Exploring Proton Pair Motion Away from the Global Proton–Tuple Energy Minimum in Yttrium-Doped Barium Zirconate
Abstract
:1. Introduction
2. Results
2.1. Lowest Energy Proton–Tuple and Single Proton Moves Away from It
2.2. Two Proton Moves Away from Global Minimum
3. Methods
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| kMC | Kinetic Monte Carlo |
| cNEB | Climbing Nudged Elastic Band |
| VASP | Vienna Ab Initio Simulations Package |
| NEB | Nudged Elastic Band |
| DFT | Density Functional Theory |
| PBE | Perdew–Burke–Ernzerhof |
References
- Regalado Vera, C.Y.; Hanping, D.; Peterson, D.; Gibbons, W.; Zhou, M.; Ding, D. A mini-review on proton conduction in BaZrO3-based perovskite electrolytes. J. Phys. Energy 2021, 3, 032019. [Google Scholar] [CrossRef]
- Hossain, M.K.; Biswas, M.C.; Chanda, R.K.; Rubel, M.H.K.; Khan, M.I.; Hashizume, K. A review on experimental and theoretical studies of perovskite barium zirconate proton conductors. Emergent Mater. 2021, 4, 999–1027. [Google Scholar] [CrossRef]
- Vignesh, D.; Sonu, B.K.; Rout, E. Factors Constituting Proton Trapping in BaCeO3 and BaZrO3 Perovskite Proton Conductors in Fuel Cell Technology: A Review. Energy Fuels 2022, 36, 7219–7244. [Google Scholar] [CrossRef]
- Toyoura, K.; Fujii, T.; Hatada, N.; Han, D.; Uda, T. Carrier–Carrier Interaction in Proton-Conducting Perovskites: Carrier Blocking vs Trap-Site Filling. J. Phys. Chem. 2019, 123, 26823–26830. [Google Scholar] [CrossRef]
- Ding, J.; Balachandran, J.; Sang, X.; Guo, W.; Anchell, J.S.; Veith, G.M.; Bridges, C.A.; Cheng, Y.; Rouleau, C.M.; Poplawsky, J.D.; et al. The Influence of Local Distortions on Proton Mobility in Acceptor Doped Perovskites. Chem. Mater. 2018, 30, 4919–4925. [Google Scholar] [CrossRef]
- Buannic, L.; Sperrin, L.; Dervisoglu, R.; Blanc, F.; Grey, C.P. Proton distribution in Sc-doped BaZrO3: A solid state NMR and first principle calculations analysis. Phys. Chem. Chem. Phys. 2018, 20, 4317–4328. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, Y.; Blanc, F.; Okuyama, Y.; Buannic, L.; Lucio-Vega, J.C.; Grey, C.P.; Haile, S.M. Proton trapping in yttrium-doped barium zirconate. Nat. Mater. 