On the Question of the Complex Processing of Pyrite Cinders
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Research Methods
3.2. Methods of Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rare Elements: The Market Gives the Go-Ahead. Editor. Review. Kazakhstan Int. Bus. Mag. 2013, 3, 56–58. Available online: http://investkz.com/journals/91/1108.html (accessed on 22 October 2022).
- Abikak, E.B.; Kenzhaliyev, B.K. Development of an integrated technology intended to process pyrite cinders using Chemical pre-activation. NEWS Natl. Acad. Sci. Repub. Kazakhstan Ser. Geol. Tech. Sci. 2022, 3, 32–51. [Google Scholar] [CrossRef]
- Lebedev, B.N. Chloride distillation is a new extraction method FOR noble metals from pyrite cinders. Sov. Zolotopromyshlennos 1936, 6, 45–47. [Google Scholar]
- Beregovsky, V.I.; Bergman, R.V.; Danilova, L.A.; Kozyrev, B.C.; Tarasov, B.E.; Teper, B.C.; Fominykh, E.G. Complex Use of Pyrite Cinders; Metallurgizdat: Moscow, Russia, 1963; 70p. [Google Scholar]
- Shaari, H.R.; Azlan, M.N.; Azlina, Y.; Hajer, S.S.; Nazrin, S.N.; Umar, S.A.; Kenzhaliyev, B.K.; Boukhris, I.; Al-Hada, N.M. Investigation of Structural and Optical Properties of Graphene Oxide-Coated Neodymium Nanoparticles Doped Zinc-Tellurite Glass for Glass Fiber. J. Inorg. Organomet. Polym. 2021, 31, 4349–4359. [Google Scholar] [CrossRef]
- Larin, V.K.; Diachenko, A.N.; Kraydenko, R.I.; NechaYev, Y. Processing of Pyrite Cinders. Technol. Tomsk. Polytech. University. Available online: https://news.vtomske.ru (accessed on 22 December 2022).
- Litvinenko, L.G.; Litvinenko, V.G.; Shchelkonogov, M.A.; Morozov, A.A. The Method of Complex Processing of Pyrite Cinders. Patent 2623948 RU, 6 December 2017. [Google Scholar]
