorbent. Chemosphere 2006, 62, 45–52. [Google Scholar] [CrossRef]
- Wang, S.; Hseu, R.; Chang, R.; Chiang, P.; Chen, J.; Tzou, Y. Adsorption and thermal desorption of Cr(VI) on Li/Al layered double hydroxide. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 277, 8–14. [Google Scholar] [CrossRef]
- Murayama, N.; Sakamoto, D.; Shibata, J.; Valix, M. Removal of Harmful Anions in Aqueous Solution with Various Layered Double Hydroxides. Resour. Process. 2013, 60, 131–137. [Google Scholar] [CrossRef]
- Irawan, C.; Sari, A.R.; Yulianingtias, A.; Melinda, R.A.; Mirwan, A. Removal of Arsenic from Synthetic Acid Mine Drainage using Mn-Fe Layered Double Hydroxide Adsorbent. J. Rekayasa Kim. Lingkung. 2021, 16, 45–51. [Google Scholar] [CrossRef]
- Kameda, T.; Fubasami, Y.; Yoshioka, T. Kinetics and equilibrium studies on the treatment of nitric acid with Mg–Al oxide obtained by thermal decomposition of NO3--intercalated Mg–Al layered double hydroxide. J. Colloid Interface Sci. 2011, 362, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Socías-Viciana, M.M.; Ureña-Amate, M.D.; González-Pradas, E.; García-Cortés, M.J.; López-Teruel, C. Nitrate Removal by Calcined Hydrotalcite-Type Compounds. Clays Clay Miner. 2008, 56, 2–9. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Enhanced Arsenic Removal by Hydrothermally Treated Nanocrystalline Mg/Al Layered Double Hydroxide with Nitrate Intercalation. Environ. Sci. Technol. 2009, 43, 2537–2543. [Google Scholar] [CrossRef]
- Yoshida, M.; Koilraj, P.; Qiu, X.; Hirajim, T.; Sasaki, K. Sorption of arsenate on MgAl and MgFe layered double hydroxides derived from calcined dolomite. J. Environ. Chem. Eng. 2015, 3, 1614–1621. [Google Scholar] [CrossRef]
- Goswamee, R.L.; Sengupta, P.; Bhattacharyya, K.G.; Dutta, D.K. Adsorption of Cr(VI) in layered double hydroxides. Appl. Clay Sci. 1998, 13, 21–34. [Google Scholar] [CrossRef]
- Koilraj, P.; Kannan, S. Phosphate uptake behavior of ZnAlZr ternary layered double hydroxides through surface precipitation. J. Colloid Interface Sci. 2010, 341, 289–297. [Google Scholar] [CrossRef]
- Châttelet, L.; Bottero, J.; Yvon, J. Competition between monovalent and divalent anions for calcined and uncalcined hy-drotalcite: Anion exchange and adsorption sites. Colloids Surf. A Physicochem. Eng. Asp. 1996, 111, 167–175. [Google Scholar] [CrossRef]
- Peligro, F.R.; Pavlovic, I.; Rojas, R.; Barriga, C. Removal of heavy metals from simulated wastewater by in situ formation of layered double hydroxides. Chem. Eng. J. 2016, 306, 1035–1040. [Google Scholar] [CrossRef]
- Pérez, M.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Hermosín, M.; Ulibarri, M. Uptake of Cu2+, Cd2+ and Pb2+ on Zn–Al layered double hydroxide intercalated with edta. Appl. Clay Sci. 2006, 32, 245–251. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, B.; Wang, P.; Wang, Y.; Li, Z.; Wang, Y.; Feng, L.; Li, X.; Du, S. From waste to waste treatment: Mesoporous magnetic NiFe2O4/ZnCuCr-layered double hydroxide composite for wastewater treatment. J. Alloys Compd. 2020, 819, 153053. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, W. Magnetic Fe3O4 based layered double hydroxides (LDHs) nanocomposites (Fe3O4/LDHs): Recent review of progress in synthesis, properties and applications. J. Nanostruct. Chem. 2018, 8, 393–412. [Google Scholar] [CrossRef]
- Valente, J.; Rodriguez-Gattorno, G.; Valle-Orta, M.; Torres-Garcia, E. Thermal decomposition kinetics of MgAl layered double hydroxides. Mater. Chem. Phys. 2012, 133, 621–629. [Google Scholar] [CrossRef]
