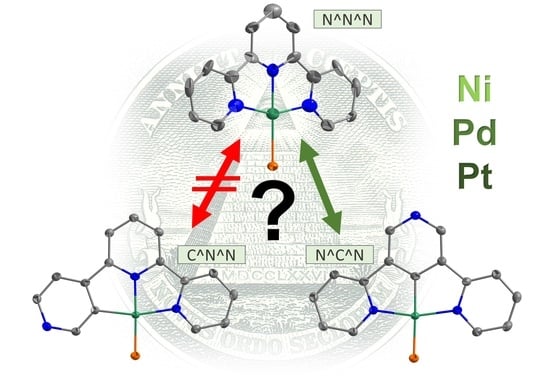
C–H Metalation of Terpyridine Stereoisomers with Ni(II), Pd(II), and Pt(II)
Abstract
:1. Introduction
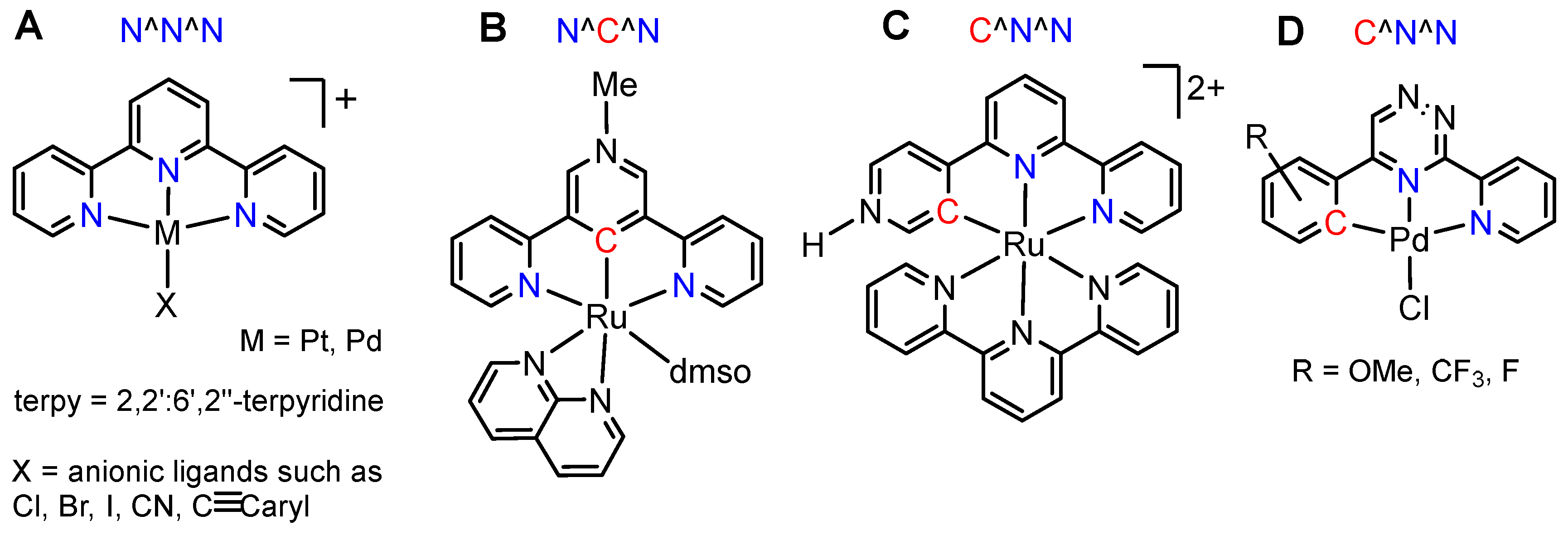
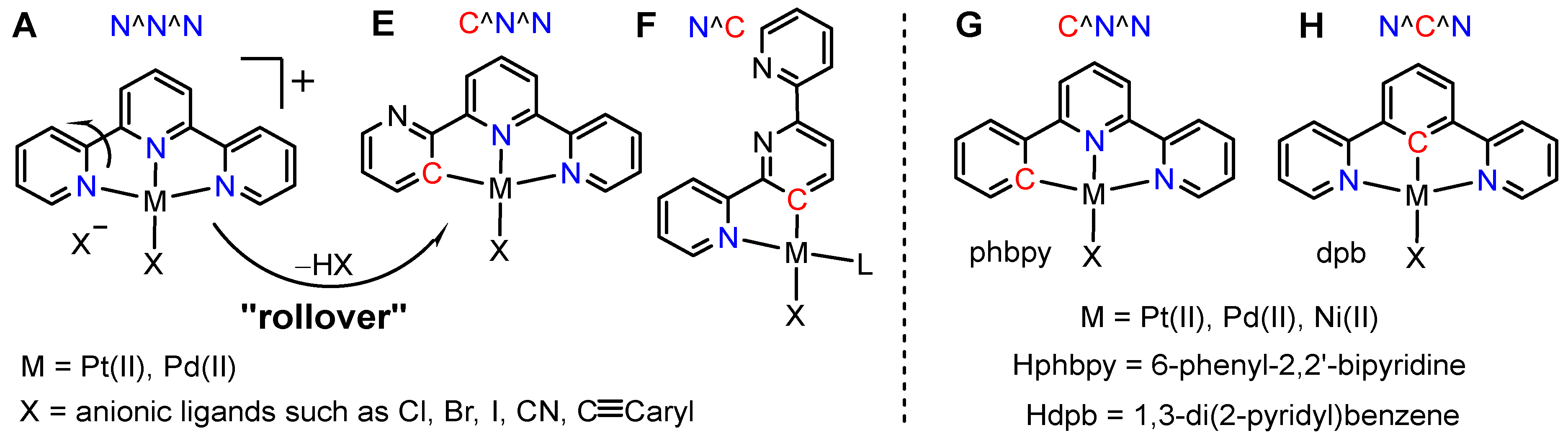
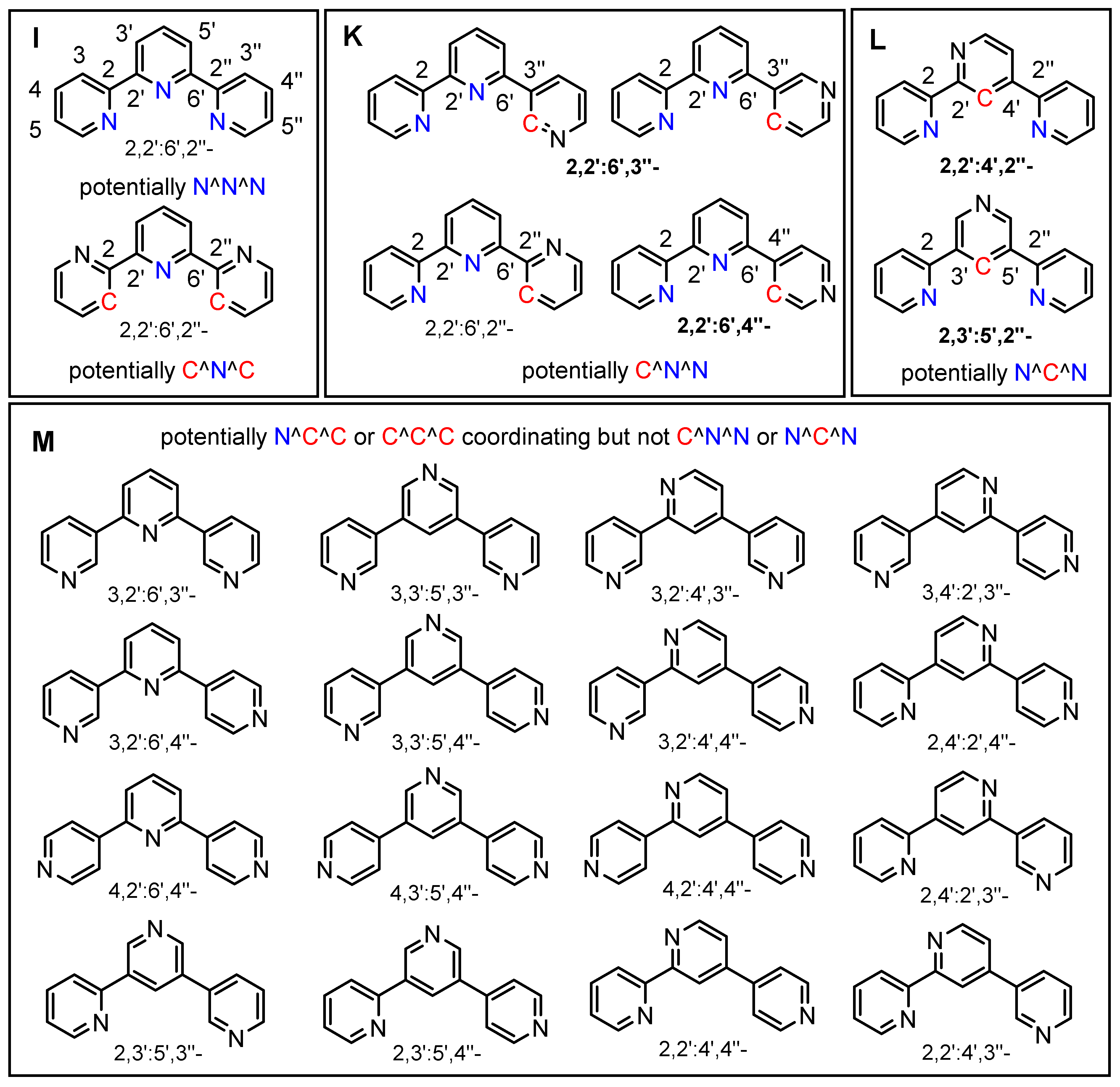
2. Results and Discussion
2.1. Preparation and Analytical Characterisation
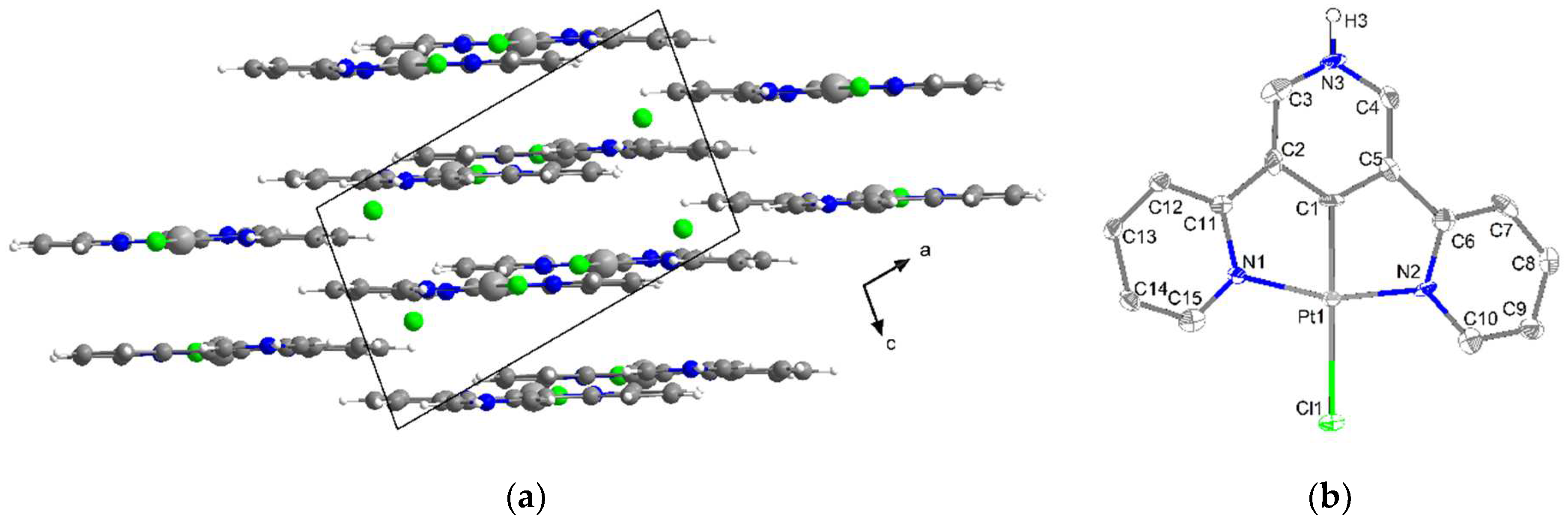
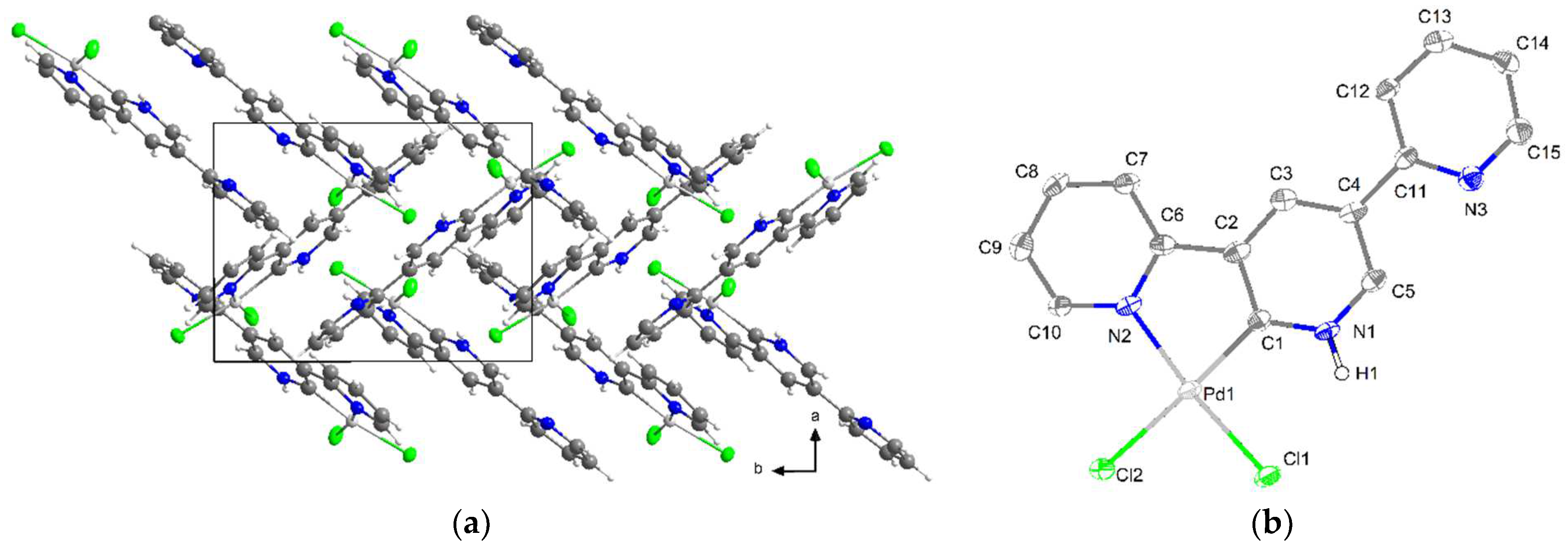
2.2. Crystal Structures
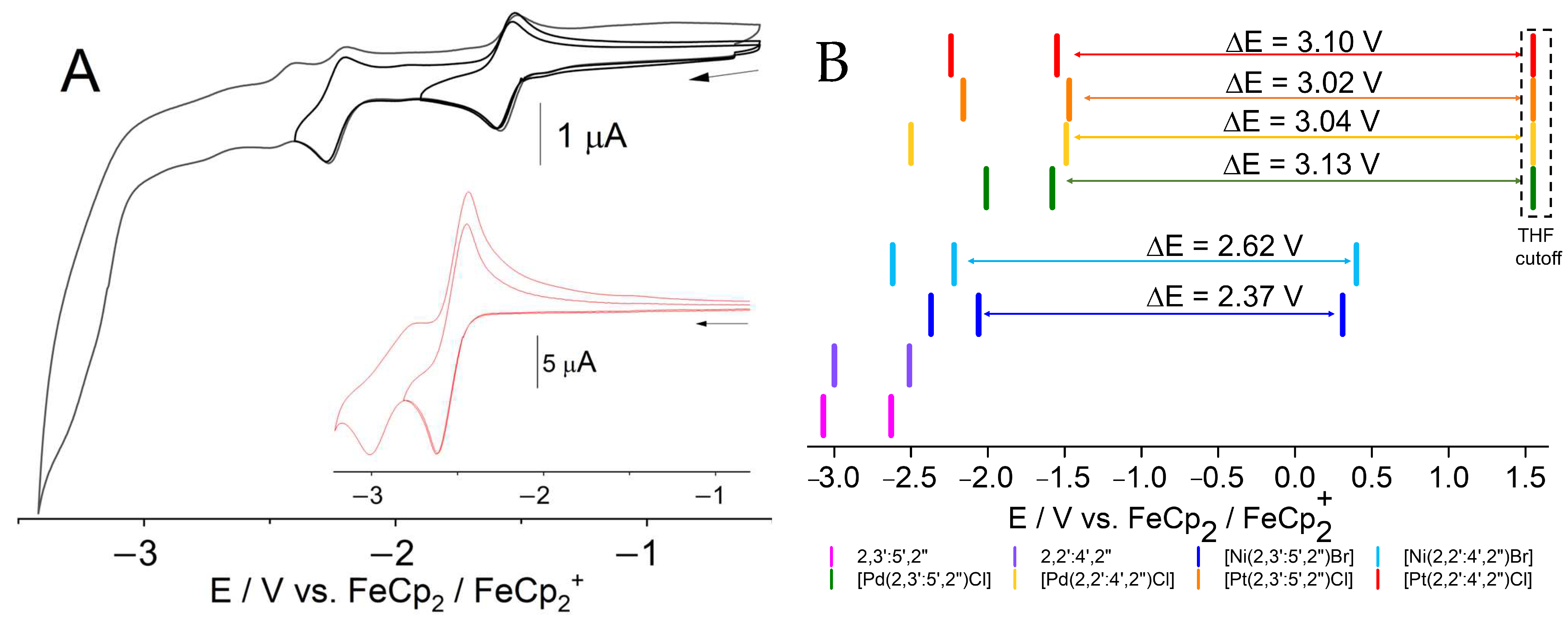
2.3. Electrochemistry
2.4. UV-Vis Absorption Spectroscopy
3. Materials and Methods
3.1. Instrumentation
3.2. Single Crystal Structure Analysis by X-ray Diffractometry (XRD)
3.3. Materials
3.4. Syntheses
3.4.1. Syntheses of Ligand Precursors and Terpy Ligands
3.4.2. Synthesis of the Ni(II) Complexes [Ni(2,3′:5′,2″-terpy)Br] and [Ni(2,2′:4′,2″-terpy)Br]—General Description
3.4.3. Synthesis of the Pd(II) Complexes [Pd(2,3′:5′,2″-terpy)Cl] and [Pd(2,2′:4′,2″-terpy)Cl]—General Description
3.4.4. Synthesis of the Pt(II) Complexes [Pt(2,3′:5′,2″-terpy)Cl] and [Pt(2,2′:4′,2″-terpy)Cl]—General Description
3.4.5. Synthesis of the Pt(II) Complexes [Pt(2,3′:5′,2″-terpy)(C≡CPh)] and [Pt(2,2′:4′,2″-terpy)(C≡CPh)]—General Description
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogge, T.; Kaplaneris, N.; Chatani, N.; Kim, J.; Chang, S.; Punji, B.; Schafer, L.L.; Musaev, D.G.; Wencel-Delord, J.; Roberts, C.A.; et al. C–H activation. Nat. Rev. Methods Primers 2021, 1, 43. [Google Scholar] [CrossRef]
- Corio, A.; Gravier-Pelletier, C.; Busca, P. Regioselective Functionalization of Quinolines through C-H Activation: A Comprehensive Review. Molecules 2021, 26, 5467. [Google Scholar] [CrossRef] [PubMed]
- Rej, S.; Das, A.; Chatani, N. Strategic evolution in transition metal-catalyzed directed C–H bond activation and future directions. Coord. Chem. Rev. 2021, 431, 213683. [Google Scholar] [CrossRef]
- Zucca, A.; Pilo, M.I. Rollover Cyclometalation as a Valuable Tool for Regioselective C–H Bond Activation and Functionalization. Molecules 2021, 26, 328. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, O. Multiple Catalytic C–H Bond Functionalization for Natural Product Synthesis. Angew. Chem. Int. Ed. 2020, 59, 17798–17809. [Google Scholar] [CrossRef]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef]
- Zakis, J.M.; Smejkal, T.; Wencel-Delord, J. Cyclometallated complexes as catalysts for C–H activation and functionalization. Chem. Commun. 2022, 58, 483–490. [Google Scholar] [CrossRef]
- Winter, A.; Schubert, U.S. Metal-Terpyridine Complexes in Catalytic Application—A Spotlight on the Last Decade. ChemCatChem 2020, 12, 2890–2941. [Google Scholar] [CrossRef]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef]
- Budnikova, Y.H.; Vicic, D.A.; Klein, A. Exploring mechanisms in Ni terpyridine catalysed C–C cross coupling reactions—a review. Inorganics 2018, 6, 18. [Google Scholar] [CrossRef]
