Keggin Heteropolyacid Salt Catalysts in Oxidation Reactions: A Review
Abstract
:1. Introduction
2. Main Routes to Synthesize POMs Salts
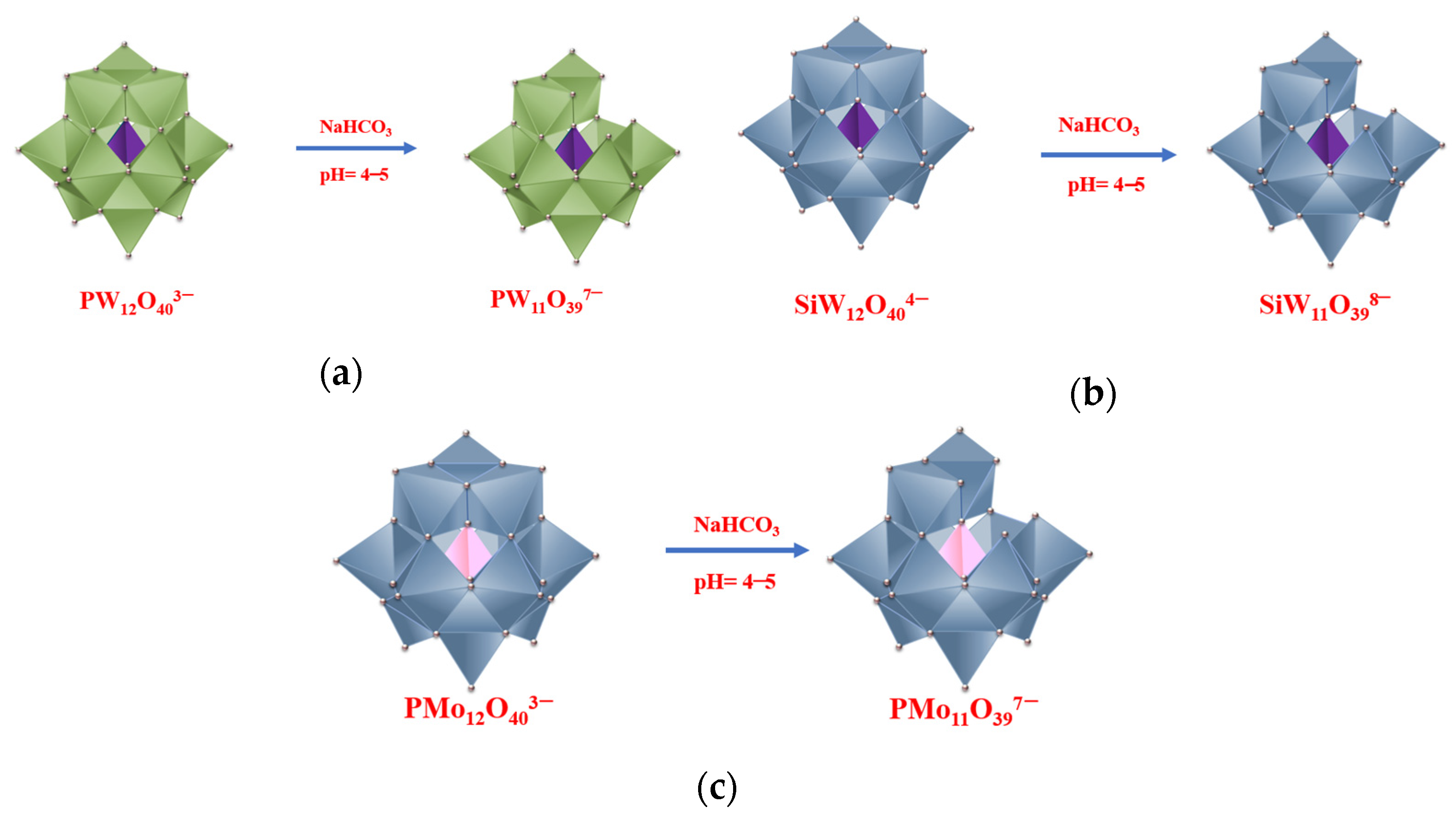
2.1. Synthesis of Metal-Exchanged Phosphotungstic, Phosphomolybdic, and Silicotungstic Acid Salts
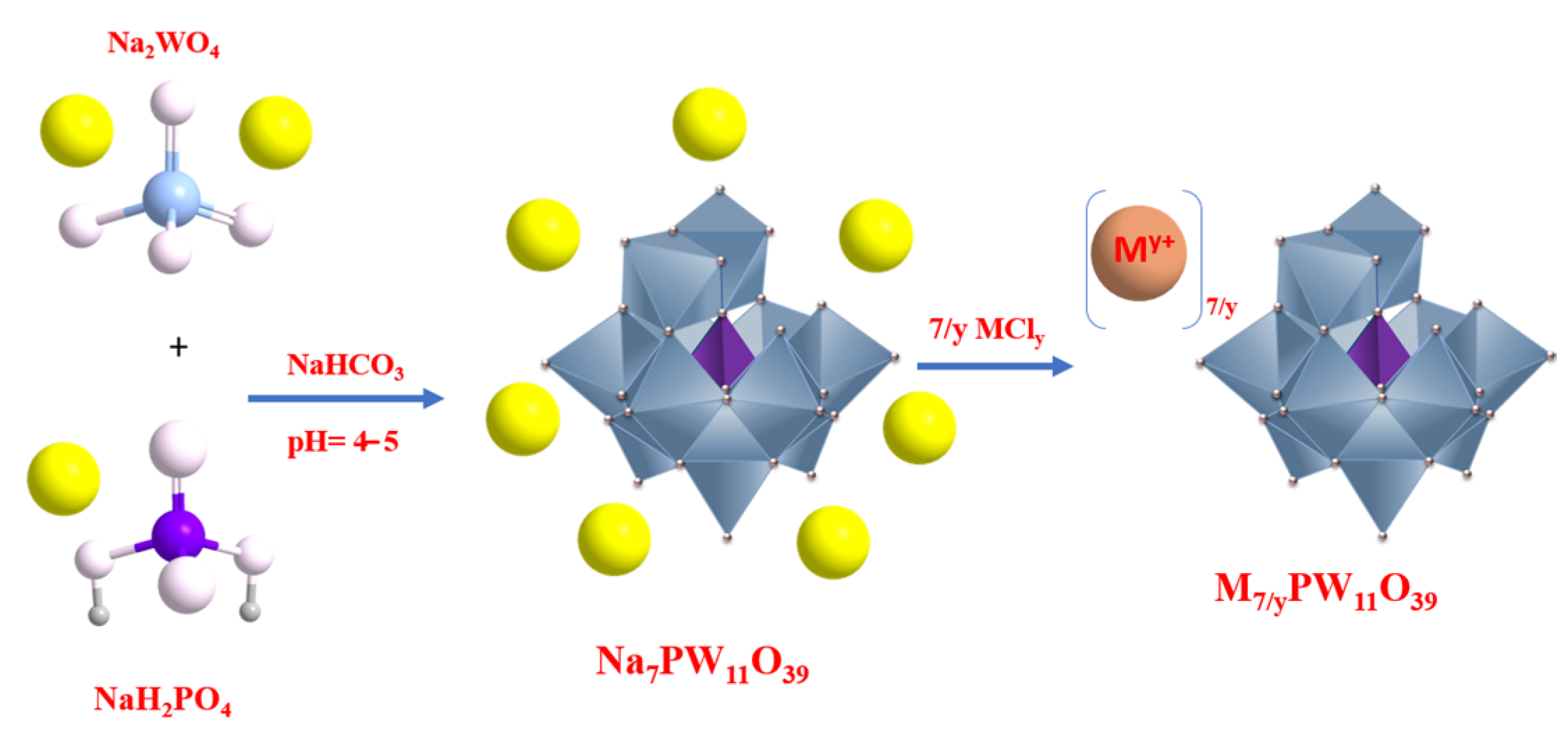
2.2. Synthesis of Phosphotungstic, Phosphomolybdic, and Silicotungstic Acids Lacunar Salts
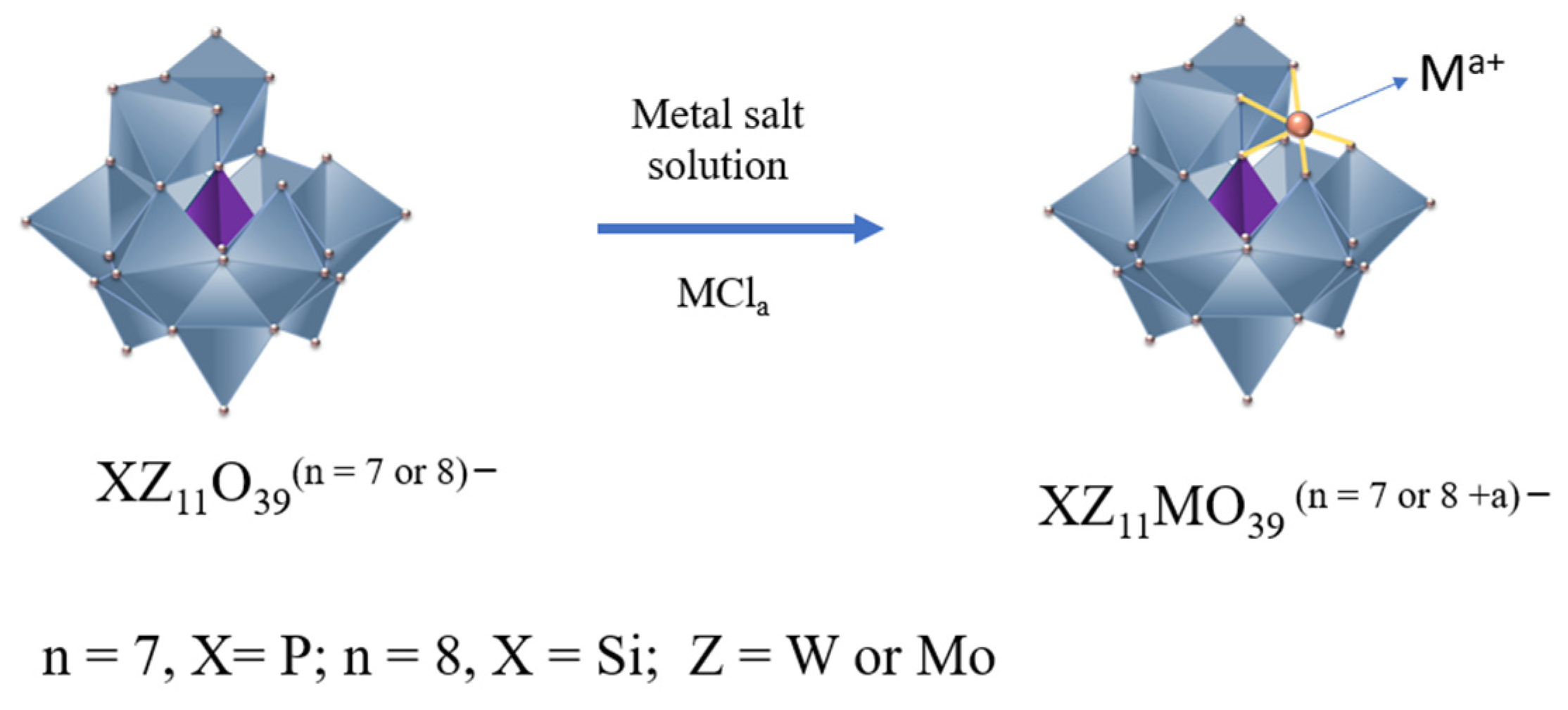
2.3. Synthesis of Metal-Substituted Lacunar HPA Salts
3. Characterization Techniques of Keggin HPA Salts
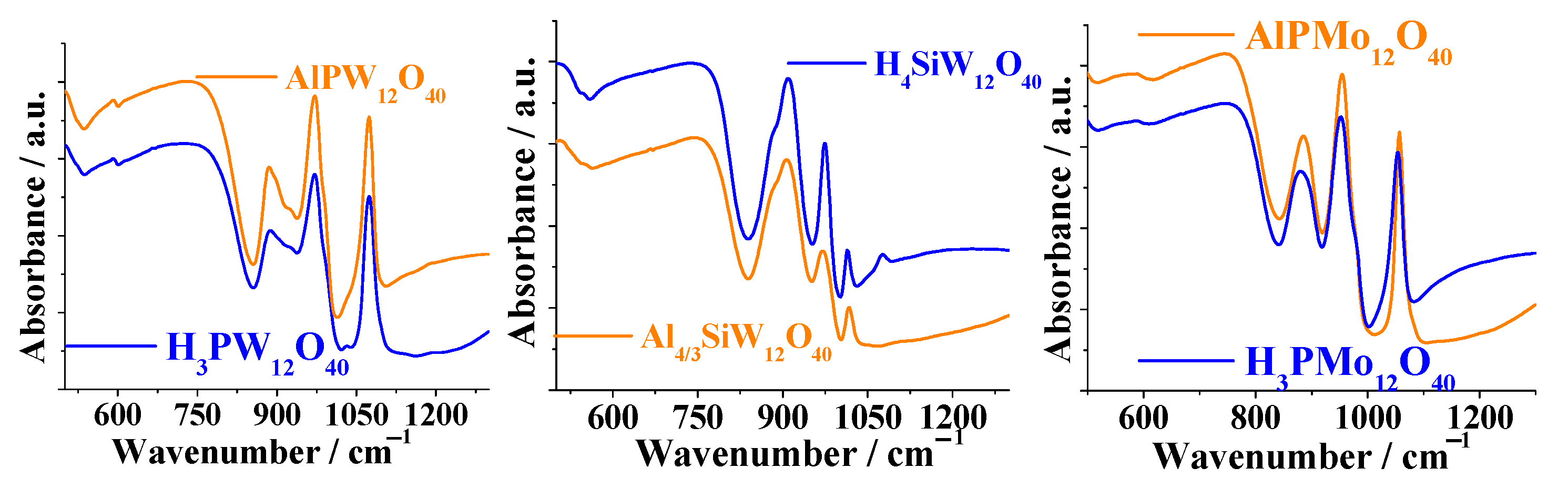
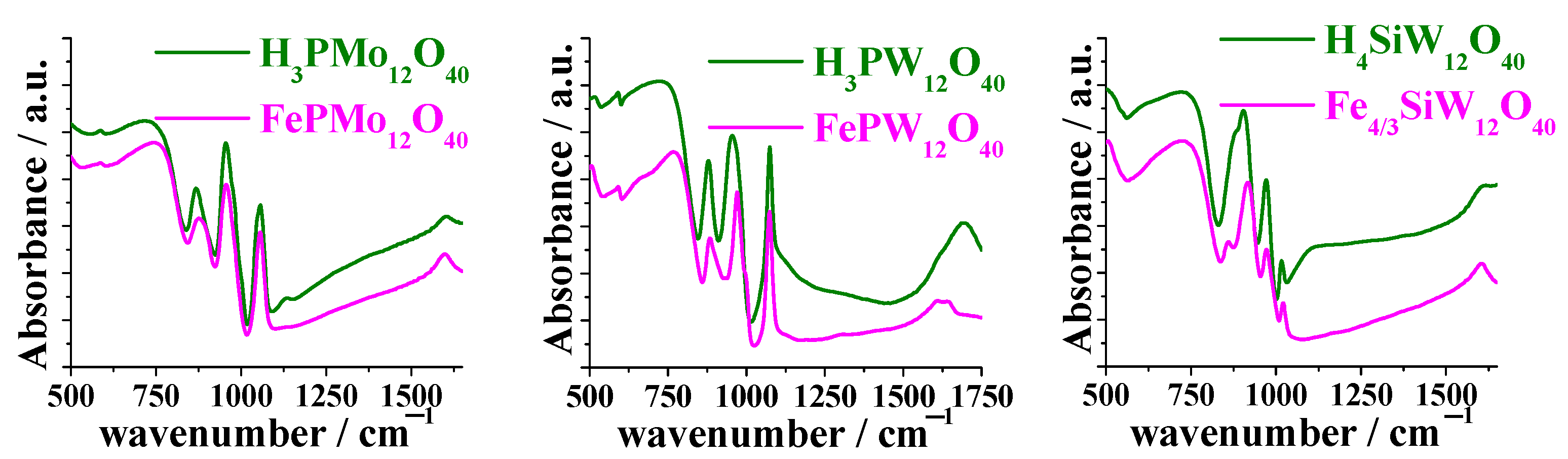
3.1. Infrared Spectroscopy
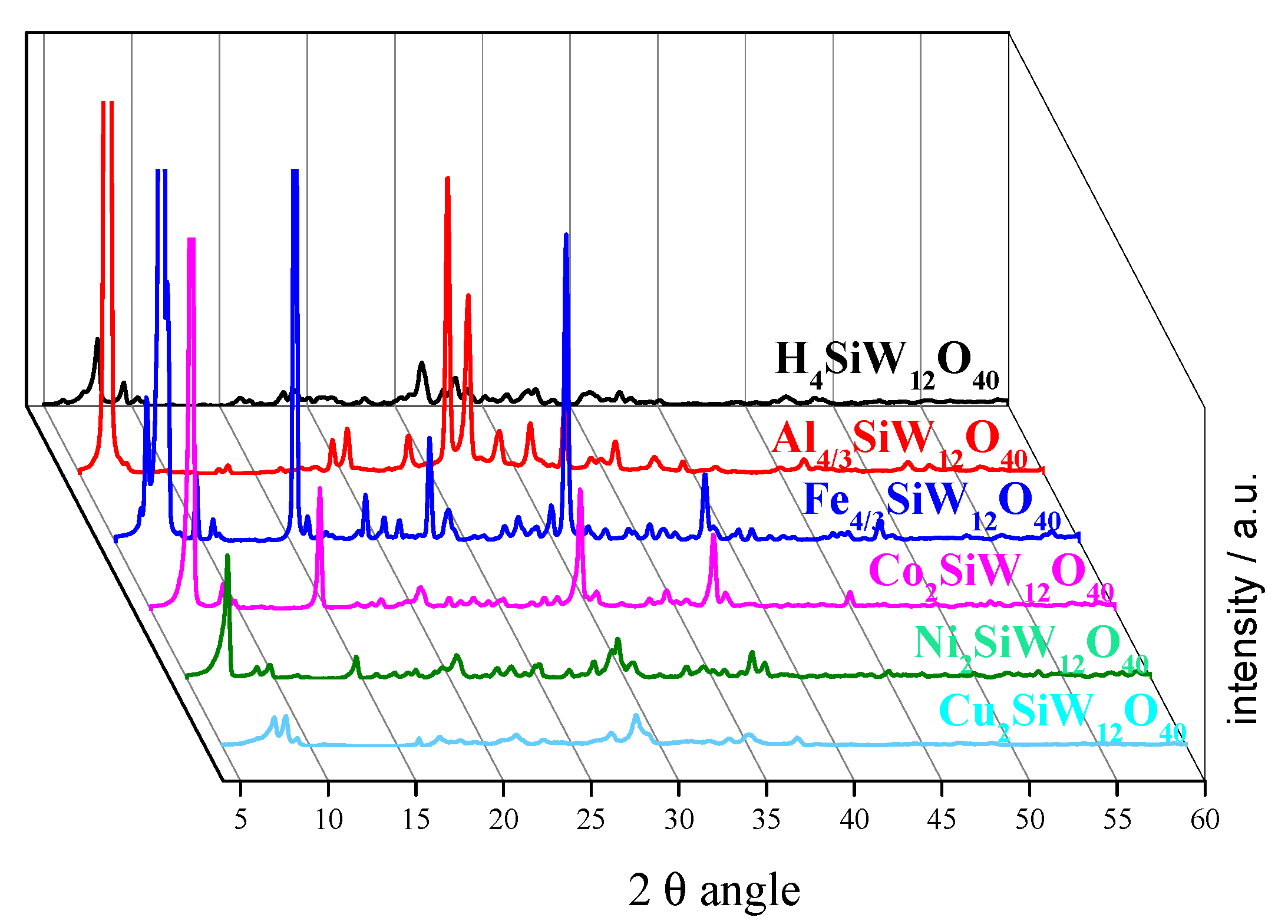
3.2. Powder X-rays Diffraction Patterns
3.3. Acidity Properties
4. Keggin HPA Salts as Catalysts in Oxidation Reactions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, A.M.; Hensley, J.E.; Medlin, J.W. Bifunctional Catalysts for Upgrading of Biomass-Derived Oxygenates: A Review. ACS Catal. 2016, 6, 5026–5043. [Google Scholar] [CrossRef]
- Tang, C.; Cheng, Y.; Min, X.; Jiang, S.P.; Wang, S. Bifunctional Catalysts for Reversible Oxygen Evolution Reaction and Oxygen Reduction Reaction. Chem. Eur. J. 2020, 26, 3906–3929. [Google Scholar] [CrossRef]
- Tan, K.B.; Zhan, G.; Sun, D.; Huang, J.; Li, Q. The development of bifunctional catalysts for carbon dioxide hydrogenation to hydrocarbons via the methanol route: From a single component to integrated components. J. Mater. Chem. A 2021, 9, 5197–5231. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef]