- Singh, K.; Ohlan, A.; Saini, P.; Dhawan, S.K. Poly (3,4-ethylenedioxythiophene)γ-Fe2O3 polymer composite–super paramagnetic behavior and variable range hopping 1D conduction mechanism–synthesis and characterization. Polym. Adv. Technol. 2008, 19, 229–236. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Wang, Q.; Wilkie, C.A.; O’Hare, D. Flame retardant polymer/layered double hydroxide nanocomposites. J. Mater. Chem. A 2014, 2, 10996–11016. [Google Scholar] [CrossRef]
- Zammarano, M.; Franceschi, M.; Bellayer, S.; Gilman, J.W.; Meriani, S. Preparation and flame resistance properties of revolutionary self-extinguishing epoxy nanocomposites based on layered double hydroxides. Polymer 2005, 46, 9314–9328. [Google Scholar] [CrossRef]
- Chen, J.-S.; Poliks, M.D.; Ober, C.K.; Zhang, Y.; Wiesner, U.; Giannelis, E. Study of the interlayer expansion mechanism and thermal–mechanical properties of surface-initiated epoxy nanocomposites. Polymer 2002, 43, 4895–4904. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Y.; Zhang, Q.; Wang, F.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient Flame-Retardant and Smoke-Suppression Properties of Mg–Al-Layered Double-Hydroxide Nanostructures on Wood Substrate. ACS Appl. Mater. Interfaces 2017, 9, 23039–23047. [Google Scholar] [CrossRef]
- Wang, P.-J.; Hu, X.-P.; Liao, D.-J.; Wen, Y.; Hull, T.R.; Miao, F.; Zhang, Q.-T. Dual Fire Retardant Action: The Combined Gas and Condensed Phase Effects of Azo-Modified NiZnAl Layered Double Hydroxide on Intumescent Polypropylene. Ind. Eng. Chem. Res. 2017, 56, 920–932. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Chen, D.; Su, S.; Wilkie, C.A. The effects of intralayer metal composition of layered double hydroxides on glass transition, dispersion, thermal and fire properties of their PMMA nanocomposites. Thermochim. Acta 2009, 495, 63–71. [Google Scholar] [CrossRef]
- Pradhan, S.; Costa, F.; Wagenknecht, U.; Jehnichen, D.; Bhowmick, A.; Heinrich, G. Elastomer/LDH nanocomposites: Synthesis and studies on nanoparticle dispersion, mechanical properties and interfacial adhesion. Eur. Polym. J. 2008, 44, 3122–3132. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Songtipya, P.; Manias, E.; Jimenez-Gasco, M.D.M.; Hossenlopp, J.M.; Wilkie, C.A. Polymer nanocomposites using zinc aluminum and magnesium aluminum oleate layered double hydroxides: Effects of the polymeric compatibilizer and of composition on the thermal and fire properties of PP/LDH nanocomposites. Polym. Degrad. Stab. 2009, 94, 2042–2054. [Google Scholar] [CrossRef]
- Nyambo, C.; Chen, D.; Su, S.; Wilkie, C.A. Does organic modification of layered double hydroxides improve the fire performance of PMMA? Polym. Degrad. Stab. 2009, 94, 1298–1306. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Wang, D.; Hossenlopp, J.M.; Wilkie, C.A. Aluminum-containing layered double hydroxides: The thermal, mechanical, and fire properties of (nano)composites of poly(methyl methacrylate). J. Mater. Chem. 2008, 18, 3091–3102. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Songtipya, P.; Manias, E.; Jimenez-Gasco, M.M.; Hossenlopp, J.M.; Wilkie, C.A. Polymer nanocomposites using zinc aluminum and magnesium aluminum oleate layered double hydroxides: Effects of LDH divalent metals on dispersion, thermal, mechanical and fire performance in various polymers. Polymer 2009, 50, 3564–3574. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Wang, D.; Hossenlopp, J.M.; Wilkie, C.A. The role of the trivalent metal in an LDH: Synthesis, characterization and fire properties of thermally stable PMMA/LDH systems. Polym. Degrad. Stab. 2009, 94, 705–711. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Wang, D.Y.; George, J.J.; Stockelhuber, K.W.; Boldt, R.; Leuteritz, A.; Heinrich, G. Fire-safe and environmentally friendly nanocomposites based on layered double hydroxides and ethylene propylene diene elastomer. RSC Adv. 2016, 6, 26425–26436. [Google Scholar] [CrossRef]