- Leist, M.; Kerner, C.; Taghizadeh Ghoochany, L.; Farsadpour, S.; Fizia, A.; Neu, J.P.; Schön, F.; Sun, Y.; Oelkers, B.; Lang, J.; et al. Roll-over cyclometalation: A versatile tool to enhance the catalytic activity of transition metal complexes. J. Organomet. Chem. 2018, 863, 30–43. [Google Scholar] [CrossRef]
- Winter, A.; Hager, M.D.; Newkome, G.R.; Schubert, U.S. The Marriage of Terpyridines and Inorganic Nanoparticles: Synthetic Aspects, Characterization Techniques, and Potential Applications. Adv. Mater. 2011, 23, 5728–5748. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The terpyridine isomer game: From chelate to coordination network building block. Chem. Commun. 2020, 56, 10786–10794. [Google Scholar] [CrossRef]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Adapting (4,4) Networks through Substituent Effects and Conformationally Flexible 3,2′:6′,3”-Terpyridines. Molecules 2021, 26, 6337. [Google Scholar] [CrossRef]
- Agosti, A.; Kuna, E.; Bergamini, G. Divergent Terpyridine-Based Coordination for the Construction of Photoactive Supramolecular Structures. Eur. J. Inorg. Chem. 2019, 577–584. [Google Scholar] [CrossRef]
- Gao, Z.; Han, Y.; Gao, Z.; Wang, F. Multicomponent Assembled Systems Based on Platinum(II) Terpyridine Complexes. Acc. Chem. Res. 2018, 51, 2719–2729. [Google Scholar] [CrossRef]
- Chen, Z.; Chan, M.H.-Y.; Yam, V.W.-W. Stimuli-Responsive Two-Dimensional Supramolecular Polymers Based on Trinuclear Platinum(II) Scaffolds: Reversible Modulation of Photoluminescence, Cavity Size, and Water Permeability. J. Am. Chem. Soc. 2020, 142, 16471–16478. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Chan, A.K.-W.; Hong, E.Y.-H. Charge- transfer processes in metal complexes enable luminescence and memory functions. Nat. Rev. Chem. 2020, 4, 528–541. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Barker, N.M.; Taylor, S.D.; Ferguson, E.; Krause, J.A.; Oliver, A.G.; Connick, W.B.; Zhang, P. Water’s Role in Polymorphic Platinum(II) Complexes. Inorg. Chem. 2021, 60, 14731–14743. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Law, A.S.-Y. Luminescent d8 metal complexes of platinum(II) and gold(III): From photophysics to photofunctional materials and probes. Coord. Chem. Rev. 2020, 414, 213298. [Google Scholar] [CrossRef]
- Petersen, A.R.; Taylor, R.A.; Vicente-Hernández, I.; Mallender, P.R.; Olley, H.; White, A.J.P.; Britovsek, G.J.P. Oxygen Insertion into Metal Carbon Bonds: Formation of Methylperoxo Pd(II) and Pt(II) Complexes via Photogenerated Dinuclear Intermediates. J. Am. Chem. Soc. 2014, 136, 14089–14099. [Google Scholar] [CrossRef] [PubMed]
- McGuire, R., Jr.; Clark McGuire, M.; McMillin, D.R. Platinum(II) polypyridines: A tale of two axes. Coord. Chem. Rev. 2010, 254, 2574–2583. [Google Scholar] [CrossRef]