- Mukhamedshin, I.K.; Bashlykova, T.V.; Fadina, I.B.; Zhivaeva, A.B. Method for Deep Processing of Pyrite Cinders. Patent 2397260 RU, 13 March 2020. [Google Scholar]
- Kurdyumov, G.E.; Galeru, K.E.; Parshin. Method of Processing Pyrite Stubs. Patent 2716440 RU, 20 March 2020. [Google Scholar]
- Abdykirova, G.Z.; Kenzhaliev, B.K.; Koyzhanova, A.K.; Magomedov, D.R. Low-sulfide gold-quartz ore concentration potential study. Obogashchenie Rud. 2020, 3, 14–18. [Google Scholar] [CrossRef]
- Gilmutdinova, R.A.; Michurin, S.V.; Kovtunenko, S.V.; Elizarieva, E.N. On the issue of the use and processing of waste from mining and processing plants of the Southern Urals. Successes Mod. Nat. Sci. 2017, 2, 68–73. [Google Scholar]
- Levchenko, G.N.; Kiselev, M.Y.; Timofeev, K.L.; Voinkov, R.S.; Krayuhin, S.A. Flotation enrichment of Kirovgrad pyrite cinders. Sci. Bases Pract. Process. Ores Technog. Raw Mater. In Proceedings of the XXVI National Scientific and Technical Conference, Voronezh, Russia, 14–16 April 2020; Volume 1, pp. 173–177. [Google Scholar]
- Zhang, X.; Chen, G.; Cai, X.; Fu, J.; Liu, M.; Zhang, P.; Yu, H. Leaching behavior of copper and iron extracted from pyrite cinder of reduction roasting. J. Hazard. Mater. 2021, 420, 126561. [Google Scholar] [CrossRef] [PubMed]
- Kenzhaliyev, B.; Surkova, T.; Yessimova, D.; Baltabekova, Z.; Abikak, Y.; Abdikerim, B.; Dosymbayeva, Z. Extraction of Noble Metals from Pyrite Cinders. ChemEngineering 2023, 7, 14. [Google Scholar] [CrossRef]
- Seitkan, A.; Redfren, S. Arsenic in refractory gold ore processing. Kompleks. Ispolz. Miner. Syra 2021, 317, 5–13. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching by copper (II) in ammoniacalthiosulphate solutions in the presence of additives. Pt, I.A review of hard-soft and Lewis acid-base properties and interactions of ions. Hydrometallurgy 2012, 115–116, 1–20. [Google Scholar] [CrossRef]