- Costache, M.C.; Heidecker, M.J.; Manias, E.; Camino, G.; Frache, A.; Beyer, G.; Gupta, R.K.; Wilkie, C.A. The influence of carbon nanotubes, organically modified montmorillonites and layered double hydroxides on the thermal degradation and fire retardancy of polyethylene, ethylene–vinyl acetate copolymer and polystyrene. Polymer 2007, 48, 6532–6545. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Das, A.; Leuteritz, A.; Mahaling, R.; Jehnichen, D.; Wagenknecht, U.; Heinrich, G. Structural characteristics and flammability of fire retarding EPDM/layered double hydroxide (LDH) nanocomposites. RSC Adv. 2012, 2, 3927–3933. [Google Scholar] [CrossRef]
- Colonna, S.; Bastianini, M.; Sisani, M.; Fina, A. CO2 adsorption and desorption properties of calcined layered double hydroxides: Effect of metal composition on the LDH structure. J. Therm. Anal. Calorim. 2018, 133, 869–879. [Google Scholar] [CrossRef]
- Garcia-Gallastegui, A.; Iruretagoyena, D.; Gouvea, V.; Mokhtar, M.; Asiri, A.M.; Basahel, S.N.; Al-Thabaiti, S.A.; Alyoubi, A.O.; Chadwick, D.; Shaffer, M.S.P. Graphene Oxide as Support for Layered Double Hydroxides: Enhancing the CO2 Adsorption Capacity. Chem. Mater. 2012, 24, 4531–4539. [Google Scholar] [CrossRef]
- Ulibarri, M. Adsorption of anionic species on hydrotalcite-like compounds: Effect of interlayer anion and crystallinity. Appl. Clay Sci. 2001, 18, 17–27. [Google Scholar] [CrossRef]
- Ulibarri, M.; Pavlovic, I.; Hermosín, M.; Cornejo, J. Hydrotalcite-like compounds as potential sorbents of phenols from water. Appl. Clay Sci. 1995, 10, 131–145. [Google Scholar] [CrossRef]
- Barriga, C.; Gaitán, M.; Pavlovic, I.; Ulibarri, M.A.; Hermosĩn, M.C.; Cornejo, J. Hydrotalcites as sorbent for 2,4,6-trinitrophenol: Influence of the layer composition and interlayer anion. J. Mater. Chem. 2002, 12, 1027–1034. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.P.; Zhang, Q.; Lu, G.M.; Hao, Z.P.; Liu, S. Studies on adsorption of phenol and 4-nitrophenol on MgAl-mixed oxide derived from MgAl-layered double hydroxide. Sep. Purif. Technol. 2009, 67, 194–200. [Google Scholar] [CrossRef]
- Chaara, D.; Pavlovic, I.; Bruna, F.; Ulibarri, M.; Draoui, K.; Barriga, C. Removal of nitrophenol pesticides from aqueous solutions by layered double hydroxides and their calcined products. Appl. Clay Sci. 2010, 50, 292–298. [Google Scholar] [CrossRef]
- El Shafei, G.M. Change of Structural and Adsorption Properties Due to Isomorphous Substitution in Hydrotalcite-like Materials. Adsorpt. Sci. Technol. 2002, 20, 767–786. [Google Scholar] [CrossRef]
- Leroux, F.; Besse, J.-P. Polymer Interleaved Layered Double Hydroxide: A New Emerging Class of Nanocomposites. Chem. Mater. 2001, 13, 3507–3515. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Torres-Dorante, L.O.; Lammel, J.; Kuhlmann, H.; Witzke, T.; Olfs, H. Capacity, selectivity, and reversibility for nitrate exchange of a layered double-hydroxide (LDH) mineral in simulated soil solutions and in soil. J. Plant Nutr. Soil Sci. 2008, 171, 777–784. [Google Scholar] [CrossRef]
- Berber, M.R.; Hafez, I.; Minagawa, K.; Mori, T. A sustained controlled release formulation of soil nitrogen based on nitrate-layered double hydroxide nanoparticle material. J. Soil Sedim. 2014, 14, 60–66. [Google Scholar] [CrossRef]