- Sakamoto, R.; Katagiri, S.; Maeda, H.; Nishihara, H. Bis(terpyridine) metal complex wires: Excellent long-range electron transfer ability and controllable intrawire redox conduction on silicon electrode. Coord. Chem. Rev. 2013, 257, 1493–1506. [Google Scholar] [CrossRef]
- Hamacher, C.; Hurkes, N.; Kaiser, A.; Klein, A.; Schüren, A. On the Electrochemistry and Spectroscopy of Organometallic Terpyridine Nickel Complexes. Inorg. Chem. 2009, 48, 9947–9951. [Google Scholar] [CrossRef]
- Cummings, S.D. Platinum complexes of terpyridine: Interaction and reactivity with biomolecules. Coord. Chem. Rev. 2009, 253, 1495–1516. [Google Scholar] [CrossRef]
- Koizumi, T.-A.; Tomon, T.; Tanaka, K. Synthesis, Structures and Fluxional Behavior of Ruthenium(II) Complexes Bearing a Bidentate 1,8-Naphthyridine Ligand. Bull. Chem. Soc. Jpn. 2003, 76, 1969–1975. [Google Scholar] [CrossRef]
- Kershaw Cook, L.J.; Halcrow, M.A. Doping ruthenium complexes into a molecular spin-crossover material. Polyhedron 2015, 87, 91–97. [Google Scholar] [CrossRef]
- Zvirzdinaite, M.; Garbe, S.; Arefyeva, N.; Krause, M.; von der Stück, R.; Klein, A. Palladium(II) complexes of ambidentate and potentially cyclometalating 5-aryl-3-(2′-pyridyl)-1,2,4-triazine ligands. Eur. J. Inorg. Chem. 2017, 2011–2022. [Google Scholar] [CrossRef]
- Intille, G.M.; Pfluger, C.E.; Baker, W.A., Jr. Crystal and molecular structure of chloro(2,2′,2”-terpyridine)palladium(II)chloride dihydrate, C15H15Cl2N3O2Pd. J. Cryst. Mol. Struct. 1973, 3, 47–54. [Google Scholar] [CrossRef]
- Maidich, L.; Pilo, M.I.; Rourke, J.P.; Clarkson, G.J.; Canu, P.; Stoccoro, S.; Zucca, A. Classical vs. Non-Classical Cyclometalated Pt(II) Complexes. Molecules 2022, 27, 7249. [Google Scholar] [CrossRef]
- Nabavizadeh, S.M.; Molaee, H.; Haddadi, E.; Niroomand Hosseini, F.; Hoseini, S.J.; Abu-Omar, M.M. Tetranuclear rollover cyclometalated organoplatinum–rhenium compounds; C–I oxidative addition and C–C reductive elimination using a rollover cycloplatinated dimer. Dalton Trans. 2021, 50, 15015–15026. [Google Scholar] [CrossRef]
- Becker, Y.; Schön, F.; Becker, S.; Sun, Y.; Thiel, W.R. Structure-dependent regioselectivity of a roll-over cyclopalladation occuring at 2,2′-bipyridine-type ligands. J. Organomet. Chem. 2021, 940, 121780. [Google Scholar] [CrossRef]
- Tovar-Ramírez, M.E.; Ramírez-Zúníga, R.; Nicasio-Collazo, J.; Wrobel, K.; Alvarado-Rodríguez, J.G.; Wrobel, K.; Serrano, O. Rollover Cyclopalladation via Remote C-H Bond Activation of Br-Pyridinbenzothiazole: An Experimental Study. Chem. Select 2018, 3, 4133–4139. [Google Scholar] [CrossRef]