- Gudkov, A.S.; Zhuchkov, I.A.; Mineev, G.G. Mechanism and kinetics of sulfite-thiosulfate dissolution of gold. Izv. Vuzov. Non-Ferr. Metall. 2010, 5, 17–21. [Google Scholar] [CrossRef]
- Yessengarayev, Y.K.; Surimbayev, B.N.; Baimbetov, B.S.; Mamyachenkov, S.V.; Kanaly, T.S. Ore treatment hydrogen peroxide during heap leaching of gold. Kompleks. Ispol’zovanie Miner. Syr’a Complex Use Miner. Resour. Miner. Shikisattardy Keshendi Paid. 2021, 316, 5–14. [Google Scholar] [CrossRef]
- Koizhanova, A.; Osipovskaya, L.; Erdenova, M. Study of precious metals extraction recovery from technogenic wastes. In Proceedings of the 12th International Multidisciplinary Scientific GeoConference and EXPO—Modern Management of Mine Producing, Geology and Environmental Protection, SGEM, Albena, Bulgaria, 17–23 June 2012; Volume 1, pp. 843–846. [Google Scholar]
- Volodin, V.N.; Trebukhov, S.A.; Kenzhaliyev, B.K.; Nitsenko, A.V.; Burabaeva, N.M. Melt–Vapor Phase Diagram of the Te–S System. Russ. J. Phys. Chem. 2018, 92, 407–410. [Google Scholar] [CrossRef]
- Petrovskaya, N.V. Native Gold; Nauka: Moscow, Russia, 1973; p. 347. Available online: https://scholar.google.com/scholar_lookup?title=Native+Gold&author=Petrovskaya,+N.V.&publication_year=1973 (accessed on 22 October 2022). (In Russian)
- He, B.; Tian, X.; Sun, Y.; Yang, C.; Zeng, Y.; Wang, Y.; Zhan, S.; Pi, Z. Recovery of iron oxide concentrate from high-sulfur and low-grade pyrite cinder using an innovative beneficiating process. Hydrometallurgy 2010, 104, 241–246. [Google Scholar] [CrossRef]