2013, 12, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Zou, J.; Shan, L.; Jiang, X.; Ni, Y.; Li, X.; Qian, X.; Chen, W.; Zhou, Y.; Zhang, W.; et al. Conductivities in Yttrium-Doped Barium Zirconate: A First-Principles Study. Crystals 2023, 13, 401. [Google Scholar] [CrossRef]
- Draber, F.M.; Denninger, J.R.; Müller, P.C.; Sommerfeld, I.K.; Martin, M. The Impact of Nanoscale Percolation in Yttrium-Doped BaZrO3 on the Oxygen Ion and Proton Conductivities: A Density Functional Theory and Kinetic Monte Carlo Study. Adv. Energy Sustain. Res. 2022, 3, 2200007. [Google Scholar] [CrossRef]
- Gomez, M.A.; Brooks-Randall, S.; Cai, G.; Glass-Klaiber, J.; Jiang, Y.; Jo, S.; Lin, Z.; Lin, S.; Marcellus, M.; Nguyen, H.A.; et al. Graph analysis of proton conduction pathways in scandium-doped barium zirconate. J. Chem. Phys. 2021, 154, 074711. [Google Scholar] [CrossRef]
- Draber, F.M.; Ader, C.; Arnold, J.P.; Eisele, S.; Grieshammer, S.; Yamaguchi, S.; Martin, M. Nanoscale percolation in doped BaZrO3 for high proton mobility. Nat. Mater. 2020, 19, 338–346. [Google Scholar] [CrossRef]
- Brieuc, F.; Dezanneau, G.; Hayoun, M.; Dammak, H. Proton diffusion mechanisms in the double perovskite cathode material GdBaCo2O5.5: A molecular dynamics study. Solid State Ionics 2017, 309, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Krueger, R.; Haibach, F.; Fry, D.; Gomez, M. Centrality measures highlight proton traps and access points to proton highways in kinetic Monte Carlo trajectories. J. Chem. Phys. 2015, 142, 154110. [Google Scholar] [CrossRef] [Green Version]
- Hermet, J.; Torrent, M.; Bottin, F.; Dezanneau, G.; Geneste, G. Hydrogen diffusion in the protonic conductor BaCe1-xGdxO3-x/2 from density functional theory. Phys. Rev. B 2013, 87, 104303. [Google Scholar] [CrossRef]
- Gomez, M.A.; Chunduru, M.; Chigweshe, L.; Foster, L.; Fensin, S.J.; Fletcher, K.M.; Fernandez, L.E. The effect of yttrium dopant on the proton conduction pathways of BaZrO3, a cubic perovskite. J. Chem. Phys. 2010, 132, 214709. [Google Scholar] [CrossRef]