- Haque, A.; Xu, L.; Al-Balushi, R.A.; Al-Suti, M.K.; Ilmi, R.; Guo, Z.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Cyclometallated tridentate platinum(II) arylacetylide complexes: Old wine in new bottles. Chem. Soc. Rev. 2019, 48, 5547–5563. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-Covalent Intramolecular Interactions through Ligand-Design Promoting Efficient Luminescence from Transition Metal Complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Cebrián, C.; Mauro, M. Recent advances in phosphorescent platinum complexes for organic light-emitting diodes. Beilstein J. Org. Chem. 2018, 14, 1459–1481. [Google Scholar] [CrossRef]
- Williams, J.A.G. The coordination chemistry of dipyridylbenzene: N-deficient terpyridine or panacea for brightly luminescent metal complexes? Chem. Soc. Rev. 2009, 38, 1783–1801. [Google Scholar] [CrossRef]
- Krause, M.; von der Stück, R.; Brünkink, D.; Buss, S.; Doltsinis, N.L.; Strassert, C.A.; Klein, A. Platinum and palladium complexes of tridentate −C^N^N (phen-ide)-pyridine-thiazol ligands—A case study involving spectroelectrochemistry, photoluminescence spectroscopy and TD-DFT calculations. Inorg. Chim. Acta 2021, 518, 120093. [Google Scholar] [CrossRef]
- Garoni, E.; Boixel, J.; Dorcet, V.; Roisnel, T.; Roberto, D.; Jacquemin, D.; Guerchais, V. Controlling the emission in flexibly-linked (N^C^N)platinum dyads. Dalton Trans. 2018, 47, 224–232. [Google Scholar] [CrossRef]
- Schulze, B.; Friebe, C.; Jäger, M.; Görls, H.; Birckner, E.; Winter, A.; Schubert, U.S. PtII Phosphors with Click-Derived 1,2,3-Triazole-Containing Tridentate Chelates. Organometallics 2018, 37, 145–155. [Google Scholar] [CrossRef]
- Li, K.; Tong, G.S.M.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Highly phosphorescent platinum(II) emitters: Photophysics, materials and biological application. Chem. Sci. 2016, 7, 1653–1673. [Google Scholar] [CrossRef] [PubMed]
- Kletsch, L.; Jordan, R.; Köcher, A.S.; Buss, S.; Strassert, C.A.; Klein, A. Photoluminescence of Ni(II), Pd(II), and Pt(II) Complexes [M(Me2dpb)Cl] Obtained from C–H Activation of 1,5-Di(2-pyridyl)-2,4-dimethylbenzene (Me2dpbH). Molecules 2021, 26, 5051. [Google Scholar] [CrossRef]
- Eskelinen, T.; Buss, S.; Petrovskii, S.K.; Grachova, E.V.; Krause, M.; Klein, A.; Strassert, C.A.; Koshevoy, I.O.; Hirva, P. Photophysics and Excited State Dynamics of Cyclometalated [M(C^N^N)(CN)] (M = Ni, Pd, Pt) Complexes: A Theoretical and Experimental Study. Inorg. Chem. 2021, 60, 8777–8789. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.-S.; Tang, M.-C.; Ng, M.; Yam, V.W.-W. Toward the Design of Phosphorescent Emitters of Cyclometalated Earth-Abundant Nickel(II) and Their Supramolecular Study. J. Am. Chem. Soc. 2020, 142, 7638–7646. [Google Scholar] [CrossRef]
- Cardenas, D.J.; Echavarren, A.M.; Ramirez de Arellano, M.C. Divergent Behavior of Palladium(II) and Platinum(II) in the Metalation of 1,3-Di(2-pyridyl)benzene. Organometallics 1999, 18, 3337–3341. [Google Scholar] [CrossRef]
- Ogata, K.; Sasano, D.; Yokoi, T.; Isozaki, K.; Yoshida, R.; Takenaka, T.; Seike, H.; Ogawa, T.; Kurata, H.; Yasuda, N.; et al. Synthesis and Self-Assembly of NCN-Pincer Pd-Complex-Bound Norvalines. Chem.–Eur. J. 2013, 19, 12356–12375. [Google Scholar] [CrossRef]