- Zhuchkov, I.A.; Bubeyev, P.P. Comparative assessment of methods intended for silver extraction from thiosulfate solutions. Tsvetnaya Metall. 1990, 3, 62–66. [Google Scholar]
- Breuer, P.L.; Jeffrey, M.I. Thiosulfate leaching kinetics of gold in the presence of copper and ammonia. Miner. Eng. 2000, 13, 1071–1081. [Google Scholar] [CrossRef]
- Cao, C.; Hu, L.; Gong, Q. Leaching gold by low concentration thiosulfate solution. Trans. Non-ferrous Metals Soc. China 1992, 2, 1113–1117. [Google Scholar]
- Perez, A.; Galaviz, H. Method for Recovery of Precious Metals from Difficult Ores with Copper-Ammonium Thiosulfate. U.S. Patent No. 4654078, 31 March 1987. [Google Scholar]
- Dementiev, V.E.; Byvaltsev, V.L.; Mullov, V.M. Method of Extraction of Noble Metals from a Solution. Patent USSR No. 1356480, 30 September 1986. [Google Scholar]
- Zhuchkov, I.A.; Bubeev, P.P. Study of the kinetics of gold dissolution in acidic thiosulfate solutions. Non-ferrous metallurgy. Univ. News 1992, 4, 47–48. [Google Scholar]
- Smolyakov, A.R. Release of minerals during ore grinding. Gornyi Zhurnal. 2007, 8, 224–228. [Google Scholar]
- Begalinov, A.B.; Yakovlev, A.P.; Akhmedzhanov, T.K.; Medeuov, C.K. Thiosulfate leaching of gold. In Theory and Practice; Almaty, Republican State Enterprise on the Right of Economic Management «National Center for Biotechnology» Study of the Ministry of Education and Science of the Republic of Kazakhstan; Ministry of Education and Science of the Republic of Kazakhstan: Nur-Sultan City, Kazakhstan, 2001; 254p. [Google Scholar]
- Begalinov, A.B.; Shautenov, M.R.; Almenov, T.M.; Bektur, B.K.; Iskakov, E.E. Cyanide-Free Technology for Processing Gold-Bearing Mineral Raw Materials; KazNITU Publishing House: Almaty, Kazakhstan, 2020; 420p. [Google Scholar]