- Robinson, A.M.; Ferguson, G.A.; Gallagher, J.R.; Cheah, S.; Beckham, G.T.; Schaidle, J.A.; Hensley, J.E.; Medlin, J.W. Enhanced Hydrodeoxygenation of m-Cresol over Bimetallic Pt–Mo Catalysts through an Oxophilic Metal-Induced Tautomerization Pathway. ACS Catal. 2016, 6, 4356–4368. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Fu, Q.; Deng, W.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Activity and stability of low-content gold–cerium oxide catalysts for the water–gas shift reaction. Appl. Catal. B 2005, 56, 57–68. [Google Scholar] [CrossRef]
- Yoshida, K.; Inokuma, T.; Takasu, K.; Takemot, Y. Catalytic Asymmetric Synthesis of Both Enantiomers of 4-Substituted 1,4-Dihydropyridines with the Use of Bifunctional Thiourea-Ammonium Salts Bearing Different Counterions. Molecules 2010, 15, 8305–8326. [Google Scholar] [CrossRef] [Green Version]
- Villa-Marcos, B.; Li, C.; Mulholland, K.R.; Hogan, P.J.; Xiao, J. Bifunctional Catalysis: Direct Reductive Amination of Aliphatic Ketones with an Iridium-Phosphate Catalyst. Molecules 2010, 15, 2453–2472. [Google Scholar] [CrossRef]
- Amaya, J.; Moreno, S.; Molina, R. Heteropolyacids supported on clay minerals as bifunctional catalysts for the hydroconversion of decane. Appl. Cat. B Environ. 2021, 297, 120464–120479. [Google Scholar] [CrossRef]
- Pamin, K.; Połtowicz, J.; Pronczuk, M.; Krysciak-Czerwenka, J.; Karcz, R.; Serwick, E.M. Keggin-Type Heteropoly Salts as Bifunctional Catalysts in Aerobic Baeyer-Villiger Oxidation. Materials 2018, 11, 1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirpsza, A.; Lalik, E.; Mordarski, G.; Micek-Ilnicka, A. Catalytic properties of carbon nanotubes-supported heteropolyacids in isopropanol conversion. Appl. Catal. A 2018, 549, 254–262. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Heteropoly acids and related compounds as catalysts for fine chemical synthesis. Catal. Rev. 1995, 37, 311–352. [Google Scholar] [CrossRef]