- Soro, B.; Stoccoro, S.; Minghetti, G.; Zucca, A.; Cinellu, M.A.; Manassero, M.; Gladiali, S. The first pincer-aryl [M–(NCN)] complexes {M = Pd(II); Pt(II)} with chiral pyridine donors: Synthesis and catalytic activity in C–C bond formation. Inorg. Chim. Acta 2006, 359, 1879–1888. [Google Scholar] [CrossRef]
- Soro, B.; Stoccoro, S.; Minghetti, G.; Zucca, A.; Cinellu, M.A.; Gladiali, S.; Manassero, M.; Sansoni, M. Synthesis of the First C-2 Cyclopalladated Derivatives of 1,3-Bis(2-pyridyl)benzene. Crystal Structures of [Hg(N-C-N)Cl], [Pd(N-C-N)Cl], and [Pd2(N-C-N)2(µ-OAc)]2[Hg2Cl6]. Catalytic Activity in the Heck Reaction. Organometallics 2005, 24, 53–61. [Google Scholar] [CrossRef]
- Kletsch, L.; Hörner, G.; Klein, A. Cyclometalated Ni(II) complexes [Ni(N^C^N)X] of the tridentate 2,6-di(2-pyridyl)-phen-ide ligand. Organometallics 2020, 39, 2820–2829. [Google Scholar] [CrossRef]
- Vogt, N.; Sivchik, V.; Sandleben, A.; Hörner, G.; Klein, A. Direct Base-Assisted C–H Cyclonickelation of 6-Phenyl-2,2′-bipyridine. Molecules 2020, 25, 997. [Google Scholar] [CrossRef]
- Vogt, N.; Sandleben, A.; Kletsch, L.; Schäfer, S.; Chin, M.T.; Vicic, D.A.; Hörner, G.; Klein, A. On the Role of the X Coligands in Cyclometalated [Ni(Phbpy)X] Complexes (HPhbpy = 6-phenyl-2,2′-bipyridine). Organometallics 2021, 40, 1776–1785. [Google Scholar] [CrossRef]
- Mousa, A.H.; Chakrabarti, K.; Isapour, G.; Bendix, J.; Wendt, O.F. Enhancing the Stability of Aromatic PCN Pincer Nickel Complexes by Incorporation of Pyridine as the Nitrogen Side Arm. Eur. J. Inorg. Chem. 2020, 4270–4277. [Google Scholar] [CrossRef]
- Manfroni, G.; Capomolla, S.S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Isomeric 4,2′:6′,4”- and 3,2′:6′,3”-Terpyridines with Isomeric 4′-Trifluoromethylphenyl Substituents: Effects on the Assembly of Coordination Polymers with [Cu(hfacac)2] (Hhfacac = Hexafluoropentane-2,4-dione). Inorganics 2021, 9, 54. [Google Scholar] [CrossRef]
- Rocco, D.; Novak, S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Manipulating the Conformation of 3,2′:6′,3″-Terpyridine in [Cu2(µ-OAc)4(3,2′:6′,3″-tpy)]n 1D-Polymers. Chemistry 2021, 3, 182–198. [Google Scholar] [CrossRef]
- Feuerstein, W.; Breher, F. Synthetic access to a phosphorescent non-palindromic pincer complex of palladium by a double oxidative addition—comproportionation sequence. Chem. Commun. 2020, 56, 12589–12592. [Google Scholar] [CrossRef]
- Okamura, T.-a.; Ishikawa, M.; Onitsuka, K. Unsymmetrical Metallosupramolecular Polycondensation of Expanded L-Amino Acid Containing Platinum(II) Complex in Nonpolar Solvents. ACS Appl. Polym. Mater. 2022, 4, 9472–9481. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Zhu, Y.; Zhang, C.; Cao, H.; Ma, W.; Tian, X.; Wu, J.; Zhou, H.; Tian, Y. Functional Platinum(II) Complexes with Four-Photon Absorption Activity, Lysosome Specificity, and Precise Cancer Therapy. Inorg. Chem. 2021, 60, 2362–2371. [Google Scholar] [CrossRef]