- Terry, P.A. Removal of Nitrates and Phosphates by Ion Exchange with Hydrotalcite. Environ. Eng. Sci. 2009, 26, 691–696. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Yang, Q.; Evans, D.G.; Forano, C.; Duan, X. Study on adsorption of glyphosate (N-phosphonomethyl glycine) pesticide on MgAl-layered double hydroxides in aqueous solution. J. Hazard. Mater. 2005, 125, 89–95. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Bullo, S.; Hussein, M.Z. Inorganic nanolayers: Structure, preparation, and biomedical applications. Int. J. Nanomed. 2015, 10, 5609–5633. [Google Scholar] [CrossRef] [PubMed]
- Kuthati, Y.; Kankala, R.K.; Lee, C.-H. Layered double hydroxide nanoparticles for biomedical applications: Current status and recent prospects. Appl. Clay Sci. 2015, 112–113, 100–116. [Google Scholar] [CrossRef]
- Nakayama, H.; Hatakeyama, A.; Tsuhako, M. Encapsulation of nucleotides and DNA into Mg–Al layered double hydroxide. Int. J. Pharm. 2010, 393, 105–112. [Google Scholar] [CrossRef]
- Senapati, S.; Thakur, R.; Verma, S.P.; Duggal, S.; Mishra, D.P.; Das, P.; Shripathi, T.; Kumar, M.; Rana, D.; Maiti, P. Layered double hydroxides as effective carrier for anticancer drugs and tailoring of release rate through interlayer anions. J. Control. Release 2016, 224, 186–198. [Google Scholar] [CrossRef]
- De Melo, J.V.; Cosnier, S.; Mousty, C.; Martelet, C.; Jaffrezic-Renault, N. Urea Biosensors Based on Immobilization of Urease into Two Oppositely Charged Clays (Laponite and Zn-Al Layered Double Hydroxides) the effect of the buffer capacity of the outer medium. Anal. Chem. 2002, 74, 4037–4043. [Google Scholar] [CrossRef]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2017, 28, 1703868. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Toghill, K.; Compton, R. Electrochemical Non-enzymatic Glucose Sensors: A Perspective and an Evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Kulandaivalu, S.; Azman, N.H.N.; Sulaiman, Y. Advances in Layered Double Hydroxide/Carbon Nanocomposites Containing Ni2+ and Co2+/3+ for Supercapacitors. Front. Mater. 2020, 7, 147. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Cheng, J.; Ying, J.; Zhang, X. Enhanced performance of nickel–aluminum layered double hydroxide nanosheets carbon nanotubes composite for supercapacitor and asymmetric capacitor. J. Alloys Compd. 2015, 635, 225–232. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Zhang, X.B.; Cheng, J.P. A comparative study of Ni–Mn layered double hydroxide/carbon composites with different morphologies for supercapacitors. Chem. Phys. 2016, 18, 30068–30078. [Google Scholar] [CrossRef]
- Liang, R.; Xu, S.; Yan, D.; Shi, W.; Tian, R.; Yan, H.; Wei, M.; Evans, D.G.; Duan, X. CdTe Quantum Dots/Layered Double Hydroxide Ultrathin Films with Multicolor Light Emission via Layer-by-Layer Assembly. Adv. Funct. Mater. 2012, 22, 4940–4948. [Google Scholar] [CrossRef]
- Liang, R.; Yan, D.; Tian, R.; Yu, X.; Shi, W.; Li, C.; Wei, M.; Evans, D.G.; Duan, X. Quantum Dots-Based Flexible Films and Their Application as the Phosphor in White Light-Emitting Diodes. Chem. Mater. 2014, 26, 2595–2600. [Google Scholar] [CrossRef]
- Cho, S.; Jung, S.; Jeong, S.; Bang, J.; Park, J.; Park, Y.; Kim, S. Strategy for Synthesizing Quantum Dot-Layered Double Hydroxide Nanocomposites and Their Enhanced Photoluminescence and Photostability. Langmuir 2013, 29, 441–447. [Google Scholar] [CrossRef]
- Wang, X.R.; Lu, J.; Yan, D.; Wei, M.; Evans, D.G.; Duan, X. A photochromic thin film based on salicylideneaniline derivatives intercalated layered double hydroxide. Chem. Phys. Lett. 2010, 493, 333–339. [Google Scholar] [CrossRef]