- Merinov, B.; Goddard, W. Proton diffusion pathways and rates in Y-doped BaZrO3 solid oxide electrolyte from quantum mechanics. J. Chem. Phys. 2009, 130, 194707. [Google Scholar] [CrossRef] [Green Version]
- Björketun, M.E.; Sundell, P.G.; Wahnström, G. Effect of acceptor dopants on the proton mobility in BaZrO3: A density functional investigation. Phys. Rev. B 2007, 76, 054307. [Google Scholar] [CrossRef]
- Björketun, M.E.; Sundell, P.G.; Wahnström, G.; Engberg, D. A kinetic Monte Carlo study of proton diffusion in disordered perovskite structured lattices based on first-principles calculations. Solid State Ionics 2005, 176, 3035–3040. [Google Scholar] [CrossRef]
- Munch, W.; Kreuer, K.D.; Seifert, G.; Maier, J. Proton diffusion in perovskites: Comparison between BaCeO3, BaZrO3, SrTiO3, and CaTiO3 using quantum molecular dynamics. Solid State Ionics 2000, 136-7, 183–189. [Google Scholar] [CrossRef]
- Du, P.; Chen, Q.; Fan, Z.; Pan, H.; Haibach, F.G.; Gomez, M.A.; Braun, A. Cooperative origin of proton pair diffusivity in yttrium substituted barium zirconate. Commun. Phys. 2020, 3, 200. [Google Scholar] [CrossRef]
- Ziqing, L.; Lin, S.; Tian, Y.; Van Bokkelen, A.; Valerio, M.; Gomez, M.A. Oxygen Vacancies Altering the Trapping in the Proton Conduction Landscape of Doped Barium Zirconate. J. Phys. Chem. 2020, 124, 27954–27964. [Google Scholar] [CrossRef]
- Torayev, A.; Sperrin, L.; Gomez, M.A.; Kattirtzi, J.A.; Merlet, C.; Grey, C.P. Local Distortions and Dynamics in Hydrated Y-Doped BaZrO3. J. Phys. Chem. Nanomater. Interfaces 2020, 124, 16689–16701. [Google Scholar] [CrossRef]
- Gomez, M.; Fry, D.; Sweet, M. Effects on the proton conduction and limiting barriers and trajectories in BaZr0.875Y0.125O3 due to the presence of other protons. J. Korean Ceram. Soc. 2016, 53, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Braun, A.; Chen, Q. Experimental neutron scattering evidence for proton polaron in hydrated metal oxide proton conductors. Nat. Commun. 2017, 8, 15830. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, N.; Du, P.; Yang, W.; Chen, Q. Influence of Lattice Dynamics on the Proton Transport in BaZrY-oxide perovskite under high pressure. J. Phys. Chem. 2020, 124, 22376–22382. [Google Scholar] [CrossRef]