- Balbinot, L.; Schuchardt, U.; Vera, C.; Sepulveda, J. Oxidation of cyclohexanol to epsilon-caprolactone with aqueous hydrogen peroxide on H3PW12O40 and Cs2.5H0.5PW12O40. Catal. Commun. 2008, 9, 1878–1881. [Google Scholar] [CrossRef]
- Yang, Z.W.; Xu, X.Q.; Li, T.J.; Zhang, N.N.; Zhao, X.; Chen, W.L.; Liang, X.X.; He, X.L.; Ma, H.C. Preparation and Catalytic Property of Multi-walled Carbon Nanotubes Supported Keggin-Typed Tungstosilicic Acid for the Baeyer-Villiger Oxidation of Ketones. Catal. Lett. 2015, 145, 1955–1960. [Google Scholar] [CrossRef]
- Karaman, B.P.; Oktar, N.; Doğu, G.; Dogu, T. Heteropolyacid Incorporated Bifunctional Core-Shell Catalysts for Dimethyl Ether Synthesis from Carbon Dioxide/Syngas. Catalysts 2022, 12, 1102. [Google Scholar] [CrossRef]
- Narkhede, N.; Patel, A. Efficient synthesis of biodiesel over a recyclable catalyst comprising a monolacunary silicotungstate and zeolite Hβ. RSC Adv. 2014, 4, 64379–64387. [Google Scholar] [CrossRef]
- Ma, Q.G.; Zhao, J.R.; Xing, W.Z.; Peng, X.H. Baeyer-Villiger Oxidation of Cyclic Ketones Using Aqueous Hydrogen Peroxide Catalyzed by Heteropolyacids in Solvent-free System. J. Adv. Oxid. Technol. 2014, 17, 212–216. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigation on heterogeneously catalyzed condensation of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Blanco, M.N. A contribution to the physicochemical characterization of nonstoichiometric salts of tungstosilicic acid. Microporous Mesoporous Mater. 2007, 103, 40–47. [Google Scholar] [CrossRef]