- Voiloshnikov, G.I. Comparison of the effectiveness of oxidizing agents in the extraction of gold from gravity concentrates. Zolotodobycha 2012, 164, 14–19. [Google Scholar]

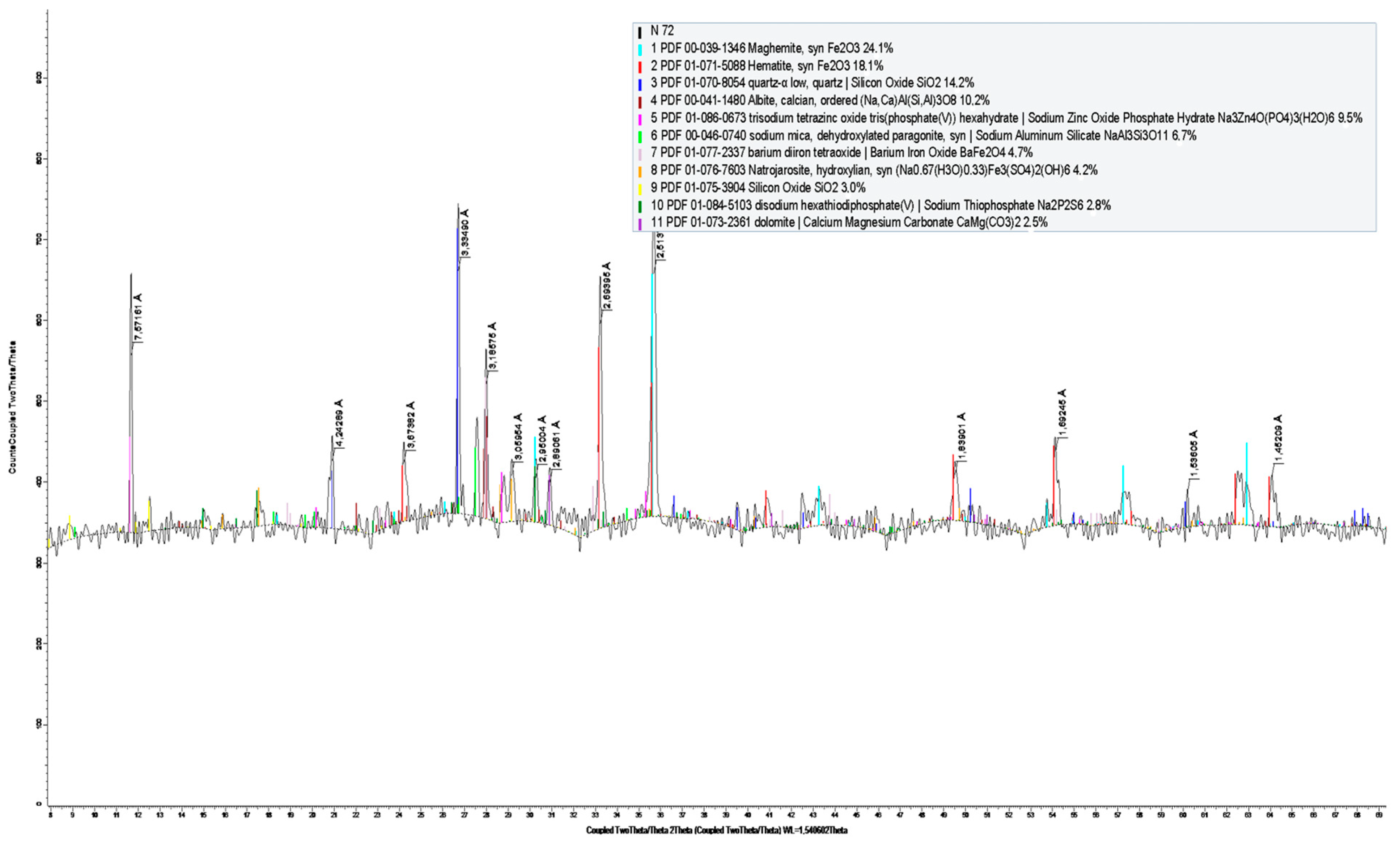
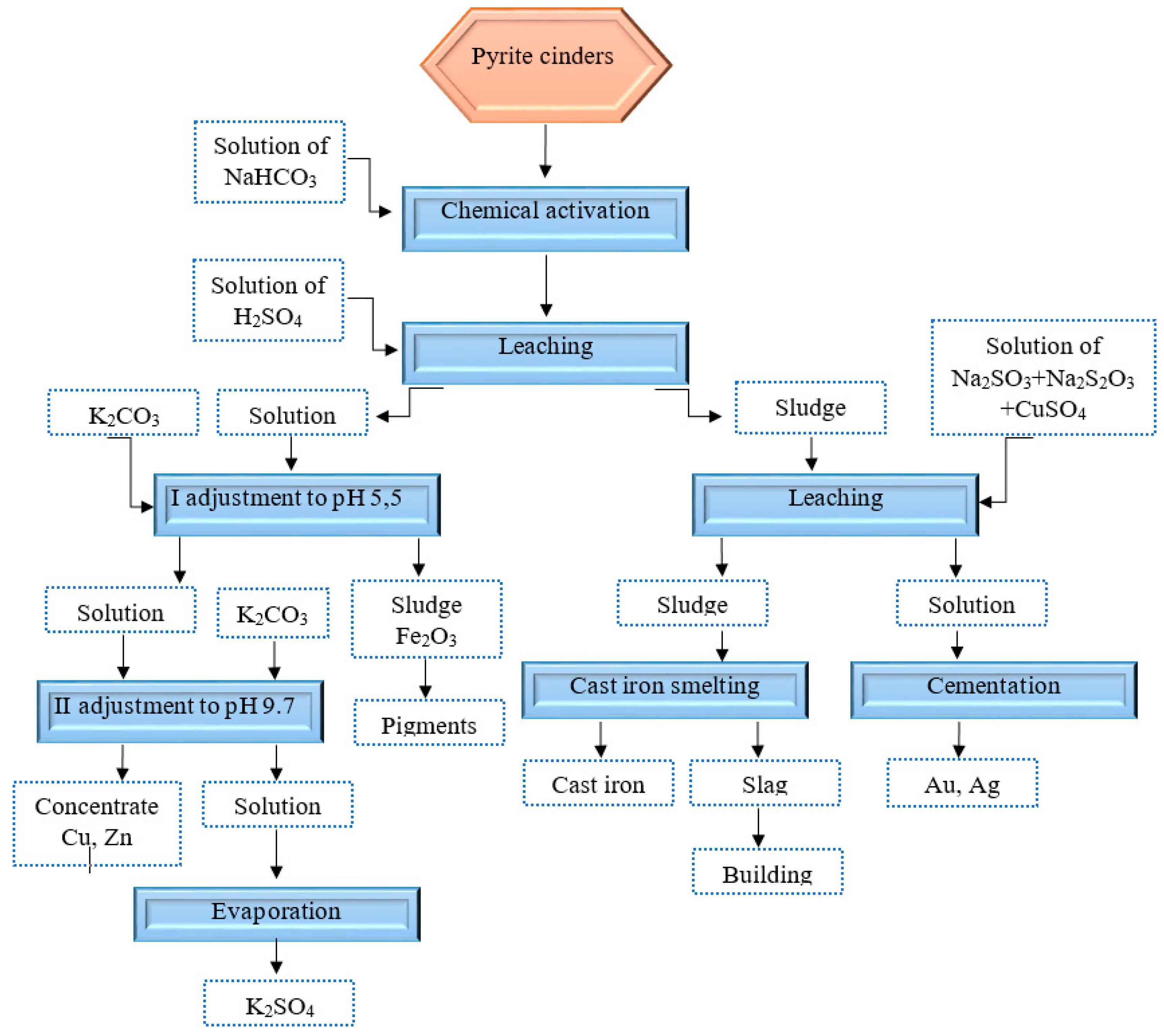

| Name | Formula | % |
|---|---|---|
| Magemite | Fe2O3 | 25.1 |
| Hematite | Fe2O3 | 19.1 |
| Quartz | SiO2 | 18.0 |
| Albite | Na(AlSi3O8) | 10.2 |
| Trisodium phosphate zinc oxide hydrate | Na3Zn4O(PO4)3(H2O)6 | 9.5 |
| Sodium aluminum silicate | NaAl3Si3O11 | 6.7 |
| Barium ferrite | BaFe2O4 | 4.7 |
| Natrozharozit | (Na0.67(H3O)0.33)Fe3(SO4)2(OH)6 | 4.2 |
| Dolomite | CaMg(CO3)2 | 2.5 |
| Name | Formula | % |
|---|---|---|
| Magemite | Fe2O3 | 30.7 |
| Hematite | Fe2O3 | 21.7 |
| Quartz | SiO2 | 13.3 |
| Albite | Na(AlSi3O8) | 5.8 |
| Sodium aluminum silicate | NaAl3Si3O11 | 7.8 |
| Barium ferrite | BaFe2O4 | 7.0 |
| Natrozharozit | (Na0.67(H3O)0.33)Fe3(SO4)2(OH)6 | 4.8 |
| Sodium thiophosphate | Na2P2S6 | 3.5 |
| Magnesium aluminum silicate | (MgAl2Si3O10)0.6 | 2.4 |
| Calcium silicate | CaSiO3 | 1.8 |
| Minerals | The Ratio of Minerals in the Residue, % |
|---|---|
| Pyrite | 9.2 |
| Gold | 0.3 |
| Chalcopyrite | 0.1 |
| Silver | 9.4 |
| Hematite | 30.6 |
| Cerussite | 1.3 |
| Magnetite | 5.8 |
| Goethite–hydrogoethite | 43.3 |
| Forms of Gold | % |
|---|---|
| Gold in the free state | 58.33 |
| Gold in association with hematite | 25.0 |
| Gold in association with goethite–hydrogoethite | 16.67 |
| «Rusty gold» | 33.33 |
| № | Component Concentrations, g/dm3 | S:L | Temperature, °C | Time, h | Extraction, % | |
|---|---|---|---|---|---|---|
| Au | Ag | |||||