- Samgin, A.L. Lattice-assisted proton motion in perovskite oxides. J. Phys. Chem. Solids 2013, 74, 1661–1668. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G. Ab Initio Molekular Dynamik für Flüssige Metalle. Ph.D. Thesis, Technische Universität at Wien, Vienna, Austria, 1993. [Google Scholar]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics for liquid-metals. Phys. Rev. B 1993, 47, RC558. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Payne, M.; Teter, M.P.; Ailan, D.C. Iterative minimization techniques for ab initio total energy calculation: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Oikawa, I.; Takamura, H. Atomistic Insight into the Correlation among Oxygen Vacancies, Protonic Defects, and the Acceptor Dopants in Sc-Doped BaZrO3 Using First-Principles Calculations. J. Phys. Chem. 2018, 122, 6501–6507. [Google Scholar] [CrossRef]
- Blanc, F.; Sperrin, L.; Lee, D.; Dervisoglu, R.; Yamazaki, Y.; Haile, S.M.; De Paëpe, G.; Grey, C.P. Dynamic nuclear polarization NMR of low-gamma nuclei: Structural Insights into hydrated yttrium-doped BaZrO3. J. Phys. Chem. Lett. 2014, 5, 2431–2436. [Google Scholar] [CrossRef] [Green Version]
- Kreuer, K.D.; Adams, S.; Munch, W.; Fuchs, A.; Klock, U.; Maier, J. Proton conducting alkaline earth zirconates and titanates for high drain electrochemical applications. Solid Sate Ionics 2001, 145, 295–306. [Google Scholar] [CrossRef]
- Bohn, H.G.; Schober, T. Electrical conductivity of the high-temperature proton conductor BaZr0.90Y0.1O2.95. J. Am. Ceram. Soc. 2000, 83, 768–772. [Google Scholar] [CrossRef]
- Gomez, M.A.; Kwan, G.; Zhu, W.; Chelliah, M.; Zuo, X.; Eshun, A.; Blackmer, V.; Huynh, T.; Huynh, M. Ordered yttrium concentration effects on barium zirconate structure, proton binding sites and transition states. Solid State Ionics 2017, 304, 126–134. [Google Scholar] [CrossRef]