- Ranieri, A.M.; Vezzelli, M.; Leslie, K.G.; Huang, S.; Stagni, S.; Jacquemin, D.; Jiang, H.; Hubbard, A.; Rigamonti, L.; Watkin, E.L.J.; et al. Structure illumination microscopy imaging of lipid vesicles in live bacteria with naphthalimide appended organometallic complexes. Analyst 2021, 146, 3818–3822. [Google Scholar] [CrossRef]
- Garbe, S.; Krause, M.; Klimpel, A.; Neundorf, I.; Lippmann, P.; Ott, I.; Brünkink, D.; Strassert, C.A.; Doltsinis, N.L.; Klein, A. Cyclometalated Pt Complexes of CNC Pincer Ligands: Luminescence and Cytotoxic Evaluation. Organometallics 2020, 39, 746–756. [Google Scholar] [CrossRef]
- Kergreis, A.; Lord, R.M.; Pike, S.J. Influence of Ligand and Nuclearity on the Cytotoxicity of Cyclometallated C^N^C Platinum(II) Complexes. Chem.–Eur. J. 2020, 26, 14938–14946. [Google Scholar] [CrossRef] [PubMed]
- Hamidizadeh, P.; Nabavizadeh, S.M.; Hoseini, S.J. Effects of the number of cyclometalated rings and ancillary ligands on the rate of MeI oxidative addition to platinum(II)–pincer complexes. Dalton Trans. 2019, 48, 3422–3432. [Google Scholar] [CrossRef]
- Campillo, D.; Escudero, D.; Baya, M.; Martín, A. Heteropolymetallic Architectures as Snapshots of Transmetallation Processes at Different Degrees of Transfer. Chem.–Eur. J. 2022, 28, e202104538. [Google Scholar] [CrossRef] [PubMed]
- Niazi, M.; Klein, A. DFT Investigation of the Molecular Properties of the Dimethylglyoximato Complexes [M(Hdmg)2] (M = Ni, Pd, Pt). Inorganics 2021, 9, 47. [Google Scholar] [CrossRef]
- Haseloer, A.; Jordan, R.; Denkler, L.M.; Reimer, M.; Olthof, S.; Schmidt, I.; Meerholz, K.; Hörner, G.; Klein, A. Ni, Pd, and Pt complexes of a tetradentate dianionic thiosemicarbazone-based O^N^N^S ligand. Dalton Trans. 2021, 50, 4311–4322. [Google Scholar] [CrossRef]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1976, 1–24. [Google Scholar] [CrossRef]
- Lai, S.-W.; Cheung, T.-C.; Chan, M.C.W.; Cheung, K.-K.; Peng, S.-M.; Che, C.-M. Luminescent Mononuclear and Binuclear Cyclometalated Palladium(II) Complexes of 6-Phenyl-2,2‘-bipyridines: Spectroscopic and Structural Comparisons with Platinum(II) Analogues. Inorg. Chem. 2000, 39, 255–262. [Google Scholar] [CrossRef]
- Janzen, D.E.; Mann, K.R. Red and Orange Polymorphs of [Pt(terpy)Cl]Cl·2H2O. J. Chem. Crystallogr. 2013, 43, 292–298. [Google Scholar] [CrossRef]
- Deka, R.; Sarkar, A.; Butcher, R.J.; Junk, P.C.; Turner, D.R.; Deacon, G.B.; Singh, H.B. Isolation of Homoleptic Dicationic Tellurium and Monocationic Bismuth Analogues of Non-N-Heterocyclic Carbene Derivatives. Organometallics 2020, 39, 334–343. [Google Scholar] [CrossRef]