| 1 | c (Na2SO3)—40; c (NH4OH)—15; c (CuSO4)—8. | 1:5 | 20 | 4 | 20 | 7.9 |
| 2 | c (Na2SO3) = 80; c (NH4OH) = 15; c (CuSO4) = 8 | 1:5 | 20 | 4 | 18 | 7.9 |
| 3 | c (Na2SO3) = 40; c (NH4OH) = 15; c (CuSO4) = 8 | 1:5 | 60 | 4 | 35 | - |
| 4 | c (Na2SO3) = 100; c (Na2S2O3) = 50; c (CuSO4) = 2.5 | 1:10 | 20 | 4 | 65.12 | 11.84 |
| 5 | c (Na2SO3) = 100; c (Na2S2O3) = 50; c (CuSO4) = 2.5 | 1:10 | 20 | 4 | 56.51 | 10.50 |
| № | Component Concentrations, g/dm3 | S:L | Temperature, °C | Time, h | Extraction, % | |
|---|---|---|---|---|---|---|
| Au | Ag | |||||
| 1 | c (Na2SO3) = 100; c (Na2S2O3) = 50; c (CuSO4) = 2.5 | 1:10 | 20 | 7 | 72 | 55 |
| 2 | c (Na2SO3) = 40; c (NH4OH) = 15; c (CuSO4) = 8 | 1:5 | 20 | 7 | 70 | 45 |
| 3 | c (Na2S2O3) = 80; c (NH4OH) = 15; c (CuSO4) = 8 | 1:5 | 20 | 7 | 62 | 48 |
| 4 | c (Na2S2O3) = 80; c (NH4OH) = 15; c CuSO4) = 8 | 1:5 | 60 | 7 | 67 | 42 |
| 5 | c (Na2SO3) = 100; c (Na2S2O3) = 50; c (CuSO4) = 2.5 | 1:10 | 60 | 7 | 68.4 | 90.8 |
| 6 | c(Na2SO3) = 100; c (Na2S2O3) = 50; c (CuSO4) = 2.5 | 1:10 | 20 | 7 | 67.1 | 98.5 |
| 7 | c (Na2SO3) = 100; c (Na2S2O3) = 50; c (CuSO4) = 2.5 | 1:10 | 60 | 7 | 87.2 | 75.1 |
| 8 | c (Na2SO3) = 100; c (Na2S2O3) = 50; c (CuSO4) = 2.5 | 1:10 | 20 | 7 | 67.3 | 87.2 |
| Leaching Conditions | S:L | Temperature, °C | Time, h | Extraction, % | |
|---|---|---|---|---|---|
| Au | Ag | ||||
| Stage I leaching Leaching with 15% sodium hypochlorite solution, PH = 9.5 | 3:1 | 20 °C | 4 | - | - |
| Stage II leaching c (Na2SO3) = 100 g/dm3; c (Na2S2O3) = 50 g/dm3; c(CuSO4) = 2.5 g/dm3; PH = 9.5 | 10:1 | 20 °C | 7 | 84.1 | 36.1 |
| Name | pH | S:L | Time, h | Concentration MnO2, in Solution, g/dm3. | E, % Au | E, % Ag |
|---|---|---|---|---|---|---|