- Dias, J.A.; Caliman, E.; Dias, S.C.L. Effects of cesium ion exchange on the acidity of 12-tungstophophoric acid. Microporous Mesoporous Mater. 2004, 76, 221–232. [Google Scholar] [CrossRef]
- Díaz, J.; Pizzio, L.R.; Pecchi, G.; Campos, C.H.; Azócar, L.; Briones, R.; Romina Romero, R.; Henríquez, A.; Gaigneaux, E.M.; Contreras, D. Tetrabutyl Ammonium Salts of Keggin-Type Vanadium-Substituted Phosphomolybdates and Phosphotungstates for Selective Aerobic Catalytic Oxidation of Benzyl Alcohol. Catalysts 2022, 12, 507. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Lopes, N.P.G.; Ferreira, S.O.; Da Silva, R.C.; Natalino, R.; Chaves, D.M.; Texeira, M.G. Monoterpenes etherification reactions with alkyl alcohols over cesium partially exchanged Keggin heteropoly salts: Effects of the catalyst composition. Chem. Pap. 2021, 75, 153–168. [Google Scholar] [CrossRef]
- Park, H.W.; Park, S.; Park, D.R.; Choi, J.H.; Song, I.K. Decomposition of phenethyl phenyl ether to aromatics over CsxH3.0− xPW12O40 (X= 2.0–3.0) heteropolyacid catalysts. Catal. Commun. 2010, 12, 35. [Google Scholar] [CrossRef]
- Vilanculo, C.B.; Da Silva, M.J. Unraveling the role of the lacunar Na7PW11O39 catalyst on the oxidation of terpene alcohols with hydrogen peroxide at room temperature. New J. Chem. 2020, 44, 2813–2820. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Rodrigues, A.A. Metal silicotungstate salts as catalysts in furfural oxidation reactions with hydrogen peroxide. Mol. Catal. 2020, 493, 111104–111114. [Google Scholar] [CrossRef]