| Anion | Metal Ions | Pollutant | Removal Mechanism | Cm (mg/g) | Reference |
|---|---|---|---|---|---|
| CO3−2 | Mg-Al | CrO42− | Reconstruction | 276 | [108] |
| CO3−2 | Mg-Al | AsO43− | Anion exchange | 276 | [109] |
| CO3−2 | Mg-Al | NO3− | Reconstruction adsorption | 147 | [103] |
| CO3−2 | Mg-Al | AsO43− | Reconstruction adsorption | 116 | [104] |
| CO3−2 | Mg-Al | CrO42− | Reconstruction | 248 | [106] |
| CO3−2 | Mg-Al | CrO42− | Reconstruction | 280 | [106] |
| CO3−2 | Ni-Al | CrO42− | Reconstruction | 86 | [106] |
| CO3−2 | Zn-Al | PO43− | Weathering/precipitation | 273 | [107] |
| CO3−2 | Zn-Al | PO43− | Weathering/precipitation | 189 | [107] |
| CO3−2 | Zn-Al | PO43− | Weathering/precipitation | 75 | [107] |
| NO3− | Mg-Al | NO3− | Reconstruction adsorption | 236 | [102] |
| NO3− | Mg-Al | AsO43− | Anion exchange | 31 | [104] |
| Cl− | Mg-Al | SO32− | Anion exchange | 495 | [110] |
| Cl− | Mg-Al | SO32− | Anion exchange | 415 | [110] |
| Cl− | Mn-Fe | SO32− | Coprecipitation | 310 | [110] |
| Cl− | Mg-Al | AsO43− | Anion exchange | 222 | [105] |
| Cl− | Mg-Fe | AsO43− | Anion exchange | 373 | [105] |
| Organic Anions | LDH Metal Ions | Polymer * | Reduction in PHRR | Reference |
|---|---|---|---|---|
| Undecenoate | Zn-Al | PMMA | 46% (10 wt %) | [119] |
| Undecenoate | Ni-Al | PMMA | 16% (10 wt %) 41% (10 wt %) 25.7% (10 wt %) | [119] |
| Undecenoate | Ca-Al | PMMA | 36% (6 wt %) 24% (6 wt %) 16% (6 wt %) 36% (6 wt %) 7% (6 wt %) | [126] |
| Oleate | Mg-Al | PP | 5% (2 wt %) | [129] |
| Oleate | Zn-Al | PP PMMA PE EVA | 25% (4 wt %) 28% (10 wt %) 58% (10 wt %) 33% (10 wt %) | [129] |
| Oleate | Zn-Mg-Al | PP | 38% (4 wt %) | [129] |
| ASA | Mg-Al | EVA | 39% (3 wt %) | [132] |
| DBS | Mg-Al | EPDM | 25% (40 wt %) | [133] |
| DBS | Mg-Al | PMMA | 45% (10 wt %) | [129] |
| Cn | Mg-Al | PS | 20–55% (10 wt %) | [127] |
| Anion | Metal Ion | Pollutant | Removal Mechanism | Cm (mg/g) | Reference |
|---|---|---|---|---|---|
| CO3−2 | Mg-Al | TNP | Anion exchange | 185 | [137] |
| CO3−2 | Mg-Al | TNP | Reconstruction | 1330 | [137] |
| CO3−2 | Mg-Al | TCP | Anion exchange | 2 | [138] |
| CO3−2 | Mg-Al | TCP | Reconstruction | 8 | [138] |
| CO3−2 | Mg-Al | 4-NP | Reconstruction | 370 | [139] |
| CO3−2 | Mg-Al | Phenol | Reconstruction | 47 | [139] |
| Cl− | Mg-Al | DNP | Anion exchange | 714 | [140] |
| Cl− | Mg-Al | DNOP | Anion exchange | 503 | [140] |
| Cl− | Mg-Al | DNOP | Anion exchange | 440 | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kameliya, J.; Verma, A.; Dutta, P.; Arora, C.; Vyas, S.; Varma, R.S. Layered Double Hydroxide Materials: A Review on Their Preparation, Characterization, and Applications. Inorganics 2023, 11, 121. https://doi.org/10.3390/inorganics11030121
Kameliya J, Verma A, Dutta P, Arora C, Vyas S, Varma RS. Layered Double Hydroxide Materials: A Review on Their Preparation, Characterization, and Applications. Inorganics. 2023; 11(3):121. https://doi.org/10.3390/inorganics11030121
Chicago/Turabian StyleKameliya, Jitendra, Aazad Verma, Partha Dutta, Charu Arora, Shweta Vyas, and Rajender S. Varma. 2023. "Layered Double Hydroxide Materials: A Review on Their Preparation, Characterization, and Applications" Inorganics 11, no. 3: 121. https://doi.org/10.3390/inorganics11030121
APA StyleKameliya, J., Verma, A., Dutta, P., Arora, C., Vyas, S., & Varma, R. S. (2023). Layered Double Hydroxide Materials: A Review on Their Preparation, Characterization, and Applications. Inorganics, 11(3), 121. https://doi.org/10.3390/inorganics11030121