| Leaching of pyrite cinders with MnO2 | 9.5 | 1:10 | 7 | 5 | 65.53 | 31.04 |
| Leaching of pyrite cinders with MnO2 | 9.7 | 1:10 | 7 | 10 | 90.1 | 59.5 |
| Leaching of pyrite cinders with MnO2 | 9.4 | 1:10 | 7 | 15 | 71.2 | 27.14 |
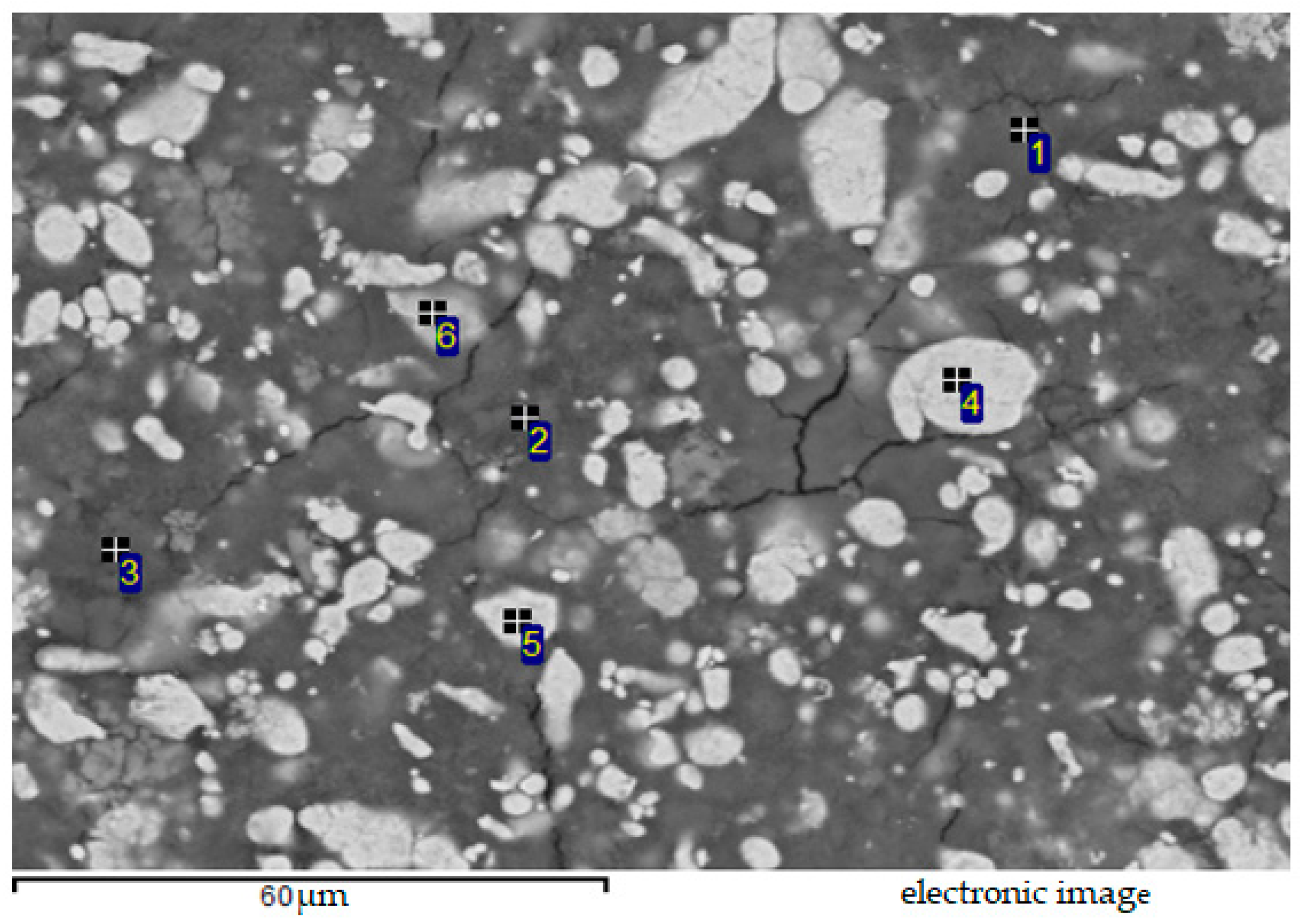
| Spectrum | O | Si | S | Fe | Cu | Zn | Ag | Au |
|---|---|---|---|---|---|---|---|---|
| 1 | 37.98 | 11.11 | 18.36 | 0.05 | 2.52 | 29.24 | 0.00 | 0.74 |
| 2 | 46.18 | 17.33 | 19.92 | 0.32 | 1.01 | 14.63 | 0.02 | 0.58 |
| 3 | 37.01 | 7.74 | 24.40 | 0.03 | 17.52 | 12.36 | 0.01 | 0.93 |
| 4 | 8.30 | 2.33 | 0.56 | 0.03 | 0.42 | 88.30 | 0.02 | 0.05 |
| 5 | 3.05 | 0.45 | 0.14 | 0.07 | 0.97 | 95.28 | 0.03 | 0.00 |
| 6 | 18.51 | 8.09 | 1.36 | 0.02 | 0.51 | 71.45 | 0.04 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenzhaliyev, B.; Surkova, T.; Yessimova, D.; Abikak, Y.; Mukhanova, A.; Fischer, D. On the Question of the Complex Processing of Pyrite Cinders. Inorganics 2023, 11, 171. https://doi.org/10.3390/inorganics11040171
Kenzhaliyev B, Surkova T, Yessimova D, Abikak Y, Mukhanova A, Fischer D. On the Question of the Complex Processing of Pyrite Cinders. Inorganics. 2023; 11(4):171. https://doi.org/10.3390/inorganics11040171
Chicago/Turabian StyleKenzhaliyev, Bagdaulet, Tatiana Surkova, Dinara Yessimova, Yerkezhan Abikak, Ainur Mukhanova, and Dametken Fischer. 2023. "On the Question of the Complex Processing of Pyrite Cinders" Inorganics 11, no. 4: 171. https://doi.org/10.3390/inorganics11040171
APA StyleKenzhaliyev, B., Surkova, T., Yessimova, D., Abikak, Y., Mukhanova, A., & Fischer, D. (2023). On the Question of the Complex Processing of Pyrite Cinders. Inorganics, 11(4), 171. https://doi.org/10.3390/inorganics11040171