- Vilanculo, C.B.; Da Silva, M.J.; Ferreira, S.O.; Teixeira, M.G. A rare oxidation of camphene to acid and aldehyde in the presence of Lacunar Keggin heteropoly salts. Mol. Catal. 2019, 478, 110589–110596. [Google Scholar] [CrossRef]
- Vilanculo, C.B.; Da Silva, M.J.; Teixeira, M.G.; Villarreal, J.A. One-pot synthesis at room temperature of epoxides and linalool derivative pyrans in monolacunary Na7PW11O39-catalyzed oxidation reactions by hydrogen peroxide. RSC Adv. 2020, 10, 7691–7697. [Google Scholar] [CrossRef] [Green Version]
- Coronel, N.C.; Da Silva, M.J.; Ferreira, S.O.; Da Silva, R.C.; Natalino, R. K5PW11NiO39-catalyzed oxidation of benzyl alcohol with hydrogen peroxide. Chem. Select. 2019, 4, 302–310. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Fournier, M. Catalysis by polyoxometalates. Part 3—Influence of Vanadium(V) on the thermal stability of 12-metallophosphoric acids from in situ infrared studies. J. Chem. Soc. Faraday Trans. 1991, 87, 3913–3920. [Google Scholar] [CrossRef]
- Mansilla, D.S.; Torviso, M.R.; Alesso, E.N.; Vazquez, P.G.; Caceres, C.V. Synthesis and characterization of copper and aluminium salts of H3PMo12O40 for their use as catalysts in the eco-friendly synthesis of chromanes. Appl. Catal. A 2010, 375, 196–204. [Google Scholar] [CrossRef]
- Pathan, S.; Patel, A.; Tilani, H. Selective Oxidation of Alcohols and Alkenes with Molecular Oxygen Catalyzed by Highly Dispersed Cobalt (II) Decorated 12-Tungstosilicic Acid-Modified Zirconia. Catalysts 2022, 12, 1622. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Leles, L.C.A.; Teixeira, M.G. Lewis acid metal cations exchanged heteropoly salts as catalysts in β-pinene etherification. React. Kinet. Mech. Catal. 