- Bercaw, J.E.; Durrell, A.C.; Gray, H.B.; Green, J.C.; Hazari, N.; Labinger, J.A.; Winkler, J.R. Electronic Structures of PdII Dimers. Inorg. Chem. 2010, 49, 1801–1810. [Google Scholar] [CrossRef]
- Niedzielska, D.; Pawlak, T.; Wojtczak, A.; Pazderski, L.; Szlyk, E. Structural and 1H, 13C, 15N NMR spectroscopic studies of Pd(II) chloride organometallics with 2-phenylpyridine and ammonia, pyridine or its methyl derivatives. Polyhedron 2015, 92, 41–51. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Chang, X.; Han, J.-T.K.; Lim, M.S.; Lee, S.W. Cyclometallated Pd(II) azido complexes containing 6-phenyl-2,2′-bipyridyl or 2-phenylpyridyl derivatives: Synthesis and reactivity toward organic isocyanides and isothiocyanates. Dalton Trans. 2004, 21, 3699–3708. [Google Scholar] [CrossRef]
- Williams, J.A.G.; Beeby, A.; Davies, E.S.; Weinstein, J.A.; Wilson, C. An Alternative Route to Highly Luminescent Platinum(II) Complexes: Cyclometalation with NˆCˆN-Coordinating Dipyridylbenzene Ligands. Inorg. Chem. 2003, 42, 8609–8611. [Google Scholar] [CrossRef]
- Poirier, S.; Lynn, H.; Reber, C.; Tailleur, E.; Marchivie, M.; Guionneau, P.; Probert, M.R. Variation of M···H−C Interactions in Square-Planar Complexes of Nickel(II), Palladium(II), and Platinum(II) Probed by Luminescence Spectroscopy and X-ray Diffraction at Variable Pressure. Inorg. Chem. 2018, 57, 7713–7723. [Google Scholar] [CrossRef]
- Wang, Z.; Turner, E.; Mahoney, V.; Madakuni, S.; Groy, T.; Li, J. Facile Synthesis and Characterization of Phosphorescent Pt(N∧C∧N)X Complexes. Inorg. Chem. 2010, 49, 11276–11286. [Google Scholar] [CrossRef]
- Chen, Y.; Li, K.; Lu, W.; Chui, S.S.-Y.; Ma, C.-W.; Che, C.-M. Photoresponsive Supramolecular Organometallic Nanosheets Induced by PtII⋅⋅⋅PtII and C–H⋅⋅⋅π Interactions. Angew. Chem. Int. Ed. 2009, 48, 9909–9913. [Google Scholar] [CrossRef]
- APEX3—Software Suite for Crystallographic Programs, Bruker AXS, Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
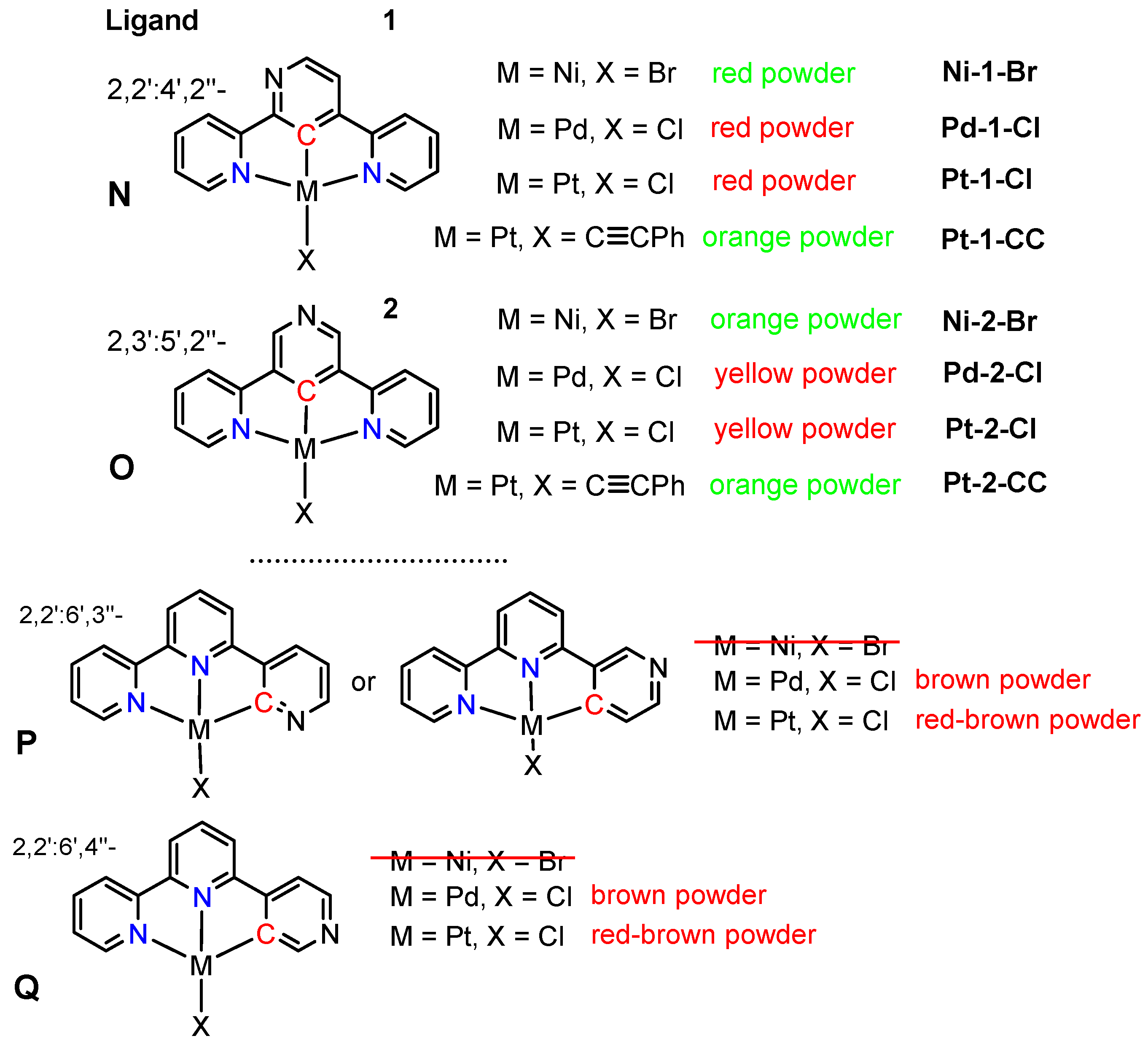



| Terpy Ligand | Precursor | Conditions | Product |
|---|---|---|---|
| 2,2′:6′,3″ | NiBr2 | KOAc/K2CO3, p-xylene, reflux, 67 h | no conversion, ligand re-isolated |
| 2,2′:6′,3″ | [Ni(DME)Br2] | NEt3, THF, reflux, 16 h | no conversion, ligand re-isolated |
| 2,2′:6′,4″ | NiBr2 | KOAc/K2CO3, p-xylene, reflux, 69 h | red–brown powder c |
| 2,2′:6′,3″ | K2PdCl4 | MeCN/H2O, reflux, 18 h | brown powder (12%) a |
| 2,2′:6′,4″ | K2PdCl4 | MeCN/H2O, reflux, 19 h | no conversion, ligand re-isolated |
| 2,2′:6′,4″ | K2PdCl4 | DMF, reflux, 65 h | brown powder (7%) a,b |
| 2,2′:6′,3″ | K2PtCl4 | Glacial HOAc, reflux, 19 h | red–brown powder (24%) a |
| 2,2′:6′,4″ | K2PtCl4 | Glacial HOAc, reflux, 67 h | red–brown powder (33%) a |
| [Ni(2,3′:5′,2″-terpy)Br0.13/OAc0.87].H2O | [Ni(dpb)Br] b | [Ni(dpb)(OAc)] b | [Pt(2,3′:5′,2″-terpyH)Cl].Cl (Pt-H2-ClCl) | [Pt(terpy)Cl]Cl.H2O c | [Pt(dpb)Cl] d | |
|---|---|---|---|---|---|---|