- Geneste, G. Proton transfer in barium zirconate: Lattice reorganization, Landau-Zener curve-crossing approach. Solid State Ionics 2018, 323, 172–202. [Google Scholar] [CrossRef]
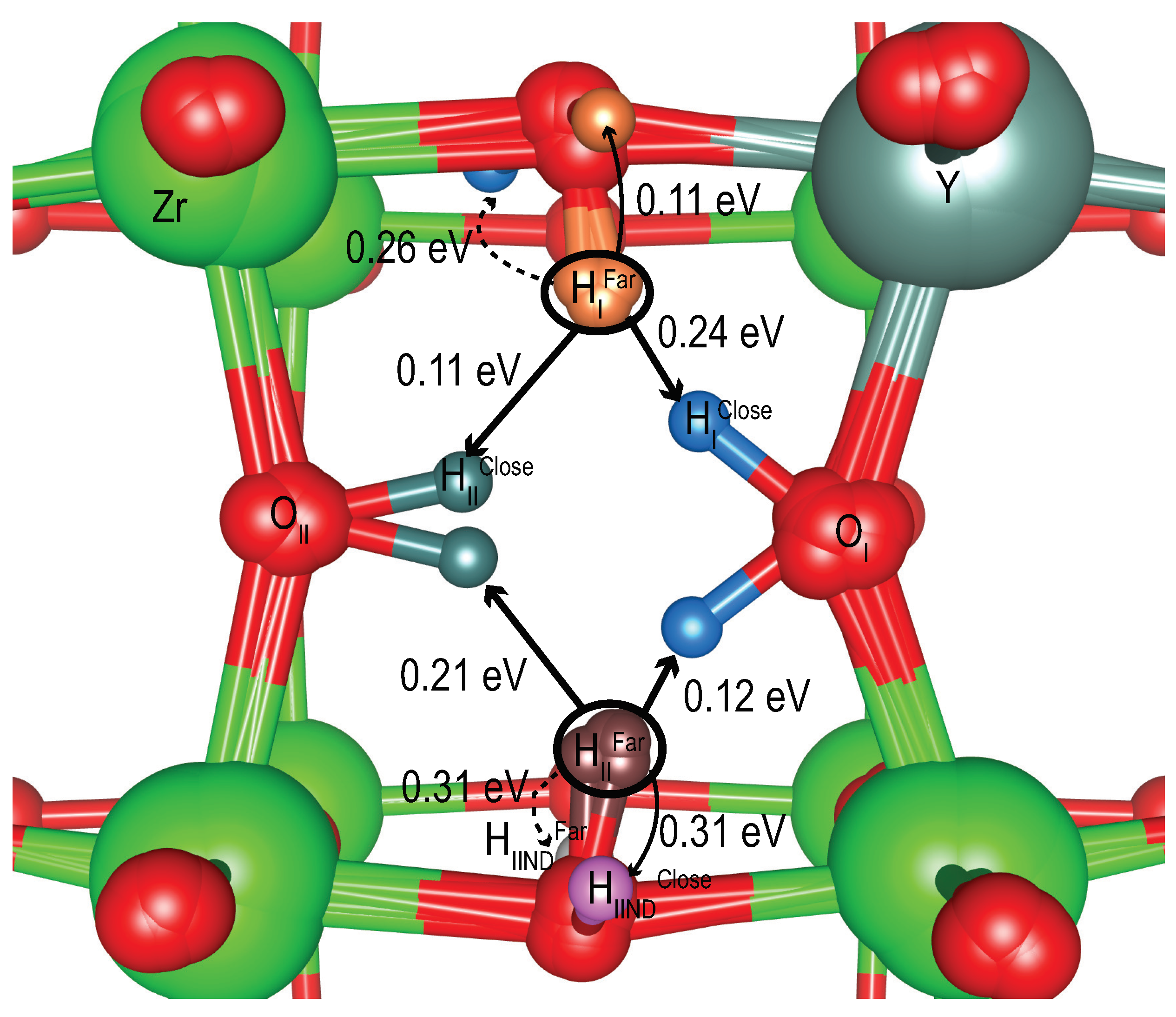
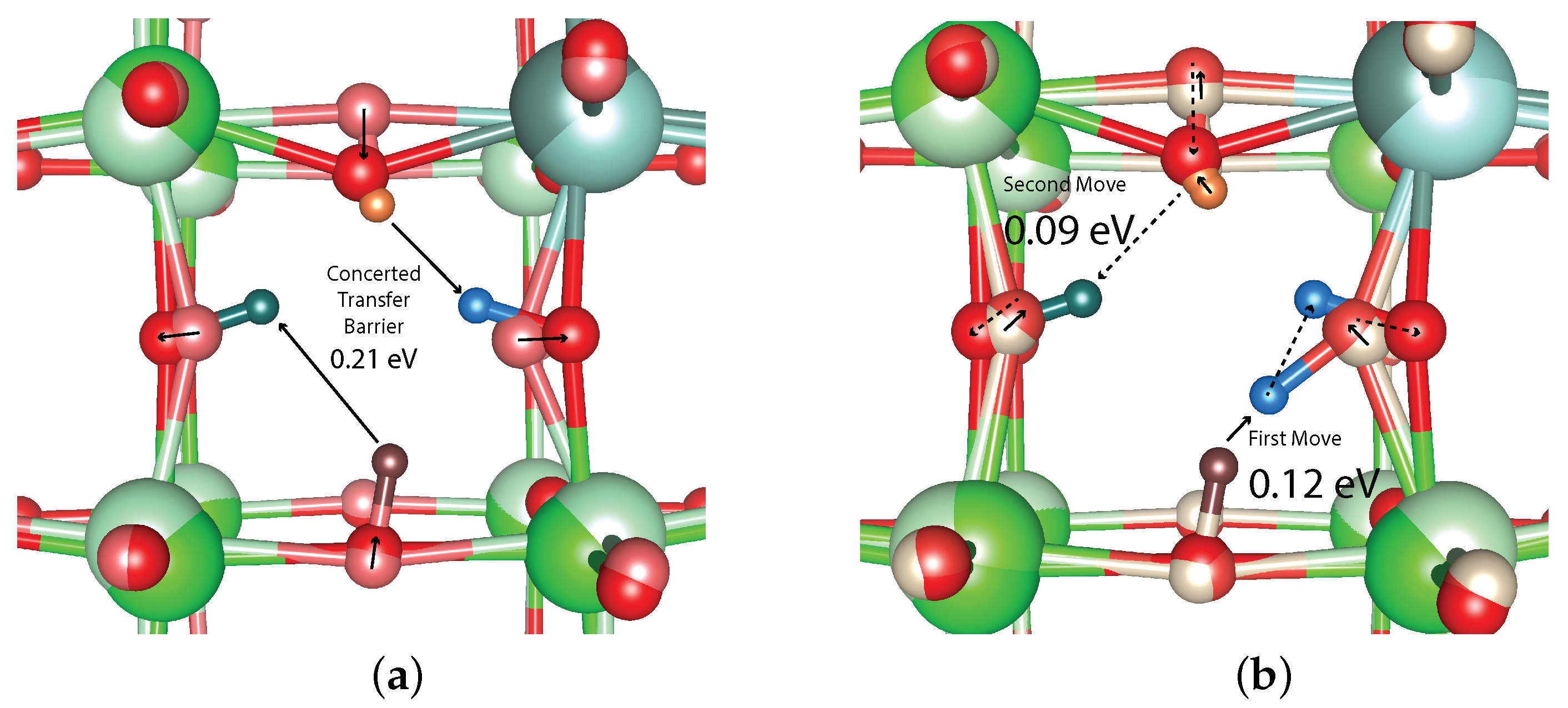

| Tuple | Relative Energy (eV) | Move From Global Minimum | Move Barrier (eV) |
|---|---|---|---|
| 0.11 | T | 0.11 | |
| 0.11 | R | 0.11 | |
| 0.16 | R | 0.26 | |
| 0.23 | T | 0.24 | |
| 0.11 | T | 0.12 | |
| 0.20 | T | 0.21 | |
| 0.31 | R | 0.31 | |
| 0.30 | R | 0.31 |
| Move | Barrier (eV) | Nearby Proton | One Proton Barrier |
|---|---|---|---|
| T | 0.11 | H | 0.14 [10], 0.32 [15] |
| 0.09 | H | ||
| R | 0.11 | H | 0.02 [10], 0.12 [15] |
| 0.25 | H | ||
| T | 0.24 | H | 0.24 [10], 0.25 [15] |
| R | 0.26 | H | 0.10 [10], 0.22 [15] |
| T | 0.12 | H | 0.11 [10], 0.17 [15] |
| 0.18 | H | ||
| 0.23 | H | ||
| T | 0.21 | H | 0.15 [10], 0.43 [15] |
| 0.26 | H | ||
| R | 0.31 | H | 0.24 [10,21], 0.24 [15] |
| 0.34 | H | ||
| 0.34 | H | ||
| 0.35 | H | ||
| 0.44 | H | ||
| R | 0.31 | H | 0.25 [10,21] 0.27 [15] |
| 0.33 | H | ||
| 0.37 | H | ||
| 0.41 | H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Hoang, M.T.; Mansoor, S.; Gomez, M.A. Exploring Proton Pair Motion Away from the Global Proton–Tuple Energy Minimum in Yttrium-Doped Barium Zirconate. Inorganics 2023, 11, 160. https://doi.org/10.3390/inorganics11040160
Pan Y, Hoang MT, Mansoor S, Gomez MA. Exploring Proton Pair Motion Away from the Global Proton–Tuple Energy Minimum in Yttrium-Doped Barium Zirconate. Inorganics. 2023; 11(4):160. https://doi.org/10.3390/inorganics11040160
Chicago/Turabian StylePan, Yiqing, Minh Tam Hoang, Sanaa Mansoor, and Maria Alexandra Gomez. 2023. "Exploring Proton Pair Motion Away from the Global Proton–Tuple Energy Minimum in Yttrium-Doped Barium Zirconate" Inorganics 11, no. 4: 160. https://doi.org/10.3390/inorganics11040160
APA StylePan, Y., Hoang, M. T., Mansoor, S., & Gomez, M. A. (2023). Exploring Proton Pair Motion Away from the Global Proton–Tuple Energy Minimum in Yttrium-Doped Barium Zirconate. Inorganics, 11(4), 160. https://doi.org/10.3390/inorganics11040160