2020, 131, 875–887. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Leles, L.C.A.; Ferreira, S.O.; Da Silva, R.C.; Viveiros, K.d.V.; Chaves, D.M.; Pinheiro, P.F. A Rare Carbon Skeletal Oxidative Rearrangement of Camphene Catalyzed by Al-Exchanged Keggin Heteropolyacid Salts. ChemSelect 2019, 4, 7665–7672. [Google Scholar] [CrossRef]
- Patel, A.; Patel, J. Nickel salt of phosphomolybdic acid as a bi-functional homogeneous recyclable catalyst for the base free transformation of an aldehyde into ester. RSC Adv. 2020, 10, 22146–22155. [Google Scholar] [CrossRef]
- Patel, A.; Patel., J. Exchanged Supported Phosphomolybdic Acid: Synthesis, Characterization and Low-Temperature Water Mediated Hydrogenation of Cyclohexene. Catal. Lett. 2022, 152, 2716–2728. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M.; Okuhara, T. Catalytic Chemistry of Heteropoly Compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar] [CrossRef]
- Méndez, L.; Torviso, R.; Pizzio, L.; Blanc, M. 2-Methoxynaphthalene acylation using aluminium or copper salts of tungstophosphoric and tungstosilicic acids as catalysts. Catal. Today 2011, 173, 32–37. [Google Scholar] [CrossRef]
- Shringarpure, P.; Patel, A. Cobalt (II) exchanged supported 12-tungstophosphoric acid: Synthesis, characterization, and non-solvent liquid phase aerobic oxidation of alkenes. J. Mol. Catal. A 2010, 321, 22–26. [Google Scholar] [CrossRef]