| Distances/Å | ||||||
| M1–C1 | 1.805(2) | 1.8298(2) | 1.8136(7) | 1.896(8) | 1.941(4) (N) e | 1.907(8) |
| M1–N1 | 1.923(2) | 1.9489(1) | 1.9203(2) | 2.039(8) | 2.033(5) | 2.033(6) |
| M1–N2 | 1.923(2) | 1.9536(1) | 1.9232(2) | 2.025(8) | 2.035(4) | 2.041(6) |
| M1–O1 | 1.929(2) | - | 1.9794(2) | - | - | - |
| M1–O2 | 2.962(3) | - | 3.0331(2) | - | - | - |
| M1–X1 | 2.487(8) | 2.3963(3) | - | 2.382(2) | 2.303(2) | 2.417(2) |
| Angles/° | ||||||
| C7–M1–N1 | 82.37(9) | 81.94(7) | 82.59(8) | 81.2(4) | 81.32(2) (N) e | 80.1(3) |
| C7–M1–N2 | 82.24(9) | 81.85(7) | 82.48(8) | 80.9(4) | 80.97(2) (N) e | 80.9(3) |
| N1–M1–O2 | 99.28(8) | - | 98.31(7) | - | - | - |
| N2–M1–O2 | 96.05(8) | - | 96.61(7) | - | - | - |
| N1–M1–X1 | 93.49(2) | 97.83(4) | - | 99.1(2) | 98.63(2) | 99.1(2) |
| N2–M1–X1 | 101.08(2) | 98.39(4) | - | 98.8(2) | 99.09(2) | 99.8(2) |
| N1–M1–N2 | 164.43(8) | 163.78(6) | 165.00(7) | 162.1(3) | 162.29(2) | 161.1(2) |
| C7–M1–X1 | 160.03(2) | 179.48(5) | - | 179.6(3) | 179.9(2) (N) e | 179.0(2) |
| Sum/° f | 359.94(2) | 360.01 | 359.99 | 360.00 | 360.01 | 359.90 |
| [M(Y-terpy)X] | λ (ε) | ∆Eopt (eV) | ΔEredox (eV) | ΔΔ |
|---|---|---|---|---|
| M = Ni, X = Br | ||||
| Y = 2,3′:5′,2″ (Ni-1-Br) | 466 (1.7) | 2.48 | 2.37 | 0.11 |
| Y = 2,2′:4′,2″ (Ni-2-Br) | 431 (6.4) | 2.87 | 2.62 | 0.25 |
| [Ni(dpb)Br] b | 433 (5.6) | 2.86 | 2.37 | 0.49 |
| M = Pd, X = Cl | ||||
| Y =2,3′:5′,2″(Pd-1-Cl) | 389 (2.6) | 3.19 | 3.13 | 0.06 |
| Y = 2,2′:4′,2″(Pd-2-Cl) | 355 (6.6) | 3.49 | 3.04 | 0.45 |
| [Pd(Me2dpb)Cl] c | 375 (1.2) | 3.31 | 3.08 | 0.23 |
| M = Pt, X = Cl | ||||
| Y = 2,3′:5′,2″ (Pt-1-Cl) | 396 (0.8) | 3.13 | 3.02 | 0.11 |
| Y = 2,2′:4′,2″ (Pt-2-Cl) | 427 (0.4) | 2.90 | 3.10 | 0.20 |
| [Pt(dpb)Cl] d | 402 (7.0) | 3.08 | 2.49 | 0.59 |
| M = Pt, X = C≡CPh | ||||
| Y = 2,3′:5′,2″ (Pt-1-CC) | 395 (1.9) | 3.14 | ||
| Y = 2,2′:4′,2″ (Pt-2-CC) | 424 (4.6) | 2.92 | ||
| [Pt(dpb)(C≡CPh)] e | 391 (9.3) | 3.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payen, L.; Kletsch, L.; Lapić, T.; Wickleder, M.; Klein, A. C–H Metalation of Terpyridine Stereoisomers with Ni(II), Pd(II), and Pt(II). Inorganics 2023, 11, 174. https://doi.org/10.3390/inorganics11040174
Payen L, Kletsch L, Lapić T, Wickleder M, Klein A. C–H Metalation of Terpyridine Stereoisomers with Ni(II), Pd(II), and Pt(II). Inorganics. 2023; 11(4):174. https://doi.org/10.3390/inorganics11040174
Chicago/Turabian StylePayen, Leo, Lukas Kletsch, Tobias Lapić, Mathias Wickleder, and Axel Klein. 2023. "C–H Metalation of Terpyridine Stereoisomers with Ni(II), Pd(II), and Pt(II)" Inorganics 11, no. 4: 174. https://doi.org/10.3390/inorganics11040174
APA StylePayen, L., Kletsch, L., Lapić, T., Wickleder, M., & Klein, A. (2023). C–H Metalation of Terpyridine Stereoisomers with Ni(II), Pd(II), and Pt(II). Inorganics, 11(4), 174. https://doi.org/10.3390/inorganics11040174