- Karcz, R.; Niemiec, P.; Pamin, K.; Połtowicz, J.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Michalik-Zym, A.; Witko, M.; Tokarz-Sobieraj, R.; Serwicka, E.M. Effect of cobalt location in Keggin-type heteropoly catalysts on aerobic oxidation of cyclooctane: Experimental and theoretical study. Appl. Catal. A 2017, 542, 317–326. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Andrade, P.H.S.; Sampaio, V.F.C. Transition metal-substituted potassium silicotungstate salts as catalysts for oxidation of terpene alcohols with hydrogen peroxide. Catal. Lett. 2021, 151, 2094–2106. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Leles, L.C.A.; Natalino, R.; Ferreira, S.O.; Coronel, N.C. An efficient benzaldehyde oxidation by hydrogen peroxide over metal-substituted lacunary Potassium heteropolyacid Salts. Catal. Lett. 2018, 148, 1202–1214. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A. Nickel exchanged supported 12-tungstophosphoric acid: Synthesis, characterization and base free one-pot oxidative esterification of aldehyde and alcohol. RSC Adv. 2019, 9, 1460–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Fang, Y.; Xie, K.; Chen, Y.; Liu, Y.; Zuo, H.; Lu, W.; Liu, B. Lacunary silicotungstic heteropoly salts as high-performance catalysts in the oxidation of cyclopentene. Chin. J Eng. Chem. 2022, 56, 152–159. [Google Scholar] [CrossRef]
- Tahar, A.; Benadji, S.; Mazari, T.; Dermeche, L.; Marchal-Roch, C.; Rabia, C. Preparation, Characterization and Reactivity of Keggin Type Phosphomolybdates, H3-2xNixPMo12O40 and (NH4)3-2x NixPMo12O40, for Adipic Acid Synthesis. Catal Lett. 2015, 145, 569–575. [Google Scholar] [CrossRef]

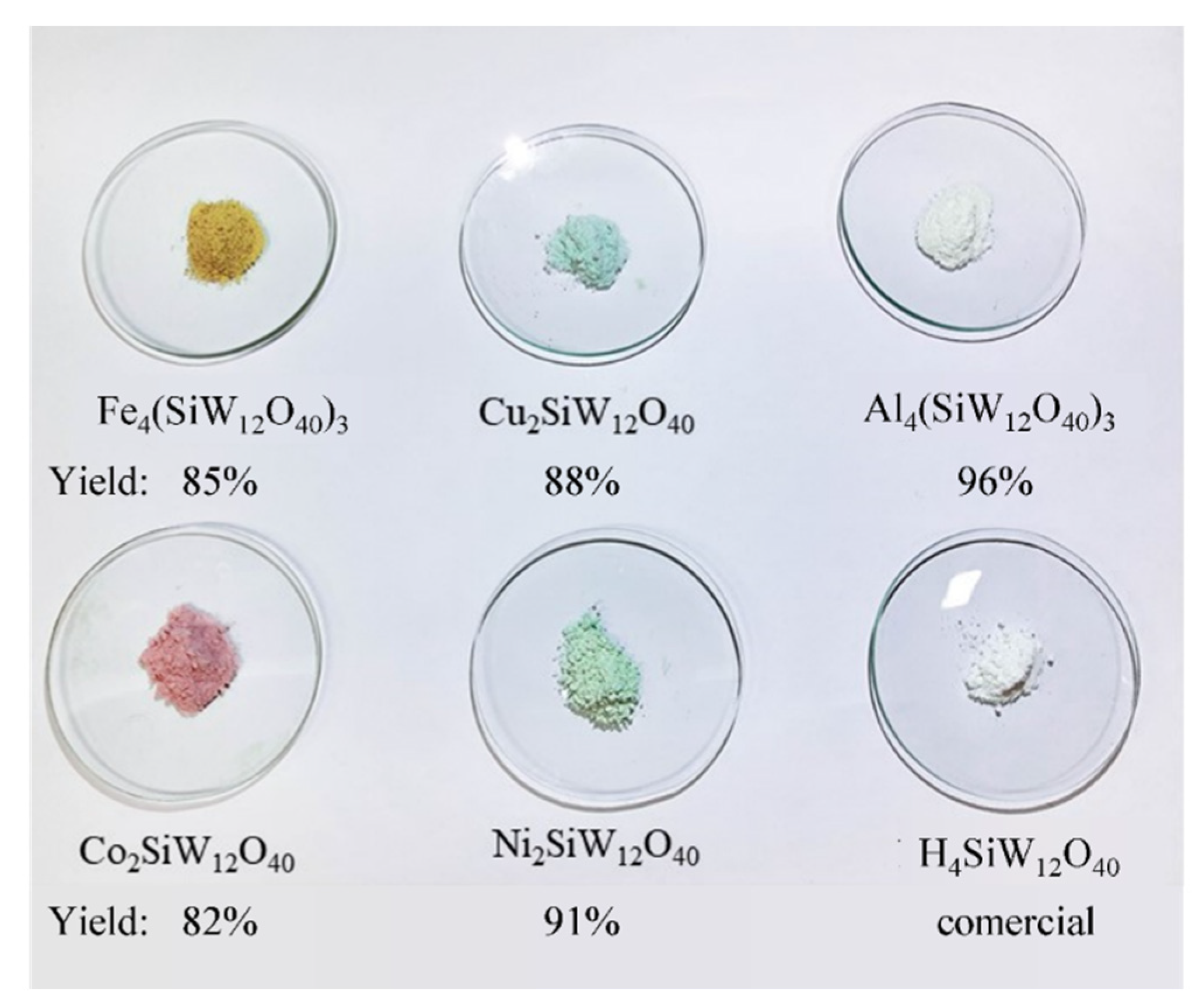






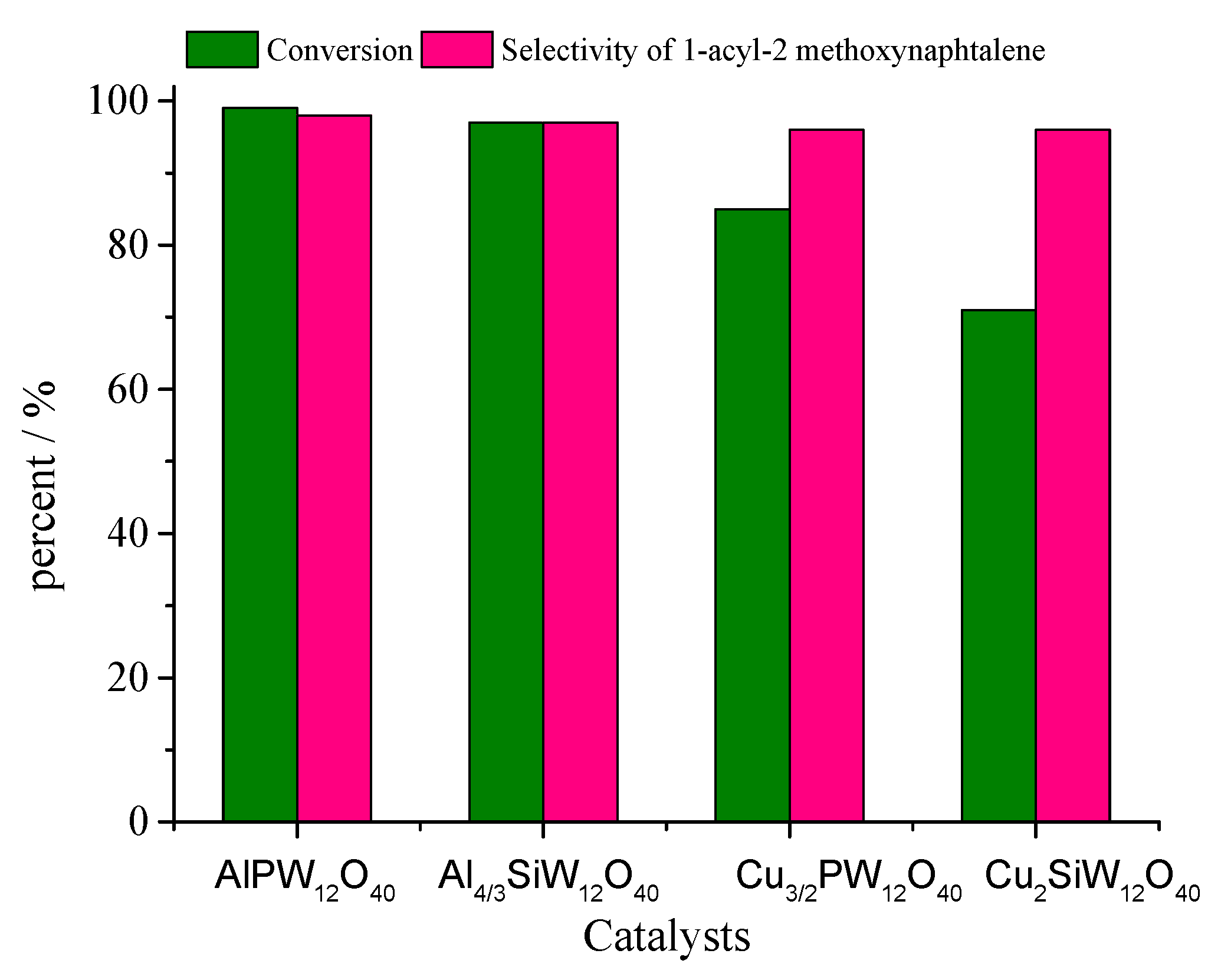
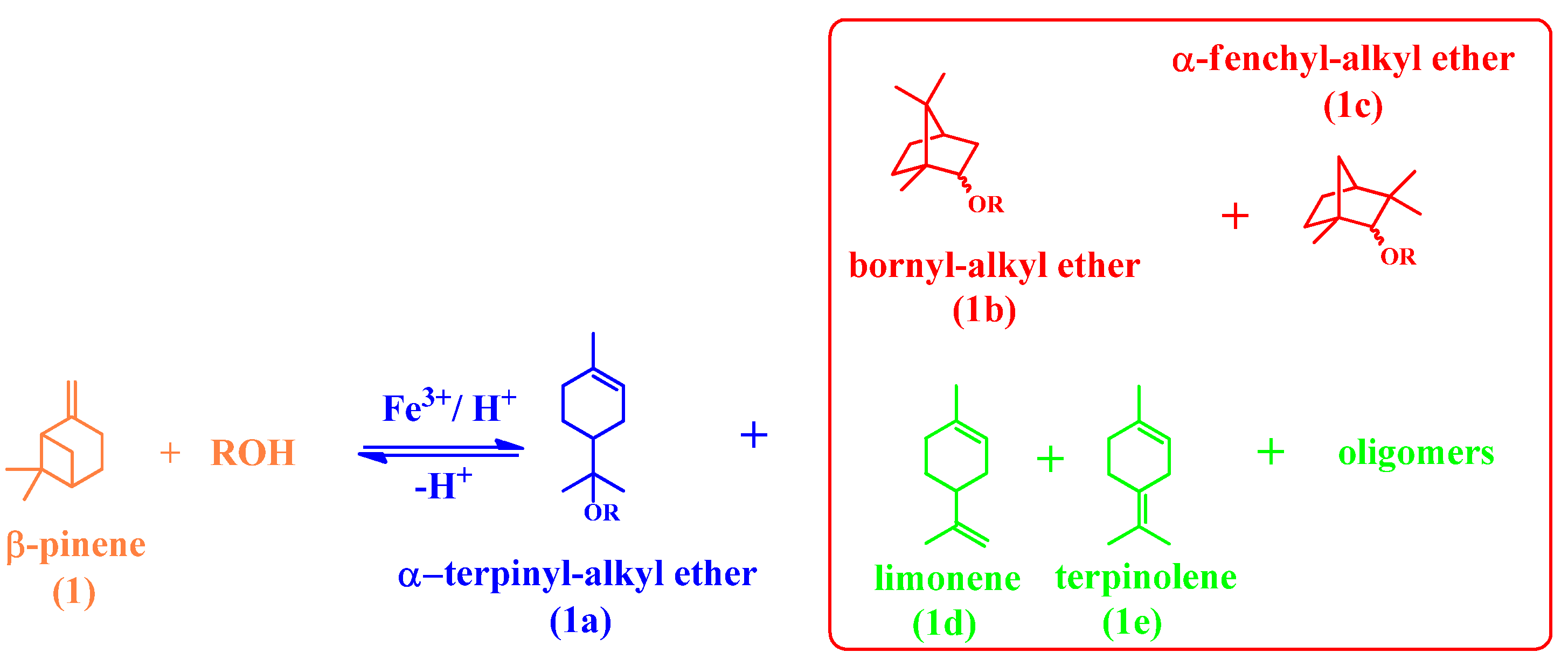
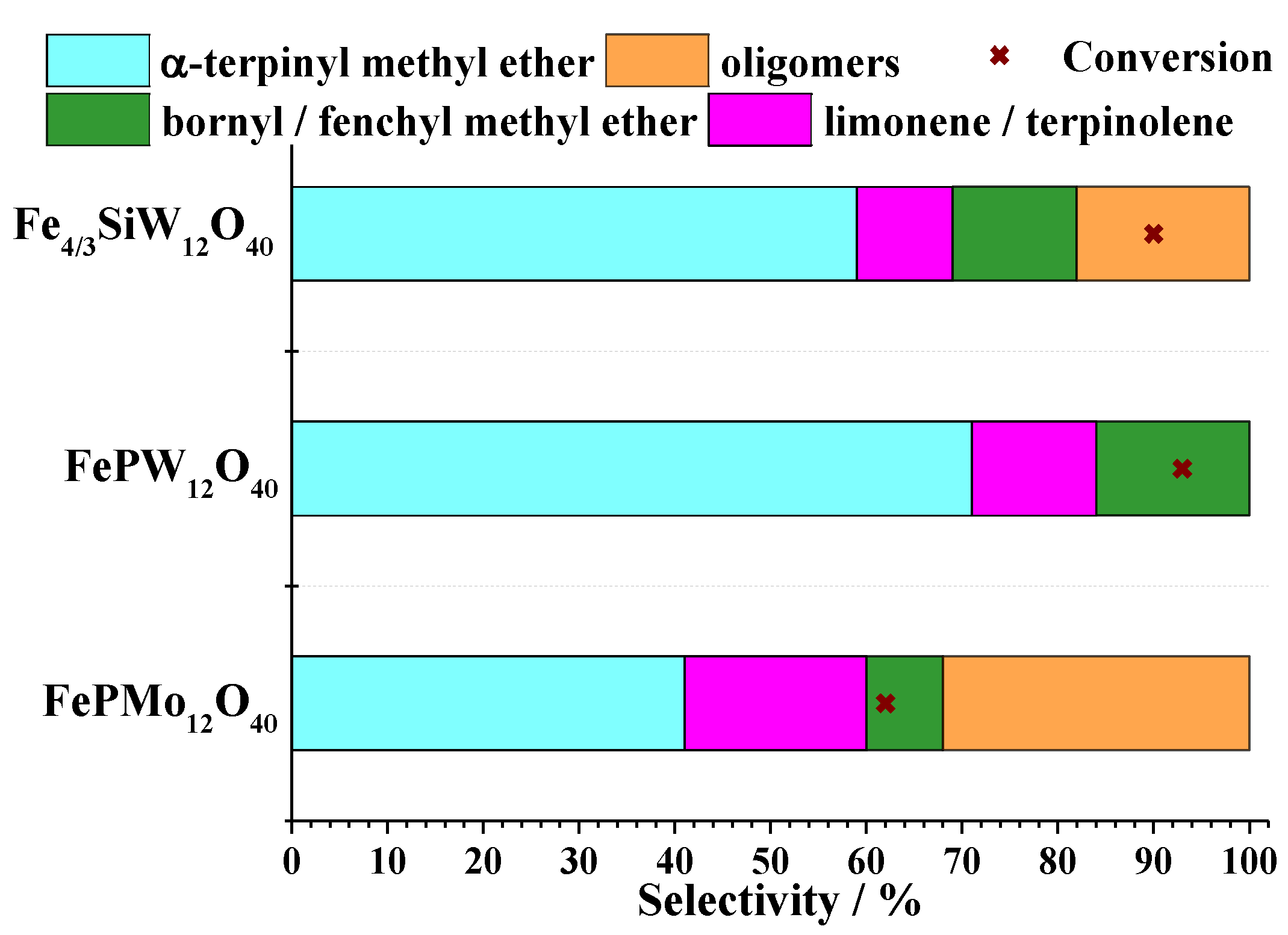
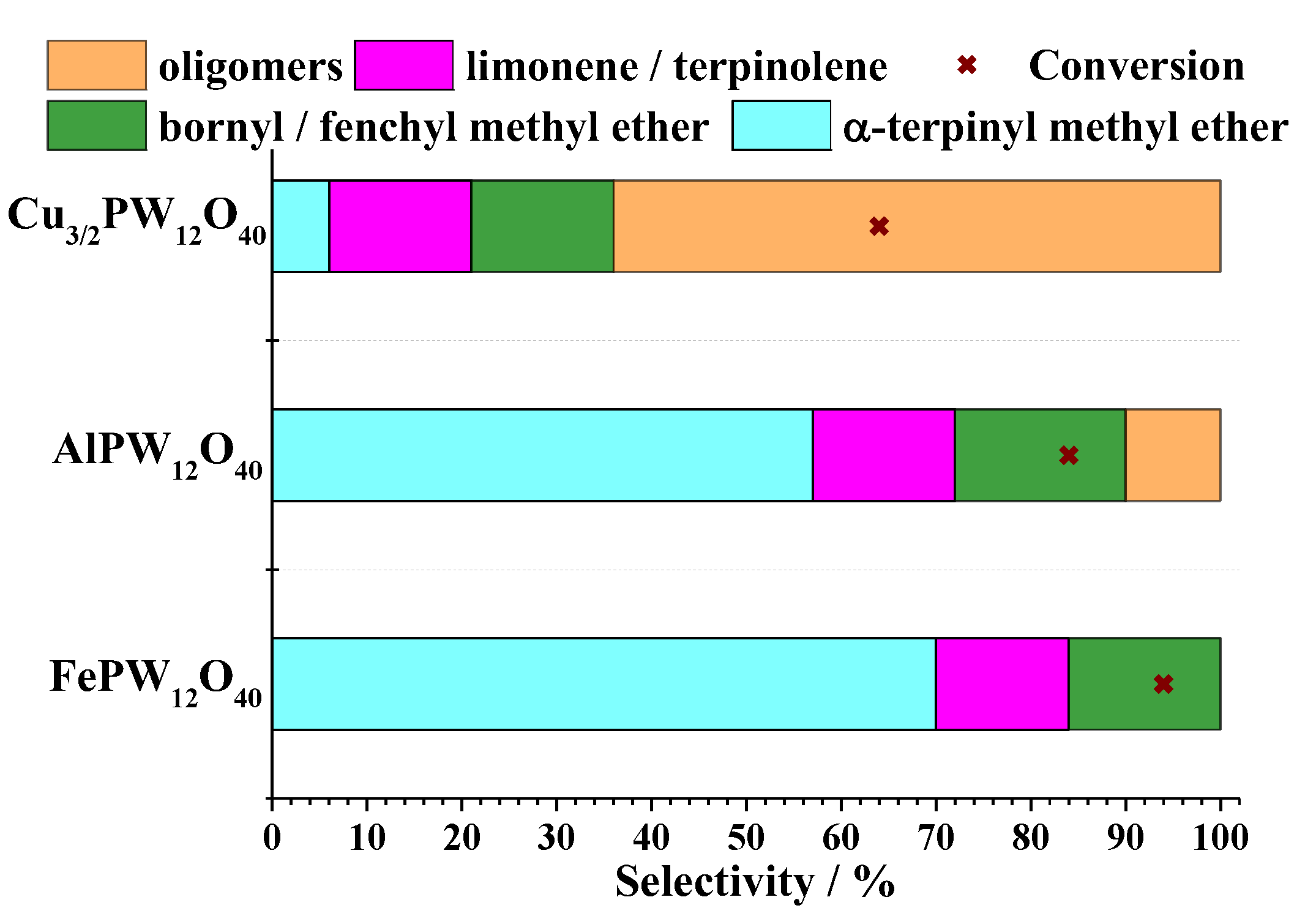

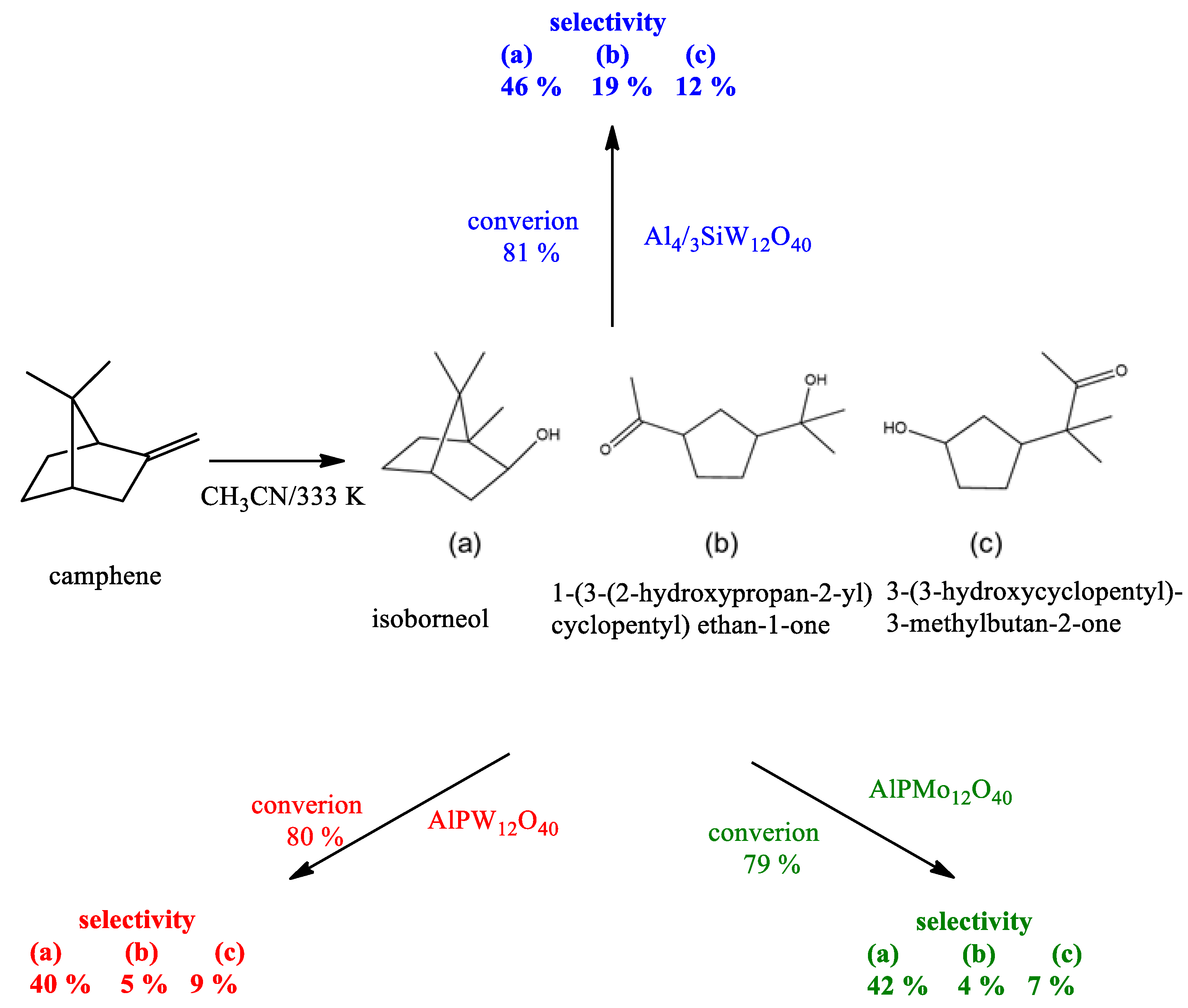
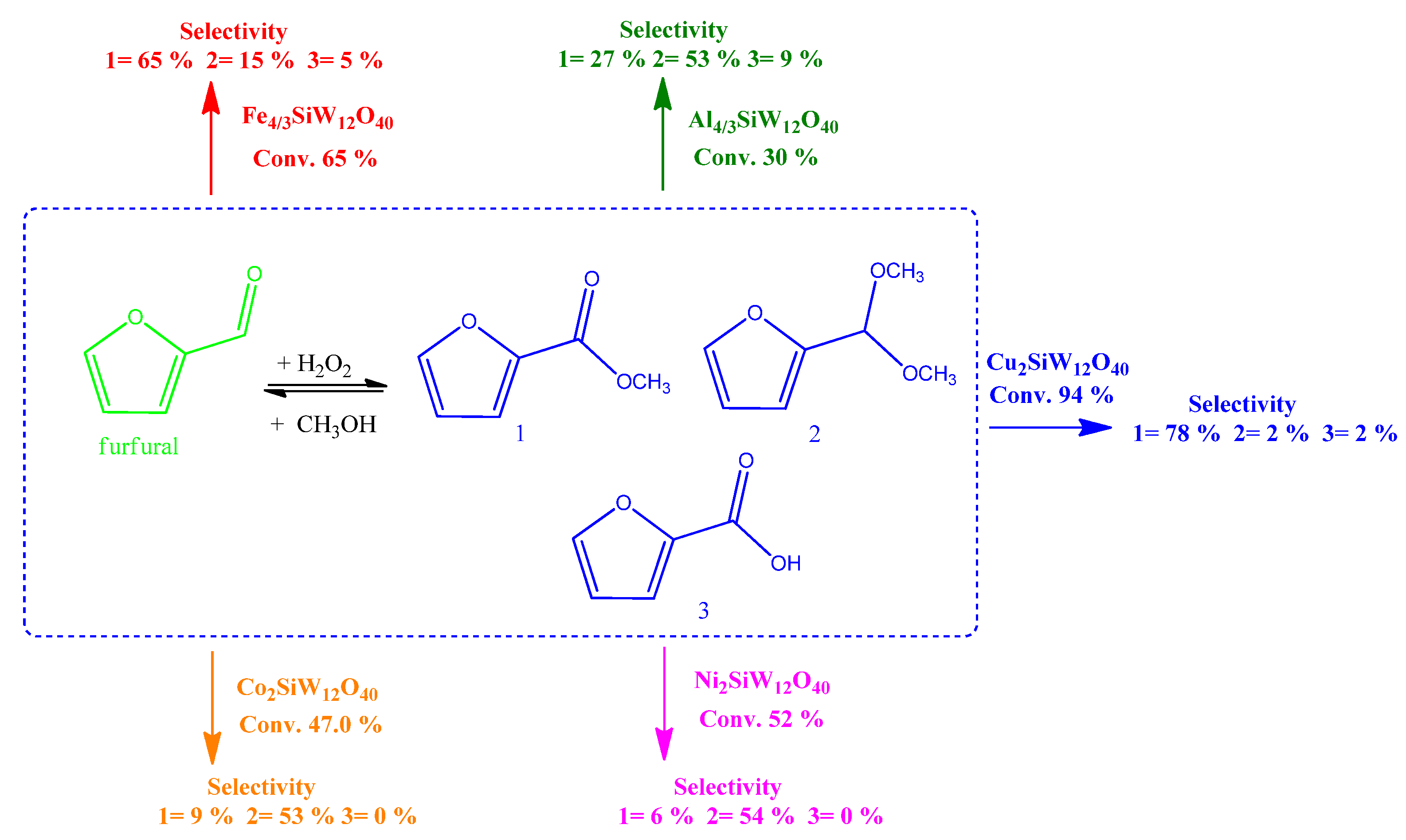


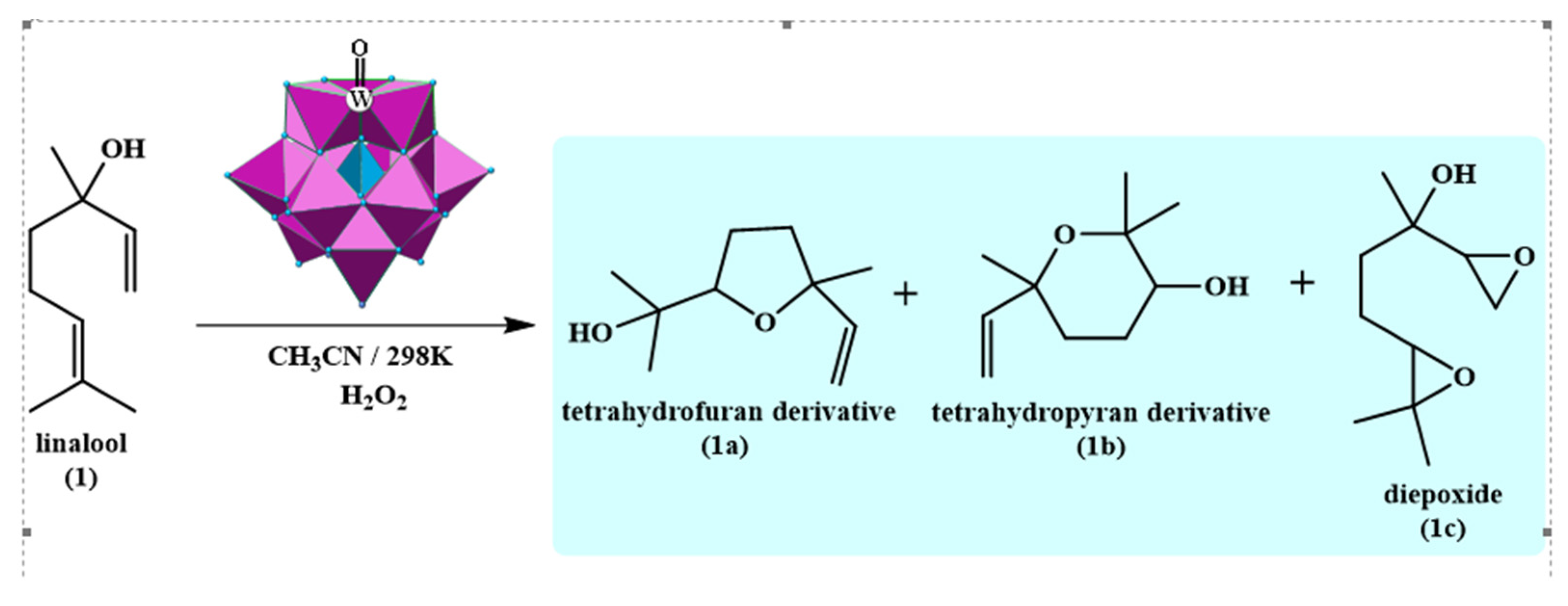
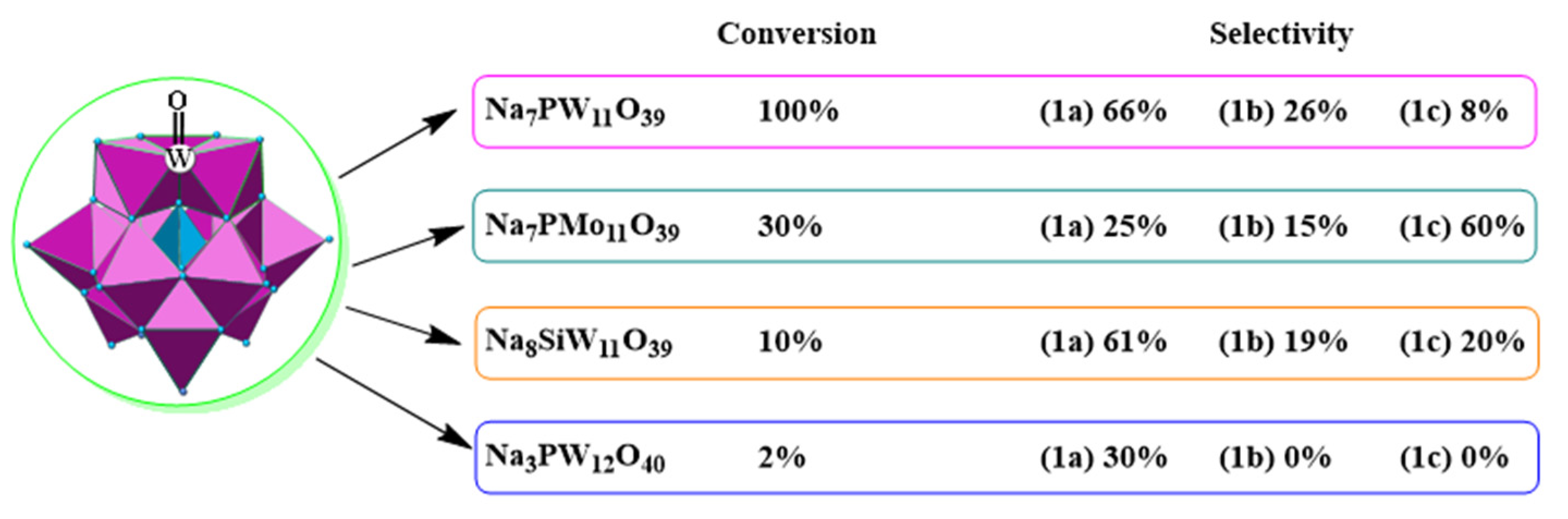

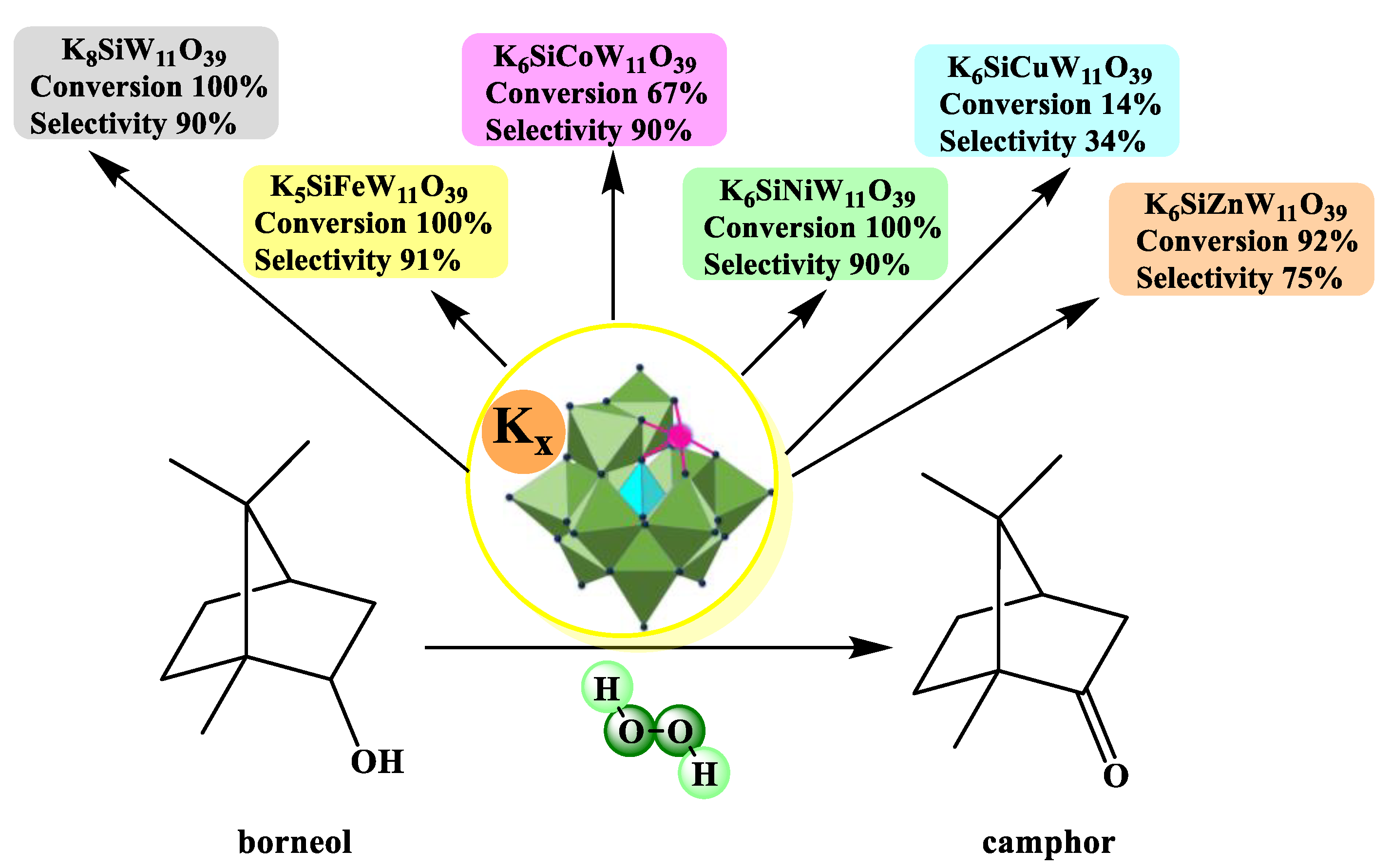
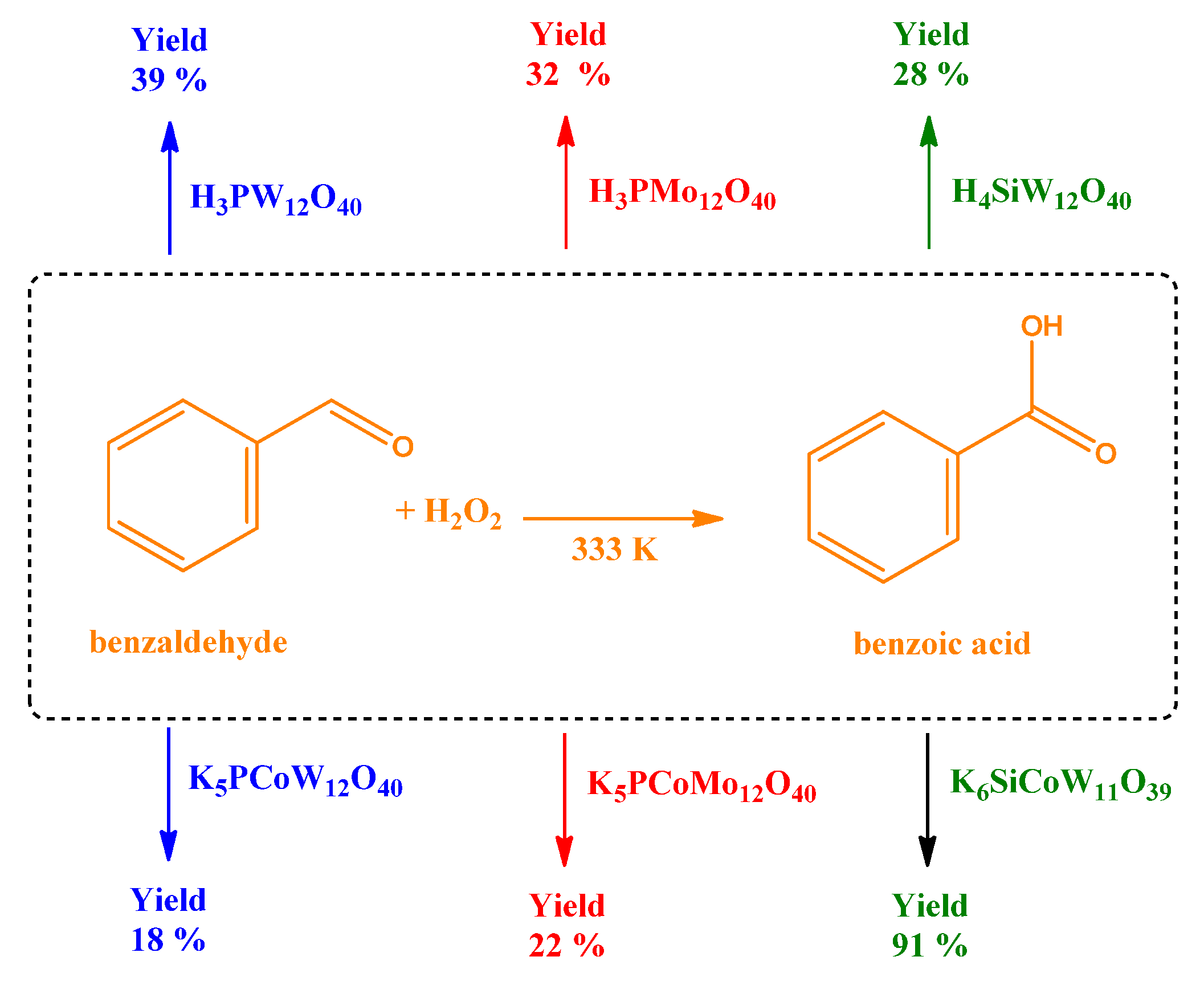
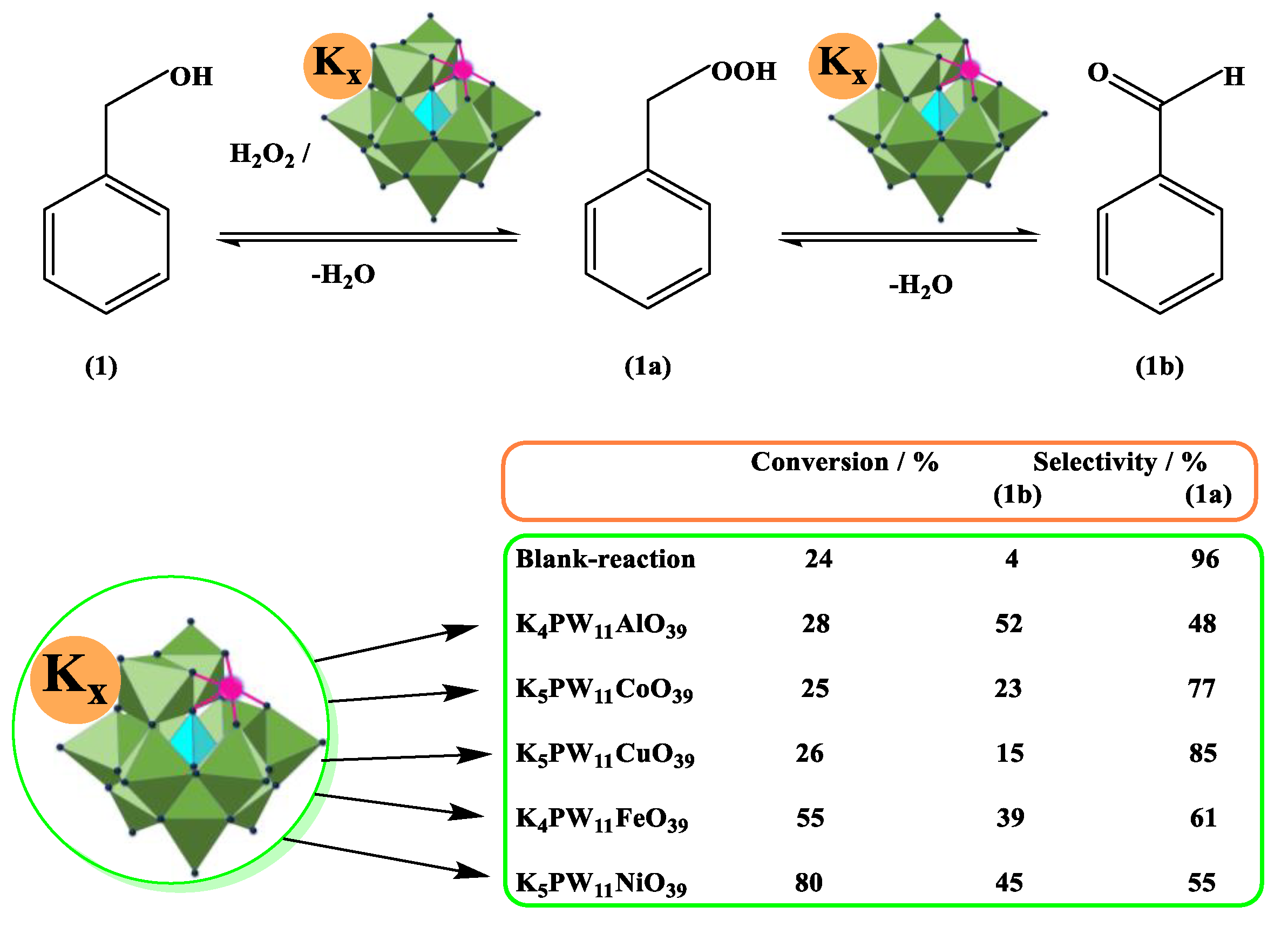
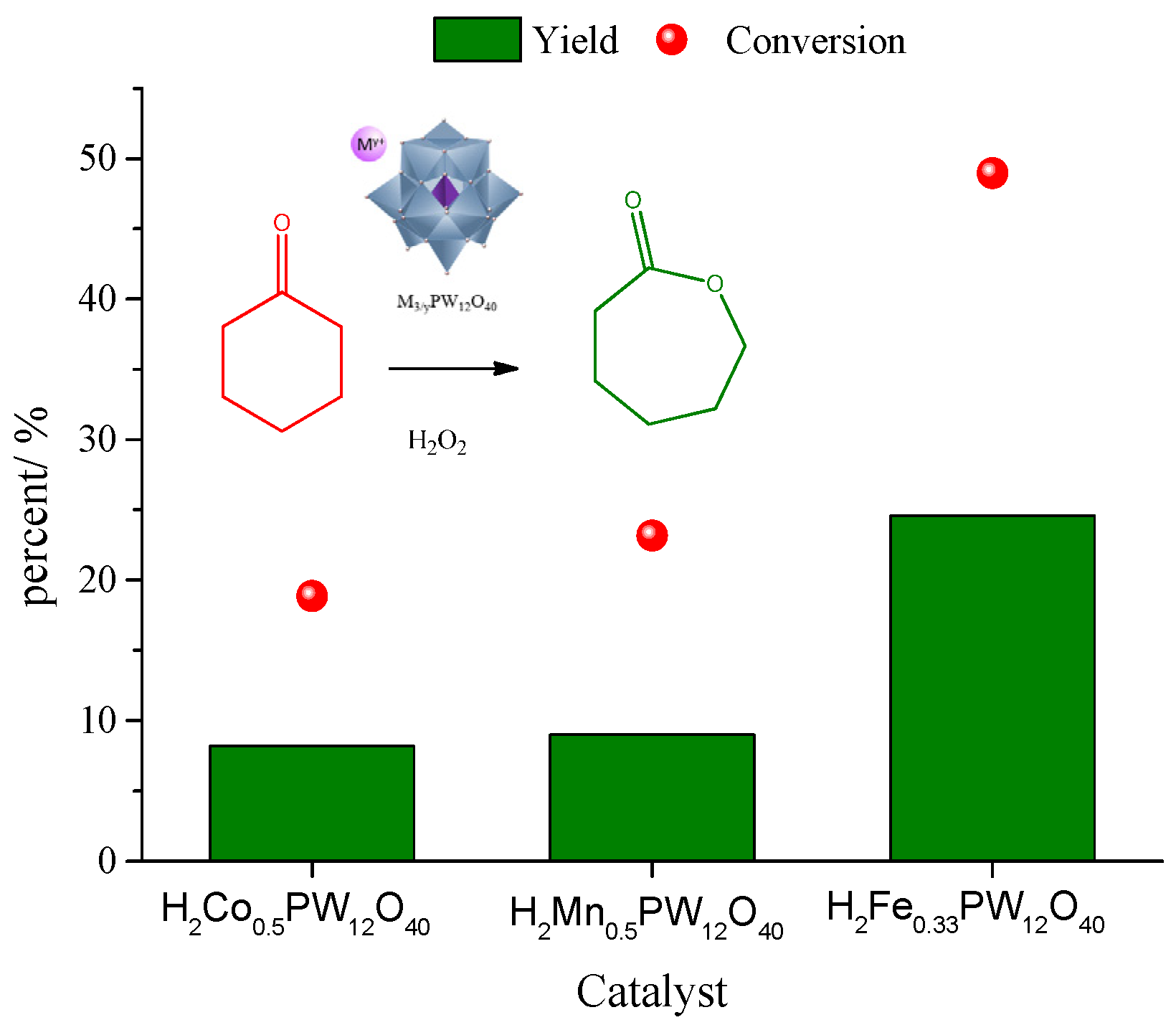
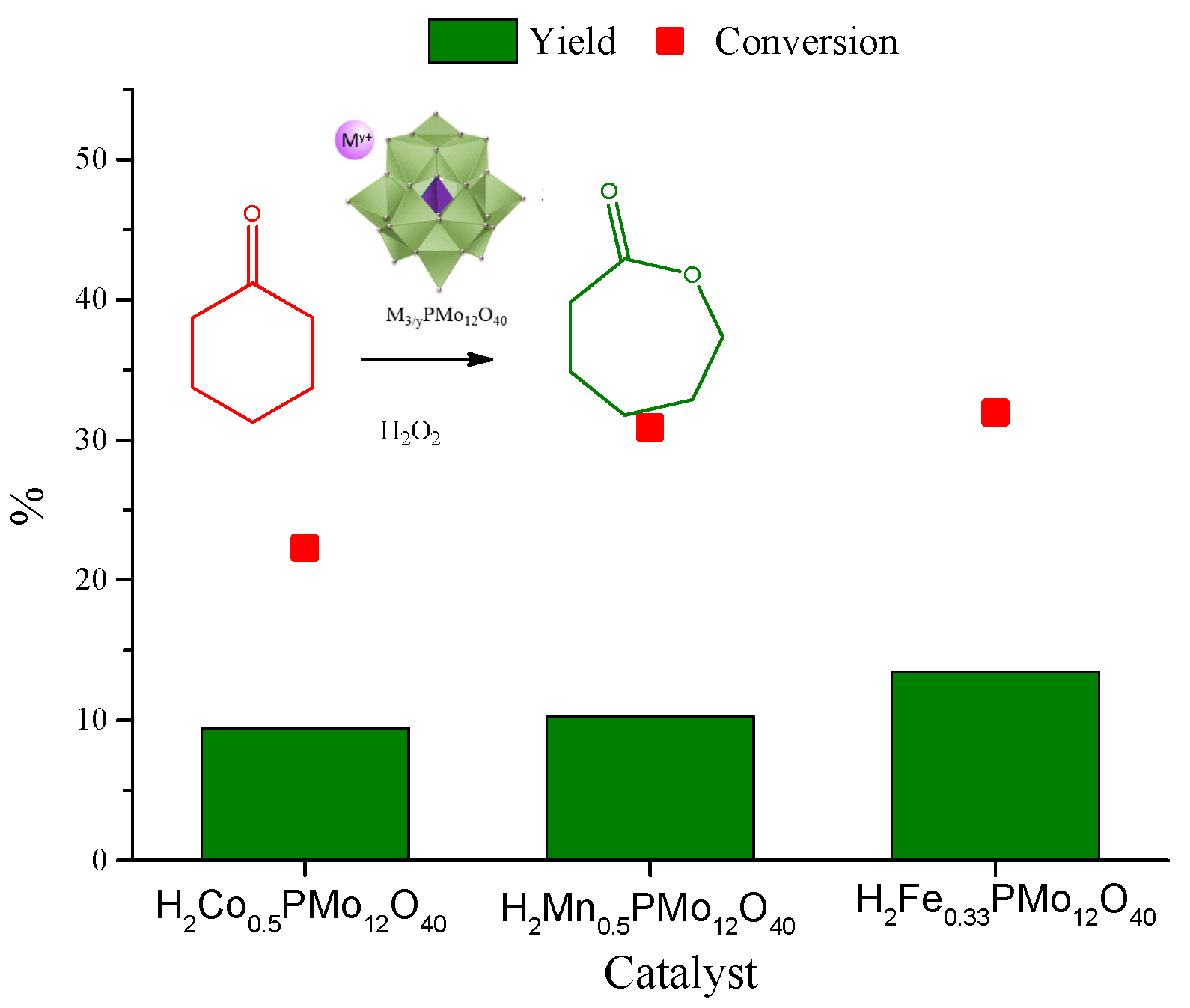
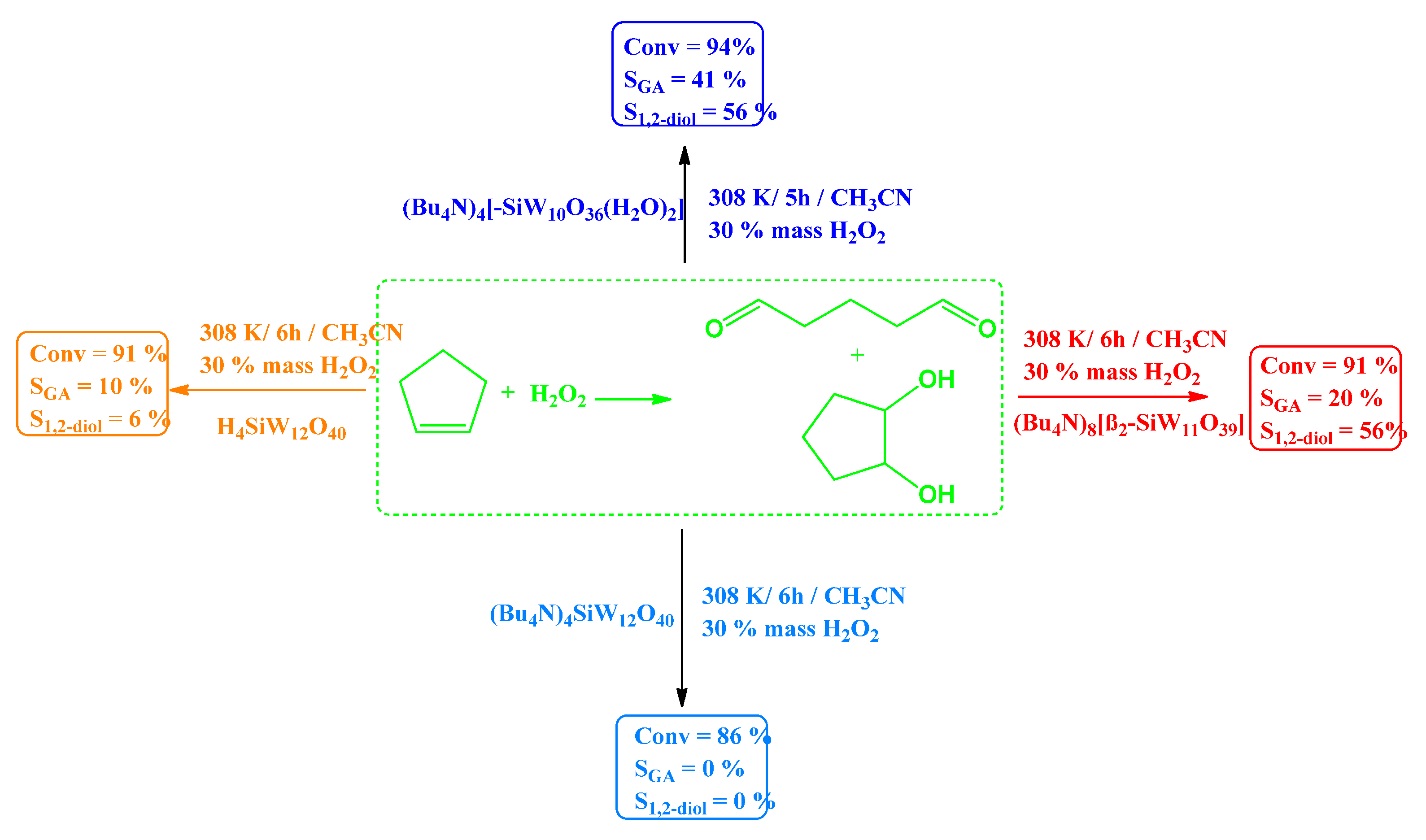
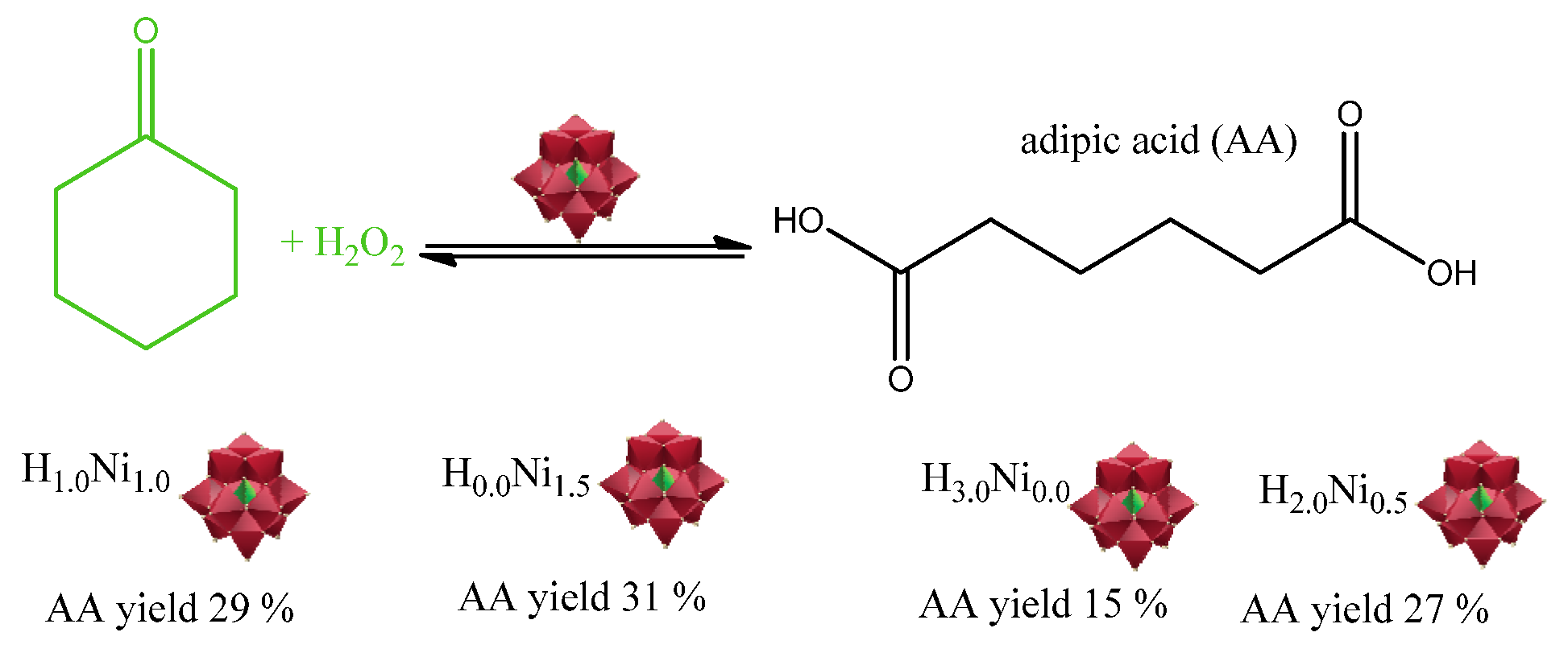
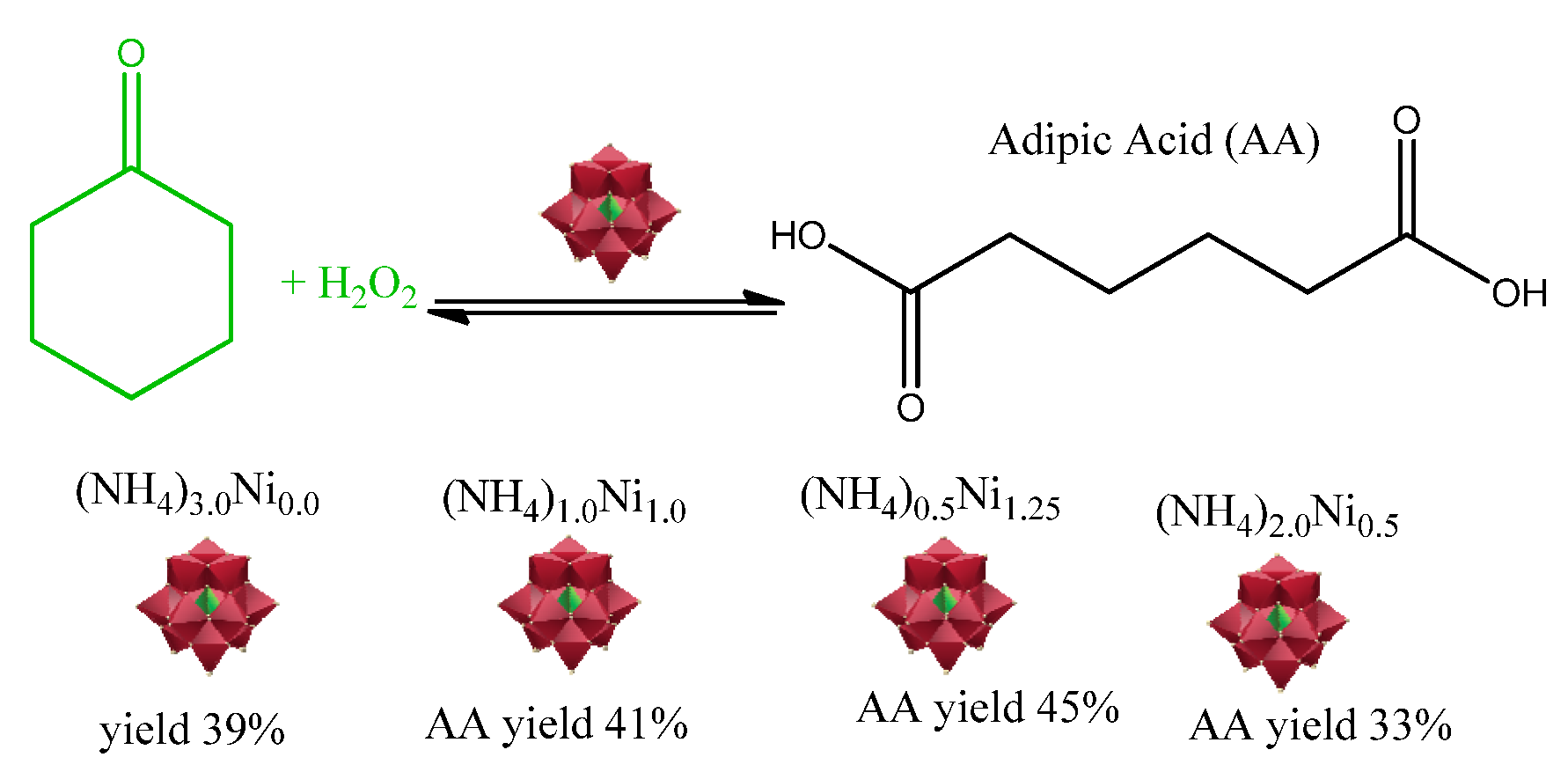
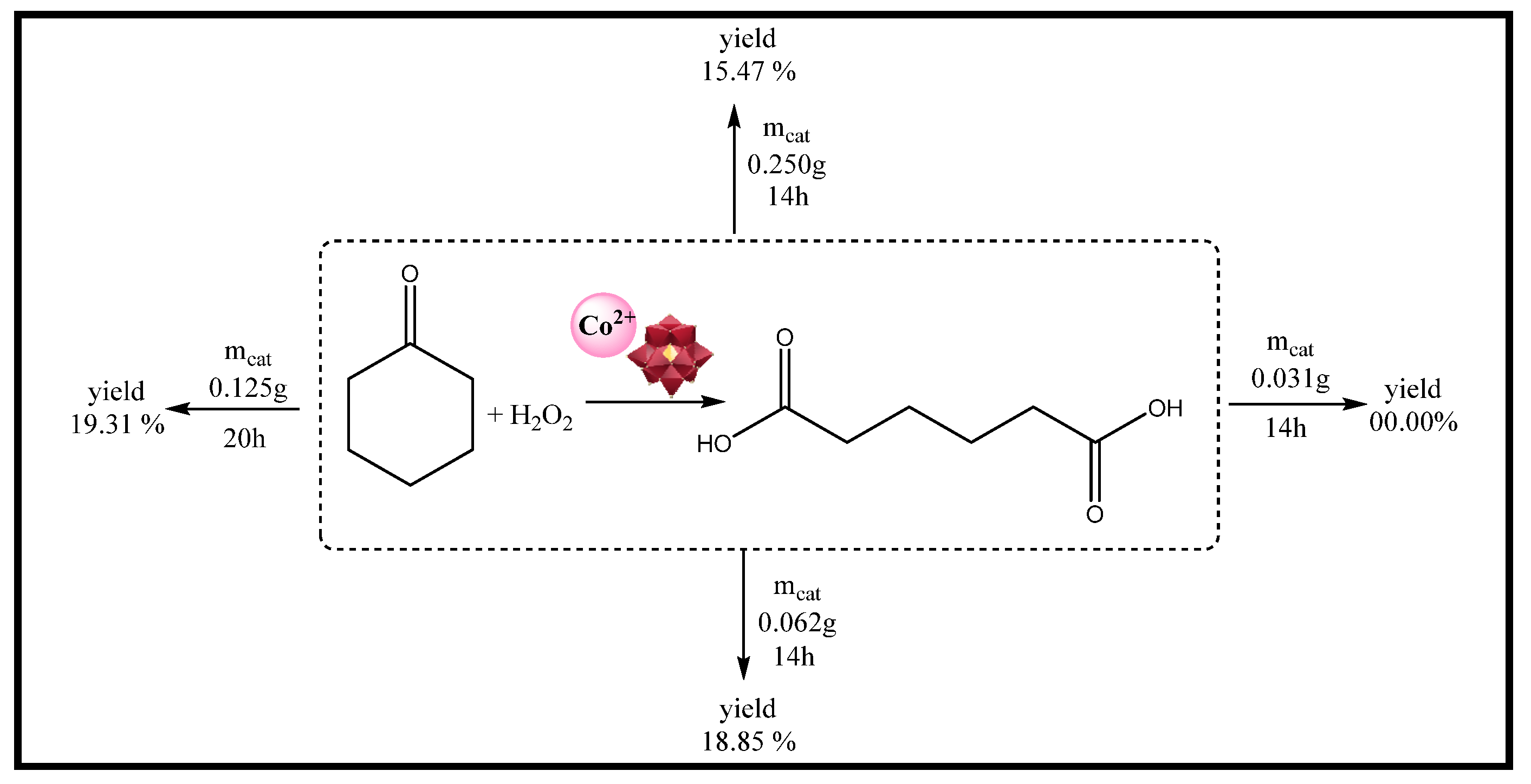
| Catalyst | Water/Catalyst mol |
|---|---|
| H4SiW12O40 | 11 |
| Ni2SiW12O40 | 7 |
| Cu2SiW12O40 | 7 |
| Co2SiW12O40 | 10 |
| Fe4/3SiW12O40 | 13 |
| Al4/3SiW12O40 | 8 |
| Cycle | Catalyst Recovery (%) | Conversion (%) |
|---|---|---|
| 1 | 92 | 97 |
| 2 | 92 | 93 |
| 3 | 92 | 92 |
| 4 | 91 | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.J.; Rodrigues, A.A.; Lopes, N.P.G. Keggin Heteropolyacid Salt Catalysts in Oxidation Reactions: A Review. Inorganics 2023, 11, 162. https://doi.org/10.3390/inorganics11040162
da Silva MJ, Rodrigues AA, Lopes NPG. Keggin Heteropolyacid Salt Catalysts in Oxidation Reactions: A Review. Inorganics. 2023; 11(4):162. https://doi.org/10.3390/inorganics11040162
Chicago/Turabian Styleda Silva, Marcio Jose, Alana Alves Rodrigues, and Neide Paloma Gonçalves Lopes. 2023. "Keggin Heteropolyacid Salt Catalysts in Oxidation Reactions: A Review" Inorganics 11, no. 4: 162. https://doi.org/10.3390/inorganics11040162
APA Styleda Silva, M. J., Rodrigues, A. A., & Lopes, N. P. G. (2023). Keggin Heteropolyacid Salt Catalysts in Oxidation Reactions: A Review. Inorganics, 11(4), 162. https://doi.org/10.3390/inorganics11040162